Abstract
Diffuse WHO grade II (GIIG) may be unresectable when involving critical structures. To assess the feasibility and functional tolerance (cognition and quality of life) of an original therapeutic strategy combining neoadjuvant chemotherapy followed by surgical resection for initially inoperable GIIG. Ten patients underwent Temozolomide for unresectable GIIG, as initial treatment or at recurrence after previous partial resection, due to invasion of eloquent areas or bi-hemispheric diffusion preventing a total/subtotal removal. Functional outcome after both treatments was assessed, with evaluation of seven cognitive domains. Chemotherapy induced tumor shrinkage (median volume decrease 38.9%) in ipsilateral functional areas in six patients and in the contralateral hemisphere in four. Only four patients had a 1p19q codeletion. The tumor shrinkage made possible the resection (mean extent of resection 93.3%, 9 total or subtotal removals) of initially inoperable tumors. Postoperatively, three patients had no deficits, while verbal episodic memory and executive functions were slightly impaired in seven patients. However, global quality of life was roughly preserved on the EORTC QLQ C30 + BN 20 (median score: 66.7%). Role functioning score was relatively reduced (median score: 66.7%) whereas KPS was preserved (median score: 90, range 80–100). Seven patients became seizure-free while three improved. This combined treatment is feasible, efficient (surgery made possible by neoadjuvant chemotherapy) and well-tolerated (preservation of quality of life, no serious cognitive disturbances). Cognitive deficits seem mostly related to tumor location. Because KPS is not reliable enough, a detailed neuropsychological assessment should be systematically performed in GIIG.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diffuse World Health Organization (WHO) grade II gliomas (GIIG) are slow growing tumors which migrate within the brain, with an ineluctable risk of anaplastic transformation. In the recent literature, the extent of resection has been demonstrated to have a significant impact on the natural history of the disease, by delaying malignant transformation and increasing overall survival [1–4]. Thus, in the guidelines of the European associations of neuro-oncology and neurology, surgical resection is now the first therapeutic option [5]. Nevertheless, diffusion of GIIG, especially along the white matter pathways, may limit their surgical removal. As a consequence, in cases with an extensive involvement of eloquent areas or contra-lateral hemisphere, non-surgical treatments could represent a valuable therapeutic alternative. However, a phase III trial showed that early radiotherapy in GIIG has no impact on overall survival [6] while it may induce a worsening of cognitive functions and quality of life (QoL) in long-term survivors [7].
On the other hand, a growing number of authors recently reported the likely role of chemotherapy on GIIG [8]. Nonetheless, its optimal timing remains to be determined. The first chemotherapy regimen used in GIIG was PCV [9–11]. Despite the effectiveness, it was associated with significant hematological, neurological and general toxicity. Preliminary results with Temozolomide (TMZ), a more recent oral alkylating agent, have supported its efficacy and a good tolerance [12–15], but few studies [9, 12, 16] only have performed an extensive assessment of cognition and QoL after chemotherapy.
More recently, some authors have suggested that chemotherapy might play a major role in the management of GIIG in combination with surgical treatment. They reported the first case in which an inoperable GIIG invading the contralateral hemisphere through the corpus callosum was treated with neoadjuvant Temozolomide and shrank significantly. Then, a complete surgical resection was possible in a second stage, with preservation of QoL [17]. The feasibility of such new strategy, combining neoadjuvant chemotherapy followed by surgical resection, has nevertheless to be fully evaluated. In particular, beyond its oncological impact, the assessment of QoL and cognitive status following this original management is essential, due to the young age and long-term survival of the majority of patients bearing GIIG.
The aim of the present study is, for the first time to our best knowledge, to assess the feasibility and tolerance of this novel approach in a series of 10 patients with GIIG (two with microfoci of anaplastic transformation), using a systematic extensive neuropsychological assessment (NPA).
Materials and methods
Patient population
This series consisted of 10 patients fulfilling the following inclusion criteria: age >18 years; an inoperable diffuse cortico-subcortical supra-tentorial glioma due to involvement of eloquent regions or due to bilateral diffusion (newly diagnosed or at recurrence following partial resection)—of note, “unresectable tumor” means that the chance to perform at least 80% of glioma removal (demonstrated as a statistical threshold significantly correlated with the impact on the natural history of the tumor [4]) was very low, according to the preoperative estimation made on the probabilistic map of residual volume that we previously validated [18, 19]; histological diagnosis of WHO grade II astrocytoma, oligodendroglioma or oligoastrocytoma, without or with microfoci of anaplastic transformation (following biopsy or resection); combined therapeutic approach, consisting of neoadjuvant chemotherapy inducing tumor shrinking and making a priori possible a subsequent total or subtotal surgical resection. Any subject that received previous radiation therapy was excluded from the study. All participants provided proof of their agreement to participate.
A retrospective examination of medical files from a computerized database shared by two French neurooncological centers (Montpellier and Nancy), allowed the retrieval of the following clinical characteristics: gender, age at diagnosis and at the NPA, Karnofsky performance scores before and after the combined treatment, intervals of time between the end of chemotherapy and the surgical procedure as well as between the operation and the NPA, location and volume of the glioma, use of antiepileptic drugs and length of follow-up.
Chemotherapy
Patients in this series were treated with TMZ administered orally from days 1 through 5 at a starting dose of 200 mg/m2/day. Treatment cycles were repeated every 28 days. If no disease progression and no unacceptable toxicity were observed, the patients continued to receive TMZ. The exact doses, timing, number of cycles, toxicity, impact on seizures and on professional life were recorded. Toxicity and adverse effects were graded according to the national cancer institute—common toxicity criteria (NCI-CTC v4.0).
Neuroimaging
The location and volume of the tumor were assessed on 1.5 T magnetic resonance imaging (MRI) examinations consisting of at least an enhanced T1-weighted and a non-enhanced (T2-weighted or fluid-attenuated inversion recovery—FLAIR) acquisitions in three different orthogonal planes. They were performed at five different times during the follow-up: at diagnosis, before and after chemotherapy, and before and after tumor resection (within 6 h, at 3 months and then every 6 months after surgery). The impact of chemotherapy on the tumor volume was estimated using Volume Viewer® software, on a 4.4 Advantage Windows workstation (General Electrics GE Healthcare, Milwaukee, WI, USA). The tumors were delineated on 5.5 mm axial images, and then the corresponding volumes were measured. FLAIR sequences were chosen because they appropriately show the zones of infiltration. For exams in which only printed images were accessible, a three diameters technique was used. The volume variation induced by chemotherapy was calculated using the following formula: Vpre−Vpost/Vpre, where Vpre and Vpost correspond to the pre- and the post-chemotherapy volumes, respectively. MRIs allowed to assess whether the glioma was too infiltrating initially to propose a primary surgical treatment; to evaluate the shrinking after chemotherapy; and, finally, to verify if surgical removal seemed possible after chemotherapy. The person doing the volumetric measurements for pre- and post-operative chemotherapy was blinded.
Surgical procedure
The patients underwent surgery using a method of intraoperative functional mapping previously described by the authors [20, 21]. A wide craniotomy was performed and the tumor margins were verified in relation to the brain surface anatomy using intraoperative ultrasound. Sterile tags labeled with letters marked the glioma boundaries. Prior to resection, the cortex was mapped for language and sensorimotor sites (or only motor sites for patients in whom a procedure under general anesthesia was performed) in order to avoid any damage to eloquent areas. A bipolar electrode (Nimbus; Newmedic, Labège, France) with 5 mm tip spacing was utilized for mapping to apply electrostimulation with a biphasic current intensity between 1.5 and 4 mA (60 Hz pulse frequency, 1 ms single pulse phase and approximately 4 s of tissue contact) while the patient performed the functional tasks. In a patient at rest, involuntary movements or paresthesis were induced when the primary motor or sensory areas were stimulated, respectively. Intraoperative language tasks consisted of counting and picture naming. For naming tasks, we used DO 80 tests [22, 23], which consists of eighty different black and white pictures selected according to patient’s familiarity, age and level of education. A speech therapist present in the operating room performed on-line analysis of the language responses. A cortical site of 5 × 5 mm was considered positive when any interference in sensorimotor or language functions was observed at three non-sequential stimulations followed by normalization [24]. The same site was never stimulated twice successively in order to avoid generating seizures. Sterile numbered tags marked the positive stimulation sites in order to obtain the cortical map. Then, the resection was started during which the subcortical structures, especially the white matter tracts, were systematically stimulated. The resection cavity was extended up to the functional boundaries, with no margin, so that maximal glioma resection was obtained while preserving essential cortical and subcortical eloquent structures [25, 26].
Neuropathology and molecular genetic methods
Besides the confirmation of the histological diagnosis and the WHO grade, the index of proliferation MIB1 was recorded for each case. Blood and tumor DNA samples were screened for the loss of heterozygosity on chromosomes 1p and 19q using the following polymorphic markers: D1S2660, D1S450, D1S507, D1S507, D1S234, D1S2890, D1S230, D1S207, D1S206 and D19S414, D19S420, D19S903, D19S571. All patients gave their written informed consent for this analysis.
Neuropsychological assessment
A set of neuropsychological examinations was carried out after completion of the combined treatment (neoadjuvant chemotherapy and surgical removal). It was aimed of evaluating the global efficiency, the premorbid intelligence, the laterality in handedness, and seven cognitive domains: information processing/psychomotor speed, attention, episodic memory (verbal and non verbal), working memory (verbal and non verbal), language, visuo-spatial abilities and executive functions. A complete set took from 120 to 150 min and involved eighteen tests, which are listed in Table 1. The assessment could be performed at the patient’s home or in the hospital. Scores on all test parameters were converted to z scores by comparison with the mean and standard deviation of a reference based on healthy control groups, who were matched individually for age, sex, and educational level. All tests have been standardized for the French population. A deficient score was defined as a value of at least two standard deviations (SDs) under the corresponding value for a healthy control group or inferior to the fifth percentile.
Assessment of QoL
At the end of neuropsychological evaluation, two questionnaires were delivered to the patients. The first one (EORTC QLQ C30) pertained to the quality of life and was associated to a Brain Module BN-20 [27]. The second one, the beck depression inventory (BDI), was aimed of detecting symptoms of depression [28].
EORTC QLQ C30 is a 30-items questionnaire comprising several domains: physical functioning, role functioning, cognitive functioning, emotional functioning, social functioning, fatigue, pain, nausea/vomiting and global quality of life scale. In addition, five questions assess symptoms that are commonly reported by oncological patients such as dyspnea, appetite loss, sleeping troubles, constipation and diarrhea. Finally, the same questionnaire asks the patient to declare the financial impact of his/her disease.
The QLQ-BN20 is a module comprising 20 items of different domains: future uncertainty, visual disorders, motor dysfunctions, and communication deficits. Also, seven isolated items assess the occurrence of headache, seizure, drowsiness, hair loss, itchy skin, lower limb weakness and bladder control.
In these two questionnaires, most items were scored using the 4-point Likert system, from “not at all” to “very much”. Only two questions concerning QoL (EORTC QLQ C30) used a 7-point visual analogue scale, ranging from “very poor” to “excellent”. For analysis purposes, all data were linearly transformed into a 0–100 range. It is worth noting that only in the scales of functional status and global QoL higher scores represented a better condition. Besides the application of these tools, the examiner could also appreciate the QoL of a given patient during the interview, by assessing the following additional criteria: social occupations, professional status, driving status and frequency of seizures.
The BDI includes a series of questions aiming to estimate the presence and the severity of depression. In this study, the long version was applied, which comprises 21 items. The maximal possible global score is 63. A subject was classified as presenting with minor depression if the score was between 4 and 7, with mild depression if it was between 8 and 15 and with severe depression if it was superior to 16.
Results
Patient population
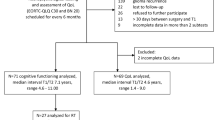
All evaluations were performed in the period from January 2010 to May 2010. A total of 9 men and 1 female were included. The median age was 42.3 years (range 21.9–51) at diagnosis and 48.1 years (range 26.2–55.7) at the NPA. The median follow-up from diagnosis was 61.3 months (31.3–89.6) and the median period of time between the operative procedure and the NPA was 17.9 months (range 3.1–70.1). The presenting symptoms were partial seizures in three patients, secondarily generalized seizures in six and an episode of status epilepticus in the remaining one. The anatomical location of the tumor was frontal in five cases with a large involvement of the central area (3 right, 2 left), fronto-temporo-insular in four cases (1 right, 3 left) and temporo-insular (left) in one. Seven patients had their first neuropathological diagnosis by means of stereotactic biopsy (Fig. 1), two were diagnosed following a previous craniotomy (partial resection) and one was already operated on twice in another institution (with partial resection at each time) for the same tumor before being included in the study.
After maximal surgical resection has been performed in the context of this combined therapeutic approach (i.e., following tumor shrink induced by chemotherapy), the results of neuropathological examination were as follow: WHO grade II oligodendroglioma in six cases, WHO grade II astrocytoma in two cases, and WHO grade II oligoastrocytoma in two cases. Of note, in the two more voluminous tumors (206.8 and 140 cc), two microfoci of anaplastic transformation were detected on the neuropathological examination, which analyzed 90.6 and 97.1% of the volumes, respectively.
The characteristics of the study population are detailed in Table 2.
Chemotherapy
In this series, chemotherapy has been prescribed in the context of tumor inoperability and independent of 1p19q status. The agent used was Temozolomide during the whole period in nine cases. In the remaining patient it had to be replaced by Fotemustine (100 mg/m2/day, every 4 weeks, intravenous) because of side effects (arthromyalgia) induced by Temozolomide. In seven patients, chemotherapy was the initial treatment (neoadjuvant) for an unresectable tumor while in three patients, it was administered at recurrence after a previous partial surgical resection. The median number of chemotherapy cycles was 18.5 (range 8–24).
The regimen was well tolerated. However, one patient required a 25% dose reduction because of a grade 3 thrombocytopenia (Table 3). Six others presented grade 1 nausea and nine complained on grade 1–2 fatigue. There was no case of rash, cutaneous toxicity or pneumocystosis. Three patients continued to work during chemotherapy while six did not work because they could not drive, two because of side effects and two given the impossibility of job adaptation. One patient had already quit his job before the diagnosis.
The severity and frequency of seizures were reduced during chemotherapy in nine cases, being in five patients seizure-free. After completion of the combined treatment (chemotherapy followed by tumor removal), a total of seven patients were free of seizures until the end of the follow-up (one could interrupt antiepileptic agents) and three presented with reduced frequency and severity.
At the occasion of the NPA, one out of the ten patients had re-started Temozolomide because of tumor re-growth.
Neuroimaging
The evaluation of tumor volumes from the diagnosis to the end of the follow-up period is detailed in Table 4. Eight out of the fifty volume estimations were performed using manual measurements on printed MRIs, because of the unavailability of digital images. The median tumor volume at diagnosis was 78.9 cc (range 13–276). The median pre-chemotherapy volume was 109 cc (range 24–240) and the median post-chemotherapy volume was 52.3 cc (range 11.6–206.8). The calculated median volume variation induced by chemotherapy was 32.7 cc (38.9%) and varied from 8 to 100 cc (5.6–54.1%).
In addition, the tumor shrinkage occurred from the periphery to the center of the glioma, thus with a decrease of the infiltration at the level of eloquent areas or a reduction of the contra-lateral infiltration—thus making a surgical resection possible in the 10 cases.
Surgical procedure after chemotherapy
Eight patients were operated on with the aid of cortical and sub-cortical brain maps, using an asleep-awake-asleep technique. Two patients were operated on under general anesthesia, because the tumor location (right frontal lobe with initial involvement of the central area before chemotherapy) did not impose language and sensory mapping. In these cases, intraoperative electrical stimulation was nonetheless used for identifying eloquent motor areas both at the cortical and subcortical levels.
Surgical removal of the glioma was made possible because of the reduction of the tumor infiltration—within ipsilesional functional areas in six patients. In the other four cases, a significant reduction of the infiltration within the contralateral hemisphere through the corpus callosum was observed. The time interval between the completion of the chemotherapy and the operative procedure was 5.1 ± 4.7 months (mean ± SD). Indeed, all the patients underwent surgery when the tumor became stable, i.e., with no more shrink, as demonstrated on 2 consecutive MRIs spaced by 3–6 months following the arrest of the chemotherapy.
The median tumor volume was estimated at 52.3 cc before (range 11.6–211.7) and 4 cc after (range 0–20) the surgical removal. The calculated extent of resection varied from 82.8 to 100% (median 91.4, mean 93.3 ± 5.5). Three patients benefited from a gross total tumor removal; six underwent subtotal (<10 cm3 residue) resection; and one underwent partial (≥10 cm3 residue) resection according to the postoperative FLAIR-weighted MRI (Figs. 2, 3).
Case illustration a Axial FLAIR-weighted MRI before chemotherapy showing a left low-grade glioma involving the fronto-temporo-insular structures, in a right-handed patient. b Axial FLAIR-weighted MRI after chemotherapy (24 cycles of Temozolomide), showing a reduction of the tumoral infiltration (despite the lack of 1p19q deletion), especially with a shrink of the glioma at the level of the anterior perforating substance, i.e., a part of the brain inoperable. c Axial FLAIR-weighted MRI 14 months after surgery (subtotal resection), showing no recurrence without any complementary treatment
Case illustration a Axial FLAIR-weighted MRI before chemotherapy showing a right frontal low-grade glioma involving the internal capsule, in a right-handed patient. b Axial FLAIR-weighted MRI after chemotherapy, showing a reduction of the tumoral invasion, especially with a shrink of the glioma at the level of the internal capsule, i.e., a part of the brain inoperable. c Axial FLAIR-weighted MRI 15 months after surgery, showing a subtotal resection with no recurrence without any complementary treatment
Of note, there was no any correlation between extent of response to chemotherapy and extent of surgical resection. For instance, the patient 8 who demonstrated only an 8 cc response to chemotherapy had an invasion of the contralateral hemisphere through the corpus callosum. This shrinkage allowed to perform a 100% extent of resection, because the residual tumor after chemotherapy was located in only one hemisphere.
Molecular genetics
Because of a technical problem, results of molecular genetic analysis (Table 4) were not available for one patient. In four cases a 1p19q deletion was detected. In the others with absence of 1p19q codeletion, a tendency to an increased response to chemotherapy was observed, with higher levels of tumor volume reduction (mean volume reduction 41.9 vs. 29.5%, ns). However, there was no significant correlation between 1p19q status and subsequent extent of resection.
Neuropsychological assessment
Nine patients have been evaluated at hospital and one at his domicile. Nine of them were right-handed and one left-handed. No one presented with premorbid intelligence deficit, according to the fNART test (QIt range 89–113). Thus, NPA could be applied to all subjects (Table 5). The mean MMS was 28.7 ± 1.4 (mean ± SD). Overall results are detailed in Table 5.
Three patients did not show any neuropsychological deficit. One of them received chemotherapy again because of a recurrence, but he was still working at the moment of the NPA. Seven patients failed at three or less out of the eighteen cognitive tests that were applied. The three others failed at least four tests.
The analysis of information processing/psychomotor speed using the Trail Making Test-A, revealed no significant deficit. One patient showed a tendency to slowness, presenting with results between the 5th and the 10th percentile. In the digit-symbol substitution test, a significant decline was observed in three cases.
Concerning visuo-spatial attention (Bells’ test), three patients presented with scores under normal values. The errors observed did not predominate in any side, which suggested the absence of spatial neglect.
A decline in verbal episodic memory was underlined in five subjects. A frontal profile of disturbances (i.e., of executive aspects of memory) was observed in three cases: one with a right frontal, one with a left frontal and one with a left fronto-temporo-insular tumor. A hippocampal profile was observed in two cases: one with a left temporo-insular and one with a fronto-temporo-insular tumor. No significant non verbal episodic memory impairment was detected.
Two patients showed a significant deficit in working memory competences: one (harboring a left temporo-insular tumor) for visuo-spatial span and the other (harboring a left frontal tumor) for both visuo-spatial and verbal spans (letter-digit sequencing). The decline in non-verbal working memory observed in these two patients might be secondary to attention disturbances and executive impairments.
Regarding language abilities, one patient with a left temporo-insular lesion exhibited a significant deficit in the naming test associated to a decline in verbal initiation. Two patients (one with a left frontal and another with a fronto-temporo-insular lobe glioma) presented a decline only in verbal fluency, secondary to the impairment of executive functions.
Finally, examination of visuo-spatial abilities revealed no significant deficit.
In the assessment of executive functions, one patient showed a significant deficit in flexibility abilities (Trail Making Test-B), four patients in inhibition domain (SCWT) and three in verbal initiation (Verbal Fluency). No impairment in verbal abstraction abilities or rules deduction was observed.
In summary, the main cognitive domains where deficits were observed were episodic memory, especially verbal modality (five patients), and executive functions (five patients). Interestingly, the patients who did not continue to work were not the same who presented the most severe cognitive impairment.
Assessment of QoL
The results of QoL assessments are detailed in Table 6. The Karnofsky Performance Scale (KPS) scores ranged from 80 to 100 (median 90) and were globally stable during the whole follow-up period (Table 2).The main domain that presented with significant impairments in the QoL assessment was role functioning (feeling of independence and socio-professional life) with a median score of 66.7% (range 50-100). The global QoL score was moderately preserved for most patients with a median value of 66.7% (range 33.3–83.3). Cognitive, emotional, physical and social well-being scores were also relatively preserved (medians 83.3, 79.2, 100 and 100%, respectively).
Among the general symptoms, the main complains were fatigue (median score 33.3% range 11.1–100) and pain (median score 16.6%, range 0–66.7) (some patients exhibited painful disease like osteoarthritis and arteriopathy). Sleeping troubles (mean score 20 ± 30.6%), financial impact (mean score 23.3 ± 39.6%) and digestive troubles (mean score 20 ± 30.6%) seemed to have a moderate influence on the QoL.
No patient reached the cut-off of 15 in the inventory for signs or symptoms of depression (BDI), with a mean score of 8.7 ± 3.6. However, seven subjects showed a tendency for “mild depression”, characterized by a score between 8 and 14.
Of note, there was no any correlation between extent of response to chemotherapy and functional outcomes.
Discussion
Neoadjuvant chemotherapy followed by surgical resection: proposal of a novel therapeutic strategy
In the past 10 years, the management of GIIG switched from a classical “wait and see” policy to a more therapeutic management. According to European guidelines [5], surgery represents the first treatment option, with the aim of performing a maximal tumor mass removal whenever possible while minimizing the long-term morbidity. Likewise, chemotherapy has a role at recurrence after surgery or radiotherapy, or for patients with large postoperative residues, or even as initial treatment for unresectable tumors, delaying the risk of neurotoxicity from large field radiotherapy [29]. However, except for two previous cases [17, 30] reported in the literature (one from our team), to our knowledge there is no series assessing the feasibility of neoadjuvant chemotherapy in GIIG [17]. The use of neoadjuvant chemotherapy has usually been reported for malignant neoplasias, such as breast [31], ovarian [32] and oral [33] cancers, with the aim of reducing lymphatic and microscopic dissemination, and thus optimizing surgery.
In the present series, seven patients received true neoadjuvant chemotherapy (initial treatment after biopsy) and three were treated at tumor recurrence after partial removal. A complete tumor resection after a significant shrinkage of tumor due to chemotherapy was achieved in three cases, a subtotal resection was performed in six patients and only a partial resection was obtained in one case. Such results were possible because a substantial tumor volume reduction was demonstrated with Temozolomide, with a mean initial value of 110.4 cm3 and a mean pre-operative volume of 70.2 cm3. As preoperative chemotherapy may render GIIG more resectable, and since it was demonstrated that the extent of resection was significantly related to the overall survival [1, 3, 4], we can suggest that this strategy might have a potential impact on long-term survival by delaying the occurrence of anaplastic transformation. Interestingly, in this series, chemotherapy seemed to induce tumor shrinkage even beyond the completion of cycles, as the mean tumor volume continued to reduce even after the arrest of Temozolomide. This is the reason why surgery was decided only when demonstration of a stabilization of the tumor size was obtained on repeated MRIs spaced by 3–6 months.
From a functional point of view, conversely to surgery [34], the exact relationship between chemotherapy and cerebral plasticity is not completely understood. Since Temozolomide may reduce tumor volume and infiltration from functional areas, it could be hypothesized that the development of brain plasticity might be facilitated. Beyond the interest concerning the quality of life, an additional benefit of inducing functional redistribution could be the maximization of the extent of resection. Indeed, the more eloquent areas are redistributed far from the glioma, the less are the risks to generate post-operative permanent deficits, while increasing the possibilities of performing near-total resection. Such optimization of the benefit-to-risk ratio of surgery has already been demonstrated to be directly related to the brain plastic potential at the individual level [34]. Therefore, it could be useful in future studies to appreciate such functional reorganization when adopting a dynamic therapeutic strategy, especially by performing longitudinal functional neuroimaging studies (before and after chemotherapy), as previously performed before and after surgical resections [34]. Indeed, a better understanding of the concomitant effects of chemotherapy (anti-tumoral and promoter of cerebral plasticity) might modify the management of GIIG by introducing new therapeutic options aiming of both controlling the tumor while improving the quality of life.
From a molecular point of view, it is puzzling to note that, in the present study, patients who did not present with 1p19q codeletion tended to exhibit a better response to chemotherapy. Despite the fact that this is a small series, we nevertheless suggest that the 1p19q status should be currently used with caution, as it is insufficiently reliable to predict response to chemotherapy and thus to allow to take the best decision concerning therapeutic management at the individual level.
Finally, we have to acknowledge that the follow-up period is short for some patients. However, tumor volumes progress very slowly in the post-operative period. Thus, it is possible that patients undergoing a pre-operative chemotherapy still benefit from a prolonged effect after surgery. Long-term follow-up is thus mandatory to draw definitive conclusions.
Combined therapy: assessing the functional tolerance, quality of life and cognition
The therapeutic strategy described above was associated with an acceptable tolerance when assessing the neurological status, cognitive functions and QoL. It is important to detail such neuropsychological effects in consideration of the young age and relatively long outcome of glioma patients. Additionally, a combined strategy may be an argument to delay radiotherapy and reduce the risks of permanent cognitive impairment [7].
In the initial phases of utilization of this novel approach, exhaustive neuropsychological evaluations seem required. Mini Mental State Examination showed to be insufficient to assess the neurocognitive status [35]. Indeed, MMS scores were within the normal range for all patients, whereas seven patients presented significant cognitive deficits in at least one domain. Executive functions and verbal episodic memory were the main cognitive functions attempted. Executive functions and verbal episodic memory impairments have often been described with WHO grade II gliomas [36]. These deficits seem to be related to the tumor location in the present study. Executive deficits were mostly observed in patients harboring frontal lesions, whereas a hippocampal profile of memory deficits was associated to a temporal location. In a particular patient, significant executive deficits were observed despite the presence of a mesio-temporal tumor. We can hypothesize that these findings were related to the disruption of wide subcortical networks, in particular fronto-temporal connections.
Beyond the cognitive status, we consider that comprehensive evaluation of QoL is mandatory in the follow-up of patients with gliomas, and takes an integral part of their oncological management. KPS, as well as MMSE scores, seem to lack sensitivity and thus to be inadequate in GIIG gliomas patients, especially for appreciation of mood disorders [37]. Indeed, in this series, despite the fact that KPS was preserved, it was not always the case for the QoL scores. Functional aspects such as social and professional activities, as well as the feeling of independence seemed to be the major factors related to the level of QoL in the applied tests. The control of seizure is also a very important factor influencing QoL. Interestingly, chemotherapy allowed, in the majority of cases, an improvement of the epileptic status results that are in line with previous studies on GIIG chemotherapy [13, 38–40]. Tumor removal also induced additional reduction in frequency and severity of seizures.
Important issues in preserving the professional activity are the difficulty to adapt the job, and the impossibility to drive in cases of persistence of seizure. Moreover, fatigue, a multi-dimensional symptom, has to be taken into account because of its interaction with neurocognitive functions (like attention, motivation and concentration [16]) and QoL. However, this is a very subjective parameter, and it is difficult to quantify this variable. Finally, sleep disturbances were often reported and may interact with QoL.
In all cases, distinguishing chemotherapy, surgery, antiepileptic or tumor-related cognitive dysfunctions is a very complex issue. As all patients presented with low scores in the BDI, we can suggest that NPA were only little or not at all influenced by mood disorders or depression in the present series. Nonetheless, the evaluation of the changes over time of neuropsychological scores was not possible here, given the absence of a baseline NPA before the combined treatment.
Conclusion
On the basis of our findings, we suggest that a novel therapeutic approach combining neoadjuvant chemotherapy followed by tumor resection may be of great utility in cases of initially unresectable WHO grade II gliomas. This data is to remind us that patients with “inoperable” lesions should be continually evaluated during the course of their chemotherapy for surgical intervention.
From an oncological point of view, because the extent of resection has been demonstrated in the recent literature to be an independent prognostic factor significantly related to overall survival, the combined approach could have a possible impact on the long-term survival. Of course, since the overall cohort size is small, further standardized studies with larger populations of patients bearing initially inoperable GIIG and longer follow-up are needed, to validate the actual efficacy of such original therapeutic strategy.
In addition, from a functional point of view, we showed that this strategy was associated with an acceptable quality of life. No serious cognitive disturbances were observed after extensive neuropsychological assessments which tested several domains of cognition. Cognitive deficits seemed mostly related to tumor location. Finally, we have to underline that tools such as MMSE or KPS are not sensitive enough, and thus are not adapted for patients with GIIG. A more detailed neurocognitive assessment should be systematically performed in relation to the different therapeutic options.
Although a randomized trial of chemotherapy alone versus neoadjuvant chemotherapy followed by surgery for eloquent regions GIIG would be the best way to objectively evaluate the impact of each treatment on the quality of life, such trial is nonetheless impractical. However, a prospective study might be proposed with a higher number of patients and a longer follow-up.
References
Berger MS, Deliganis AV, Dobbins J, Keles GE (1994) The effect of extent of resection on recurrence in patients with low grade cerebral hemisphere gliomas. Cancer 74:1784–1791
Duffau H, Lopes M, Arthuis F et al (2005) Contribution of intraoperative electrical stimulations in surgery of low grade gliomas: a comparative study between two series without (1985–96) and with (1996–2003) functional mapping in the same institution. J Neurol Neurosurg Psychiatry 76:845–851
Keles GE, Lamborn KR, Berger MS (2001) Low-grade hemispheric gliomas in adults: a critical review of extent of resection as a factor influencing outcome. J Neurosurg 95:735–745
Smith JS, Chang EF, Lamborn KR et al (2008) Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol 26:1338–1345
Soffietti R, Baumert BG, Bello L et al (2010) Guidelines on management of low-grade gliomas: report of an EFNS-EANO* Task Force. Eur J Neurol 17:1124–1133
van den Bent MJ, Afra D, de Witte O et al (2005) Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet 366:985–990
Douw L, Klein M, Fagel SS et al (2009) Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol 8:810–818
Whittle IR (2004) The dilemma of low grade glioma. J Neurol Neurosurg Psychiatry 75:31–36
Buckner JC, Gesme D Jr, O’Fallon JR et al (2003) Phase II trial of procarbazine, lomustine, and vincristine as initial therapy for patients with low-grade oligodendroglioma or oligoastrocytoma: efficacy and associations with chromosomal abnormalities. J Clin Oncol 21:251–255
Mason WP, Krol GS, DeAngelis LM (1996) Low-grade oligodendroglioma responds to chemotherapy. Neurology 46:203–207
Soffietti R, Rudà R, Bradac GB, Schiffer D (1998) PCV chemotherapy for recurrent oligodendrogliomas and oligoastrocytomas. Neurosurgery 43:1066–1073
Brada M, Viviers L, Abson C et al (2003) Phase II study of primary temozolomide chemotherapy in patients with WHO grade II gliomas. Ann Oncol 4:1715–1721
Hoang-Xuan K, Capelle L, Kujas M et al (2004) Temozolomide as initial treatment for adults with low-grade oligodendrogliomas or oligoastrocytomas and correlation with chromosome 1p deletions. J Clin Oncol 22:3133–3138
Pace A, Vidiri A, Galie E et al (2003) Temozolomide chemotherapy for progressive low-grade glioma: clinical benefits and radiological response. Ann Oncol 14:1722–1726
Quinn JA, Reardon DA, Friedman AH et al (2003) Phase II trial of temozolomide in patients with progressive low-grade glioma. J Clin Oncol 21:646–651
Liu R, Solheim K, Polley MY et al (2009) Quality of life in low-grade glioma patients receiving temozolomide. NeuroOncol 11:59–68
Duffau H, Taillandier L, Capelle L (2006) Radical surgery after chemotherapy: a new therapeutic strategy to envision in grade II glioma. J Neurooncol 80:171–176
Mandonnet E, Jbabdi S, Taillandier L et al (2007) Preoperative estimation of residual volume for WHO grade II glioma resected with intraoperative functional mapping. Neuro-Oncology 9:63–69
Ius T, Angelini E, Thiebaut de Schotten M, Mandonnet E, Duffau H (2011) Evidence for potentials and limitations of brain plasticity using an atlas of functional resectability of WHO grade II gliomas: towards a “minimal common brain”. NeuroImage 56:992–1000
Duffau H, Capelle L, Sichez N et al (2002) Intraoperative mapping of the subcortical language pathways using direct stimulations. An anatomo-functional study. Brain 125:199–214
Duffau H (2005) Intraoperative cortico-subcortical stimulations in surgery of low-grade gliomas. Expert Rev Neurother 5:473–485
Deloche G, Hannequin D (1997) Test de dénomination orale de 80 images : DO80. Edition du Centre de Psychologie Appliquée, Paris
Metz-Lutz MN, Kremin H, Deloche G et al (1991) Standardisation d’un test de dénomination orale : contrôle de l’âge, du sexe et du niveau de scolarité chez des sujets adultes normaux. Rev Neuropsychol 1:73–95
Ojemann G, Ojemann J, Lettich E, Berger M (1989) Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. J Neurosurg 71:316–326
Duffau H, Gatignol P, Mandonnet E, Capelle L, Taillandier L (2008) Intraoperative subcortical stimulation mapping of language pathways in a consecutive series of 115 patients with Grade II glioma in the left dominant hemisphere. J Neurosurg 109:461–471
Gil-Robles S, Duffau H (2010) Surgical management of World Health Organization grade II gliomas in eloquent areas: the necessity of preserving a margin around functional structures? Neurosurg Focus 28(2):E8
Aaronson NK, Ahmedzai S, Bergman B et al (1993) The European organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85:365–376
Beck AT, Steer RA, Brown GK (1998) Inventaire de Dépression de Beck (BDI-II), deuxième ed. Éditions du Centre de Psychologie Appliquée (ECPA), Paris (version 2)
Schiff D, Brown PD, Giannini C (2007) Outcome in adult low-grade glioma: the impact of prognostic factors and treatment. Neurology 69:1366–1373
Spena G, Garbossa D, Barletta L, Prevost C, Versari P (2010) Preoperative chemotherapy for infiltrative low-grade oligoastrocytoma: a useful strategy to maximize surgical resection -case report. Neurol Med Chir (Tokyo) 50:410–413
Kaufmann M, Hortobagyi GN, Goldhirsch A et al (2006) Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: an update. J Clin Oncol 24:1940–1949
Vergote I, van Gorp T, Amant F, Neven P, Berteloot P (2005) Neoadjuvant chemotherapy for ovarian cancer. Oncology (Williston Park) 19:1615–1622
Klug C, Wutzl A, Kermer C et al (2005) Preoperative radiochemotherapy and radical resection for stages II–IV oral and oropharyngeal cancer: outcome of 222 patients. Int J Oral Maxillofac Surg 34:143–148
Duffau H (2008) Brain plasticity and tumors. Adv Tech Stand Neurosurg 33:3–33
Brown PD, Buckner JC, O’Fallon JR et al (2003) Effects of radiotherapy on cognitive function in patients with low-grade glioma measured by the folstein mini-mental state examination. J Clin Oncol 21:2519–2524
Klein M, Heimans JJ, Aaronson NK et al (2002) Effect of radiotherapy and other treatment-related factors on mid-term to long-term cognitive sequelae in low-grade gliomas: a comparative study. Lancet 360:1361–1368
Mackworth N, Fobair P, Prados MD (1992) Quality of life self-reports from 200 brain tumor patients: comparisons with Karnofsky performance scores. J Neurooncol 14:243–253
Kaloshi G, Benouaich-Amiel A, Diakite F et al (2007) Temozolomide for low-grade gliomas: predictive impact of 1p/19q loss on response and outcome. Neurology 68:1831–1836
Taillandier L, Duffau H (2009) Epilepsy and insular grade II gliomas: an interdisciplinary point of view from a retrospective monocentric series of 46 cases. Neurosurg Focus 27(2):E8
Rudà R, Trevisan E, Soffietti R (2010) Epilepsy and brain tumors. Curr Opin Oncol 22:611–620
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatry Res 12(3):189–198 (Traduction française) : version consensuelle GRECO, Derouesne C, Poitreneau J, Hugonot L, Kalafat M, Dubois B, Laurent B, Presse Med. 1912, pp 1141–1148, 1999
Derouesné C, Poirteneau J, Hugonot L, Kalafat M, Dubois B, Laurent B (1999) Le mini-mental state examination (MMSE) : un outil pratique pour l’évaluation de l’état cognitif des patients par le clinicien. Presse Med 28:1141–1148
Mackinnon A, Mulligan R (2005) The estimation of premorbid intelligence levels in French speakers. Encephale 31:31–43
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113
Wechsler D (2000) WAIS-III. Echelle d’intelligence de Wechsler pour adultes, Troisième edition edn. Edition du Centre de Psychologie Appliquée, Paris
Reitan RM, Wolfson D (1985) The Halstead–Reitan neuropsycholgical test battery: therapy and clinical interpretation. Neuropsychological Press, Tucson
Godefroy O. GREFEX. Fonctions exécutives et pathologies neurologiques et psychiatriques. Évaluation en pratique clinique. Solal Marseille, 2008
Gauthier L, Dehaut F, Joanette Y (1989) The bells test: a quantitative and qualitative test for visual neglect. Int J Clin Neuropsychol 11:49–53
Rousseaux M, Beis JM, Pradat-Diehl P et al (2001) Presenting a battery for assessing spatial neglect. Norms and effects of age, educational level, sex, hand and laterality. Rev Neurol (Paris) 157:1385–1400
Rey A (1941) L’examen psychologique dans les cas d’encéphalopathie traumatique. Arch Psychol 28:328–336
Delbecq-Derouesné J, Beauvois M (1989) Memory processes and aging: a defect of automatic rather than controlled processes? Arch Gerontol Geriatrics 1:121–150
Buschke H (1973) Selective reminding for analysis of memory and learning. J Verbal Learning Verbal Behav 12:543–550
Van der Linden M, Coyette F, Poitrenaud J (2004) L’épreuve de rappel libre/rappel indicé à 16 items (RL/RI-16). In M. Van der Linden, S. Adam, A. Agniel, C. Baisset-Mouly & al, members of GRENEM (Eds). L’évaluation des troubles de la mémoire: Présentation de quatre tests de mémoire épisodique (avec leur étalonnage). Marseille, Solal pp 5–47
Grégoire J, Van der Linden M (1997) The effect of age on forward and backward digit spans. Aging, Neuropsychol Cogn 4:140–149
Thuillard F, Assal G (1991) Données neuropsychologiques chez le sujet âgé normal. In: Habib M, Joanette Y, Puel M (eds) Démences et syndromes démentiels, approche neuropsychologique. Masson, Paris, pp 125–133
Warrington EK, James M (1991) The visual object and space perception battery (VOSP). Bury St Edmunds, England
Agniel A, Joanette Y, Doyon B, Duchein C (1987) Protocole d’évaluation des gnosies visuelles Montréal-Toulouse (PEGV) Laboratoire Th-Alajouanine. Centre de recherche du Centre Hospitalier Côte-des-Neiges, Montréal
Burgess P, Shallice T (1997) The Hayling and Brixton Tests. Test manual Bury St Edmunds. Thames Valley Test Company, UK
Stroop JR (1935) Studies of interference in serial verbal reactions. J Exp Psychol 6:643–662
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Blonski, M., Taillandier, L., Herbet, G. et al. Combination of neoadjuvant chemotherapy followed by surgical resection as a new strategy for WHO grade II gliomas: a study of cognitive status and quality of life. J Neurooncol 106, 353–366 (2012). https://doi.org/10.1007/s11060-011-0670-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-011-0670-x