Abstract
There is a growing evidence of using Temozolomide as upfront therapy for progressive low grade gliomas. No data exist on the efficacy of nitrosoureas as an alternative to radiotherapy in those patients who progress after Temozolomide. We retrospectively reviewed 30 patients with median age of 46 years. Twenty-one patients had pure oligodendrogliomas. Thirteen patients had a non-enhancing tumor at progression after Temozolomide. The chromosomes 1p/19q were co-deleted in 5 cases and retained in 10 cases. Response rate was 10% (3 minor responses achieved in non-enhancing tumors). Tolerance was acceptable (17% grade III and IV myelosupression). Median PFS was 6.5 months. Median OS from start of salvage treatment was 23.4 months. Tumors without contrast enhancement demonstrated a better prognosis than those with contrast enhancement both in term of PFS (P = 0.0003) and OS (P = 0.0006). Chromosomes 1p/19q codeletion was not predictive for objective response to salvage treatment but correlated with a better PFS (P = 0.02). In conclusion, salvage NU chemotherapy provide disappointing results in TMZ-pretreated low grade gliomas (LGG), which should be treated in priority by conventional radiotherapy especially in LGG that display contrast enhancement at progression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Temozolomide (TMZ) chemotherapy has recently been shown to represent an alternative approach to radiotherapy as initial treatment for low grade gliomas (LGG) [1, 2]. Unfortunately, these patients will progress. Radiotherapy is the standard treatment at this time. However, elderly patients or those with large tumors are exposed to delayed neurotoxicity. Hence, some of these patients may be treated by a second line nitrosourea-based chemotherapy. The aim of this study was to evaluate the activity of nitrosourea (NU) chemotherapy as salvage treatment in progressive LGG previously treated with TMZ.
Methods
Since 1999, all patients in our institution with progressive diffuse LGG were offered up-front TMZ chemotherapy [3]. In the present study, we have retrospectively reviewed all the patients who met the following criteria: (1) progressive disease after TMZ treatment; (2) NU-based chemotherapy used as second line treatment; (3) measurable disease on MRI; and (4) absence of prior radiotherapy. Response was assessed by MRI every 3 months, as previously reported [1, 2]. Survival rates were estimated using the Kaplan–Meier technique. Progression-free survival (PFS) was defined as the time from start of NU treatment until the date of progressive disease, and overall survival (OS) was calculated from the time from start of NU treatment to death. Log-rank test was used to compare survival according to prognostic factors. The World Health Organization (WHO) scale for toxicity was used.
Results
We identified 30 patients who were evaluable for response to salvage NU chemotherapy (Table 1). Median age was 46 years (range: 30–81) and median Karnofsky performance status was 90 (range: 40–100). Histological subtypes included 21 pure oligodendrogliomas and 9 diffuse astrocytomas and mixed gliomas. Seventeen patients demonstrated a mild contrast-enhancing tumor and 13 patients had a non-enhancing tumor at progression after TMZ. The chromosome 1p/19q status of the initial tumor was available for 15 patients (1p/19q were co-deleted in 5 cases and retained in 10 cases). None of the LGG patients had a biopsy at progression for histological analysis.
Twenty-three patients received a PCV chemotherapy at conventional dose and schedule (procarbazine 60 mg/m2 p.o. days 8–22; CCNU 110 mg/m2 p.o. day 1; vincristine 1.4 mg/m2 i.v. days 8 and 29; each cycle delivered every 6 weeks) and 7 patients received a BCNU chemotherapy (150–200 mg/m2 i.v. every 8 weeks) with a median number of 6 cycles (range 1–6). The tolerance was acceptable with a 17% rate of grade III and IV myelosupression.
Only 3 patients achieved an objective minor response (10%), 20 patients had a stable disease (67%) and 7 patients progressed (23%). All 3 responding patients had non-enhancing tumors. At the time of analysis, the median follow-up from the start of NU treatment was 41 months. Interestingly, the 3 responding patients to NU previously responded to TMZ (3 out of 13 patients) while none of the non-responding patients to TMZ responded to NU (0 out of 17 patients) (P = 0.03).
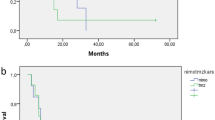
Median PFS was 6.5 months (95% CI 3.9–11.8 months) and the 1-year PFS rate was 27.6% (95% CI 16–42%). Median OS from start of salvage treatment was 23.4 months (95% CI 13.3–29.4 months) and the 2-year survival rate was 44% (95% CI 30–60%) (Fig. 1). The OS from diagnosis was 47.5 months (CI 36.2–94.8 months). Tumors without contrast enhancement demonstrated a better prognosis than those with contrast enhancement in term of PFS (13.8 vs 4.7 months; P = 0.0003), OS from start of NU (34.5 vs 15 months; P = 0.0006), as well as OS from diagnosis (96.6 vs 44.4 months, P = 0.006). On the other hand, the patients who progressed some time after the discontinuation of TMZ had a higher response rate (30% vs 0%, P = 0.002) and a longer PFS and OS than refractory patients (P < .0001 in both PFS and OS). The 1p/19q co-deletion was not predictive for objective response to salvage treatment, but correlated with a better PFS (15.4 vs 4.7 months; P = 0.02). It was also associated with a trend for a longer overall survival (32.5 vs 15.5 months); however, this difference did not reach statistical significance (P = 0.12).
At progression under NU chemotherapy, patients were offered a conventional radiotherapy (20 patients) or a third line chemotherapy (7 patients; platine- or etoposide-based) or palliative treatment (3 patients). The treatment was entirely and individually decided by the referent physicians of the patients. The reasons for not proposing RT as third line treatment were as follows: too widely infiltrative tumor (secondary gliomatosis) which would require a whole brain RT and expose them to a high risk of neurotoxicity, especially in elderly patients, patients in poor physical and neurological conditions, and patient refusal. Most of these reasons cited above were already those why NU was preferred as second line treatment to RT. After RT (n = 20), median PFS was 11.2 months and median OS was 13.3 months. After third line chemotherapy (n = 10), median PFS was 2.3 months and median OS was 3.9 months.
Discussion
Our study suggests that NU has a limited activity in TMZ-pretreated LGG. The low response rate contrasts with those reported with NU used as neoadjuvant chemotherapy [4] and as first line salvage chemotherapy after radiotherapy in oligodendroglial tumors [5]. This may be likely related to the development of an acquired cross-resistance to alkylating agents. The response rate and better survival profile found in non-refractory patients could confirm this hypothesis.
Even if we failed to show a prognostic impact of contrast enhancement at onset of TMZ chemotherapy in our previously published series of progressive low grade gliomas [3], here, once chemotherapy was initiated, we can validate the presence of contrast enhancement, as a marker of a transformation to higher grade tumor and consequently of a worse prognosis.
Combined loss of 1p/19q has been shown to be highly predictive of response to first line NU chemotherapy in anaplastic oligodendroglial tumors (AOT) [6]. Although our series is not capable of answering this question, our results suggest that its predictive impact seems much lower when NU is used as second line chemotherapy after TMZ, although it remains correlated with improved PFS, as previously shown in recurrent AOT [7].
In conclusion, salvage NU chemotherapy provides disappointing results in TMZ-pretreated LGG, which should be treated, in the absence of any available controlled study, in priority by conventional radiotherapy especially in LGG that display contrast enhancement at progression.
References
Brada M, Viviers L, Abson C et al (2003) Phase II study of primary temozolomide chemotherapy in patients with WHO grade II gliomas. Ann Oncol 14:1715–1721
Hoang-Xuan K, Capelle L, Kujas M et al (2004) Temozolomide as initial treatment for adults with low-grade oligodendrogliomas or oligoastrocytomas and correlation with chromosome 1p deletions. J Clin Oncol 22:3133–3138
Kaloshi G, Benouaich-Amiel A, Diakite F et al (2007) Temozolomide for low-grade gliomas: predictive impact of 1p/19q loss on response and outcome. Neurology 68(21):1831–1836
Buckner JC, Gesme D Jr, O’Fallon JR et al (2003) Phase II trial of procarbazine, lomustine, and vincristine as initial therapy for patients with low-grade oligodendroglioma or oligoastrocytoma: efficacy and associations with chromosomal abnormalities. J Clin Oncol 21:251–255
Van den Bent MJ, Kros JM, Heimans JJ et al (1998) Response rate and prognostic factors of recurrent oligodendroglioma treated with procarbazine, CCNU, and vincristine chemotherapy. Dutch Neuro-oncology Group. Neurology 51(4):1140–1145
Cairncross JG, Ueki K, Zlatescu MC et al (1998) Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst 90:1473–1479
Kouwenhoven MC, Kros JM, French PJ et al (2006) 1p/19q loss within oligodendroglioma is predictive for response to first line temozolomide but not to salvage treatment. Eur J Cancer 42:2499–2503
Acknowledgement
G. Kaloshi is recipient of a grant of ARTC (Association pour la Recherche sur les Tumeurs Cérébrales).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaloshi, G., Sierra del Rio, M., Ducray, F. et al. Nitrosourea-based chemotherapy for low grade gliomas failing initial treatment with temozolomide. J Neurooncol 100, 439–441 (2010). https://doi.org/10.1007/s11060-010-0197-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-010-0197-6