Abstract
Pancreatic cancer is the tenth most common cancer and is the fourth leading cause of cancer mortality. While surgery remains the current potentially curative treatment of choice, few cancers are resectable at presentation. Chemotherapy and radiation play a vital role in locally advanced non-metastatic pancreatic cancer. Systemic therapy is vital in these patients and often, when they remain non-metastatic after induction therapy radiation improves local control. Conventional chemoradiation is delivered in 5–6 weeks. Stereotactic body radiotherapy (SBRT) has been used in patients with locally advanced pancreas cancer, in fewer treatments, without significantly affecting systemic therapy, thereby maximizing systemic and local control. SBRT has also been used to boost positive margins, local recurrences after prior radiation, and in oligometastatic pancreatic cancer.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
10.1 Introduction
With an estimated 45,220 new cases, resulting in 38,460 deaths, in the United States in 2013, pancreatic cancer is the fourth-leading cause of cancer-related deaths [1]. Despite many new treatment approaches, pancreatic cancer survival has not improved in the past 25 years [2]. Surgical resection remains the only treatment approach with the potential for providing long-term survival for patients without metastatic disease at presentation, but between 40 and 50 % of patients have locally advanced inoperable cancer at presentation or at surgical exploration [3]. The 5-year overall survival rate for patients with inoperable pancreatic cancer is less than 5 % [4–6].
Radiation therapy and chemotherapy, used either alone or in combination, are the only treatment options available for patients with locally advanced unresectable disease [6, 7]. However these treatments only marginally improve the median survival time to between 8 and 14 months [8–15]. Conventional radiation therapy requires approximately 6 weeks of daily treatments, which thus may take a significant fraction of the patient’s limited life expectancy. In addition, side effects from radiation therapy can be substantial. Studies using chemotherapy alone have suggested outcomes equivalent to those of chemoradiation, raising further doubts about the value of a long course of conventional radiation therapy given the additional toxicity [16–23].
Stereotactic body radiotherapy (SBRT) is a minimally invasive treatment option that has been shown in prospective Phase I and Phase II studies and single institution studies to be a safe, quick and feasible approach for the treatment of locally advanced pancreatic cancer [24–36].
10.2 Patient Selection
Patients with biopsy proven non-metastatic locally advanced unresectable pancreatic cancer who are referred for conventional chemoradiation are also ideal potential candidates for SBRT. Patients with gastric or duodenal obstruction are generally excluded. Patients with borderline resectable disease can also be considered for this hypofractionated approach. A radiologist and experienced pancreatic surgeon should review a pancreas-specific multiphasic CT angiogram, to determine inoperability using standard CT criteria [37]. Patients who appear potentially resectable by axial imaging and considered for surgery and those found unresectable at the time of surgery would also be candidates. EUS (Endoscopic Ultrasound) has further increased diagnostic yield to identify patients with unresectable pancreas cancer and use the opportunity to obtain histological diagnosis and placement of fiducials [38].
Most patients eventually succumb to metastatic disease. This fact and randomized trials which did not show the benefit of addition of radiation therapy to systemic treatment [9] emphasizes the role of systemic therapy. As with conventional chemoradiation [12], it is now acceptable practice to use systemic therapy as induction therapy prior to SBRT for pancreas cancer. Single institution prospective studies have indeed validated tis approach [31].
10.3 Patient Work-Up
In addition to the history and clinical examination, Carbohydrate antigen 19–9 (CA19–9] measurements, complete blood counts, and a biochemistry panel including liver function tests are performed in all patients prior to treatment and at follow-up. As mentioned above a multiphasic CT scan of the abdomen with oral and IV contrast is mandatory in the evaluation of these patients.
10.4 Treatment Planning
For image guidance, three to five fiducial seeds are commonly placed in and around the tumor percutaneously using CT guidance, at laparotomy, or (more commonly) under endoscopic ultrasound guidance. CT planning images with oral and IV contrast are obtained at least 1 week after fiducial placement, to allow for potential fiducial migration. Patients were imaged and treated in the supine position, with their arms down, lying in memory foam placed over a customized Vac-LokTM (CIVCO Medical Solutions, Orange City, IA) immobilization cradle to ensure a comfortable and reproducible position. The CT images are transferred to a SBRT planning workstation and the target volume (visible gross disease) and critical structures, including the stomach, duodenum, kidneys, liver and spinal cord, are contoured. The clinical target volume is generally defined as the gross disease. No expansion margin is used where the tumor was in contact with the bowel (stomach or duodenum); otherwise, a 5 mm or smaller expansion margin (extending up to the outer bowel wall) is usually included to determine the planning target volume (PTV) [31].
10.5 Stereotactic Body Radiotherapy Dose Prescription
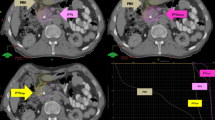
The SBRT dose is commonly prescribed to a conformal isodose line generally covering at least 95 % of the target volume (usually 70—80 %). While some institutions use a single fixed dose prescription [25–27, 29, 30, 34, 39], others use a radiation dose based on the relationship between the tumor location and the gastroduodenal loop, in order to limit toxicity (Fig. 10.1) [32]. However, when patients have had a palliative gastro-duodenal bypass, such duodenal constraints may have a lesser significance. The suggested maximal point tolerance dose of the duodenum is three fractions of 10 Gy each [40, 41]. In one institutional scheme [32], if the tumor approximated one-third or more of the circumference of the duodenum or stomach, then a dose of 24 Gy (three fractions of 8 Gy each) is used. If the tumor abutted the bowel in only one area and/or the space between the tumor and the bowel wall was less than 3 mm, then a dose of 30 Gy (three fractions of 10 Gy each) is prescribed. Finally, if the gap between the tumor and the duodenum was 3 mm or wider, a dose of 36 Gy (three fractions of 12 Gy each) is used. A representative treatment plan is shown in Fig. 10.2.
Normal tissue constraints are respected during treatment planning. When treating in three fractions, the volumes of liver receiving 21 Gy (V21) or more and 15 Gy (V15) or more are kept below 30 and 50 %, respectively. The corresponding doses to these volumes in single fraction would be V12 and V 7 [42]. The volume of each kidney receiving 12 Gy or more will be maintained below 25 %. The total maximal spinal cord limit is usually 12 Gy and the maximum point dose to the bowel is less 10 Gy per fraction.
10.6 Treatment Delivery
The CyberKnife Robotic Radiosurgery system (Accuray Incorporated, Sunnyvale, CA) with Synchrony motion tracking has been commonly used as a SBRT system to treat locally advanced pancreas cancer. Other SBRT modalities with image guidance and respiratory gating or dampening (E.g. Novalis, TrueBeam) can be equally applicable to treat these patients. Patients are generally premedicated with H2 receptor blockers or proton pump inhibitors, and antiemetics.
10.7 Systemic Chemotherapy
Patients with unresectable, locally advanced pancreatic cancer often progress with metastatic disease as they harbor micrometastasis at presentation. Hence systemic therapy plays a vital role in the management of these patients. By utilizing a strategy of delivering upfront systemic chemotherapy it is possible to select those patients who are more likely to benefit from local therapy [12, 31]. Intensified chemotherapy regimens with proven improved response rates in the metastatic setting, like FOLFIRINOX, or Gemcitabine and nab-Paclitaxel is now being routinely used in patients with locally advanced pancreas cancer.
The use of single- and multiple-fraction SBRT has been shown to be feasible and safe for patients with locally advanced pancreatic cancer. The results of published data on the use of SBRT in locally advanced pancreas cancer is presented in Table 10.1 [25–27, 29–36, 39, 43]. In contrast to 5–6 weeks of conventional chemoradiation, SBRT can be performed in only 1–3 days, resulting in only a minimal delay in initiating systemic therapy. When SBRT is selectively used for patients who have been treated with systemic therapy, it appears to benefit them most without immediate overt development of metastasis when used upfront [31, 44].
Initial experience with single fraction SBRT with or without external beam radiation has been fraught with acute and chronic toxicity [25, 27, 29, 30]. Similarly high fixed doses of SBRT without accounting for respiratory motion has be associated with significant toxicity [27]. More recently tolerance based moderate doses of hypo fractionated radiation, with respiratory motion tracking and in the setting of systemic therapy has proven to be an acceptable regime [31]. Assuming an α/β ratio of 10 for pancreatic tumor response, Chang et al. calculated their scheme to be equivalent to 74 Gy delivered in 1.8-Gy fractions of conventional radiation. The Hoyer et al. study gave a dose equivalent to 95 Gy. In the study from Mahadevan et al., the equivalent dose was 51–76 Gy, comparable to a conventional radiation dose. While this may potentially appear to decrease the likelihood of local control it likely provides a better therapeutic ratio. The RBE (Relative Biological Effectiveness) and the equivalent doses for tumor control, acute and late toxicity in these series are presented in Table 10.2 [25, 27, 32].
10.8 Toxicity
Most patients experience fatigue and some nausea and temporary loss of appetite. Flare pain at the tumour site can also sometime occur and is usually self-limiting. Given the high doses of radiation per fraction, and the close association of the gaswPhase I and Phase II SBRT toxicity data for liver tumors suggest that the maximum point dose to the duodenum should be kept below the equivalent of three fractions each of 10 Gy (equivalent to 130 Gy in 1.8-Gy fractions, assuming an α/β ratio of 3) [40]. Other Dose Volume constraints have been proposed [45, 46]. The acute and long-term toxicity of the Hoyer study was higher than either the Stanford or the Boston groups. Assuming an α/β ratio of 3, the Chang et al., Hoyer et al. and Mahadevan et al. treatment schemes are equivalent to 233 Gy, 270 Gy, and 88–180 Gy in 1.8-Gy fractions, respectively. While this may explain the toxicity, a tolerance based (gastroduodenal tolerance) approach could decrease toxicity. This strategy has been used to reduce toxicity in other cancers treated with SBRT [47, 48].
10.9 Review of Literature
The treatment of patients with non-metastatic locally advanced pancreatic cancer continues to evolve. While the data is conflicting, the general standard of care appears to be concurrent chemoradiation in addition to systemic chemotherapy [49]. However, the effectiveness of the addition of chemoradiation to a chemotherapy treatment plan has been questioned. In addition there are significant side effects associated with 5–6 weeks of upper abdominal radiation, which are particularly problematic for these patients with short life expectancies. Nevertheless, randomized trials have shown a survival benefit to giving radiation therapy to such patients, as in other gastrointestinal cancers, and radiotherapy may be particularly helpful in local control and palliating local symptoms [50–52].
The use of single- and multiple-fraction SBRT has been shown to be feasible and safe for patients with locally advanced pancreatic cancer in several series. In contrast to 5–6 weeks of conventional chemoradiation, SBRT can be performed in only 1–3 days, resulting in only a minimal delay in initiating systemic therapy. Table 10.1 summarizes the outcomes for published studies of SRS and SBRT. Initial experience with single fraction SBRT with or without external beam radiation has been fraught with acute and chronic toxicity. Similarly high fixed doses of SBRT without accounting for respiratory motion has been associated with significant toxicity. More recently tolerance based moderate doses of hypo fractionated radiation, with respiratory motion tracking and in the setting of systemic therapy has proven to be an acceptable regime. While fractionation may decrease the likelihood of local control it potentially provides a better therapeutic ratio. The RBE (Relative Biological Effectiveness) and the equivalent doses for tumor control, acute and late toxicity in these series are presented in Table 10.2. Assuming an α/β ratio of 10 for pancreatic tumor response, 25 Gy single fraction is equivalent to 74 Gy delivered in 1.8-Gy fractions of conventional radiation. Similarly 45 Gy in three fractions delivers a dose equivalent to 95 Gy and the 24–36 Gy equivalent doses are 51–76 Gy, comparable to a conventional radiation dose. This along with consideration of equivalent doses for long-term toxicity as described above may explain the differences in outcomes.
10.10 Other Potential Roles of SBRT in Pancreas Cancer
Local failure is a significant problem in resected cancers even after an R0 resection. It is particularly relevant in patients with positive margins. A stereotactic boost to areas of known positive margins in addition to standard adjuvant therapy may provide additional local control and even a survival benefit [53]. In Boston fiducial seeds are routinely placed during pancreaticoduodenectomy at the uncinate, retroperitoneal, superior mesenteric and pancreatic margins, and pathology guided stereotactic boost of 10 Gy is delivered in addition to standard 50 Gy of postoperative chemoradiation for R1 resections.
Young patients with good performance status and isolated oligo metastasis (e.g. liver metastasis) presenting synchronously or metachronously, may also benefit from local control with SBRT in addition to systemic therapy. Yet another rare indication would include re-irradiation for local failure after prior radiation therapy in the absence of controlled metastatic disease.
10.11 Conclusion
SBRT can be delivered safely and quickly to potentially benefit patients with locally advanced unresectable pancreatic cancer. The toxicity and outcomes appear comparable or more favorable than those of conventional chemoradiation. A randomized trial will be required to answer whether SBRT plus chemotherapy will improve progression-free survival, overall survival, and patients’ quality of life compared to chemotherapy with or without conventional chemoradiation. SBRT may have a role in patients with positive margins, oligometastasis and local recurrence after prior radiation.
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. PubMed PMID: 23335087.
Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378(9791):607–20. PubMed PMID: 21620466.
Staley CA, Cleary KR, Abbruzzese JL, Lee JE, Ames FC, Fenoglio CJ, et al. The need for standardized pathologic staging of pancreaticoduodenectomy specimens. Pancreas. 1996;12(4):373–80. PubMed PMID: 8740405.
Cardenes HR, Chiorean EG, Dewitt J, Schmidt M, Loehrer P. Locally advanced pancreatic cancer: current therapeutic approach. Oncologist. 2006;11(6):612–23. PubMed PMID: 16794240.
Ducreux M, Boige V, Goere D, Deutsch E, Ezra P, Elias D, et al. The multidisciplinary management of gastrointestinal cancer. Pancreatic cancer: from pathogenesis to cure. Best Pract Res Clin Gastroenterol. 2007;21(6):997–1014. PubMed PMID: 18070700.
Yip D, Karapetis C, Strickland A, Steer CB, Goldstein D. Chemotherapy and radiotherapy for inoperable advanced pancreatic cancer. Cochrane Database Syst Rev. 2006;3, CD002093. PubMed PMID: 16855985.
Morganti AG, Massaccesi M, La Torre G, Caravatta L, Piscopo A, Tambaro R, et al. A systematic review of resectability and survival after concurrent chemoradiation in primarily unresectable pancreatic cancer. Ann Surg Oncol. 2010;17(1):194–205. PubMed PMID: 19856029.
Blackstock AW, Tepper JE, Niedwiecki D, Hollis DR, Mayer RJ, Tempero MA. Cancer and leukemia group B (CALGB) 89805: phase II chemoradiation trial using gemcitabine in patients with locoregional adenocarcinoma of the pancreas. Int J Gastrointest Cancer. 2003;34(2–3):107–16. PubMed PMID: 15361643.
Chauffert B, Mornex F, Bonnetain F, Rougier P, Mariette C, Bouche O, et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000–01 FFCD/SFRO study. Ann Oncol. 2008;19(9):1592–9.
Crane CH, Varadhachary G, Pisters PW, Evans DB, Wolff RA. Future chemoradiation strategies in pancreatic cancer. Semin Oncol. 2007;34(4):335–46. PubMed PMID: 17674962.
Crane CH, Winter K, Regine WF, Safran H, Rich TA, Curran W, et al. Phase II study of bevacizumab with concurrent capecitabine and radiation followed by maintenance gemcitabine and bevacizumab for locally advanced pancreatic cancer: Radiation Therapy Oncology Group RTOG 0411. J Clin Oncol. 2009;27(25):4096–102. PubMed PMID: 19636002. Pubmed Central PMCID: 2734421.
Huguet F, Andre T, Hammel P, Artru P, Balosso J, Selle F, et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol. 2007;25(3):326–31. PubMed PMID: 17235048.
Magnino A, Gatti M, Massucco P, Sperti E, Faggiuolo R, Regge D, et al. Phase II trial of primary radiation therapy and concurrent chemotherapy for patients with locally advanced pancreatic cancer. Oncology. 2005;68(4–6):493–9. PubMed PMID: 16020980.
Okusaka T, Furuse J, Funakoshi A, Ioka T, Yamao K, Ohkawa S, et al. Phase II study of erlotinib plus gemcitabine in Japanese patients with unresectable pancreatic cancer. Cancer Sci. 2011;102(2):425–31. PubMed PMID: 21175992.
Okusaka T, Ito Y, Ueno H, Ikeda M, Takezako Y, Morizane C, et al. Phase II study of radiotherapy combined with gemcitabine for locally advanced pancreatic cancer. Br J Cancer. 2004;91(4):673–7. PubMed PMID: 15226765. Pubmed Central PMCID: 2364779.
Andre T, Noirclerc M, Hammel P, Meckenstock R, Landi B, Cattan S, et al. Phase II study of leucovorin, 5-fluorouracil and gemcitabine for locally advanced and metastatic pancreatic cancer (FOLFUGEM 2). Gastroenterol Clin Biol. 2004;28(8–9):645–50. PubMed PMID: 15646530.
El-Rayes BF, Zalupski MM, Shields AF, Vaishampayan U, Heilbrun LK, Jain V, et al. Phase II study of gemcitabine, cisplatin, and infusional fluorouracil in advanced pancreatic cancer. J Clin Oncol. 2003;21(15):2920–5. PubMed PMID: 12885810.
Heinemann V, Quietzsch D, Gieseler F, Gonnermann M, Schonekas H, Rost A, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol. 2006;24(24):3946–52. PubMed PMID: 16921047.
Louvet C, Andre T, Lledo G, Hammel P, Bleiberg H, Bouleuc C, et al. Gemcitabine combined with oxaliplatin in advanced pancreatic adenocarcinoma: final results of a GERCOR multicenter phase II study. J Clin Oncol. 2002;20(6):1512–8. PubMed PMID: 11896099.
Louvet C, Labianca R, Hammel P, Lledo G, Zampino MG, Andre T, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005;23(15):3509–16. PubMed PMID: 15908661.
O’Reilly EM, Abou-Alfa GK. Cytotoxic therapy for advanced pancreatic adenocarcinoma. Semin Oncol. 2007;34(4):347–53. PubMed PMID: 17674963.
Reni M, Passoni P, Panucci MG, Nicoletti R, Galli L, Balzano G, et al. Definitive results of a phase II trial of cisplatin, epirubicin, continuous-infusion fluorouracil, and gemcitabine in stage IV pancreatic adenocarcinoma. J Clin Oncol. 2001;19(10):2679–86. PubMed PMID: 11352960.
Stathopoulos GP, Syrigos K, Aravantinos G, Polyzos A, Papakotoulas P, Fountzilas G, et al. A multicenter phase III trial comparing irinotecan-gemcitabine (IG) with gemcitabine (G) monotherapy as first-line treatment in patients with locally advanced or metastatic pancreatic cancer. Br J Cancer. 2006;95(5):587–92. PubMed PMID: 16909140. Pubmed Central PMCID: 2360678.
Chang BW, Saif MW. Stereotactic body radiation therapy (SBRT) in pancreatic cancer: is it ready for prime time? JOP. 2008;9(6):676–82. PubMed PMID: 18981547.
Chang DT, Schellenberg D, Shen J, Kim J, Goodman KA, Fisher GA, et al. Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer. 2009;115(3):665–72. PubMed PMID: 19117351.
Schellenberg D, Kim J, Christman-Skieller C, Chun CL, Columbo LA, Ford JM, et al. Single-fraction stereotactic body radiation therapy and sequential gemcitabine for the treatment of locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2011;81(1):181–8. PubMed PMID: 21549517.
Hoyer M, Roed H, Sengelov L, Traberg A, Ohlhuis L, Pedersen J, et al. Phase-II study on stereotactic radiotherapy of locally advanced pancreatic carcinoma. Radiother Oncol. 2005;76(1):48–53. PubMed PMID: 15990186.
Schellenberg D, Goodman KA, Lee F, Chang S, Kuo T, Ford JM, et al. Gemcitabine chemotherapy and single-fraction stereotactic body radiotherapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2008;72(3):678–86. PubMed PMID: 18395362.
Koong AC, Le QT, Ho A, Fong B, Fisher G, Cho C, et al. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;58(4):1017–21. PubMed PMID: 15001240.
Koong AC, Christofferson E, Le QT, Goodman KA, Ho A, Kuo T, et al. Phase II study to assess the efficacy of conventionally fractionated radiotherapy followed by a stereotactic radiosurgery boost in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2005;63(2):320–3. PubMed PMID: 16168826.
Mahadevan A, Miksad R, Goldstein M, Sullivan R, Bullock A, Buchbinder E, et al. Induction gemcitabine and stereotactic body radiotherapy for locally advanced nonmetastatic pancreas cancer. Int J Radiat Oncol Biol Phys. 2011;81(4):e615–22. PubMed PMID: 21658854.
Mahadevan A, Jain S, Goldstein M, Miksad R, Pleskow D, Sawhney M, et al. Stereotactic body radiotherapy and gemcitabine for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2010;78(3):735–42. PubMed PMID: 20171803.
Polistina F, Costantin G, Casamassima F, Francescon P, Guglielmi R, Panizzoni G, et al. Unresectable locally advanced pancreatic cancer: a multimodal treatment using neoadjuvant chemoradiotherapy (gemcitabine plus stereotactic radiosurgery) and subsequent surgical exploration. Ann Surg Oncol. 2010;17(8):2092–101. PubMed PMID: 20224860.
Rwigema JC, Parikh SD, Heron DE, Howell M, Zeh H, Moser AJ, et al. Stereotactic body radiotherapy in the treatment of advanced adenocarcinoma of the pancreas. Am J Clin Oncol. 2011;34(1):63–9. PubMed PMID: 20308870.
Tozzi A, Comito T, Alongi F, Navarria P, Iftode C, Mancosu P, et al. SBRT in unresectable advanced pancreatic cancer: preliminary results of a mono-institutional experience. Radiat Oncol. 2013;8(1):148. PubMed PMID: 23799996. Pubmed Central PMCID: 3707803.
Chuong MD, Springett GM, Freilich JM, Park CK, Weber JM, Mellon EA, et al. Stereotactic body radiation therapy for locally advanced and borderline resectable pancreatic cancer is effective and well tolerated. Int J Radiat Oncol Biol Phys. 2013;86(3):516–22. PubMed PMID: 23562768.
Raptopoulos V, Steer ML, Sheiman RG, Vrachliotis TG, Gougoutas CA, Movson JS. The use of helical CT and CT angiography to predict vascular involvement from pancreatic cancer: correlation with findings at surgery. AJR Am J Roentgenol. 1997;168(4):971–7. PubMed PMID: 9124153.
Morris-Stiff G, Escofet X, Barry JD, Lewis WG, Puntis MC, Roberts SA. Selective use of endoscopic ultrasound in the evaluation of carcinomas of the pancreatic head. Dig Surg. 2011;28(5–6):373–8. PubMed PMID: 22134196.
Goyal K, Einstein D, Ibarra RA, Yao M, Kunos C, Ellis R, et al. Stereotactic body radiation therapy for nonresectable tumors of the pancreas. J Surg Res. 2012;174(2):319–25. PubMed PMID: 21937061.
Rusthoven KE, Kavanagh BD, Cardenes H, Stieber VW, Burri SH, Feigenberg SJ, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. 2009;27(10):1572–8. PubMed PMID: 19255321.
Schefter TE, Kavanagh BD, Timmerman RD, Cardenes HR, Baron A, Gaspar LE. A phase I trial of stereotactic body radiation therapy (SBRT) for liver metastases. Int J Radiat Oncol Biol Phys. 2005;62(5):1371–8. PubMed PMID: 16029795.
Pan CC, Kavanagh BD, Dawson LA, Li XA, Das SK, Miften M, et al. Radiation-associated liver injury. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S94–100. PubMed PMID: 20171524.
Didolkar MS, Coleman CW, Brenner MJ, Chu KU, Olexa N, Stanwyck E, et al. Image-guided stereotactic radiosurgery for locally advanced pancreatic adenocarcinoma results of first 85 patients. J Gastrointest Surg. 2010;14(10):1547–59. PubMed PMID: 20839073.
Gurka MK, Collins SP, Slack R, Tse G, Charabaty A, Ley L, et al. Stereotactic body radiation therapy with concurrent full-dose gemcitabine for locally advanced pancreatic cancer: a pilot trial demonstrating safety. Radiat Oncol. 2013;8:44. PubMed PMID: 23452509. Pubmed Central PMCID: 3607991.
Taniguchi CM, Murphy JD, Eclov N, Atwood TF, Kielar KN, Christman-Skieller C, et al. Dosimetric analysis of organs at risk during expiratory gating in stereotactic body radiation therapy for pancreatic cancer. Int J Radiat Oncol Biol Phys. 2013;85(4):1090–5. PubMed PMID: 23273994.
Murphy JD, Christman-Skieller C, Kim J, Dieterich S, Chang DT, Koong AC. A dosimetric model of duodenal toxicity after stereotactic body radiotherapy for pancreatic cancer. Int J Radiat Oncol Biol Phys. 2010;78(5):1420–6. PubMed PMID: 20399033.
Lee MT, Kim JJ, Dinniwell R, Brierley J, Lockwood G, Wong R, et al. Phase I study of individualized stereotactic body radiotherapy of liver metastases. J Clin Oncol. 2009;27(10):1585–91. PubMed PMID: 19255313.
Tse RV, Hawkins M, Lockwood G, Kim JJ, Cummings B, Knox J, et al. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. 2008;26(4):657–64. PubMed PMID: 18172187.
Richter J, Saif MW. Locally advanced pancreatic adenocarcinoma: where are we and where are we going? Highlights from the “2010 ASCO Gastrointestinal Cancers Symposium”. Orlando, FL, USA. January 22–24, 2010. JOP. 2010;11(2):139–43.
Ko AH, Crane CH. Radiation therapy in operable and locally advanced pancreatic cancer. J Natl Compr Canc Netw. 2010;8(9):1022–31. PubMed PMID: 20876542.
Minn AY, Koong AC, Chang DT. Stereotactic body radiation therapy for gastrointestinal malignancies. Front Radiat Ther Oncol. 2011;43:412–27. PubMed PMID: 21625166.
Savir G, Huber KE, Saif MW. Locally advanced pancreatic cancer. Looking beyond traditional chemotherapy and radiation. JOP. 2013;14(4):337–9. PubMed PMID: 23846922.
Rwigema JC, Heron DE, Parikh SD, Zeh 3rd HJ, Moser JA, Bahary N, et al. Adjuvant stereotactic body radiotherapy for resected pancreatic adenocarcinoma with close or positive margins. J Gastrointest Cancer. 2012;43(1):70–6. PubMed PMID: 20809393.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag London
About this chapter
Cite this chapter
Mahadevan, A., Gaya, A.M. (2015). Stereotactic Body Radiotherapy (SBRT) for Pancreatic Cancer. In: Gaya, A., Mahadevan, A. (eds) Stereotactic Body Radiotherapy. Springer, London. https://doi.org/10.1007/978-0-85729-597-2_10
Download citation
DOI: https://doi.org/10.1007/978-0-85729-597-2_10
Published:
Publisher Name: Springer, London
Print ISBN: 978-0-85729-596-5
Online ISBN: 978-0-85729-597-2
eBook Packages: MedicineMedicine (R0)