Abstract
Interventional radiologists continue to expand the diagnostic and therapeutic options available for pediatric oncology patients. Percutaneous radiofrequency ablation (RFA) and transarterial chemoembolization (TACE) are performed safely in children, providing new adjuncts in the armamentarium of therapies for childhood cancer. Percutaneous image-guided radiofrequency ablation (RFA) provides a viable and effective therapeutic option in the treatment of benign and malignant solid tumors in a variety of locations to include the skeleton, liver, spleen, kidney, adrenal gland, lung, and pancreas. Primary and metastatic malignancies as large as 8 cm in children are now effectively treated with RFA. The most common indications for RFA in children include hepatoblastoma, hepatocellular carcinoma (HCC), metastatic osteosarcoma, and Wilms tumor. TACE is an additional therapy, safely performed in children, providing effective therapy options for children with multifocal hepatoblastoma, paraganglioma, neuroblastoma, HCC, osteogenic sarcoma, and Wilms tumor. When TACE and surgery are combined, blood loss and operative time are reduced, while providing more efficacious therapy when combined to surgery alone. In conclusion, RFA and TACE provide children with safe and effective options for treatment of pediatric malignancy.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Osteogenic Sarcoma
- Systemic Chemotherapy
- Orthotopic Liver Transplantation
- Gelatin Sponge
- Limb Salvage Surgery
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Interventional radiologists continue to expand the diagnostic and therapeutic options available for pediatric oncology patients. Pediatric oncologists and surgeons increasingly rely on partnership with pediatric interventional radiology for precise and effective percutaneous biopsy, tumor ablation, drainage, and vascular access procedures. With specific reference to image-guided therapy procedures and protocols, discussions of percutaneous radiofrequency ablation and transarterial chemoembolization in children will be the focus of this chapter.
Radiofrequency Tumor Ablation
Percutaneous image-guided radiofrequency ablation (RFA) provides a viable and effective therapeutic option in the treatment of benign and malignant solid tumors in a variety of locations to include the skeleton, liver, spleen, kidney, adrenal gland, lung, and pancreas [1–31]. In these applications, the basic concepts of radiofrequency tumor ablation are similar: delivery of localized and contained heat induces focal coagulative necrosis and cell death. Cytotoxicity is best induced when regional temperatures are maintained between 50 °C and 100 °C. In the recent decade, the majority of RFA experience documented in pediatric and adult medical literature focuses on primary applications of RFA in the treatment of hepatocellular carcinoma (HCC) and osteoid osteoma [1–6]. Percutaneous RFA has expanding indications in the treatment of pediatric malignancy, most evolving in the treatment of multifocal, recurrent, or metastatic malignancies of bone, liver, kidney, and face with successful treatment of children as young as 1 year old [8–12]. This section will review details of pediatric unique care issues in percutaneous RFA with respect to general patient care and organ-specific RFA applications in the treatment of pediatric malignancy.
General Procedural Issues
Although adult percutaneous RFA in organs such as the liver, lung, and kidney may be successfully performed in adults with conscious sedation, children do not tolerate similar pain experiences, and thus, general anesthesia is required for RFA procedures in children. In the authors’ clinical experience, pain, even with general anesthesia, can be significant enough to cause elevations in blood pressure and heart rate when the RFA heat contacts sensitive structures such as the pleura during lung RF ablation.
The use of prophylactic antibiotics is variable among authors and in specific tissue applications, especially when treating focal hepatic and renal malignancies [2, 5, 6, 8]. In the authors’ pediatric experience, a single dose of preprocedural antibiotics is administered when treating focal liver, bone, and lung lesions, and to date, none of our cases have been complicated by abscess formation or sepsis.
The potential for skin burns exists with RFA due to heat distribution at the skin site of grounding pads used with monopolar RFA systems or if coaxial needles are not fully withdrawn adjacent to the insulated portion of the RFA needle. Thermal burns are best avoided with the use of large surface area foil-grounding pads, placed with the longest surface edge facing the RFA electrode, at a distance of 25–50 cm from the electrode [22]. In larger children, the thigh is often recommended as a good location for placement of up to four large (100 cm2 each) foil pads. In young children, the thighs may be too small for placement of large foil pads. As an alternative large surface area structure, the buttocks serve well as a site for the foil pads when RFA is performed in the upper body.
Young children present with smaller body surface areas than their adult counterparts. Algorithms have been published that predict the range of core temperature elevation with RFA [13]. Given the relatively greater ratio of tumor volume to body surface area, the potential exists of greater total body-heating effect when large surface areas are treated with RF in children [8, 13]. In the authors’ experience, total body temperature elevation has not been encountered in the treatment of focal liver tumors. On the other hand, body core temperature elevation as high as 40°C has been documented when treating large metastatic lung lesions, responding well to the use of a hypothermic blanket. When treating large volume tumors (5–8 cm diameter), patients are placed on a hypothermic blanket prior to beginning the RFA procedure. The use of a hypothermic blanket as cool as 20°C maintains body temperature at or below 38°C during prolonged RFA treatment of large volume malignancy in the chest [8, 13].
Pain control is critical in children, requiring a plan for each specific organ system and lesion being treated with RFA. In bone, liver, and lung, pain from RFA usually requires the administration of intravenous narcotic analgesics for effective pain management. Pain following RFA is reported to be greatest 12–24 h following treatment, usually subsiding after a few days and is well managed with narcotic analgesia [8].
Hepatic Tumor Ablation
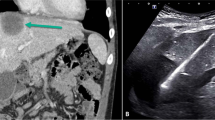
Hepatoblastoma and hepatocellular carcinoma are the most common hepatic malignancies in children [14–16]. In adults, the primary focus of hepatic tumor RFA is in treatment of hepatocellular carcinoma (HCC) or metastatic disease from organs such as the colon, pancreas, lung, and breast [1, 2, 5, 6]. Radiofrequency ablation treatment of hepatic malignancy (hepatoblastoma, HCC, metastatic leiomysarcoma) in children has been sporadically reported [8–10]. Hepatoblastoma patients that present the greatest clinical challenge are those with Beckwith-Wiedemann, with multiple hepatoblastomas developing over time. These patients are ideal candidates for RFA, given the risks of repeated surgeries for resection (Fig. 62.1). A percutaneous approach is used in the majority of reported pediatric hepatic tumor RF ablations. Overlapping RFA burns is performed in larger tumors, each site reaching a target temperature of at least 60°C over 12 min.
Two-year-old male with Beckwith-Wiedemann syndrome and focal HB, treated with RFA. (a) Pretreatment CT (arrow indicates the focal HB). (b) Ultrasound with cursors 1 and 2 delineating the focal HB. (c) Sonogram during RF ablation with watercooled electrode (straight arrow)-inducing echogenic RF coagulation necrosis (curved arrow) in the tumor. (d) CT image at 1 month follow-up demonstrating complete ablation (arrow) of the focal HB
In the treatment of focal liver tumors, the authors would apply guidelines similar to adult literature, such that we expect complete tumor RFA in 90 % of patients with tumors smaller than 2.5 cm. Tumors 2.5–3.5 cm can be ablated in 70–90 % of cases; ablation of tumors 3.5–5.0 cm will be effectively ablated in 50–70 % of cases [2, 5, 25]. In large tumors (greater than 3.5 cm), and with conditions such as Beckwith-Wiedemann with recurrent hepatoblastoma, palliative RF ablation is a reasonable goal, if treatment can prolong life expectancy with limited systemic symptoms and few minor complications. Radiofrequency ablation, in combination with chemoembolization, is effective in the treatment of adult patients with HCC larger than 3.5 cm [27] and should be considered in similar situations in the pediatric patient.
Lung and Renal Malignancy Ablation
Radiofrequency ablation is an effective percutaneous option for treatment of solid lung malignancies in adults and children. The most common indication for lung RFA in children is metastasis such as osteogenic sarcoma primarily for patients who are not operative candidates [2, 8, 9, 28–30]. Both ultrasound and CT guidance for lung RF ablation cases have been utilized for metastatic lesions as large as 8 cm (Fig. 62.2), with CT most useful when lung precludes an effective sonographic window. In large bilateral lung tumor ablations, core body temperature elevations are maintained at 38–39°C with the use of a hypothermic blanket system (Medi-Therm II; Gaymar, Orchard Park, NY), with blanket cooling temperatures as low as 20°C [8]. Although initial experience shows that RFA ablation in the pediatric lung is well tolerated, the potential for fatal complications is reported in adult lung RFA treatment [28, 29].
Twelve-year-old male undergoing palliative RFAfor bilateral pulmonary metastatic ostoesarcoma. (a) CT image of the chest demonstrating bilateral pulmonary metastatic lesions (arrows), measuring 5 cm (right) and 8 cm (left). (b) Sonogram of the osteosarcoma (curved arrows) adjacent to normal lung (straight arrow) prior to RFA. (c) Sonogram during US-guided RF ablation (straight arrow-RF electrode) of the left metastatic lesion, demonstrating early coagulation necrosis echogenicity (curved arrow) during RFA. (d) CT image during second palliative RFA session demonstrating significant bilateral necrosis following the first RFA (arrows)
The limited reported experience with percutaneous RFA of Wilms tumor and renal cell carcinoma developing in children with von Hippel-Lindau disease is promising [8, 19–21]. In these situations, percutaneous RFA is well tolerated, providing an effective nephron-sparing alternative to surgery especially for recurrent and multifocal Wilms tumor and Wilms tumor in patients with solitary kidney. Recent literature has shown that RF ablation of renal lesions during renal artery balloon occlusion results in larger effective zones of ablation, with a higher rate of infarction of normal renal tissue peripheral to the treated focus [31].
RFA Conclusion
Radiofrequency tumor ablation in children is rapidly evolving and is proving to offer effective therapeutic options in a variety of pediatric disorders. Radiofrequency ablation is well tolerated in children with few complications. As the pediatric experience grows, new opportunities for interdisciplinary treatment regimens that include RF ablation will develop, defining clear roles for this versatile minimally invasive therapy.
Chemoembolization in Children
Chemoembolization is uncommonly performed in children. Reports are confined to case reports and case studies, with no randomized controlled studies available [32]. Pediatric chemoembolization has been performed for primary and metastatic hepatic malignancies (Fig. 62.3), osteogenic sarcoma, and Wilms tumor [33–44].
Nine-month-old male with residual hepatoblastoma following partial hepatectomy, effectively treated with TACE (with no recurrence). (a) and (b) CT images demonstrating the two residual foci of HB (arrows). (c) Arteriogram with arrows indicating two foci of HB with well-defined arterial supply. (d) Microcatheter access with lipiodol contrast defining one of two foci treated with TACE (arrow). (e) Posttreatment CT demonstrating dense contrast in shrunken and necrotic focus (arrow) of previously treated HB
The basic principles of transarterial chemoembolization (TACE) are the same in both children and adults. Direct injection of the chemotherapeutic agent into the tumor allows for a greater concentration of the drug into the tumor with fewer systemic toxicities, while the embolization provides a longer dwell time and produces ischemia.
Pediatric Liver TACE
Indications for hepatic TACE in children include primary liver malignancies (predominately hepatoblastoma (HB) and hepatocellular carcinoma (HCC)) and metastatic disease such as sarcomas or neuroblastoma [33–44]. Hepatoblastoma accounts for 1 % of all pediatric malignancies [38]. While overall survival from HB is 63 %, a 90 % cure rate is possible if the tumor is resectable and the patient has no metastatic disease at diagnosis [36]. Complete surgical resection is the key [33]. Unfortunately, the tumor is initially unresectable in approximately 50 % [40, 42]. Systemic chemotherapy may shrink the tumor and allow later resection in up to 70 % of those patients [35, 36]. Unresectable patients receiving orthotopic liver transplantation (OLT) have a survival of 20–40 % and a recurrence rate of 50 % [35]. Otherwise, the outcome is dismal in tumors that remain unresectable. Due to toxicities of systemic chemotherapy, several investigators have attempted TACE to provide a greater tumor response while avoiding these complications [32, 33, 35–42]. The indications for TACE are not well established. TACE has been advocated for treating unresectable patients after failed systemic chemotherapy, for replacing primary systemic chemotherapy, as a bridge to OLT, and for palliative care [32, 35–40]. Initial attempts at TACE with HB were in patients with unresectable tumors despite systemic chemotherapy. Subsequently, TACE has been studied as the primary treatment for liver-only disease.
Arcement et al. reported on seven patients with unresectable HB treated by TACE [36]. These patients received cisplatin (90–150 mg/m2) and/or adriamycin (30 mg/m2) with gelatin sponge embolization in five of these patients. Lipiodol was not included in the TACE. One of these patients had significant reduction in the tumor size, but none became resectable. Three patients survived to the point of receiving OLT, with two still alive at the conclusion of the study. An additional patient was alive and awaiting OLT at 18 months. They concluded that TACE could be used as a bridge to OLT in unresectable patients who do not respond to systemic chemotherapy.
Malogolowkin et al. treated six children with HB, all of which remained unresectable after systemic chemotherapy [35]. Their protocol consisted of cisplatin (100 mg) and doxorubicin (30 mg), with one patient also receiving mitomycin (30 mg). This was mixed with bovine collagen (Angiostat, Regional Therapeutics, Inc, Pacific Palisades, CA) as an embolic agent and nonionic contrast to a volume of 8.75 ml. They did not use lipiodol and limited treatment to no more than 70 % of total liver volume and a maximum injected volume of 8.75 ml. All six children had a partial response (>50 % reduction in tumor volume). Three patients became resectable by imaging criteria, although two of these had residual microscopic disease. The patient with complete resection died of a recurrence in the residual liver. Both of the patients with microscopic residual disease survived, one after additional systemic chemotherapy and one after OLT.
Czanderna et al. reported on four patients with unresectable HB treated with TACE [39]. All patients had received systemic chemotherapy, using cisplatin (60 mg/m2), doxorubicin (30 mg/m2), and mitomycin (20 mg/m2) mixed with 10 ml or less of lipiodol, and embolized with gelatin sponge. Patients received 1–3 courses of treatment. Three of the patients had a reduction in volume of between 25 % and 33 % and a decrease AFP 83–99 %. One patient died of systemic myelotoxicity before response could be assessed. One patient received OLT, and two were resected. One patient died after resection, presumably as a cardiac complication from prior systemic chemotherapy.
Xuewu and colleagues reported on eight patients with unresectable HB [38]. They performed 1–3 TACE procedures on each patient with adriamycin (20 mg/m2), vincristine (1.5 mg/m2), and cisplatin (40 mg/m2) mixed with 5–10 ml of lipiodol. They performed coil embolization afterward. Six of the eight (75 %) became resectable after the first TACE procedure, and the two others became resectable after further TACE procedures. One patient died of pneumonia. Six had surgery, and one patient refused surgery but elected to have TACE 3 times and no longer has detectable disease. All were disease-free 15–49 months after TACE.
Li et al. reported on 16 patients with unresectable HB treated with TACE [37]. Their protocol included cisplatin (40–50 mg/m2), adriamycin (20–30 mg/m2), and lipiodol. After infusing the chemotherapy, embolization was performed with gelatin sponge particles. Total treatment was limited to <70 % of the liver volume and no more than 10 ml of solution. Patients received 1–3 treatments. The tumors demonstrated a 19–82 % reduction in size (mean = 59.2 %), with reduction in the alpha fetoprotein (AFP) of 29–99 % (mean = 60 %). The tumor became completely resectable in 13/16 patients, with the additional 3/16 patients having a partial resection 4 weeks after the last TACE. Survival at 1, 2, and 5 years was 87.5 %, 68.7 %, and 50 %, respectively, with all receiving systemic chemotherapy after surgery. No patients had chemotoxicity from the TACE. The authors thought TACE may be a first-line treatment in patients without metastatic disease.
Ohtsuka and colleagues were the first to report on using TACE as the primary treatment for HB without metastatic disease [40]. They did not limit TACE to unresectable patients, with seven children in their study. Four patients were without metastatic disease and did not receive systemic chemotherapy. The three children with metastases received systemic chemotherapy prior to TACE. TACE used pirarubicin (30 mg/m2) mixed with lipiodol (1 ml/maximum tumor diameter with 15 ml being the maximum volume given) and gelatin sponge embolization. Tumor volume reduced by 12–57 %. Complications included transient liver insufficiency in one patient who recovered in 6 days and pulmonary artery embolization of lipiodol in one patient who recovered in 2 weeks (lipiodol volume of 0.8 ml/maximum tumor diameter). They stated they believe the optimum lipiodol volume is 0.6 ml/maximum tumor diameter. All patients without metastatic disease at diagnosis had subsequent resection and were disease-free at the time of the report. The patients with metastatic disease all died, two of their metastatic disease and one from a secondary malignancy.
A similar study by Oue and colleagues had eight patients with HB [42]. Six patients had primary TACE and two had prior systemic chemotherapy before TACE. Only one patient had metastatic disease at diagnosis. This patient received systemic chemotherapy. The additional patient with systemic chemotherapy received it at an outside hospital prior to referral for TACE. TACE consisted of adriamycin (20–30 mg/m2) and cisplatin (4–60 mg/m2) mixed with lipiodol followed by gelatin sponge embolization. Tumor shrinkage varied from 0.9 % to 45 % with a mean of 25.8 %; after resection, pathology showed 71.1 % necrosis. All patients received systemic chemotherapy after surgery. Of the eight patients, six were disease-free and alive at the time of the report, although three received bone marrow transplantation due to elevated AFP levels. Two patients died of metastatic disease.
A case report by Xianliang et al. demonstrated a patient with an unresectable tumor treated solely by TACE and systemic chemotherapy [41]. The original tumor occupied 90 % of the total liver volume. TACE consisted of doxorubicin (20 mg/m2), vincristine (1.5 mg/m2), and cisplatin (40 mg/m2) in 10 ml of lipiodol followed by coil embolization. Tumor volume reduction was 75 % after the first TACE, and after three TACE sessions, no discernable residual tumor was present. He then had six courses of systemic chemotherapy (vincristine and cisplatin) followed by an additional TACE. His disease-free survival was 33 months at the time of the study.
These reports and an additional case report demonstrate that TACE has utility in treating patients with HB [33]. Response rates seem to be better when lipiodol is used in the TACE procedure. The role of TACE as a primary treatment option after failed systemic chemotherapy, bridge to OLT, or for palliation cannot be known due to the lack of randomized control trials.
While HB is the most reported liver tumor in pediatric TACE, other tumors have been treated with this technique. Hepatocellular carcinoma (HCC), sarcomas, neuroblastoma, and a paraganglioma have been treated as well with TACE [34–36, 39, 43, 44]. In one study, three patients with HCC and two patients with undifferentiated sarcoma were treated with TACE [35]. All HCC patients had a partial response. Two became resectable with one survivor. The other resectable patient had microscopic residual, received two additional course of TACE, and died of liver failure. There was no response in the sarcoma patients, both of which died. Arcement in the same study as above had seven patients with HCC [36]. Four received intra-arterial chemotherapy. All of these patients died, two after OLT. Three patients had gelatin sponge embolization after intra-arterial chemotherapy. One patient had OLT and was alive at 14 months. A single patient with HCC was treated by TACE in another study; this patient died from pulmonary embolization of lipiodol [39]. There is a case report on TACE for neuroblastoma; this patient had Stage 4S and was treated with TACE due to the large mass effect from the tumor causing pulmonary and hepatic compromise [43]. This patient received 10 mg of cisplatin and 7 mg of doxorubicin with polyvinyl alcohol particle embolization. The tumor reduced in volume by 50 % and improved symptoms. Another case report showed TACE for metastatic paraganglioma [34]. This patient had anemia unresponsive to erythropoietin and iron supplementation from a paraneoplastic syndrome and would not receive blood products due to religious beliefs. He had intra-arterial infusion of 5-fluorouracil and then two rounds of TACE with doxorubicin and cisplatin mixed with lipiodol. Gelatin sponge embolization was then performed. His hemoglobin increased from 5.6 mg/dL to 17 mg/dL. He died of a postsurgical complication after resection of his primary chest mass.
TACE for Osteogenic Sarcoma
Osteogenic sarcoma is the most common primary bone malignancy in children. Limb salvage surgery is the preferred treatment, supplemented with chemotherapy. Intra-arterial administration of the chemotherapy directed into bone tumors has been described [45]. In addition, case series have been published describing TACE in patients with osteogenic sarcoma [46, 47]. In one series, pirarubicin (30–50 mg) and cisplatin (40–80 mg) were infused into the arteries supplying the primary tumor followed by gelatin sponge embolization [46]. Limb salvage surgery was then performed within a week. In this series of 47 patients, the authors found that there was significantly less blood loss, the operating time was less, and the resection was easier as the tumors had developed a pseudocapsule of fibrous edematous tissue in 43/47 patients. Mean tumor necrosis was 82.9 %. Follow-up was short, and it is unknown what will be the effect on local recurrence. The only complication was skin blistering in three patients.
Another study of 32 patients received an infusion of methotrexate (1–2 gm), pharmorubicin (30–50 mg), and cisplatin (60–100 mg) [47]. The embolic agents included adriblastine gelatin microspheres, anhydrous alcohol with lipiodol, common bletilla tuber with lipiodol, and gelatin sponge. Necrosis occurred in 85.5 % of patients with extent being 81.6–87.9 %, although gelatin sponge results were significantly lower than the others. All patients also received systemic chemotherapy and had limb salvage surgery. The surgery was aided by the stable to decreased tumor size and less blood loss. Survival at 1, 2, and 5 years was 95.5 %, 72 %, and 42 %, respectively. Local recurrence occurred in three between 3 and 6 months after the procedure.
TACE for Wilms Tumor
Two reports are published on TACE for Wilms tumor, although both are on the same patient population [48, 49]. They treated 24 patients with TACE and compared them to a control group of 20. Their protocol was doxorubicin (20 mg/m2), cisplatin (50 mg/m2) mixed with lipiodol (0.5 ml/kg), and saline (5–15 ml) with gelatin sponge embolization. Mean tumor size reduction was 48.2 %. At 2-year follow-up, their conclusion was that TACE and surgery was superior to surgery alone. Survival at 2 years was 83.3 % in the TACE group and 10 % in the surgical group. At 1 year, 16.6 % of the TACE group was alive and disease-free as compared to the control group with only 40 %. Pathological specimens showed tumor cell necrosis, degeneration, and apoptosis; increased interstitial fibrous tissue hyperplasia; and lymphocyte infiltration [49].
References
Goldberg SN, Dupuy DE. Image-guided radiofrequency tumor ablation: challenges and opportunities-part I. J Vasc Interv Radiol. 2001;12:1021–32.
Dupuy DE, Goldberg SN. Image-guided radiofrequency tumor ablation: challenges and opportunities-part II. J Vasc Interv Radiol. 2001;12:1135–48.
Rosenthal DI, Hornicek FJ, Wolfe MW, Jennings LC, Gephart MC, Mankin HJ. Changes in the management of osteoid osteoma. J Bone Joint Surg. 1998;80:815–21.
Woertler K, Vestring T, Boettner F, Winkelmann W, Heindel W, Lindner N. Osteoid osteoma: CT-guided percutaneous radiofrequency ablation and follow-up in 47 patients. J Vasc Interv Radiol. 2001;12:717–22.
Ahmed M, Goldberg SN. Thermal ablation therapy for hepatocellular carcinoma. J Vasc Interv Radiol. 2002;13:S231–43.
Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radiofrequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441–51.
Rocourt DV, Shiels WE, Hammond S, Besner GE. Contemporary management of benign hepatic adenoma using percutaneous radiofrequency ablation. J Pediatr Surg. 2006;41(6):1149–52.
Shiels WE, Brown SD. Radiofrequency tumor ablation in children. In: van Sonnenberg E, McMullen W, Solbiati L, editors. Tumor ablation: principles and practice. New York: Springer; 2005. p. 488–95.
Hoffer FA. Pediatric applications of radiofrequency ablation. Semin Interv Radiol. 2003;20(4):323–31.
Jingjing Y, Qiang S, Minju L, Tian-an J. Percutaneous radiofrequency ablation for treatment of hepatoblastoma recurrence. Pediatr Radiol. 2008;38(9):1021–3.
Nashida Y. Radiofrequency ablation used for the treatment of frequently recurrent rhabdomyosarcoma in the masticator space in a 10-year-old girl. J Pediatr Hematol/Oncol. 2007;29:640–2.
Brown SD, van Sonnenberg E, Morrison PR, Diller L, Shamberger R. CT-guided radiofrequency ablation of pediatric Wilms tumor in a solitary kidney. Pediatr Radiol. 2005;35(9):923–8.
Sawada M, Watanabe S, Tsuda H, Kano T. An increase in body temperature during radiofrequency ablation of liver tumors. Anesth Analg. 2002;94(6):1416–20.
Katzenstein HM, Krailo MD, Malogolowkin MH, et al. Hepatocellular carcinoma in children and adolescents: results from the pediatric oncology group and the children’s cancer group intergroup study. J Clin Oncol. 2002;20:2789–97.
Bellani FF, Massimino M. Liver tumors in childhood: epidemiology and clinics. J Surg Oncol. 1993;53:119–21.
Exelby PR, Filler RM, Grosfeld JL. Liver tumors in children in the particular reference to hepatoblastoma and hepatocellular carcinoma. American Academy of Pediatrics Surgical Section Survey 1974. J Pediatr Surg. 1975;10:329–37.
Iannitti DA, Dupuy DE, Mayo-Smith WW, Murphy B. Hepatic radiofrequency ablation. Arch Surg. 2002;137:422–6. discussion 427.
Gervais DA, McGovern FJ, Arellano RS, McDougal WS, Mueller PR. Renal cell carcinoma: clinical experience and technical success with radiofrequency ablation of 42 tumors. Radiology. 2003;226:417–24.
Gervais DA, McGovern FJ, Wood BJ, et al. Radiofrequency ablation of renal cell carcinoma: early clinical experience. Radiology. 2000;217:665–72.
Roy-Choudhury SH, Cast JE, Cooksey G, Puri S, Breen DJ. Early experience with radiofrequency ablation of small solid renal masses. AJR Am J Roentgenol. 2003;180:1055–61.
Goldberg SN, Solbiati L, Halpern EF, Gazelle GS. Variables affecting proper system grounding for radiofrequency ablation in an animal model. J Vasc Interv Radiol. 2000;11:1069–75.
Ramnath RR, Rosenthal DI, Cates J, Gebhardt M, Quinn RH. Intracortical chondroma simulating osteoid osteoma treated by radiofrequency. Skeletal Radiol. 2002;31(10):597–602.
Chopra S, Dodd GD, Chanin MP, Chintapalli KN. Radiofrequency ablation of hepatic tumors adjacent to the gallbladder; feasibility and safety. AJR Am J Roentgenol. 2003;180:697–701.
Livraghi T, Lazzaroni S, Meloni F. Radiofrequency thermal ablation of hepatocellular carcinoma. Eur J Ultrasound. 2001;13:159–66.
Ahmed M, Lobo SM, Weinstein J, Kruskai JB, Gazelle GS, Halpern EF, Afzal SK, Lenkinski RE, Goldberg SN. Improved coagulation with saline solution pretreatment during radiofrequency tumor ablation in a canine model. J Vasc Interv Radiol. 2002;13:717–24.
Bloomston M, Binitie O, Fraiji E, et al. Transcatheter arterial chemoembolization with or without radiofrequency ablation in the management of patients with advanced hepatic malignancy. Am Surg. 2002;68:827–31.
Shankar S, van Sonnenberg E, Silverman SG, Tuncali KT, Morrison PR. Combined radiofrequency and direct alcohol infusion for percutaneous tumor ablation. Presented at the 88th Scientific Assembly and Annual meeting, RSNA. Chicago; 2002 Dec.
Dupuy DE, Zagoria RJ, Akerley W, Mayo-Smith WM, Kavanagh PV, Safran H. Percutaneous radiofrequency ablation of malignancies in the lung. AJR Am J Roentgenol. 2000;174:57–9.
Simon CJ, Dupuy DE, DiPetrillo TA, et al. Pulmonary radiofrequency ablation: long-term safety and efficacy in 153 patients. Radiology. 2007;243:268–75.
Nakamura T, Matsumine A, Yamakado K, et al. Lung radiofrequency ablation in patients with pulmonary metastases from musculoskeletal sarcomas. Cancer. 2009;115(16):3774–81.
Kariya Z, Yamakado K, Nakatuka A, Onaoda M, Kobayasi S, Takeda K. Radiofrequency ablation with and without balloon occlusion of the renal artery: an experimental study in porcine kidneys. J Vasc Interv Radiol. 2003;14:241–5.
Guvnén P. Liver embolizations in oncology; a review. Part I. Arterial (chemo) embolizations. Med Oncol. 2008;25(1):1–11.
Tashjian DB, Moriarty KP, Courtney RA, Bean MS, Steele DA. Preoperative chemoembolization for unresectable hepatoblastoma. Pediatr Surg Int. 2002;18(2–3):187–9.
Mutabagani KH, Klopfenstein KJ, Hogan MJ, Caniano DA. Metastatic paraganglioma and paraneoplastic-induced anemia in an adolescent: treatment with hepatic arterial chemoembolization. J Pediatr Hematol Oncol. 1999;21(6):544–7.
Malogolowkin MH, Stanley P, Steele DA, Ortega JA. Feasibility and toxicity of chemoembolization for children with liver tumors. J Clin Oncol. 2000;18(6):1279–84.
Arcement CM, Towbin RB, Meza MP, Gerber DA, Kaye RD, Mazariegos GV, Carr BI, Reyes J. Intrahepatic chemoembolization in unresectable pediatric liver malignancies. Pediatr Radiol. 2000;30(11):779–85.
Li JP, Chu JP, Yang JY, Chen W, Wang Y, Huang YH. Preoperative transcatheter selective arterial chemoembolization in treatment of unresectable hepatoblastoma in infants and children. Cardiovasc Intervent Radiol. 2008;31(6):1117–23.
Xuewu J, Jianhong L, Xianliang H, Zhongxian C. Combined treatment of hepatoblastoma with transcatheter arterial chemoembolization and surgery. Pediatr Hematol Oncol. 2006;23(1):1–9.
Czauderna P, Zbrzezniak G, Narozanski W, Korzon M, Wyszomirska M, Stoba C. Preliminary experience with arterial chemoembolization for hepatoblastoma and hepatocellular carcinoma in children. Pediatr Blood Cancer. 2006;46(7):825–8.
Ohtsuka Y, Matsunaga T, Yoshida H, Kouchi K, Okada T, Ohnuma N. Optimal strategy of preoperative transcatheter arterial chemoembolization for hepatoblastoma. Surg Today. 2004;34(2):127–33.
Xianliang H, Jianhong L, Xuewu J, Zhongxian C. Cure of hepatoblastoma with transcatheter arterial chemoembolization. J Pediatr Hematol Oncol. 2004;26(1):60–3.
Oue T, Fukuzawa M, Kusafuka T, Kohmoto Y, Okada A, Imura K. Transcatheter arterial chemoembolization in the treatment of hepatoblastoma. J Pediatr Surg. 1998;33(12):1771–5.
Weintraub M, Bloom AI, Gross E, Revel-Vilk S, Shahroor S, Koplewitz BZ, Freeman AI. Successful treatment of progressive state 4s hepatic neuroblastoma in a neonate with intra-arterial chemoembolization. Pediatr Blood Cancer. 2004;43(2):148–51.
Uemura S, Todani T, Watanabe Y, Toki A, Sato Y, Morotomi Y, Ohkawa M, Kojima K, Seo H. Successful left hepatectomy for hepatocellular carcinoma in a child after transcatheter arterial chemoembolization; report of a survival. Eur J Pediatr Surg. 1993;3(1):54–6.
Wang MQ, Dake MD, Wang ZP, et al. Isolated lower extremity chemotherapeutic infusion for treatment of osteogenic sarcoma; experimental study and preliminary clinical report. J Vasc Interv Radiol. 2001;12:731–7.
Zhang HJ, Yang JJ, Lu JP, Lai CJ, Sheng J, Li YX, Hao Q, Zhang SM, Gupta S. Use of intra-arterial chemotherapy and embolization before limb salvage surgery for osteosarcoma of the lower extremity. Cardiovasc Intervent Radiol. 2009;32(4):672–8.
Chu JP, Chen W, Li JP, Zhuang WQ, Huang YH, Huang ZM, Yang JY. Clinicopathologic features and results of transcatheter arterial chemoembolization for osteosarcoma. Cardiovasc Intervent Radiol. 2007;30(2):201–6.
Liu WG, Gu WZ, Zhou YB, Tang HF, Li MJ, Ma WX. The prognostic relevance of preoperative transcatheter arterial chemoembolizaton (TACE) and PCNA/VEGF expression in patients with Wilms’ tumour. Eur J Clin Invest. 2008;38(12):931–8.
Li JP, Chu JP, Oh P, Li Z, Chen W, Huang YH, Yang JY. Characterizing clinicopathological findings of transarterial chemoembolization for Wilms tumor. J Urol. 2010;183(3):1138–44.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this entry
Cite this entry
Shiels, W.E., Hogan, M.J. (2013). Percutaneous Image-Guided Treatment of Pediatric Malignancy. In: Dupuy, D., Fong, Y., McMullen, W. (eds) Image-Guided Cancer Therapy. Springer, New York, NY. https://doi.org/10.1007/978-1-4419-0751-6_64
Download citation
DOI: https://doi.org/10.1007/978-1-4419-0751-6_64
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4419-0750-9
Online ISBN: 978-1-4419-0751-6
eBook Packages: MedicineReference Module Medicine