Abstract
Background
Locally advanced unresectable pancreatic adenocarcinoma is characterized by poor survival despite chemotherapy and conventional radiation therapy (RT). Recent advances in real-time image-guided stereotactic radiosurgery (SRS) have made it possible to treat these cancers in two to four fractions followed by systemic chemotherapy.
Aims
The aims of this study includes the following: (1) obtain local control of the disease; (2) improve the survival of these unresectable patients; (3) evaluate the toxicity of SRS; and (4) report results of the largest series from a single center.
Methods
Pancreatic SRS involves delivery of high doses of accurately targeted radiation given non-invasively in two to four fractions. We treated 85 consecutive patients with locally advanced and recurrent pancreatic adenocarcinoma from February 2004 to November 2009. Age range: 36–88 years, median 66 years; sex: 50 males, 35 females; race: 79 Caucasian, five African American, one Asian; histology: 80 adenocarcinoma, three islet cell, two other. Pre-SRS staging: T3–4 85; N+ 16, Nx 57, N0 12; M0 64, M1 21. All patients were unresectable at the time of SRS. Seventy-one had no prior surgical resection, and 14 had local recurrence after prior surgical resection. Twenty-nine patients had progression of disease after prior conventional RT. Location of the tumor: head, 57; body and tail, 28. Pre-SRS chemotherapy was given in 48 patients. All patients received gemcitabine-based chemotherapy regimen after SRS. Median tumor volume was 60 cm3. PET/CT scans done in 55 patients were positive in 52 and negative in three patients. Average maximum standard uptake value was 6.9. Pain score on a scale of 1–10 was: 0–3 in 54, 4–7 in 18, and 8–10 in 13 patients. SRS doses ranged from 15 to 30 Gy with a mean dose of 25.5 Gy delivered in 3 days divided in equal fractions. Mean conformality index was 1.6, and mean isodose line was 80%.
Results
Tumor control: complete, partial, and stable disease were observed in 78 patients for the duration of 3–36 months with median of 8 months. Pain relief was noted in majority of patients lasting for 18–24 weeks. Most of the patients died of distant disease progression while their primary tumor was controlled. Overall median survival from diagnosis was 18.6 months and from SRS it was 8.65 months. For the group of 35 patients with adenocarcinoma without prior surgical resection or RT and no distant metastases, the average and 1-year survival from diagnosis was 15 months and 50%, respectively, and from SRS it was 11.15 months and 30.5%, respectively.
Toxicity
A total of 19 (22.37%) patients developed grades III/IV GI toxicity including duodenitis, 12 (14.1%); gastritis, 11 (12.9%); diarrhea, three (3.5%); and renal failure was noted in one (1.2%). Three patient had both gastritis and duodenitis. Toxicity was significantly more prevalent in the first 40 patients compared with the last 45 patients (32.5 vs 13.9%).
Conclusions
SRS for unresectable pancreatic carcinoma can be delivered in three fractions with minimal morbidity and a local tumor control rate of 91.7%. The survival is comparable or better than the reported results for advanced pancreatic cancer, specifically for the group of previously untreated patients with unresectable tumors. Development of distant metastases remains a significant factor.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pancreatic cancer is the second most common gastrointestinal malignancy and although it is the ninth most common cancer amongst all sites, it is the fourth leading cause of cancer deaths in the USA. In 2009, it is estimated that 42,470 people developed pancreatic cancer and 35,240 died from it.1 Pancreatic cancer carries a grave prognosis with overall 1- and 5-year survival rates of 24% and 5%, respectively. Moreover, only 7% of cases are diagnosed at an early stage and only 15% to 20% of patients have resectable disease at diagnosis. Approximately 30–40% have locally advanced unresectable tumor and 40% have metastatic disease.2,3
The median survival of locally advanced pancreatic cancer remains 6–11 months in the majority of prospective clinical trials despite advances in chemotherapy, radiation therapy (RT) and chemo-radiation therapy (CRT) in the last two decades.4–11 Improvement in relief of pain and quality of life remains a great problem.
In the last two decades, a few noteworthy improvements in chemotherapy, RT and a combination of CRT have made only a very modest impact on the overall prognosis. Gemcitabine-based chemotherapy has improved response rate and survival.12 The addition of erlotinib to gemcitabine made a very mild improvement in response rate and survival.13 Many clinical trials of concomitant CRT showed improvement over RT or chemotherapy alone.4,5,7 Few studies showed adverse or no beneficial effect of CRT versus chemotherapy alone.8,14
All previous trials used conventional RT along with either 5-FU or gemcitabine-based chemotherapy. Improvements in conventional RT were possible because of advances in computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET). Now, 3D conformal radiation therapy is the standard way of delivery for RT. Intensity-modulated radiation therapy (IMRT) has also impacted the delivery of megavoltage photon-based therapy by concentrating on the tumor target and sparing surrounding normal tissues.
In the last 5 years, further improvement in the precise delivery of high dose RT to the tumor was made possible with the development of real-time image-guided stereotactic radiosurgery (SRS). It enables a biologically larger dosage of radiation in one to three fractions as opposed to 30 to 40 fractions used in conventional methods of delivery.15–18 With sub-millimeter accuracy of delivery of RT, the maximum dose could be delivered at the target with minimal dose to adjacent critical structures thus achieving the best therapeutic ratio.
We treated 85 patients with locally advanced or recurrent unresectable pancreatic cancer by SRS and chemotherapy with the following aims: (1) To obtain local control of the disease. (2) To improve the survival of the unresectable pancreatic cancer patients. (3) To evaluate the toxicity of SRS. (4) To compare our results with the results of other prospective studies with conventional CRT.
Materials and Methods
From 2 March 2004 to 11 May 2009, a total of 85 patients with biopsy proven locally advanced pancreatic cancer were treated with SRS at our center. Pre-SRS evaluation in all patients included complete history and physical, Karnofsky performance score, complete metabolic panel (CMP), CA19-9, and pain score recorded on severity of pain from 0 to 10. Pre-SRS tumor staging was done by triphasic or biphasic CT in all patients and by PET/CT in the latter 55 patients. All primary or recurrent tumors were unresectable by conventional criteria: (a) visceral arterial encasement, (b) extrapancreatic retroperitoneal tumor extension near aorta or vena cava, and/or (c) complete obliteration of portal or superior mesenteric vein. Age range of patients was from 36 to 88 years with the median age of 66 years. Fifty patients (58.8%) were males and 35 (41.2%) were females. Racial distribution was: Caucasian, 79; African American, five; and Asian, one. Tumor location was in the head 57 (67%) and body/tail of the pancreas in 28 (33%). Histology of tumor was adenocarcinoma in 80 (94.12%) neuroendocrine/islet cell carcinoma in three (3.53%) and other histologies in two (2.35%) patients.
Fourteen (16.5%) patients had locally recurrent (unresectable) tumor after previous surgical resection (Whipple procedure or distal pancreatectomy). Seventy-one patients (83.5%) had no prior surgical resection. Fifty-six patients (65.9%) had no prior radiation therapy. Prior conventional RT was given in 29 patients (34.1%) and they had local progression of tumor at the time of SRS. Fourteen of this group had locally recurrent disease after surgical resection and adjuvant CRT; and remaining 15 had local progression after prior conventional CRT. The range of conventional RT dose delivered prior to SRS was 36–60 Gy (median 50 Gy). Forty-eight patients (56.5%) received prior chemotherapy for their disease and they had local progression of disease prior to SRS. None of the patients received pre-SRS chemotherapy for radiosensitizing purposes.
Tumor staging at diagnosis and pre-SRS time is given in Table 1. For pre-SRS T category, all patients were surgically unresectable. The largest single tumor diameter measured by CT ranged from 1.2 to 10 cm with a median diameter of 4 cm and mean of 4.3 cm. The majority of the patients were staged Nx as CT, PET/CT or endoscopic ultrasound (EUS) could not identify nodal metastasis with certainty. Twenty-one patients who had distant metastasis were given SRS for large symptomatic pancreatic tumors. Most of these patients had severe pain and their distant metastatic disease was controlled by systemic chemotherapy.
Pain was evaluated on the scale of 0 to 10. Pre-SRS evaluation of pain showed no pain to mild pain (pain score 0–3) in 54, moderate pain (pain score 4–7) in 18, and severe pain (pain score 8–10) in 13 patients. Pre-SRS score of general performance as measured by Karnofsky method was less than 80% in 14 patients and more than 80% in 71 patients.
Pre-SRS PET/CT was positive in 52 patients and negative in three. Thirty patients in the study, mostly in the initial period did not get a PET/CT scan. Pre-SRS maximum standard uptake value (SUV) ranged from 2 to 21 with a median of 6.0 and mean of 6.9. Pre-SRS values of CA19-9 in 65 patients with adenocarcinoma ranged from two to 38,975 units (median, 234 units).
Post-SRS follow-up was done in all patients every 8–12 weeks with complete physical examination, CMP and CA19-9. CT scans were obtained every 8–12 weeks and in the latter 42 patients, PET/CT scans were obtained every 12–18 weeks. Of the 55 patients who had pre-SRS PET/CT for planning purposes, we could obtain post-SRS PET/CT in only 42 patients because either they had distant progression or we were unable to obtain studies because of insurance limitation.
All patients had post-SRS chemotherapy within 3–4 weeks after SRS. The chemotherapy regimen included gemcitabine alone or gemcitabine with erlotinib, taxol, xeloda, and bevacizumab. Post-SRS chemotherapy decisions were made by their medical oncologists. Toxicity was recorded as per NCI guidelines.19 Grades III and IV toxicity was correlated to tumor volume, prior RT, surgery, or chemotherapy and to early or late time periods of when the SRS was administered.
Response Evaluation
Response to SRS was recorded after every evaluation by CT in all and PET/CT in the latter 42 patients. Response Evaluation Criteria in Solid Tumors (RECIST) criteria were used for response evaluation.20 We modified RECIST criteria of response by utilizing PET/CT scans in evaluation. Tissue reaction producing fibrosis at the tumor site frequently made it impossible to measure complete or partial disappearance of the tumor on CT while PET/CT has been shown to be able to differentiate fibrosis from residual viable malignancy with 18F-FDG (fluorodeoxyglucose). A complete response (CR) was the disappearance of the primary tumor by CT scan and in the patients who had PET/CT, no significant uptake in the tumor bed. A partial response (PR) was defined as at least 30% decrease in the largest diameter of the tumor and reduction in maximum SUV value. Stable disease (SD) was defined as less than 30% decrease in the largest diameter of the tumor or less than 20% increase in largest tumor diameter and no increase in the maximum SUV on PET/CT. Progression of disease was defined as more than 20% increase in the largest diameter of the tumor and increase in the maximum SUV. Local progression free response (local tumor control) included all patients with CR, PR, and SD.
SRS Technical Consideration
The SRS system (CyberKnife®) is a frameless, image-guided RT system that has a 6-megavolt linear accelerator mounted on a robotic arm with 6° of freedom. The imaging system is composed of two diagnostic orthogonal X-ray sources on the ceiling paired with amorphous silicon detectors that capture digital radiographic images of the patient in real-time. It is capable of delivering a high dose of radiation with 0.12 mm accuracy. It delivers unhindered non-coplanar treatment to pancreatic tumors through 150–200 uniquely angled beams per fraction. It requires gold fiducials implanted in the tumor to track the delivery of these beams.
One to 2 weeks before the SRS, five gold fiducials were implanted in and around the pancreatic tumor 2–5 cm apart and in three different planes. For fiducial placement, in addition to the tumor site, other preferred sites were the psoas muscle, crus of the diaphragm, periosteum of the vertebral body, and the laminae. Dilated distal pancreatic duct and vessels were avoided. The fiducial placement procedure was performed by the interventional radiologist either under CT guidance or by the surgeon during laparatomy for attempted resection or biliary bypass. In cases where no extra tumoral (spine) fiducials were placed, we used XSight™ (Accuray Incorporated, Sunnyvale, CA), a spine tracking algorithm to establish 3D rotational orientation. The accuracy of XSight™ System is comparable to that of the fiducial tracking method for precision SRS delivery.21
After allowing the implanted fiducials to settle, each patient was imaged using a CT with 1.5-mm slice thickness with the patient in an immobilized position accomplished by a custom-made Vac-Loc device (Bionix Radiation Therapy, Toledo, Ohio); oral and IV contrast were always used for delineation of surrounding critical structures, except in patients allergic to IV contrast. In the latter 55 patients, PET/CT scans were done at the same time. Fusion images of CT and PET/CT scan were used for 3D reconstruction and planning. The resulting CT volume was used in the treatment planning and creation of the normal tissue constraints through contouring the tumor and adjacent critical structures. The critical structures contoured were the duodenum, stomach, liver, kidneys and spinal cord. The gross tumor volume (GTV) and the surrounding organs including the liver, stomach, spinal cord and both kidneys were contoured jointly by the surgical and radiation oncologists. The GTV included the volume that was identifiable on the planning CT and PET/CT, unless additional information was available through intraoperative or EUS sources. The size of the GTV ranged from 9.8 – 223.3 cm3 with a median of 59.7 cm3 and mean of 70.74 cm3. The planning treatment volume (PTV) included the GTV and a 3 mm margin around the tumor margin. The dose to critical structures was limited to known tolerance levels for at least 90% of the volume of the respective organs (Figs. 1, 2, 3, and 4).
A total dose of 15–30 Gy (median, 25.5 Gy) was prescribed to a median 80% isodose line (range, 75% to 88%) in one to four fractions (mean, three fractions). During the treatment, the patient was allowed to breathe freely and the motion of the target volume was tracked by Synchrony® Respiratory Tracking System (Accuray Incorporated Sunnyvale CA), in most of the patients. Synchrony® uses a correlation algorithm to generate a model of the motion of the internal fiducials and external light emitting diodes placed on the patient’s chest.22 This model algorithm was generated right before the initiation of the treatment and updated throughout the treatment each time an X-ray image was acquired.
Statistical Methods
Patient data was entered in Microsoft Access® data base retrospectively and prospectively. SAS 9.2 program was used for computing. The Kaplan–Meier Estimate (product-limit estimate) method was used for survival data.23 For calculating the p values, non-parametric methods used were log-rank test and Wilcoxon test. Chi-square test was used to detect the association between categorical variables. Survival graphs were created by software R (2.10.1) program developed by Bell Labs.
Results
Tumor Control and Duration
Local tumor control (CR + PR + SD) was obtained in 78 (91.7%) patients. Of these 78 patients, ten (11.8%) had CR, 27 (31.7%) had PR, and 41 (48.2%) had SD. The duration of response was from 3 to 36 months with the median of 8 months. Amongst the local progression free group of patients, most developed distant metastases while their local disease was under control. Five patients had progression of local disease at 1, 8, 12 16, and 25.8 months. Two patients did not get follow-up imaging studies or they were lost to follow-up.
Of the 42 patients who had both pre- and post-SRS PET/CT, 10 showed no appreciable uptake on post treatment scans and 32 demonstrated mild uptake in the tumor. They had a minimal decrease in their SUV values at post-SRS evaluation. Mean and median pre- versus post-SRS SUV values were: 6.9 and 6 (SD ± 4.3) versus 4.5 and 4 (SD ± 2.92), respectively, p = NS. Those patients who had CR by PET/CT never showed complete disappearance of the tumor by CT evaluation, suggesting the residual density on the CT represented fibrotic reaction (Figs. 5, 6, 7). The 46 patients with adenocarcinoma and M0 disease who had both pre- and post-SRS, CA19-9 evaluation showed improvement in post-SRS CA19-9 value. Median and mean values for pre- versus post-SRS were: 245 and 2,172 (SD − 6,459) versus 138 and 1,124 (SD ± 2,191.6), respectively, p = NS.
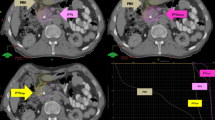
Pre-SRS PET/CT fusion images showing active tumor at the proximal body of pancreas (left panel); post-SRS PET/CT fusion images showing no activity of 18 F-FDG in tumor but no disappearance of tumor on CT images
Distant Disease Progression
Distant progression of disease was seen in 65 patients including those who had distant metastases prior to SRS. Distant progression of disease occurred from 1 to 41 months with the median time interval of 91 days. Distant disease progression occurred at multiple sites. Most common sites were peritoneum, liver, lymph nodes and lung.
Pain Control
Patients who had severe pain (score of 8–10) had relief of pain to a much lower scale and the duration of relief was up to 24 weeks from SRS. Patients who had moderate pain (score 4–7) had relief of pain lasting for 18-week period (Fig. 8).
Of the 31 patients who had pain score of more than 4, 15 had complete relief of pain lasting for more than 6 months. The remaining 16 patients had relief of pain to lower scores after SRS compared with pre-SRS pain scores.
Toxicity
A total of 19 (22.3%) patients developed multiple grades III or IV gastrointestinal toxicities. Duodenitis was seen in 12 (14.1%), gastritis in 11 (12.9%), and diarrhea in three (3.5%) patients. Of the total 19 patients who had upper GI tract toxicity, three had both gastritis and duodenitis. Furthermore, of the 12 patients who had duodenitis within 6 weeks of SRS, seven had late duodenitis as well. It resulted frequently in upper GI hemorrhage or duodenal obstruction. Tumor recurrence was seen in two patients with late duodenal toxicity.
Diarrhea was more related to post-SRS chemotherapy started within 3–4 weeks of SRS. Renal toxicity was not related to radiation to the kidneys but to deteriorating general condition with peritoneal implants and ascitis.
GI toxicity was correlated to prior RT, prior surgical resection, GTV and first 40 patients versus last 43 patients. Two patients could not be evaluated for toxicity because of noncompliance in follow-up. Statistically significant correlation of GI toxicity was noted in patients treated in early years versus latter years of the study period (Table 2).
One patient died 3 weeks after SRS treatment. The cause of death was sepsis and ascitis. The patient was on chemotherapy after SRS. We do not think the cause of death was from SRS treatment.
Surgical intervention was not needed in these patients when they developed GI toxicity. Most of these patients were treated with conservative medical management. Few patients needed duodenal stent for obstruction from progression of tumor 5–6 months after SRS.
Survival
At the end of the study period 13 patients are alive with disease, two of these having no disease progression. Sixty-one patients died of disease, nine died of other causes (sepsis, neutropenia, cardiac, or lung problems), and two patients were lost to follow-up.
Overall survival from the diagnosis in all 85 patients ranged from 6 weeks to 48 months with the mean of 22.9 months and median of 18.6 months. Mean and median overall survival from the first treatment of SRS was 13.24 and 8.65 months (Fig. 9). The survival was correlated to many factors. Median survival for patients with carcinoma in the body and tail was slightly higher than for the head of pancreas 13 versus 11.2 months but p value was not significant.
Mean survival of patients without distant disease progression was statistically better than those who had distant disease progression. Median survival for patients without distant disease progression although not reached is more than 18 versus 11.56 months in patients with distant disease progression (Fig. 10).
Post-SRS mean survival for patients who had no prior RT was better but not statistically significant than those who had prior RT (15 vs 9.21 months, Fig. 11). Prior RT did not affect survival either from diagnosis or from SRS. From the time of diagnosis, a trend of better survival was seen in the first 18–20 months in RT group because 14 patients in that group had surgical resection followed by adjuvant CRT (Fig. 11).
Most importantly, the estimated survival for the group of patients with adenocarcinoma only but without prior surgical resection, or RT or presence of distant metastases at the time of diagnosis and SRS is shown in (Fig. 12). The median, mean and 1 year survival from diagnosis was 13.4, 15.04 months (range, 2.2–30 months), and 50%; while the survival figures from the first SRS treatment were: 8.65, 11.15 months (range, 1–28.2 months), and 30.5%.
Characteristics of 49 patients who survived less than 1 year after SRS were compared with those 28 patients who survived for more than 1 year. Patients who died of other causes were excluded from the analysis.
These two groups were analyzed for tumor volume, age, gender, percentage of isodose, prior RT, histology, and PET CT scan results. No statistically significant difference was found in these two groups. A trend was seen for larger tumor volume in short survivor group compared with long survivor group (62 vs 46 cm3).
Survival was further analyzed for those patients who became PET/CT negative. Median survival for the 15 patients who became PET/CT negative was 17 months. This compares well with the overall median survival of the 8.6 months for the entire group.
Discussion
Pancreatic adenocarcinoma carries a grave prognosis. It ranks at or near the bottom of the list of all cancers in relation to patient survival from diagnosis. Resection of the tumor by pancreatico-duodenectomy or distal pancreatectomy is the only proven method to achieve improved survival. Very few patients are resectable at diagnosis. From 1985 to 1995 in the report from the National Cancer database, only 9% patients at the time of diagnosis out of 100,313 patients had surgical resection.24
In the last 25 years, even in resected pancreatic cancers, the survival reported in 5 large prospective randomized trials has not improved much.25–29 In these trials median survival for the patients receiving adjuvant CRT or chemotherapy only ranged from 16.9 to 22.1 months. In fact, from 1985 to 2008 despite better RT methods and use of gemcitabine in the last decade, the median survival remained essentially unchanged from 20–22 months. Local recurrence rates were high, from 23% to 51% after resection and distant metastasis rate was also very high 50% to 77%.
If such is the prognosis in resected patients, then the prognosis for unresectable pancreatic cancer is more discouraging. Almost 30% to 40% patients have locally advanced pancreatic cancer.30,31 They are not only unresectable, but frequently a majority of these patients will have micrometastases undetected by present available imaging techniques including PET/CT. Furthermore, many of these patients are symptomatic because of invasion of the visceral nerves and adjacent viscera. Median and 1 year survival for these patients is reported to be 7.2 months and 27%.2,3
Before 1981, these patients were treated either by chemotherapy or conventional RT and palliative procedures without any impact on survival. Since the initial reports in 1981 and 1985 by the Gastrointestinal Tumor Study Group of improved results by using of combined modality of treatment, chemotherapy and radiation therapy followed by chemotherapy, this approach has become standard not only in pancreatic but practically in all GI cancers.4,6 The standard chemotherapeutic agents used were 5-FU mainly and for radiation therapy conventional super voltage radiation of 1.8 Gy given daily 5 days per week for 30–40 days with total dose of 40 to 60 Gy.
Improvement in systemic chemotherapy with the use of gemcitabine over 5-FU made a positive impact in progression free and overall survival, and betterment of disease related symptoms.12 The addition of other chemotherapy drugs, platinum agents (cisplatin, oxaliplatin), irinotecan, capcitabine, and anitfoliates (pemetrexed) to gemcitabine has made little improvement over gemcitabine alone.32–34 Lastly, for systemic chemotherapy, the addition of erlotinib, an epidermal growth factor receptor inhibitor, has shown a very small but statistically significant survival advantage over gemcitabine alone.13
In the last 15 years, parallel to the progress in systemic chemo/molecular therapy in advanced pancreatic cancer, radiation therapy has made tremendous progress in achieving maximum therapeutic ratio with minimal dose to adjacent normal structures. 3D conformal RT has become standard. Innovations in imaging techniques, CT, MRI, and PET/CT made it easy to plan and deliver IMRT.
With the recent advances in stereotactic image-guided technology, including real-time image guidance, now it is possible to deliver high doses of radiation therapy with sub-millimeter accuracy in non-CNS body tumors. Although retroperitoneally located, the movements of the pancreas with each respiration cycle are considerable, ranging from 1.1 to 2 cm in different direction.35 Synchrony®, which utilizes respiratory gating technology, can account for such movements thereby delivering the high dose to the target without much radiation exposure to adjacent viscera. SRS can deliver 25.5 Gy dose in 1 day. This will be a biologically equivalent dose of 85.5 Gy. To deliver 87.5 Gy by conventional RT, it would take 41 days at a daily dose of 1.8 Gy. Similarly, in our study, a 25.5 Gy dose given in three fractions is biologically equivalent to 47.2 Gy. Delivery of a dose of 47.2 Gy dose by conventional method would require 22 fractions of a 1.8 Gy daily dose given over 4 to 5 weeks.36,37
Intra-operative radiation therapy (IORT) for unresectable and resected patients with pancreatic cancer did not confer any survival benefit in both randomized trials.38,39 Compared with IORT, SRS can deliver more dose in one fraction without elaborate operating room RT set up.
For any modality of therapy to succeed in locally advanced unresectable pancreatic cancer, it must address two major points:
-
a.
Since most patients develop metastatic disease an effective systemic treatment of micrometastases is most important.
-
b.
Since uncontrolled local disease causes excruciating pain, deterioration of quality of life, duodenal obstruction and bleeding, an effective local modality of treatment delivered in a short time period with minimal toxicity is equally important.
This study cannot address the first major point but it can address the second point. Local progression of disease after chemo-radiation therapy has been reported from 42% to 62% in many prospective studies utilizing modern 3D conformal RT.10,40–42 At the time of local progression, almost equal number of these patients will also have metastatic disease as well. SRS has low rate of local progression. Previous studies at other institutions and one at our institution showed local progression rate to be very low from 7–22%.15,18,43,44
Although no complete responses were observed in the Stanford series and they have not reported on partial response after SRS, we observed complete response by PET/CT and CT in ten (11.8%) patients and partial response in 27 (31.7%) patients. Local tumor control (CR + PR + SD) was observed in 91.7% patients, which is comparable to the recent reports from other centers. The duration of local progression free response was comparable to that reported by others.15,43,44
Frequently, utilization of PET/CT added a new dimension to evaluate the response to cancer therapy. The most commonly used criteria for response evaluation is RECIST.20 For intra-abdominal malignancy, CT scan is the most common imaging technology used for accurate measurement of tumor. On several occasions, we observed no disease on PET/CT imaging or considerable decrease in SUV after SRS, but CT showing no corresponding disappearance of tumor. CT showed either fibrosis or inflammatory changes making measurement of the tumor almost impossible. PET/CT has been shown to be an accurate means of assessing treatment response in many cancers, particularly lymphomas, where PET/CT can predict tumor response after one or two cycles of chemotherapy. It has been shown to be more predictive than CT. Our results suggest the same may be true for assessing treatment response following SRS. Additional studies would be helpful to quantify and confirm this.
Lasting control of the local disease can be obtained by SRS. In our series, the majority of patients developed distant metastatic disease, usually at multiple sites, while the primary tumor had no progression. Median time for duration of local response was 8 months in our series. Similar results have been reported by Stanford & Harvard Groups.15,43
Can we increase the response rate by increasing the dose of SRS to more than 25.5 Gy as administered in our series? Phase I study by Koong and associates on escalation dose of radiation therapy from 15 to 20 Gy and ultimately 25 Gy showed tolerable GI toxicity and excellent tumor control by the latter dose in the first 15 patients.17 Since the progression free local control of disease is much higher than conventional RT, we doubt that an increase in RT doses by SRS will achieve additional responses, free of toxicity.
For the last 20 years, the vast majority of patients routinely received concomitant CRT. Gastrointestinal toxicity is the most common and the most debilitating side effect after CRT. Grades III and IV GI toxicity in the form of nausea, vomiting, gastritis, duodenitis and diarrhea ranged from 20 to 48.7% in recent prospective studies.8–11,41 The toxicity increased when full dose of chemotherapy and multiple chemotherapeutic drugs were used concomitantly with RT. Additionally, local tumor response and survival remained unchanged compared with conventional CRT doses.8,9,11
In the present series, the most common toxicity was that of the gastrointestinal tract (22.3%). We correlated toxicity to multiple factors (Table 2). To our surprise, prior RT did not correlate to post-SRS GI toxicity. We thought that altered anatomic structures by prior surgery will affect proper contouring of the bowel resulting in increased GI toxicity, but in our series, prior surgery did not correlate with more GI toxicity. Similarly, volume of tumor which can affect exposure of adjacent GI structures did not correlate with more GI toxicity. The most important factor correlating the GI toxicity was the initial first 2 years period versus the latter 3 years of SRS delivery. The toxicity was statistically significant in patients treated in the first 2 years of the study period. We think two factors may have contributed to this difference. A better delineation of duodenum, stomach and small bowel in preplanning imaging studies in the latter period may have played the role. We did further analysis of duodenal exposure to RT, however, we found that the exposure of the duodenum to radiation was well below the range of the toxicity dose.
We think the other important factor in reducing GI toxicity in the latter period was the technical development in tracking respiratory motion by Synchrony® which was not used in first few patients. Although not perfect in tracking pancreatic tumor motion with respiratory movements, Synchrony® can help in tracking tumor motion in super-inferior (SI) and antero-posterior (AP) and left to right (L–R) direction. The mean pancreatic motion in one study was: SI direction 20.8 mm, L–R direction 11.3 mm, and in AP direction 13 mm.35 Along with many other improvements, this single technical improvement distinguishes SRS from 3D conformal RT, IMRT, and IORT.
Up to 85% patients with advanced pancreatic cancer have severe pain.45 Two randomized studies, one from Hopkins and the other from Mayo Clinic, showed beneficial effect of neurolytic celiac plexus block over narcotic administration only.46,47 In our study, post-SRS symptomatic pain relief was uniformly seen in all patients having moderate and severe pain. Usually, relief of pain was noted in the first week of treatment and lasted for 18–24 weeks (Fig. 8).
Our study of the patients with locally advanced pancreatic cancer has a mixture of patients, some with distant metastasis and some with recurrence after prior surgical excision and some with prior RT, which makes inferences regarding survival difficult. However, we have isolated the group of patients with adenocarcinoma only without prior RT, surgical resection and distant metastasis before SRS. Their median and 1-year survival from diagnosis is better than the reported survival from the National Cancer database of 12,981 patients with stage III cancer.3 Most of the patients in our study died of disease from distant metastasis. Despite improvements in chemotherapy and molecular based therapy, we cannot prevent or control distant micrometastases. In fact, in our study the group of patients with no progression of distant disease showed the highest survival rate compared with the group with progression of distant disease.
Since the local control cannot translate to the development of distant metastasis, the best strategy to improve the survival with any form of RT including SRS is to avoid patients who develop metastatic disease during induction chemotherapy courses prior to the delivery of SRS. SRS should be followed by systemic chemotherapy. This approach is suggested by studies from MD Anderson, and GERCOR (Groupe Coopérateur Multidisciplinaire en Oncologie) in Europe.7,42 SRS has the advantage over conventional 3D-RT because it can be administered in 1 to 3 days, rather than the typical 5–6-week course of conventional RT. It has much less grades III and IV GI toxicity compared with concurrent CRT treatment. SRS delivers much larger biologically equivalent doses in the fewest fractions. Improvements are urgently needed in treatment of micrometastases which are often present in these patients. Local disease control in these unresectable locally advanced pancreatic cancer is much better with SRS than that of conventional CRT.
References
American Cancer Society. Cancer Facts & Figures 2009 Atlanta: American Cancer Society; 2009.
Ries LAG, Young JL, Keel GE, Eisner MP, Lin YD, Horner M-J (eds). SEER Survival Monograph; Cancer Survival Among Adults: US SEER Program, 1988–2001, Patient and tumor characteristics. NCI, SEER Program, NIH Pub. No. 07-6215, Bethesda, MD, 2007.
Bilimoria KY, Bentrem DJ, Ko CY, et al. Validation of the 6th Edition AJCC Pancreatic Cancer Staging System Report from National Cancer Database. Cancer 2007;110:738–744.
Moertel CG. Frytak S. Hahn RG. O'Connell MJ. Reitemeier RJ. Rubin J. Schutt AJ. Weiland LH. Childs DS. Holbrook MA. Lavin PT. Livstone E. Spiro H. Knowlton A. Kalser M. Barkin J. Lessner H. Mann-Kaplan R. Ramming K. Douglas HO Jr. Thomas P. Nave H. Bateman J. Lokich J. Brooks J. Chaffey J. Corson JM. Zamcheck N. Novak JW. Therapy of locally unresectable pancreatic carcinoma: a randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-fluorouracil), and high dose radiation + 5-fluorouracil: The Gastrointestinal Tumor Study Group. Cancer 1981;48(8):1705–1710.
Gastrointestinal Tumor Study Group. Treatment of locally unresectable carcinoma of the pancreas: comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. Gastrointestinal Tumor Study Group. Journal of the National Cancer Institute 1988;80(10):751–755.
Gastrointestinal Tumor Study Group. Radiation therapy combined with Adriamycin or 5-fluorouracil for the treatment of locally unresectable pancreatic carcinoma. Gastrointestinal Tumor Study Group. Cancer 1985;56(11):2563–2568.
Huguet F. Andre T. Hammel P. Artru P. Balosso J. Selle F. Deniaud-Alexandre E. Ruszniewski P. Touboul E. Labianca R. de Gramont A. Louvet C. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. Journal of Clinical Oncology 2007;25(3):326–331.
Chauffert B. Mornex F. Bonnetain F. Rougier P. Mariette C. Bouche O. Bosset JF. Aparicio T. Mineur L. Azzedine A. Hammel P. Butel J. Stremsdoerfer N. Maingon P. Bedenne L. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000-01 FFCD/SFRO study. Annals of Oncology 2008;19(9):1592–1599.
Wilkowski R. Boeck S. Ostermaier S. et al. Chemoradiotherapy with concurrent gemcitabine and cisplatin with or without sequential chemotherapy with gemcitabine/cisplatin vs chemoradiotherapy with concurrent 5-fluorouracil in patients with locally advanced pancreatic cancer-a multi-center randomized phase II study. Brit J of Cancer 2009;101:1853–1859.
Haddock MG. Swaminathan R. Foster NR. Hauge MD. Martenson JA. Camoriano JK. Stella PJ. Tenglin RC. Schaefer PL. Moore DF Jr. Alberts SR. Gemcitabine, cisplatin, and radiotherapy for patients with locally advanced pancreatic adenocarcinoma: results of the North Central Cancer Treatment Group Phase II Study N9942. Journal of Clinical Oncology 2007;25(18):2567–2572.
Small W Jr. Berlin J. Freedman GM. Lawrence T. Talamonti MS. Mulcahy MF. Chakravarthy AB. Konski AA. Zalupski MM. Philip PA. Kinsella TJ. Merchant NB. Hoffman JP. Benson AB. Nicol S. Xu RM. Gill JF. McGinn CJ. Full-dose gemcitabine with concurrent radiation therapy in patients with nonmetastatic pancreatic cancer: a multicenter phase II trial. Journal of Clinical Oncology 2008;26(6):942–947.
Burris HA. Moore MJ. Andersen J. Green MR. Rothenberg ML. Modiano MR. Cripps MC. Portenoy RK. Storniolo AM. Tarassoff P. Nelson R. Dorr FA. Stephens CD. Von Hoff DD. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. Journal of Clinical Oncology 1997;15(6):2403–2413.
Moore MJ. Goldtein D. Hamm J. et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer. A phase III trial of National Cancer Institute of Canada Clinical Trial Group. J. Clin Oncol 2007;25:1960–1966.
Klaassen DJ. MacIntyre JM. Catton GE. Engstrom PF. Moertel CG. Treatment of locally unresectable cancer of the stomach and pancreas: a randomized comparison of 5-fluorouracil alone with radiation plus concurrent and maintenance 5-fluorouracil-an Eastern Cooperative Oncology Group study. Journal of Clinical Oncology 1985;3(3):373–378.
Chang DT. Schlenberg D. Shen J. et al. Stereotactic Radiotherapy for unresectable Adenocarcinoma of the pancreas. Cancer 2009;115:665–672.
Koong AC. Christofferson E. Le QT. Goodman KA. Ho A. Kuo T. Ford JM. Fisher GA. Greco R. Norton J. Yang GP. Phase II study to assess the efficacy of conventionally fractionated radiotherapy followed by a stereotactic radiosurgery boost in patients with locally advanced pancreatic cancer. International Journal of Radiation Oncology, Biology, Physics. 2005;63(2):320–323.
Koong AC. Le QT. Ho A. Fong B. Fisher G. Cho C. Ford J. Poen J. Gibbs IC. Mehta VK. Kee S. Trueblood W. Yang G. Bastidas JA. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. International Journal of Radiation Oncology, Biology, Physics 2004;58(4):1017–1021.
Hoffelt C. & Didolkar M. Stereotactic radiosurgery for unresectable adenocarcinoma of pancreas. Initial experience at Sinai Hospital of Baltimore. In: Urschel HC (ed) Robotic radiosurgery: treating tumors that move with respiration. Springer, Berlin 2007. pp. 177–192.
National Cancer Institute common toxicity criteria version 2.0. Available at: http://ctep.cancer.gov/forms/CTCv20_4-30-992.pdf. Accessed 18 Jan 2010.
Therasse P. Arbuck SG. Eisenhauer EA. Wanders J. Kaplan RS. Rubinstein L. Verweij J. Van Glabbeke M. Van Oosterom AT. Christian MC. Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. Journal of the National Cancer Institute 2000;92(3):205–216.
Fu D. Kahn R. Wang B. et al. Xsight Lung tracking system: a fiducial-less method for respiratory motion tracking. In: Urschel HC (ed) Treating tumors that move with respiration. Springer, Berlin 2007. pp. 265–282.
Sayeh S. Wang J. Main WR. Kilby W. Maurer CR: Respiratory motion tracking for robotic radiology. In: Urschel HC (ed) Treating tumors that move with respiration. Springer, Berlin 2007. pp. 15–29.
Kaplan E. Meier P. NON-parametric estimation of incomplete observations. Journal of the American Statistical Association 1958;53:457–481.
Sener SF. Fremgen A. Menck HR. Winchester DP. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985-1995, using the National Cancer Database. J Am Coll Surg 1999;189:1–7.
Kalser MH. Ellenberg SS. Pancreatic cancer: Adjuvant combined radiation and chemotherapy following curative resection. Archives of Surgery 1985;120(8):899–903.
Klinkenbijl JH. Jeekel J. Sahmoud T. van Pel R. Couvreur ML. Veenhof CH. Arnaud JP. Gonzalez DG. de Wit LT. Hennipman A. Wils J. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Annals of Surgery 1999;230(6):776–784.
Neoptolemos JP. Stocken DD. Friess H. Bassi C. Dunn JA. Hickey H. Beger H. Fernandez-Cruz L. Dervenis C. Lacaine F. Falconi M. Pederzoli P. Pap A. Spooner D. Kerr DJ. Buchler MW. European Study Group for Pancreatic Cancer. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. New England Journal of Medicine 2004;350(12):1200–1210.
Oettle H. Post S. Neuhaus P. Gellert K. Langrehr J. Ridwelski K. Schramm H. Fahlke J. Zuelke C. Burkart C. Gutberlet K. Kettner E. Schmalenberg H. Weigang-Koehler K. Bechstein WO. Niedergethmann M. Schmidt-Wolf I. Roll L. Doerken B. Riess H. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 2007;297(3):267–77.
Regine WF. Winter KA. Abrams RA. et al. Fluorouracil vs gemcitabine chemotheraphy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA 2008;299:1019–1026.
Riall TS. Nealon WH. Goodwin JS. Zhang D. Kuo YF. Townsend CM Jr. Freeman JL. Pancreatic cancer in the general population: Improvements in survival over the last decade. Journal of Gastrointestinal Surgery 2006;10(9):1212–1224.
White R. Lee C. Anscher M. Gottfried M. Wolff R. Keogan M. Pappas T. Hurwitz H. Tyler D. Preoperative chemoradiation for patients with locally advanced adenocarcinoma of the pancreas. Annals of Surgical Oncology 1999;6(1):38–45.
Wolff RA. Chemotherapy for pancreatic cancer: From metastatic disease to adjuvant therapy. Cancer J 2007;13:175–184.
Bendel J & Goldberg RM. Targeted agents in the treatment of pancreatic cancer; history and lessons learned. Current opinion in Oncology 2007;19:390–395.
Rocha-Lima CM. New directions in management of advanced cancers a review. Anti-cancer drugs 2008;19:435–446.
Minn AY. Schellenberg D. Maxim P. et al. Pancreatic tumor motion on a single planning 4D-CT does not correlate with intrafraction tumor motion during treatment. AM J Clin Oncol 2009;32:364–368.
Fowler JF. The linear–quadratic model and progression radiotherapy. Br J Radiol 1989;62: 679–694.
Whelden TE. Deehan C. Wheldon GE. et al. The linear quadratic transformation of dose-volume histogram in fractioned radiotherapy. Radiother Oncol 1998;46: 285–295.
Sindelar WF. Kinsella TJ. Studies of intraoperative radiotherapy in carcinoma of the pancreas. Annals of Oncology 1999;10 (Suppl 4):226–230.
Tepper JE. Noyes D. Krall J. Sause MD. et al. Intraoperative radiation therapy of pancreatic carcinoma: A report of RTOG-8505. Int. J. Radiation Oncology Biol Phys 1991;21:1145–1149.
Ko AH. Quivey JM. Venook AP. Bergsland EK. Dito E. Schillinger B. Tempero MA. A phase II study of fixed-dose rate gemcitabine plus low-dose cisplatin followed by consolidative chemoradiation for locally advanced pancreatic cancer. International Journal of Radiation Oncology, Biology, Physics 2007;68(3):809–816.
Murphy JD. Adusumilli S. Griffith KA. Ray ME. Zalupski MM. Lawrence TS. Ben-Josef E. Full-dose gemcitabine and concurrent radiotherapy for unresectable pancreatic cancer. International Journal of Radiation Oncology, Biology, Physics 2007;68(3):801–808.
Krishnan S. Rana V. Janjan NA. Varadhachary GR. Abbruzzese JL. Das P. Delclos ME. Gould MS. Evans DB. Wolff RA. Crane CH. Induction chemotherapy selects patients with locally advanced, unresectable pancreatic cancer for optimal benefit from consolidative chemoradiation therapy. Cancer 2007;110(1):47–55.
Mahadevan A. Jain S. Goldstein M. et al. Stereotactic body radiotherapy and gemcitabine for locally advanced pancreatic cancer. International Journal of Radiation Oncology, Biology, Physics 2010 (in press). doi:10.1016/j.ijrobp.2009.08.046.
Polistina F. Costantin G. Casamassima F. et al. Unresectable locally advanced pancreatic cancer: A multimodal treatment using neoadjuvant chemoradiotherapy (gemcitabine plus stereotactic radiosurgery) and subsequent surgical exploration. Annals of Surgical Oncology. 2010;17(8):2092–2101.
Kalser MH. Barkin J. MacIntyre JM. Pancreatic cancer: Assessment of prognosis by clinical presentation. Cancer 1985;56(2):397–402.
Lillemoe KD. Cameron JL. Kaufman HS. Yeo CJ. Pitt HA. Sauter PK. Chemical splanchnicectomy in patients with unresectable pancreatic cancer. A prospective randomized trial. Annals of Surgery 1993;217(5):447–457.
Wong GY. Schroeder DR. Carns PE. Wilson JL. Martin DP. Kinney MO. Mantilla CB. Warner DO. Effect of neurolytic celiac plexus block on pain relief, quality of life, and survival in patients with unresectable pancreatic cancer: a randomized controlled trial. JAMA 2004;291(9):1092–1099.
Acknowledgements
Many thanks to the Mangione Family Foundation that provided some of the financial support with this study. We thank Philip Mone, Radiation Physicist, for technical data.
Discussant
DR. MARK P. CALLERY (Boston, MA): Twenty-five of your 85 patients in your study were M1. What was the indication for CyberKnife radiotherapy in patients with metastases? Was it for pain control? And did these patients dilute your overall results?
Closing discussant
DR. MUKUND S. DIDOLKAR: The main indication for SRS was pain in these patients. The other indication was the progression of the primary tumor in the presence of stable or responded (CR or PR) metastatic disease on chemotherapy. To answer the second question, it did affect the overall results because the survival for the group of patients without distant metastases was much higher than the survival of the patients with distant metastases.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Didolkar, M.S., Coleman, C.W., Brenner, M.J. et al. Image-Guided Stereotactic Radiosurgery for Locally Advanced Pancreatic Adenocarcinoma Results of First 85 Patients. J Gastrointest Surg 14, 1547–1559 (2010). https://doi.org/10.1007/s11605-010-1323-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-010-1323-7