Abstract
Cartilage is an avascular tissue with a limited rate of oxygen and nutrient diffusion, resulting in its inability to heal spontaneously. Articular cartilage defects eventually lead to osteoarthritis (OA), the endpoint of progressive destruction of cartilage. In companion animals, OA is the most common joint disease, and many pain management and surgical attempts have been made to find an appropriate treatment. Pain management of OA is usually the first choice of OA therapy, which is often managed with nonsteroidal anti-inflammatory drugs (NSAIDs). To avoid known negative side effects of NSAIDs, other approaches are being considered, such as the use of anti-nerve growth factor monoclonal antibodies (anti-NGF mAB), hyaluronic acid (HA), platelet-rich plasma (PRP), and mesenchymal stem cells (MSCs). The latter is increasingly being recognized as effective in reducing or even eliminating pain and lameness associated with OA. However, the in vivo mechanisms of MSC action do not relate to their differentiation potential, but rather to their immunomodulatory functions. Achieving actual regeneration of cartilage to prevent OA from developing or even revert already existing OA condition has not yet been achieved. Several techniques have been tried to overcome cartilage’s inability to regenerate, from osteochondral transplantation, autologous chondrocyte implantation (ACI), and matrix-induced ACI (MACI). Combinatory use of MSCs unique features and biomaterials is also being investigated with the aim to as much as possible recapitulate the native microenvironment of the cartilage, yet so far none of the methods have produced reliable and truly effective results. Although OA, for now, remains an incurable disease, novel techniques are being developed, rendering hope for the future accomplishment of actual cartilage regeneration. The aim of this chapter is firstly to summarize known and developing pain management options for OA, secondly to present surgical attempts to regenerate articular cartilage, and finally to present the attempts to improve existing regenerative treatment options using mesenchymal stem cells, with the vision for the possible use of developing strategies in veterinary medicine.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Cartilage and Its (In)Ability to Heal
Cartilage is a connective tissue of mesodermal origin (Armiento et al. 2019). In the fetus, cartilage acts as a bone template and provides a structure for endochondral ossification (Chiara and Ranieri 2009). In the adult organism, cartilage remains in several areas in the body, such as joints, nose, ear, trachea, and intervertebral disks, playing a role of a supportive structure, shock absorber, flexibility, and movement (Hoshi et al. 2018). Four types of cartilaginous tissues are distinguished based on the cellularity, morphology, and extracellular matrix (ECM) composition: hyaline cartilage, fibrocartilage, and elastic and hypertrophic cartilage (Armiento et al. 2019). The development of certain cartilage type is dependent on the mechanical impact on the tissue. The most common is hyaline cartilage, the embryonic form of cartilage, present at the connection between the ribs and sternum, in the trachea, and on the joint surface where it resists compressive load and provides frictionless movement (Nürnberger et al. 2006). Major constituents of cartilage include a small number of cells, chondrocytes, and a large proportion of their product, ECM, embedded in an abundant interstitial fluid which represents the majority of tissue weight and is essential for joint lubrication and wear resistance (Bora Jr. and Miller 1987). In the vertebrate skeletal system, articular cartilage is highly organized (Nürnberger et al. 2006). Complex organization of articular cartilage rises from differentiation of the cartilage into four layers (superficial, middle, deep, and calcified zone), ECM compartmentalization (collagen type I predominating in the uppermost part of the zone and collagen type II in the middle and deep zone), and orientation of collagen fibers (Nürnberger et al. 2006) (forming Benninghoff arcades, oriented mostly parallel to the articulating surface with average fibril rotating through the tissue until the orientation of collagen fibers in the middle and deep zones near the interface with bone is perpendicular to the joint surface) (Benninghoff 1925). Despite well-established cartilaginous tissue structure, there are considerable variations between the species. For example, small species such as mice have higher cellularity than larger animals (Stockwell 1971), whereas cartilage thickness is higher in smaller animals (Stockwell 1971; Frisbie et al. 2006).
2 Osteoarthritis in Companion Animals
In the adult organism, cartilage lacks blood and lymph vessels, nerves, and perichondrium. Chondrocytes are thus sustained by nutrients, gases, and cytokines delivered by the synovial fluid (Stockwell 1978). Cartilage metabolism is relatively slow. Low rate of tissue turnover, ascribed to cartilage avascularity and limited rate of oxygen and nutrient diffusion from synovial fluid, results in cartilage inability to heal spontaneously (Hayes Jr. et al. 2001). Intrinsic repair mechanisms, even in minor cartilage defects, are insufficient for the regeneration of cartilage ad integrum (Nürnberger et al. 2006). Natural repairing process of hyaline cartilage results in mechanically inferior fibrocartilage that in comparison to hyaline cartilage contains high levels of type I collagen and only a small portion of glycosaminoglycans (GAGs) and collagen type II, making it less resilient to wear, with higher-friction motion between bones (Armiento et al. 2019). Cartilage injuries may often appear asymptomatic but symptoms appear with progressive cartilage destruction (Mehana et al. 2019; Janakiramanan et al. 2006). The loss and dysfunction of articular cartilage eventually lead to osteoarthritis (OA), a clinical and pathological endpoint of progressive cartilage destruction, affecting both humans and animals worldwide. OA is a slowly progressing degenerative joint disease characterized by whole joint structural changes including varying degrees of osteophyte formation, subchondral bone change, and synovitis, leading to pain and loss of joint function (Dieppe and Lohmander 2005; Enomoto et al. 2019). OA is the most common joint disease in companion animals, especially dogs and horses (Gencoglu et al. 2020) and also geriatric cats (Clarke et al. 2005). Risk factors for OA in dogs are associated with genetics, breed and conformational predispositions, body weight, age, and neuter status (Anderson et al. 2020). In horses, changes in composition and structure properties of cartilage result from cartilage damage due to trauma, impact injuries, abnormal joint loading, excessive wear, or aging process (Gencoglu et al. 2020). In cats, idiopathic OA mediated by congenital, traumatic, infectious, nutritional, and immune-mediated causes is prevailing (Enomoto et al. 2019). The prevalence of OA is higher in older animals, but can also occur in young animals (Gencoglu et al. 2020; Anderson et al. 2020). Although the exact etiology of OA has yet to be identified, the environmental stress followed by metabolic changes in chondrocytes may play a key role in cartilage degeneration (Zheng et al. 2021): Adverse microenvironmental conditions lead to a switch in chondrocyte metabolism from a resting regulatory state in which oxidative phosphorylation is a leading metabolic process to highly metabolically active glycolysis (Zheng et al. 2021). The consequential increase in biosynthesis of inflammatory and degrative mediators and exposure of chondrocytes to proinflammatory cytokines, hypoxia, and nutrient stress are promoting signaling pathways of catabolism. Enhanced catabolism is followed by mitochondrial dysfunction, resulting in excessive production of reactive oxygen species (ROS) and oxidative damage, a hallmark of OA (Zheng et al. 2021; Mobasheri et al. 2017). The important consequence of ROS is the activation of AMP-activated protein kinase (AMPK) and consequential upregulation of the expression of collagen type I, proinflammatory cytokines, and matrix metalloproteinases (MMP) (Zheng et al. 2021). In particular, MMP13 is known to break down collagen type II, a key structural component of cartilage ECM. Matrix degradation products further promote inflammation and prevent the cycle of degeneration to break (Bedingfield et al. 2020).
3 Pain Management of OA
3.1 Conservative Treatment
OA is currently an incurable disease (Enomoto et al. 2019) and pain management is usually the first step in cartilage therapy. In veterinary medicine, nonsteroidal anti-inflammatory drugs NSAIDs are often the first choice for the treatment of OA and can be used for long-term management of the inflammatory component of OA pain. In addition to NSAIDs, gabapentin, amantadine, and tramadol can be administered when treatment with NSAIDs is not an option. Conservative treatment of OA also relies on the use of weight management, nutritional joint support, and physical rehabilitation including laser therapy, magnetic field therapy, shock wave therapy, massage, and balneotherapy (Zylinska et al. 2018; Rychel 2010). Unfortunately, existing therapies are often associated with severe side effects, such as potential renal, gastrointestinal, or hepatic adverse reactions, and are also often not sufficiently effective (Rychel 2010).
Additional conservative treatment option for treating OA is arthrocentesis or articular puncture, performed to inject supplements such as GAGs or HA to improve the natural qualities of HA, present in the articular fluid, and to increase the mobility of the joint (Zylinska et al. 2018). IM injections of polysulfated GAGs to dogs with OA resulted in improved lameness scores in 12 out of 16 dogs. Reduced lameness was ascribed to GAGs inhibition of cartilage oligomeric matrix protein (COMP) degradation seen as a decrease in serum COMP concentration (Fujiki et al. 2007). However, these results were short-lived similar to the HA treatment. Single intraarticular injection of HA alone in dogs with naturally occurring hip OA also had only a temporary amelioration of the symptoms as measured by Canine Brief Pain Inventory. However, intraarticular injection of HA combined with corticosteroids appeared superior in positive effects compared to HA alone (Alves et al. 2020). Although intraarticular injection of HA and corticosteroids might prove useful for patients that cannot tolerate NSAIDs (Franklin and Cook 2013), based on the retrospective studies in dogs, there was weak or no evidence to support the use of HA for OA (Sanderson et al. 2009; Aragon et al. 2007). Evidence for the efficacy of HA is relatively weak due to the lack of control groups, and the limited numbers of controlled clinical studies make it difficult to suggest the superior effect of HA over the use of NSAID (Aragon et al. 2007).
3.2 Novel Pain Management Treatment Options
3.2.1 Platelet-Rich Plasma
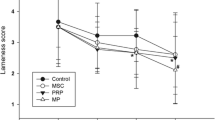
In comparison to intraarticular injection of HA combined with corticosteroids, patient-based assessment scores in lameness and pain were better with intraarticular injection of autologous conditioned platelet-rich plasma (PRP) (Franklin and Cook 2013). PRP is an autologous product, containing an increased concentration of growth factors and bioactive proteins that may enhance the healing process on a cellular level. Besides bioactive factors such as serotonin, histamine, dopamine, calcium, and adenosine that have fundamental effects on the biological aspect of wound healing, PRP contains cytokines and growth factors, including transforming growth factor-β (TGF-β), platelet-derived growth factor (PDGF), insulin-like growth factor (IGF), fibroblast growth factor (FGF), epidermal growth factor (EGF), and vascular endothelial growth factor (VEGF) that play an important role in cell chemotaxis, proliferation, differentiation, and angiogenesis and therefore represent a potential to enhance healing of tendon, ligament, muscle, and bone (Foster et al. 2009). The advantage of PRP is primarily that it is a simple, rapid, cost-effective, and safe way to obtain a clinical improvement of animals affected by OA (Catarino et al. 2020), although diverse methods and devices used to evaluate pain and lameness among different studies make the results difficult to compare (Vilar et al. 2018). Several studies have shown beneficial, albeit temporary, results of intraarticular injection of PRP in the treatment of canine OA. A single injection of PRP into OA joints of dogs was shown to have a positive effect estimated by the lameness grades (Catarino et al. 2020) or force platform gait analysis (Vilar et al. 2018; Venator et al. 2020), but these effects only lasted for 3 to 6 months. Prolonging management of pain was achieved by combining PRP treatment with physical therapy (Cuervo et al. 2020). In comparison to dogs, intraarticular administration of PRP in horses with naturally occurring OA indicates variable changes in kinetic gait parameters (Mirza et al. 2016). Due to differences in PRP concentrations used in different studies, optimization of number of enriched platelets, the volume applied, and concentration of growth factors used for clinical application is needed. Furthermore, characteristics of PRP products differ considerably in the amount of blood processed, method of PRP preparation, and the amount of PRP produced (Franklin et al. 2015). Despite mentioned promising results, there is a lack of data supporting the use of a particular PRP for a specific medical condition, and a consensus on the actual benefits of PRP has not yet been established.
3.2.2 Anti-nerve Growth Factor Monoclonal Antibodies Therapy
A potential alternative to pharmacological pain management in dogs and cats is analgesia using anti-nerve growth factor monoclonal antibodies (anti-NGF mAB) therapy. NGF is a soluble signaling protein, belonging to a family of neurotrophin molecules. During development, NGF has an essential role in the development of sensory and sympathetic neurons, whereas in the adult organism, NGF takes an important part in the sensitization of nociceptors after tissue injury (Mantyh et al. 2011). NGF is produced and released by peripheral tissues such as chondrocytes (Enomoto et al. 2019) and white adipose tissue depots (Ryan et al. 2008). NGF serum level was shown to be associated with stress-related conditions, for example, during transportation (Kawamoto et al. 1996) or exercise load (Matsuda et al. 1991; Ando et al. 2016), and was thus recognized as an important factor to evaluate stress status in an animal (Ando et al. 2020). Besides psychological stress, NGF was correlated also with the mechanical stress associated with OA. Isola et al. (Isola et al. 2011) reported that the concentration of NGF in synovial fluid in dogs with OA was significantly higher in comparison to healthy dogs, suggesting the involvement of NGF in OA inflammation. Similarly, as in dogs, NGF concentration in horses was also higher in synovial fluid from acutely inflamed joints and joints with chronic OA in comparison to healthy joints (Kendall et al. 2021). Some recent clinical studies used anti-NGF mAB to alleviate OA pain in animal patients and are limited to a few studies conducted on dogs and cats. For the treatment of inflammatory pain in dogs, rat anti-NGF mAB were fully caninized (Gearing et al. 2013). Canine-specific anti-NGF mAB were used intravenously in pilot, masked, placebo-controlled clinical studies to alleviate pain in dogs with degenerative joint disease (Lascelles et al. 2015). With 25 dogs included in the study, a positive analgesic effect, similar to that expected with NSAIDs, was recognized based on significantly improved patient-specific outcomes of pain and mobility and significantly increased objectively measured activity. Positive effects of the treatment were observed over 4 weeks after a single treatment with anti-NGF mAB (Lascelles et al. 2015). Similar observations were made in another study conducted by Webster et al. (Webster et al. 2014) where OA-associated pain was alleviated in dogs up to 4 weeks after IV treatment with anti-NGF mAB. Similarly, as in dogs, species-specific anti-NGF mAB were developed for pain treatment in cats (Gearing et al. 2016). In a study with 34 cats, feline-specific anti-NGF mAB were used subcutaneously to treat degenerative joint disease-associated pain. A positive analgesic effect was observed for 6 weeks during the study with significantly increased objectively measured activity (Gruen et al. 2016). Current evidence suggests that anti-NGF mAB therapy of OA in dogs and cats and possibly in horses could be an alternative to NSAIDs and other pharmacological drugs. The efficiency of a single injection of anti-NGF mAB seems to last 4–6 weeks, but further studies are needed to better understand the level of analgesia and to determine possible adverse side effects and the long-term safety of NGF use.
3.2.3 Mesenchymal Stem Cells/Medicinal Signaling Cells
Longer-lasting pain management effects of treating OA were accomplished using adult multipotent mesenchymal stem cells (MSCs). Stem cells are undifferentiated cells with the capability of self-renewal and differentiation into different specialized cells (Morrison et al. 1997). Compared to other stem cell types such as embryonic stem cells and induced pluripotent stem cells, MSCs were recognized as the most promising type of stem cells for therapy because of the relatively simple harvest techniques, isolation, and the absence of greater ethical concerns associated with their use (Sasaki et al. 2018). For both laboratory-based scientific investigations and preclinical studies, a set of standards to define human MSCs was proposed by the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy (ISCT) (Dominici et al. 2006). In essence, (1) MSC must be plastic-adherent when maintained in standard culture conditions using tissue culture flasks; (2) 95% of the MSC population must express CD105, CD73, and CD90 and lack the expression of CD45, CD34, CD14, or CD11b, CD79a or CD19, and HLA class II; and (3) MSCs must be able to differentiate into osteoblasts, adipocytes, and chondroblasts under standardized in vitro differentiating conditions. For the identification of animal MSCs, minimal criteria are yet to be defined. MSCs are found in numerous tissues, which, when endogenously activated, act to replace dead, injured, or diseased tissue cells (Caplan 1991). MSCs of common veterinary patients, e.g., dogs, horses, and cats, have been isolated from several tissues including the bone marrow and adipose tissue (Sasaki et al. 2018; Arevalo-Turrubiarte et al. 2019; Webb et al. 2012), umbilical cord (Zhang et al. 2018; Denys et al. 2020), umbilical cord blood (Kang et al. 2012; Koch et al. 2007), muscle and periosteum (Radtke et al. 2013; Kisiel et al. 2012), gingiva and periodontal ligament (Mensing et al. 2011), peripheral blood (Sato et al. 2016; Longhini et al. 2019), endometrium (Rink et al. 2017), and placenta (Carrade et al. 2011). MSCs have also been described in several joint tissues such as synovium (Sasaki et al. 2018), synovial fluid, and synovial membranes (Arevalo-Turrubiarte et al. 2019; Prado et al. 2015) and inside the infrapatellar fat pad. One of the important aspects of the therapeutic potential of MSCs is their ability to migrate into the damaged tissue and secrete immunomodulatory and trophic bioactive factors (Caplan 2017). Therapeutic properties of MSCs, ascribed to their immunomodulatory functions, are exhibited by paracrine action, secretion of extracellular vesicles, immunomodulation mediated by apoptosis, and mitochondrial transfer (Voga et al. 2020). In veterinary medicine, the therapeutic potential of MSCs is being exploited for the treatment of various organ systems. Musculoskeletal diseases have especially been proven indicative for MSC therapy, as was shown in horses with tendon injuries (Pacini et al. 2007; Godwin et al. 2012; Dyson 2004; Smith et al. 2013; Muir et al. 2016), bone spavin (Nicpon et al. 2013), and meniscal damages (Ferris et al. 2014). Notably, as recently reviewed by our group (Voga et al. 2020), remarkable clinical outcomes of MSC treatment have also been shown in dogs (Mohoric et al. 2016; Black et al. 2007; Vilar et al. 2013; Shah et al. 2018; Harman et al. 2016; Maki 2020; Kriston-Pal et al. 2020) and horses (Magri et al. 2019; Marinas-Pardo et al. 2018) with osteoarthritic conditions, showing as significant longer-termed reduction or even elimination of pain and lameness. Based on the results of these studies, MSC treatment for OA appears safe with promising clinical outcomes, showing reduced lameness and pain associated with OA, decreasing the need for use of anti-inflammatory drugs with their known side effects. However, in comparison to clinical evaluation, the long-term follow-ups with radiographic and CT imaging are scarce and often do not report improvements following MSC therapy, as recently reviewed by Brondeel et al. (2021). Some reduction in progression of OA, demonstrated with radiographic images, was shown in an equine model of OA in fetlock joints (Bertoni et al. 2021), but there is a need for the long-term follow-up imaging performed on actual patients where the progression of the disease is often very different from the experimentally induced pathologies. Demonstrated ability of MSCs to slow down or even stop OA progression is indicative of their well-established immunomodulatory function. One of the important features of MSCs is their tendency to home to injured or inflammation sites when administrated in vivo. However, in contrast to the initial belief that MSCs differentiate and replace damaged tissue, evidence from recent years suggest that MSCs in vivo rarely or never differentiate into the tissue at the site (Guimaraes-Camboa et al. 2017; Meirelles Lda et al. 2009) but secrete bioactive factors. The in vitro multipotency of MSCs thus cannot be directly related to their mechanisms of action in vivo. To avoid the confusion originating from the discrepancy between the name and therapeutic potential of MSCs, it was proposed by Caplan that the term “mesenchymal stem cells” should be changed into “medicinal signaling cells” (MSCs) (Caplan 2017). The actual regeneration of cartilage to prevent OA from developing or even revert already existing OA condition, therefore, remains the topic of research, which is in recent years focusing on exploiting the in vitro differentiation capabilities of MSCs as a basis for finding novel potential solutions to address this issue.
The following part of this chapter focuses on the regenerative surgical attempts to treat cartilage defects, starting with the initial MSC-free attempts to regenerate cartilage, followed by the presentation of the studies exploiting the in vitro differentiation potential of MSCs for cartilage regeneration.
4 Surgical Treatment of OA and Cartilage Defects
4.1 Conventional Treatment Options
Conventional surgical treatment of OA is indicated when conservative therapy fails or is inadequate in alleviating pain and maintaining the function of the joint (Cook and Payne 1997). In dogs, several surgical techniques for OA have been developed. Surgeries may offer treatment of the primary cause, such as cranial cruciate ligament rupture, where tibial plateau leveling osteotomy (TPLO) (Slocum and Slocum 1993), tibial tuberosity advancement (TTA) (Lafaver et al. 2007), or modified Maquet procedure (MMP) (Ness 2016) is indicated. In cases where providing pain relief and lessening the progression of future OA is needed, salvage procedures are performed, such as femoral head and neck excision (indicated in coxofemoral luxation; severe coxofemoral OA; comminuted or complicated fractures of the femoral head, neck, or acetabulum; avascular necrosis of the femoral head; or failed total hip replacement) (Harper 2017a), arthrodesis (indicated for intractable articular fractures, luxations, subluxations, or failed total joint replacement) (McCarthy et al. 2020), and total joint replacement (indicated for patients with debilitating OA secondary to trauma or joint dysplasia) (Harper 2017b).
4.2 Reparative Treatment Techniques
In contrast to salvage surgical interventions used to treat irrevocably damaged articular cartilage by removal or replacement, reparative bone marrow stimulation techniques are used to expose the subchondral bone to stimulate bone marrow and improve cartilage vascularization, enabling the diffusion of nutrients from the subchondral bone into the cartilage and stimulating bone marrow cells to reach the avascular cartilage lesion and initiate a healing response (Stupina et al. 2015). In humans, the method of bone marrow stimulation is one of the most recommended reparative surgical techniques to treat OA (Gill and Steadman 2004). It can be achieved via drilling, chondral abrasion, or microfractures. The latter are of special interest as it can be performed arthroscopically. Light scraping, but not complete removal of calcified cartilage, is indicated to facilitate attachment of the reparative tissue to exposed calcified cartilage (Breinan et al. 2000). In veterinary medicine, objective evidence documenting the efficiency of bone marrow stimulation is not available. In a canine model of OA, chondral abrasion resulted in a fibrocartilage (Altman et al. 1992). In another study using a dog model of OA, subchondral tunneling of subchondral bone together with the injection of autologous bone marrow into the canals resulted in improved cartilage vascularization and consequently improved chondrocyte metabolism and functionality of cartilage (Stupina et al. 2012). Neither method of bone marrow stimulation resulted in hyaline cartilage formation, but rather in reparation of cartilage with the formation of fibrocartilaginous tissue. Similar was shown in horses. While microfractures increased the tissue volume in the defects (Frisbie et al. 1999) and did not cause any negative effects, this technique did not seem to have clinical effects in horses with stifle lameness diagnosed with naturally occurring OA (Cohen et al. 2009). Bone marrow stimulation results in the formation of fibrocartilage, with poor structural and mechanical properties that do not provide long-term efficacy of reparative surgical treatment techniques (Zylinska et al. 2018). Moreover, poor long-term wear characteristics of fibrocartilage do not prevent the progression of OA (Lane et al. 2004). Since the prevention of degenerative joint changes over time is one of the ultimate goals in the treatment of cartilage lesions (Burks et al. 2006), the limited intrinsic ability of cartilage to heal is proposed to alter with the regenerative treatment options that are therefore at the forefront of the cartilage treatment research.
4.3 Regenerative Treatment Options
A common feature of OA is cartilage defects that may either be associated with pain and decreased function or may appear asymptomatically (Janakiramanan et al. 2006). Either way, without treatment, cartilage defects may lead to progressive joint disease (Mehana et al. 2019; Burks et al. 2006). Treatment of cartilage defects is thus directed toward the regeneration of the defective cartilage and prevention of progression of the disease. Cartilage regeneration methods include osteochondral grafting, autologous chondrocyte implantation (ACI), matrix-induced ACI (MACI), and combinatory use of MSCs and biomaterials, aiming to replace the damaged cells and extracellular matrix while preserving the microarchitecture and biomechanical functions of the cartilage (Zylinska et al. 2018).
4.3.1 Osteochondral Transplantation
Osteochondral grafting is an attractive option for cartilage reconstruction because live homologous tissue is used. In humans, osteochondral and meniscal allograft transplantation in the knee has been performed for over 40 years (Rucinski et al. 2019; Familiari et al. 2018; De Armond et al. 2021). In animals, the majority of the studies are performed on animal models. One of the indications for using osteochondral grafts as a means for cartilage reconstruction in dogs is osteochondritis dissecans (OCD). OCD is an inflammatory condition that occurs when the diseased cartilage separates from the underlying bone. The disease can increase the risk of developing OA and it is an important cause of lameness in dogs (Schreiner et al. 2020). It was previously reported that no differences were detected between the surgical and medical treatment of OCD in 19 dogs. Medical treatment resulted in an even more rapid return to normal weight-bearing. Despite some clinical improvement, in most dogs, lameness continued and the disease progressed (Bouck et al. 1995). Albeit demonstrated to be technically feasible in canine caudocentral humeral head, medial humeral, and medial femoral condyle, positive clinical outcomes of osteochondral autograft transfer in dogs with OCD were short-termed, with minimal donor site morbidity (Fitzpatrick et al. 2010; Fitzpatrick et al. 2009; Fitzpatrick et al. 2012). The osteochondral graft may not even render clinical changes, as was shown in a canine model of full-thickness cartilage defect, where phalangeal osteochondral graft did not result in significant functional difference compared to the nongrafted group of dogs 6, 12, or 20 weeks after surgery (Dew and Martin 1992). In comparison to OCD in dogs, osteochondral grafts in the case of subchondral bone cysts in horses that can also lead to osteochondrosis (Bodo et al. 2004) resulted in the reconstruction of the articular surface, subchondral decompression, and a renewed cartilage gliding surface. Promising clinical outcomes demand further investigation of the suitability of treatment of subchondral bone cysts with osteochondral grafts (Bodo et al. 2004).
Even though studies on animals are for the most part conducted on animal models and not the actual patients, up to 20% of procedures are unsatisfactory (Huang et al. 2004). The clinical success of the grafts is dependent on the viability of cartilage cells, the capacity of host bone to join graft cartilage, and the host’s immunologic tolerance. Integration of donor allograft into recipient’s bone can thus be incomplete and can cause failure (Pritzker et al. 1977). Although function and quality of life, based on owner perception, seem to improve after osteochondral grafting (Cook et al. 2008), donor site morbidity is considered a major ethical concern albeit donor sites from canine stifle are currently the only reliable available source of canine donor osteochondral autograft material (Fitzpatrick et al. 2009). Morbidity associated with autografted tissue for treating osteochondral defects could be avoided using fresh allograft tissues. In a canine model of knee cartilage defect, allografts were shown to be similar to autografts regarding bone incorporation, articular cartilage composition, and biomechanical properties (Glenn Jr et al. 2006). Despite being a promising solution for mismatch of transplanted cartilage, allografts may be immunogenic; hence the cartilage becomes vulnerable to direct injury by cytotoxic antibodies or lymphocytes or to indirect injury by inflammatory mediators and enzymes induced by the immune response. However, the literature on the immunogenicity of allografts is contradictory. In some studies, the severe immune response was demonstrated upon allograft transplantation, as shown by an induced inflammatory response, thinned, dull, and roughened cartilage of allografts, with the severely fibrotic and hyperplastic synovial membrane of the joints in dog models (Stevenson et al. 1989). In other studies, no immune response was detected (Glenn Jr et al. 2006; McCarty et al. 2016), or immune response was dependent on whether or not allografts were previously frozen or were vascularized (Stevenson et al. 1996). Freezing was reported to cause harm to the cartilage and thus lower the success rate of osteochondral transplantation (Stevenson et al. 1989). As it was demonstrated in a canine model, viable chondrocytes in osteochondral allografts at the time of transplantation are primarily responsible for the maintenance of donor articular cartilage health in the long term, confirming that not only storage but also procurement, processing, transportation, and clinical implantation are of great importance for allograft clinical use (Cook et al. 2016).
Novel systems for preserving osteochondral allografts, such as MOPS (Missouri Osteochondral Allograft Preservation System) (Cook et al. 2014), and novel methods for enhancing graft integration are being developed. A lack of osteochondral graft integration is one of the important problems in transplanting osteochondral grafts that can cause a treatment failure, especially since there is often a mismatch of transplanted cartilage regarding the contour and thickness of the injured surface (Huang et al. 2004; Hurtig et al. 2001). Also, transplantation of osteochondral grafts involves manual precise preparation of the donor graft and recipient bed. The process is user-dependent, not standardized, and subject to human error. A possible solution for bypassing the issue of insufficient supply of available donor tissue with accurate anatomical features is a fabrication of osteochondral constructs with the use of 3D printing techniques, improving the accuracy of anatomical architecture and topology, suggesting clinical relevance for large area cartilage repair (De Armond et al. 2021; Roach et al. 2015). Additionally, enhancing graft integration was attempted by using saturating grafts with bone marrow aspirate concentrate (Schreiner et al. 2020; Stoker et al. 2018) or PRP (Stoker et al. 2018), with the assumption that growth factors, cytokines, and other proteins contained in bone marrow aspirate concentrate may enhance osteoinductive, chemotactic, and neovascular signals needed for better graft integration. For example, in an in vitro study, bone marrow aspirate concentration was shown to be superior to PRP in enhancing integration potential for canine osteochondral allografts (Stoker et al. 2018). A combination of novel graft preservation and implantation techniques may therefore result in more satisfying clinical outcomes, as was demonstrated in a study where osteochondral allograft transplantation technique using fresh unicompartmental bipolar osteochondral and meniscal osteochondral allografts and application of bone marrow aspirate concentrate were used to treat medial compartment gonarthrosis in a canine model. Clinical, radiographic, and arthroscopic assessment of the graft and joint demonstrated the maintenance of the integrity of transplants and integration into the host tissue, leading to superior outcomes without early OA progression compared to NSAID controls (Schreiner et al. 2020).
While animal models provide crucial information about disease mechanisms, the artificially induced disease cannot recreate the natural in vivo environment (Cope et al. 2019). Studies conducted on actual veterinary patients are scarce, and extensive research is still needed to prove the efficacy and usefulness of osteochondral graft transplantation on actual patients. However, advancement in allograft transplantation in animal models suggests that osteochondral grafting is worthy of further investigation also in actual veterinary patients.
4.3.2 Autologous Chondrocyte Implantation
The lack of significant cellular activity in chondral defects was indicative for the researchers that chondrocytes are needed for articular cartilage regeneration (Shortkroff et al. 1996). Autologous chondrocyte implantation (ACI) was thus developed as an alternative for treating defects of articular cartilage. In humans with full-thickness cartilage defects, the procedure was described in 1994 by Peterson et al. (Brittberg et al. 1994): Cartilage slices were obtained from an uninvolved area of the injured knee during arthroscopy. Chondrocytes were then isolated and cultured for 14 to 21 days in the laboratory and then injected into the injured area under a periosteal flap taken from the proximal medial tibia. ACI seems to be advantageous over bone marrow stimulation techniques in that the cartilage that is formed is predominantly hyaline-like, containing collagen type II (Brittberg et al. 1994; Min et al. 2007; Cherubino et al. 2003). It was demonstrated by Min et al. that cartilage regeneration after ACI is correlated with at least 4-week-long survival of transplanted chondrocytes (Min et al. 2007). Fluorescently labeled chondrocytes implanted in the goat model were shown to integrate into the surrounding tissue and become a structural part of repaired tissue, rich in collagen type II and proteoglycans (Dell’Accio et al. 2003). In the canine model, ACI was shown to be superior to bone marrow stimulation techniques based on morphology, histology, and serum marker levels, with smooth surface, less fissure, and good border integration (Nganvongpanit et al. 2009). Similar as in dogs, in three horse models of cartilage lesions of fetlock joints in the forelimb, hyaline-like cartilage was formed after ACI treatment (Barnewitz et al. 2003). In the majority of animal models, ACI is investigated in full-thickness cartilage lesions. Partial-thickness cartilage lesions represent a more hostile environment for regeneration due to avascularity, poor cellularity, and smoothness of calcified cartilage. However, in patellofemoral joints in equine models, partial-thickness defects with intact calcified cartilage were proven to be a good indication for treatment with ACI. ACI improved cartilage healing (although less obviously as in full cartilage defects), as seen with improved histological, immunohistological, and biochemical scores, including defect filling with collagen type II and attachment to the surrounding cartilage (Nixon et al. 2011).
Although ACI has produced promising results, it was indicated in previous studies that the degree to which hyaline-like cartilage fills a defect is insufficient to integrate with surrounding tissue (Breinan et al. 1997). Significant effects after ACI treatment in dog models seem to be short-termed and degenerative changes are not prevented (Nixon et al. 2011). In attempts to enhance the filling of cartilage defects with the functional tissue, biomaterials were developed to serve as carriers of cells.
4.3.3 Matrix-Induced ACI (MACI)
In the original ACI technique, the periosteal cover was used since it was thought to have the chondrogenic potential (O’Driscoll and Fitzsimmons 2001) and stimulate subchondral bone remodeling (Russlies et al. 2005). However, with ACI, there are damage associated with periosteal harvest (Ueno et al. 2001), damage associated with the suturing of articular cartilage (Hunziker and Stahli 2008), and hypertrophy observed after periosteal grafting (Ueno et al. 2001). The downside of this method is also a non-homogenous distribution of chondrocytes due to the use of cellular suspension, together with the risk of leaking out in case of inadequate sealing (Haddo et al. 2004). These limitations were improved by using the matrix-induced ACI (MACI), where alternative covers, such as porcine-derived type I/III collagen membrane, are used. The bilayered structure of a membrane is cell occlusive at the compact side, protecting cells from diffusion and mechanical impact, and the porous side consists of collagen fibers, allowing for cell invasion and attachment (Haddo et al. 2004). Autologous chondrocytes are seeded onto the membrane, enabling the membrane to be attached to the defect with the fibrin glue eliminating periosteal harvest, and procedure is faster and with less extensive exposure, as surgical implantation could be achieved via arthroscopy or mini-arthrotomy (Cherubino et al. 2003). Besides facilitating the handling of the cells, scaffolds are also useful for immobilization and broader distribution of the cells (Nuernberger et al. 2011). The procedure is traditionally performed by arthrotomy (Cherubino et al. 2003), but arthroscopy was also shown to be possible, as was shown in some studies with equine models that underwent arthroscopic implantation of cell-polymer (Ibarra et al. 2006; Masri et al. 2007) or cell-collagen membrane constructs (Frisbie et al. 2008; Nixon et al. 2017). In several studies of equine joint defect models, treatment with MACI resulted in significantly improved cartilage compared to spontaneously healing empty controls, as shown by arthroscopy, gross healing, histology scores, and mechanical analysis (Nixon et al. 2017; Nixon et al. 2015; Griffin et al. 2015). Materials other than collagenous membranes were also used for MACI, for example, PGLA, used in eight horse models and were shown to efficiently contain a large number of chondrocytes without the risk of cell loss when implanted arthroscopically with the use of a fluid pump (Masri et al. 2007). Although ACI and MACI have produced promising results and MACI treatment indeed improved cartilage healing, characterization of MACI graft implant in animal models showed that formed tissue has inferior shear properties to native cartilage (Nixon et al. 2015; Griffin et al. 2015; Lee et al. 2003). The loss of chondrocyte capacity to produce hyaline cartilage might be associated with the cell dedifferentiation occurring during chondrocyte culturing (Rakic et al. 2017).
Although increasing the dose of articular chondrocytes was shown to improve articular cartilage repair in a sheep model (Guillen-Garcia et al. 2014), chondrocytes cultured in vitro are prone to spontaneous dedifferentiation, albeit less so when cultured in a 3D environment. It was shown by Sanz-Ramos et al. (2014) that chondrocytes cultured in a 3D collagen environment possessed a better chondrogenic capacity in vitro and in vivo than the cells expanded on a plastic surface (Sanz-Ramos et al. 2014). Interestingly, the extent of dedifferentiation seems to vary between species. For example, sheep chondrocytes were shown to be able of spontaneous redifferentiation into hyaline-like cartilage, whereas human chondrocytes were able to redifferentiate only when stimulated by chondrogenic inducers (Giannoni et al. 2005). In the equine model, chondrocyte redifferentiation was shown to be possible under the influence of 3D collagenous microenvironment, hypoxia, and BMP2 (bone morphogenetic protein-2) and RNA interference (Rakic et al. 2017). In comparison to human and equine chondrocytes, dog chondrocytes showed no capacity to redifferentiate regardless of the inducers present (Giannoni et al. 2005). The interspecies differences in chondrocyte characteristics in culture indicate that species should be considered when extrapolating data from one species to another and that differences between species in terms of chondrocyte phenotype stability during expansion might also result in different clinical outcomes when used in ACI. In addition to interspecies differences, chondrogenic differentiation of chondrocytes was dependent also on the number of passages and aging (De Angelis et al. 2020; Acosta et al. 2006; Veilleux et al. 2004), as well as whether the cells were osteoarthritic or not (Acosta et al. 2006). While, interestingly, adult donors showed a more stable expression of some chondrogenic markers, chondrocytes from elderly animals dedifferentiated at earlier passages, associated with a reduced proliferative capacity (De Angelis et al. 2020). Chondrocyte dedifferentiation could therefore be controlled from different aspects of donor and culture factors.
Another hurdle in using ACI/MACI for the treatment of chondral defects is a need for a two-step surgery. In 2006 the evidence that ACI could be delivered without cell expansion was presented. It was proposed that mechanical fragmentation of cartilage was sufficient to mobilize embedded chondrocytes through the increased surface of tissue area. In goats, cartilage fragments were placed on resorbable scaffold hyaline-like tissue (Lu et al. 2006). The procedure was adopted also in horse models with autologous cartilage fragments on a polymer scaffold implanted in a defect within the equine femoral trochlea. Compared to two-step ACI treatment, one-step treatment with minced cartilage achieved an even higher score in arthroscopic, histologic, and immunohistochemistry evaluation and prompted a phase 1 clinical study in humans (Frisbie et al. 2009). In a study performed in dogs, it was demonstrated that 100-μm-sized cartilage particles yielded the highest number of cells and provided the most optimal cartilage regeneration, based on the autologous intrafacial implantation of the microcartilage together with the absorbable scaffold and the slow release system of the basic fibroblast growth factor (Nishiwaki et al. 2017). Another possibility to overcome the need for two-step surgery was proposed by Bekkers et al. who showed that a one-stage procedure could be achieved by combining chondrocytes or chondrons with bone marrow mononuclear cells or MSCs. In a goat model, such implantation outperformed microfracture (Bekkers et al. 2013a, b).
Despite promising results associated with ACI/MACI for treatment of chondral defects, there are still many challenges that have not yet been overcome, such as insufficient integration of implanted chondrocytes, insufficient capacity of chondrocytes to produce hyaline cartilage, dedifferentiation of cultured chondrocytes, the need for two-step surgery, and the harvesting procedure that may result in changes in the articular cartilage that potentially represent a risk of becoming clinically relevant (Lee et al. 2000). This is why in recent years other treatment options for cartilage defects are increasingly being investigated. MSCs as possible substitute cells for chondrocytes are the focus of the most recent research. MSCs seem promising candidates for replacing chondrocytes because of their immunomodulatory properties and their ability to differentiate into several specialized cells, including chondrocytes. At the same time, many novel biomaterials are at the forefront of cartilage regeneration research, aiming to (i) resemble native cartilage tissue to provide the most optimal environment for chondrogenic differentiation of MSCs and (ii) simultaneously develop clinically relevant biocompatible material for in vivo implantation.
5 Attempts to Improve Existing Regenerative Treatment Options with the Use of Mesenchymal Stem Cells
5.1 Chondrogenic Differentiation of MSCs
MSCs have in recent years received significant interest in veterinary and human medicine due to their immunomodulatory and multilineage differentiation properties. Under appropriate culture conditions, MSCs can be induced toward differentiation into different lineages such as adipocyte, osteocyte, and chondrocyte lineages (Dennis et al. 1999). Although there are some reports on spontaneous chondrogenic differentiation of MSC ascribed to either high cell density (Bosnakovski et al. 2004; Dudakovic et al. 2014), presence (Fortier et al. 1998) or absence (Cho et al. 2018) of fetal bovine serum (FBS) in cell culture media, early passages (De Bari et al. 2001), or tissue source (Naruse et al. 2004), chondrogenesis on a standard 2D polystyrene surface is commonly induced with specific culture conditions such as chondrogenic differentiation media, high cell density, and highly humid atmosphere. Chondrogenic differentiation of MSCs is commonly performed in two ways. One technique is a pellet culture – a scaffold-free three-dimensional (3D) culture with high cellular density, where cells are grown in polystyrene conical tubes to form a spherical aggregate at the bottom of a tube (Johnstone et al. 1998). Another method is a micromass culture system where cells are placed in the microwell cell culture plate as droplets of cells with high density that become coalesced to form micromasses of cartilaginous tissue (Mello and Tuan 1999). During early chondrogenesis progenitor cells condense and express collagen type I. By the 5th day, collagen type II is detected and type X collagen is detected by the 14th day. The presence of aggrecan and link protein in the cell aggregates demonstrate that aggregating proteoglycans of the cartilaginous tissue are synthesized by the newly differentiating cells (Yoo et al. 1998). Commonly recognized markers of chondrogenesis in MSCs are SOX9, collagen type II, aggrecan, GAG, and COMP (De Angelis et al. 2020). In chondrogenic differentiating media, growth factors and hormones, namely, TGF-β and dexamethasone (Li and Pei 2018; Mwale et al. 2006), are often used to induce chondrogenesis. TGF-β upregulates chondrogenesis by enhancing SOX9 expression and inhibiting osteoblast differentiation by repressing expression of RUNX2 (Pei et al. 2009), while dexamethasone potentiates the growth factor-induced chondrogenesis of MSCs in vitro, although its influence is not indispensable for chondrogenic differentiation of MSCs as it is dependable on tissue source and microenvironment of MSCs (Shintani and Hunziker 2011). Besides TGF-β, other growth factors, namely, IHH and BMP2 (Steinert et al. 2012; An et al. 2010), FGF (Handorf and Li 2011), and IGF (An et al. 2010; Patil et al. 2012), were also shown to be inducers of chondrogenesis of human MSCs. However, the molecular mechanisms of chondrogenesis are not yet fully understood.
5.2 Hypertrophy Associated with Chondrogenic Differentiation of MSCs
Due to their rapid expansion in culture, trilineage differentiation potential, and easier retrieval that is not associated with articular cartilage damage as opposed to chondrocytes, using MSCs over articular chondrocytes is thought to be advantageous, especially since chondrogenesis of MSCs can be achieved with relatively simple procedures on a standard polystyrene surface. However, the undesirable effect of differentiating MSCs toward chondrogenic lineage is the constitutive expression of hypertrophic markers in MSCs. Hypertrophic markers include collagen type X, MMP13, VEGF (Chen et al. 2019), and a novel biomarker, thrombospondin-1 (TSP-1), known by its antiangiogenic properties and recently described as an antihypertrophic protein (Cortes et al. 2021; Gelse et al. 2011). The chondrocyte hypertrophy stage can ultimately lead to apoptosis, vascular invasion, and ossification, similarly as in the growing cartilage (Bruderer et al. 2014; Mueller and Tuan 2008). Notably, hypertrophy-related changes can also be related to pathological conditions such as OA (Tchetina et al. 2005; Walker et al. 1995; Nakase et al. 2002). Importantly, it was shown that chondrogenically differentiated MSCs with expressed hypertrophy-associated genes result in mineralization, related to endochondral ossification when transplanted to ectopic sites in severe combined immunodeficient mice (Pelttari et al. 2006). The main hesitation associated with the clinical use of MSCs is therefore their inability to recapitulate stable articular chondrocyte phenotype. Indeed, the extent of the expression of hypertrophic factors might be dependent on the protocol for induction of chondrogenesis. Micromass culture was shown to be superior to pellet culture in that induced cartilaginous tissue was larger, more homogenous, and enriched in collagen type II, while the expression of hypertrophic markers was lower than in a pellet culture (Zhang et al. 2010). Yet, MSCs cultured under either of the two chondrogenic conditions are prone to hypertrophy and matrix calcification, unlike articular chondrocytes that under the same conditions maintain a non-hypertrophy phenotype (Pelttari et al. 2006). Hypertrophy correlated with both techniques is therefore undesirable as it may cause endochondral ossification in vivo.
Reduction of chondrocyte hypertrophy is extensively being investigated by using different techniques, such as co-culturing MSCs with chondrocytes; culturing MSCs in the hypoxic atmosphere; adding hormones, proteins, or other components to the culture media; silencing hypertrophic genes; or using biomaterials to imitate the natural cell environment. Some of these techniques offer promising results, although to date none have shown clinically relevant reduction, let alone complete prevention of hypertrophic differentiation.
5.3 Attempts at Reduction of MSC Hypertrophy
5.3.1 Co-culture
Chondrogenesis of MSCs greatly depends on the microenvironment, as soluble factors from surrounding tissue/cells or direct cell-cell contact can alter gene and protein expression profiles (Grassel and Ahmed 2007). The accurate regulation of key factors involved in chondrocyte hypertrophy might enable guidance of MSCs between chondral and endochondral pathways (Dreher et al. 2020). One of the ways to reduce hypertrophic differentiation of MSCs is thus co-culturing MSCs with chondrocytes, as it was previously shown that chondrocytes provide chondrogenic signals to MSCs via paracrine secretion of soluble factors including TGF-β1, IGF-1, and BMP2 (Liu et al. 2010). Inversely, chondrocytes were also shown to be affected by paracrine secretion of MSCs, as was shown by co-culturing human adipose or bone marrow-derived MSCs, leading to reduction of hypertrophy and dedifferentiation of chondrocytes, which was partially ascribed to HGF secretion by MSCs (Maumus et al. 2013). In rats, reduced hypertrophy by MSC and chondrocyte co-culture was demonstrated by increased expression of aggrecan and collagen type II together with a reduction of collagen type X and MMP13 formation (Ahmed et al. 2014). Similarly, hypertrophy reduction was shown in 3D in vitro environment with co-cultures of bovine MSCs and ACs (Meretoja et al. 2013). Effects of hypertrophy suppression were demonstrated in several other studies where MSCs were co-cultured with chondrocytes (Fischer et al. 2010; Ramezanifard et al. 2017; Amann et al. 2017). Since there is a lack of proper chondrogenic niche, it is a great challenge to stabilize ectopic chondrogenic differentiated MSC phenotype not only in vitro but also in vivo, e.g., in subcutaneous tissue. It was previously shown that the differentiation potential of MSCs is different in vitro when compared to implantation in vivo. Yang et al. (2009) demonstrated that the proliferation rate of bone marrow-derived rat MSCs cultured in vitro in a 3D environment was similar to self-renewal capacity during in vivo implantation (Yang et al. 2009), whereas trilineage differentiation potential was suppressed in vivo in comparison to in vitro conditions. However, it was shown by Liu et al. (2010) that chondrogenic niche within subcutaneous environment could be created by co-transplantation of MSCs and articular chondrocytes, as was shown with bone marrow-derived porcine MSCs and articular chondrocytes. Chondrogenic signals were provided by the secretion of soluble factors by chondrocytes, including TGF-β1, IGF-1, and BMP2, and not by cell-cell interactions (Liu et al. 2010). Interestingly, there are some reports about the inability of articular chondrocytes to prevent hypertrophy of MSCs in pellet cultures (Giovannini et al. 2010). Similarly, nasal chondrocytes were not able to prevent MSC hypertrophy and calcification in vivo unless parathyroid hormone-related protein (PTHrP) was added to the culture (Anderson-Baron et al. 2020).
5.3.2 PTHrP
PTHrP along with its receptors is generally accepted as an inhibitor of chondrocyte development during chondrogenesis of the growth plate (Kronenberg 2003) and is a commonly reported factor to reduce hypertrophy. Fischer et al. showed that when cultured in a chondrocyte-conditioned medium together with PTHrP, expression of collagen type X, the activity of alkaline phosphatase, and matrix calcification in human MSCs were reduced. Pulsed rather than constant application of PTHrP was shown to be even more effective in the reduction of endochondral differentiation (Fischer et al. 2014). PTHrP was shown to be effective in the reduction of endochondral ossification in several other studies investigating the effect of PTHrP on human MSCs (Mwale et al. 2010; Weiss et al. 2010; Mueller et al. 2013). However, although PTHrP was shown to reduce hypertrophy, it was also reported to simultaneously reduce GAG synthesis and thus have a negative effect on chondrogenesis in human MSCs (Browe et al. 2019). Therefore, further research is needed to better understand the role of PTHrP in the chondrogenesis of MSCs.
5.3.3 Matrilin-3
Besides PTHrP, a non-collagenous ECM protein matrilin-3 (MAT3) was reported to play a regulatory role in cartilage homeostasis. It was previously shown that mutation or deletion of human MAT3 is associated with the early onset of cartilage degenerative diseases (Stefansson et al. 2003; Borochowitz et al. 2004). Indicative chondroprotective properties of MAT3 were supported in a study conducted on human and mice chondrocytes, where it was shown that MAT3 was responsible for the upregulation of cartilage matrix components such as collagen type II and aggrecan. Moreover, it was shown to slow down cartilage degeneration by downregulation of matrix-degrading enzymes, namely, collagenase MMP13 and aggrecanase ADAMTS-4 and ADAMTS-5 (Jayasuriya et al. 2012). The role of MAT3 in slowing cartilage degeneration was shown also in vivo, where MAT3 -primed MSCs suspension slowed the progression of cartilage degeneration in the medial meniscus OA mouse model (Muttigi et al. 2020). In addition to its chondroprotective role, MAT3 was also shown to significantly reduce hypertrophy in chondrocytes and MSCs. In hypertrophic chondrocytes, MAT3 acts as a BP-2 antagonist as it was shown to inhibit BMP/SMAD 1 activity leading to downregulation of collagen X expression and thus inhibition of premature chondrocyte hypertrophy (Yang et al. 2014). In hypertrophic human adipose-derived MSCs, MAT3 significantly reduced the expression of hypertrophic markers such as collagen type X, RUNX2, and ALP (Muttigi et al. 2020). In a study conducted by Liu et al. (2018) where the chondroprotective role of MAT3 was demonstrated in vivo as well as in vitro, the role of MAT3 was ascribed to its function in promoting the expression of HIF1-α. Hypoxia-inducible factor-1alpha (HIF-1α) was shown to be a key mediator in the cellular response to hypoxia (Kanichai et al. 2008) and vital in articular cartilage homeostasis (Liu et al. 2018).
5.3.4 Hypoxia
Since the articular cartilage microenvironment is relatively low in partial oxygen pressure (~ 1–5% O2) (Gale et al. 2019; Brighton and Heppenstall 1971), a low-oxygen environment for cell chondrogenic differentiation culture conditions was proposed as opposed to standard incubator culture conditions (~ 21% O2). In fetal mice forelimb organ culture, HIF-1α was shown to regulate chondrocyte differentiation and function during endochondral ossification through triggering BMP2 activation and suppressing the activity of alkaline phosphatase and suppressing collagen type X expression (Hirao et al. 2006). When combined with BMP2, hypoxia and BMP2 synergistically promote the expansion of proliferating chondrocyte zone and inhibit chondrocyte hypertrophy and ossification (Zhou et al. 2015). In chondrocytes, hypoxia promoted chondrocyte rather than osteoblast commitment by suppressing collagen type X mediated by downregulation of RUNX2 activity (Hirao et al. 2006). Interestingly, in chondrocytes, hypoxic culture conditions were shown to induce the expression of PTHrP in a HIF-1alpha-dependent manner (Pelosi et al. 2013). Combining hypoxia and exogenous PTHrP may therefore result in an additive effect in maintaining high levels of GAGs while reducing ALP activity (Browe et al. 2019). Similar effects of hypoxia that were shown with chondrocytes were also shown with MSCs. Kanichai et al. demonstrated that a hypoxic cell environment together with chondrogenic culture conditions significantly enhances collagen II expression and proteoglycan deposition in rat MSCs (Kanichai et al. 2008). HIF-1α in human and murine MSCs, similarly as in chondrocytes, potentiated the expression of BMP2-induced chondrogenic markers and inhibited expression of RUNX2 and osteogenic markers in vitro (Zhou et al. 2015). As in chondrocytes, where hypoxia was shown to induce the expression of PTHrP, hypoxia was also shown to induce PTHrP and reduce MEF2C expression in human MSCs, demonstrating a pathway by which hypoxia attenuates hypertrophy (Browe et al. 2019). Based on the published results from human and murine stem cells, hypoxia seems to enhance chondrogenesis while suppressing hypertrophy. In addition, hypoxia was shown to enhance chondrogenesis also in canine and equine MSCs (Lee et al. 2016; Ranera et al. 2013). Interestingly, in another study investigating the effect of hypoxia on chondrogenesis of equine MSCs, hypoxia did not significantly increase the chondrogenesis of either synovium or bone marrow-derived MSCs, but it did downregulate the expression of hypertrophic marker collagen type X (Gale et al. 2019). Moreover, when studying hypertrophy of bovine MSCs and ACs cultured in a 3D microenvironment under different atmospheric conditions, hypertrophy was reduced in co-cultures of MSCs and ACs in both normoxic and hypoxic conditions, whereas culturing MSCs alone even increases hypertrophic differentiation in hypoxia compared to normoxic conditions (Meretoja et al. 2013). These studies indicate the possibility that there is a difference in susceptibility of MSC to hypoxic conditions between species. The effect of hypoxic culture conditions on suppressing hypertrophy in MSC chondrogenic differentiation might also be dependent on the tissue source of MSCs (Gale et al. 2019). Further studies are therefore needed to more accurately establish the role of hypoxia in MSC chondrogenesis.
Silencing genes associated with hypertrophy is another possible approach in stabilizing chondrogenic phenotype, as was demonstrated in a study conducted on equine bone marrow-derived MSCs, where it was shown that silencing the hypertrophic genes might prevent the persistence of collagen I expression and increase the collagen type II/collagen type I ratio. Introducing siRNA to cells targeting col1a1 resulted in 50% inhibition of col1 expression, suggesting the need for further exploration of the knockout strategy to limit hypertrophic differentiation of MSCs (Branly et al. 2018).
Besides abovementioned attempts to revert hypertrophy, there are also some reports of other possible ways to reduce chondrogenic differentiation-related hypertrophy. For example, it was previously shown that TGF-β and high doses of steroid hormones together with the absence of thyroid hormones inhibit the induction of hypertrophy (Mueller and Tuan 2008; Karl et al. 2014). Pei et al. showed that TGF-β-induced chondrogenesis was enhanced when synovium-derived MSCs were transfected with histone deacetylase 4, while type X collagen expression was simultaneously reduced (Pei et al. 2009). One of the reported agents to suppress the expression of hypertrophic genes is XAT (xanthotoxin), a furanocoumarin, also named methoxsalen, otherwise used in treating various skin diseases in humans such as vitiligo and psoriasis. It was previously shown to be able to prevent bone loss in ovariectomized mice through inhibition of RANKL-induced osteoclastogenesis (Dou et al. 2016). In the following study examining the effect of XAT on chondrocyte hypertrophic differentiation, it was shown that XAT inactivates the p38-MAPK/HDAC4 signaling pathway leading to reduced degradation of HDAC4 and inhibition of RUNX2 and thus participates in maintaining chondrocyte phenotype in regenerated cartilage (Cao et al. 2017). Hypertrophy of IPSC during chondrogenesis was also reduced using lithium-containing bioceramics with bioactive ionic components (Hu et al. 2020).
Studies investigating different options to revert hypertrophy provide promising results and offer the potential for new ways of maintaining chondrogenic differentiation by suppressing endochondral ossification. However, in most of these studies, MSCs were cultured in a standard 2D environment, which is fundamentally different from their natural environment, and none of the methods described above have provided satisfactory results, preventing the application of differentiated cells in clinical use for cartilage regeneration. To further address this issue, other approaches in the induction of chondrogenic differentiation of MSCs and cartilage regeneration are being investigated, with the focus on recapitulating MSCs native environment.
5.4 Biomaterials for Mimicking Native Cartilage Tissue
5.4.1 The Influence of the 3D Structure on MSCs
The importance of mimicking cellular natural microenvironment lies in spatially and temporally complex signaling that directs the cellular phenotype. The cell, together with the ECM, growth factors, hormones, and other molecules, is connected into an entity, which guides the functioning of individual organs and the whole organism (Tibbitt and Anseth 2009). The interaction of stem cells and their niches creates a dynamic system that is being imitated by in vitro niche models to move closer to the possibility of the therapeutic use of chondrogenic differentiated MSCs. 3D cell culture mimics mechanical and biochemical properties of the natural cellular environment and consequently provides a better insight into the physiological function of MSCs (Jensen and Teng 2020), which is especially important from the therapeutic aspect of using MSCs (Egger et al. 2019). Studies investigating the influence of the 3D environment on MSCs have shown that the 3D environment provides better conditions for expressing biological mechanisms, including cell number, vitality, morphology, proliferation, differentiation, response to environmental signals, intercellular communication, migration, angiogenesis stimulation, immune system avoidance, gene expression, and protein synthesis. 3D cell environment has thus been shown to be more suitable for cell culture than 2D (Antoni et al. 2015). In 3D cultures using carriers or biomaterials, four basic groups of materials are used – polymeric, ceramic, metallic, and composite materials (Kapusetti et al. 2019) – among which the most commonly used are hydrogels, polymeric materials, hydrophilic glass fibers, and organoids (Jensen and Teng 2020).
5.4.2 Influence of Biomaterial Properties on MSCS
The mechanical, surface, and chemical properties of the biomaterial are recognized as crucial in controlling cell fate (Martino et al. 2012). Stem cells are known to be sensitive to the mechanical properties of biomaterials and can recognize a solid substrate even when they are not in direct contact with it (Schaap-Oziemlak et al. 2014). Their adhesion to the substrate depends on the elasticity of the biomaterial, suggesting that even the smallest changes in the mechanical properties of the biomaterial can affect stem cell differentiation. Thus, the different elasticities of the biomaterial have different effects on cell adhesion, proliferation, and differentiation potential. For example, higher biomaterial strength leads to greater potential for osteogenic differentiation due to increased integrin activation, and softer biomaterials increase expression of II type collagen and lipoprotein lipase, markers for adipogenic and chondrogenic differentiation, respectively (Xu et al. 2013). In addition to the mechanical properties of the biomaterial, the surface properties also play an important role in the fate of MSCs. Stem cells do not bind directly to the surface of the biomaterial. In proteinaceous solution, e.g., in cell culture medium, stem cells bind indirectly to the surface of the biomaterial by binding to pre-bound proteins because of their slower movement compared to proteins (Tamada and Ikada 1993). The binding of cells to proteins depends on the distribution and conformation of the proteins, the latter of which depends on the wettability and chemical composition of the biomaterial (Schaap-Oziemlak et al. 2014). Therefore, the manipulation of proteins bound to the surface of the biomaterial is of particular importance in controlling cell adhesion (Schaap-Oziemlak et al. 2014). The results of several studies also indicate the influence of the chemical properties of the biomaterial surface on the direction of cell differentiation (Ren et al. 2009; Curran et al. 2006; Benoit et al. 2008). The surface treatment of biomaterials with different chemical groups, e.g., methyl (-CH3), amino (-NH2), thiol (-SH), hydroxyl (-OH), or carboxyl (-COOH) groups, can have different effects on cell fate and lead MSCs to adipogenic, osteogenic, or chondrogenic differentiation (Curran et al. 2006; Benoit et al. 2008). However, the direction of cell differentiation in a 2D or 3D environment may differ with the addition of the same chemical group (Schaap-Oziemlak et al. 2014). Therefore, the 2D or 3D environment may affect the fate of MSCs differently depending on the functional chemical group.
5.4.3 General Structure of Biomaterials for Cell Encapsulation
In addition to the mechanical, surface, and chemical properties, the scaffold structure itself also importantly affects stem cells. 3D biomaterials can be microporous, nanofibrous, or composed as hydrogels. Microporous structure supports the encapsulation of cells, but due to the pore size (100 μm) being larger than the average cell diameter (10 μm), they represent a curved 2D microenvironment. Nanofibrous structures containing fibrillar ECM proteins provide a better approximation of the natural cellular environment, but their mechanical properties are too weak to handle the stress required for mechanotransduction. Hydrogels do not have these limitations, making them a suitable biomaterial for the development of an ECM-like environment. The network structure of interconnected polymer chains allows for high water content and transport of oxygen, nutrients, waste, and other soluble molecules. Hydrogels can be composed from a range of natural or synthetic materials that exhibit a wide range of different mechanical and chemical properties (Tibbitt and Anseth 2009). Compared to synthetic hydrogels, natural hydrogels not only enable but also promote their cell activities. Natural hydrogels are usually composed of ECM proteins such as collagen, fibrin, hyaluronic acid, or components from other biological sources such as chitosan (Ribeiro et al. 2017), alginate (Sun and Tan 2013), and silk (Kundu et al. 2013).
5.4.4 Natural Biomaterials to Promote MSC Chondrogenesis
For cartilage regeneration, various scaffold materials have been developed. Most commonly used biomaterials for cartilage tissue regeneration are of natural origin, which are biocompatible, contain bioactive molecules such as RGD tripeptides that enable cell adhesion, but have in most cases poor mechanical properties and high degradation rate. Natural biomaterials are composed either of polymers, for example, agarose, alginate, chitosan, and hyaluronate, or of proteins, such as collagen, gelatin, fibrin, and silk (Ge et al. 2012). On the other hand, synthetic polymers such as polyglycolic acid (PGA), polylactic acid (PLA), poly(lactic-co-glycolic acid (PLGA), or poly(ethylene glycol) (PEG) lack the binding sites for adhesion molecules and have been shown to promote the undesirable endochondral ossification (Salonius et al. 2020), but usually provide with controllable degradation rate, high reproducibility, and easy manipulation to form specific shapes (Ahmed and Hincke 2010). Due to the advantages and disadvantages of either natural or synthetic materials, hybrid materials are also thought of as promising materials for providing microenvironment resembling cartilage tissue that is suitable for induction of stem cell chondrogenesis. Below, the commonly used biomaterials for induction of chondrogenesis are described.
5.4.4.1 Collagen
One of the most extensively used biomaterials in tissue engineering is collagen as it is a key component of cartilage ECM. It is also biocompatible and easy to manipulate with. Bioactive domains in its structure allow for good adhesion of cells. Type I/III collagen membrane has been frequently used in MACI therapy (Haddo et al. 2004). However, there are several disadvantages associated with the use of collagen as a scaffold. Firstly, the use of collagen is associated with the risk of immunogenicity (Kim et al. 2020a, b). Secondly, there is also a possibility of prion transmission (Raftery et al. 2016). Thirdly, collagen does not possess suitable mechanical strength to withstand the in vivo forces (Ahmed and Hincke 2010; Raftery et al. 2016), and lastly, culturing MSCs on collagen does not prevent hypertrophic differentiation of MSCs, as shown by human bone marrow-derived MSCs cultured either on commercial type I/III membrane or collagen/polylactide composite scaffolds, both resulting in a hypertrophic state of the cells (Salonius et al. 2020).
Regarding the immunogenicity of collagen, atelocollagen – telopeptides-free collagen – provides a biomaterial with no immunogenic activity. For treatment of chondral defects in human medicine, atelocollagen combined with microdrilling is used as an enhancement of traditional microfracture technique using the off-the-shelf product (Kim et al. 2020a). Atelocollagen, obtained by salt precipitation, was also tested for chondrogenesis of MSCs. Compared to type I collagen, type I atelocollagen enhanced chondrogenic markers’ expression of human adipose-derived MSCs. Moreover, reduction of chondrogenic markers’ expression RUNX2, osterix, and MMP13 was observed in cells cultured on atelocollagen, indicating better suitability of atelocollagen compared to collagen for in vitro cartilage engineering applications (Kim et al. 2020b). As a less immunogenic alternative to collagen, gelatin is also used. It is produced from processed bovine or porcine bones and skin and is usually used in combination with other materials to combine positive properties of both (Ahmed and Hincke 2010). For example, the gelatin-alginate scaffold was used to demonstrate that the proliferation rate of bone marrow-derived rat MSCs cultured in vitro on the scaffold was similar to self-renewal capacity during in vivo implantation (Yang et al. 2009).
To avoid the risk of prion transmission, other sources of collagen, besides mammal, are being investigated, such as salmon skin. However, it was shown that salmon skin-derived collagen is inferior to bovine-derived collagen in several terms such as porosity, pore size, architecture, compressive modulus, capacity for water uptake, and rat MSC proliferation and differentiation (Raftery et al. 2016).
In structural and load-bearing performance, collagen plays a pivotal role, while surrounding polysaccharides are needed for internal stress management and elastic reinforcement of collagen and absorption of fluids due to their hydrophilic nature. A protein-polysaccharide scaffold was therefore thought of as a promising material for induction of stem cell chondrogenesis. When used either alone or cross-linked with dextran or chitosan, the PEG-chitosan construct was determined as the most appropriate in inducing chondrogenesis as well as in reducing hypertrophy in human bone marrow-derived MSCs (Sartore et al. 2021). To improve the mechanical strength of the scaffold, chitosan is also increasingly studied and often used in combination with collagen. The addition of chitosan to collagen not only improved the mechanical strength of collagen but also increased compressive strength and swelling ratio and prolonged the degradation rate (Raftery et al. 2016).
5.4.4.2 Hyaluronic Acid
In addition to collagen, hyaluronic acid (HA) is one of the promising biomaterials in use for chondrogenic induction of stem cells. Hyaluronic acid is a natural component of the cartilage ECM. However, HA is highly degradable in vivo and cannot bind proteins with high affinity because of the lack of negatively charged sulfate groups. Sulfated HA was therefore fabricated to encapsulate human MSCs. The sulfated HA exhibited slower degradation, improved protein sequestration, and promoted chondrogenesis. Furthermore, it suppressed hypertrophy in vitro and in vivo in the OA rat model, due to improved growth factor retention (Feng et al. 2017). When HA was added as a supplementation to a collagen hydrogel, it was shown to stimulate chondrogenic differentiation of adipose-derived human MSCs in a dose-dependent manner. Among different concentrations from 0 to 5%, 1% HA showed the best overall results in terms of SOX and Coll type II expression. Furthermore, exchanging 25% of human articular chondrocytes with 75% of adipose-derived human MSCs didn’t change the chondrogenic potential of MSCs, but reduced hypertrophy and improved biomechanical properties (Amann et al. 2017).
5.4.4.3 Silk Fibroin
One of the promising biomaterials for use in tissue engineering is silk fibroin, derived from the silkworm Bombyx mori. It is biocompatible, has suitable mechanical properties, and is produced in bulk in the textile industry (Kundu et al. 2013). In comparison to other natural biomaterials used for tissue engineering, SF provides a remarkable combination of strength, toughness, and elasticity that are ascribed to its crystallinity, hydrogen bonding, and numerous small β-sheet crystals (Altman et al. 1992). Another advantage of SF is its ability to take the form of different shapes such as hydrogels, tubes, sponges, composites, fibers, microspheres, and films that could be used in tissue engineering (Rockwood et al. 2011). It was previously reported that silk fibroin can aid in MSC differentiation when combined with different components. It was previously shown that silk fibroin with incorporated L-ascorbic acid 2-phosphate significantly promoted collagen type I in mouse fibroblast L929 cells (Fan et al. 2012). It was shown to promote osteogenic differentiation and mineralization of human ADMSCs (Gandhimathi 2015), and in another study, it was shown that silk fibroin scaffold combined with PRP effectively induced chondrogenesis of human ADMSCs (Rosadi et al. 2019). Interestingly, it was shown by Barlian et al. that silk fibroin combined with silk spidroin promoted better chondrogenesis of human Wharton jelly’s MSCs than silk fibroin alone and that cell culture medium supplemented with PRP promoted higher GAG accumulation in comparison with medium supplemented with ascorbic acid (Barlian et al. 2018). Contrary to mentioned studies where combining silk fibroin with other components was needed to induce chondrogenesis in MSCs, we have shown in our previous research that SF alone could also induce chondrogenesis in canine adipose-derived MSCs, possibly as a species-specific effect.
5.4.4.4 Decellularized Cartilage Matrix
Besides natural biomaterials such as collagen, hyaluronic acid, gelatin, chitosan, or silk fibroin, which have provided some promising results regarding chondrogenic differentiation of MSCs and reducing their hypertrophy phenotype, other ways for more accurate recapitulation of the cartilage microenvironment are being exploited. Among them, decellularized cartilage scaffolds have shown promise in providing the structural integrity of engineered tissues, better load-bearing ability, and functioning as a reservoir of signaling molecules, e.g., cytokines and growth factors, providing a specific microenvironment similar to native tissue. A hybrid natural ECM scaffold/artificial polymer polycaprolactone (PCL) was developed by combining ECM produced by bovine chondrocytes co-cultured with rabbit MSCs on electrospun microfibrous PCL. This hybrid scaffold was shown to have a positive effect on rabbit MSCs on aggrecan, collagen II, and collagen II/I expression compared to PCL controls (Levorson et al. 2014). Further, Yang et al. developed a cartilage ECM-derived acellular matrix by physically shattering human cartilage, followed by decellularization, freeze drying, and cross-linking techniques. They showed that ECM enabled attachment, proliferation, and chondrogenic differentiation of canine bone marrow-derived MSCs (Yang et al. 2008). ECM scaffold was also shown to be beneficial in reducing loss of chondrogenic phenotype as shown by using ECM scaffold derived from porcine chondrocytes seeded with rabbit MSCs in vivo compared with PGA scaffold (Choi et al. 2010).
In comparison to other mentioned biomaterials, decellularized cartilage ECM is advantageous in that it importantly recapitulates the native cartilage structure. However, achieving the complexity of articular cartilage structure regarding the mechanical stimulation to which the articular cartilage is constantly subjected and related orientation of collagen fibrils is especially challenging. The effect of mechanical loading and orientation of collagen fibrils on cartilage regeneration potential has been investigated in several studies.
5.4.5 Role of Mechanical Stimulation in Cartilage Regeneration
Since articular cartilage is subjected to constant movement and mechanical load, mechanical stimulation was proposed as a factor to affect ECM development. For example, it was shown in chicken micromass cultures that mechanical loading significantly augmented cartilage matrix production and upregulated expression of collagen type III, aggrecan, and hyaluronan synthases through enhanced expression of SOX9 and protein kinase A activity (Juhasz et al. 2014). Improvement of cartilage formation with reduction of hypertrophy was demonstrated to depend on several parameters, such as loading intensity, duration, and frequency of mechanical stimulation (Thorpe et al. 2012; Haugh et al. 2011; Zhang et al. 2015; O’Conor et al. 2013; Li et al. 2010; Bian et al. 2012). Optimal mechanical load, therefore, plays a crucial role during in vitro chondrogenesis of MSCs. Although mechanical forces importantly regulate MSC chondrogenic gene expression, sustained TGF-β exposure is usually also necessary for mechanically based chondrogenic improvement (Zhang et al. 2015; Huang et al. 2010; Goldman and Barabino 2016). Also, the dosage of growth factor was shown to importantly affect hypertrophy, in that only high levels of TGF-β stabilized chondrogenic phenotype (Zhang et al. 2015; Bian et al. 2012). There are, however, reports on mechanically induced proteoglycan synthesis in the absence of chondrogenic cytokines (Kisiday et al. 2009). In a study investigating the influence of mechanical load on porcine bone marrow-derived MSCs cultured on agarose or fibrin scaffolds, the mechanical load was even shown to override the influence of specific substrates, scaffolds, or hydrogels that have been shown to regulate MSC fate (Thorpe et al. 2012). In contrast to studies supporting the effectiveness of mechanical load in MSC chondrogenesis, it was shown that in the initiation stage of cartilage repair, the mechanical load may not necessarily positively affect the cell fate. In a study investigating the effect of chondrogenic priming of equine peripheral blood MSCs on adhesion and incorporation into cartilage explants, it was shown that mechanical loading reduced the adhesion of cells and altered integration of MSCs into isolated cartilage explants (Spaas et al. 2015). These results are consistent with other studies investigating the effect of biomaterial properties on cell chondrogenesis, showing that mechanical properties can influence cells in terms of their spreading, migration, and differentiation (Toh et al. 2012; Vainieri et al. 2020). This indicates that adjusting biomaterial properties to match mechanical properties, alongside composition and architecture of cartilage, may prevent the incorporation of cells into the cartilage and consequently alter initiation steps of tissue repair (Vainieri et al. 2020). In support of these data, it was also previously demonstrated that mechanical load was associated with bone formation. Mechanical load led to the expression of NGF in mice osteoblasts, followed by the activation of NGF-receptor-positive sensory neurons, resulting in osteogenic cues and bone mass formation (Tomlinson et al. 2017). The data indicate that removing the mechanical load could have a positive effect on MSC in enabling them to reestablish joint homeostasis. Due to the contradictory results from different studies investigating mechanical load on MSCs, further research of the biomechanics, especially early in the disease course, will be needed to provide the data on which MSC repair strategies are needed for optimal cartilage regeneration (McGonagle et al. 2017).
5.4.6 Importance of Biomaterial Architecture
The mechanical performance of articular cartilage directly correlates with the complexity of its structure. Scaffold geometry, recapitulating native orientation of collagen fibrils forming Benninghoff arcades (Benninghoff 1925), thus also seems to play an important role in regulating the cartilage-like activity of cells. For example, bone marrow-derived porcine MSCs expressed collagen type II and synthesized GAGs to a greater extent when cultured on aligned polycaprolactone (PCL) microfibers than on randomly oriented scaffold that was more supportive of an endochondral phenotype as indicated by higher expression of bone morphogenetic protein-2 (BMP2) and type I collagen gene (Olvera et al. 2017). Similarly, mimicking aligned structures of ECM fibrils in cartilage tissue led to better chondrogenesis of human BM-MSC in a nanofibrous scaffold compared to a scaffold with randomly aligned nanofibers (Zamanlui et al. 2018). Furthermore, it was shown that chondrocytes respond differently to geometrically different scaffolds, for example, nanofibrous poly(L-lactide) scaffold more efficiently promotes the cartilage-like activity of bovine chondrocytes than microfibrous scaffolds (Li et al. 2006). A similar tendency of cells toward favoring nanoultrastructure of the scaffold was shown for MSCs. Culturing human MSCs on nanofibrous polycaprolactone resulted in an increased expression of aggrecan compared to MSCs cultured on a microfibrous scaffold (Schagemann et al. 2013). These studies indicate that nano-topographical geometry with aligned structures is favored by cell types such as chondrocytes and MSCs.
To further improve the imitation of the complex structure of cartilage tissue, Nurnberger et al. (2021) have fabricated decellularized articular cartilage scaffold treated for GAG removal and engraved with a CO2 laser to create the well-defined structure of native cartilage. With the laser, lines and crossed lines were created allowing enough space for homogenous distribution and for the new matrix to be generated. Interestingly, it was shown that new collagen fibers perpendicularly aligned to the cartilage superficial zone, corresponding to the natural alignment of the collagen fibers, deeming superior over scaffolds that promote random matrix deposition (Nurnberger et al. 2021).
One of the novel techniques used for creating complex 3D scaffold structures is 3D bioprinting, as was shown by printing decellularized ECM cross-linked with gelatin methacrylate. Bioactive factors and cells were quantitatively and accurately placed within to form a bionic multifunctional scaffold to recognize, bind, and recruit endogenous stem cells to the site. Scaffold with implanted aptamers for specifically recognizing and recruiting adipose-derived stem cells, together with TGF-β for stem cell chondrogenesis, resulted in a great improvement of in vivo cartilage full-thickness defects in rabbit models (Yang et al. 2021). Similarly, as in rabbit models, pig models of cartilage defects were used for testing 3D-printed hybrid scaffolds made of gelatine and hydroxyapatite. Gelatine-hydroxyapatite scaffolds, compared to gelatine scaffolds or blank controls, were shown to be the best in reducing hypertrophic markers and repairing cartilage injuries (Huang et al. 2021). 3D bioprinting allows for the fabrication of complicated yet stable structures of tissue analogs and is thus considered a very promising technology, holding considerable potential for articular cartilage repair.
The architectural complexity of cartilage tissue and its constant subjection to mechanical forces demands an understanding of complex mechanisms required for induction of stable chondrogenic phenotype with minimizing the upregulation of hypertrophic genes. Challenges faced in scaffold fabrication are achieving a layered structure mimicking highly specific hierarchical ultrastructure arrangement of ECM of cartilage, mechanical environment for cells resembling native cartilage, and providing physical and biochemical cues to control the biological environment of cells. Mimicking native mechanotransduction pathways may thus be a promising way in creating the desired environment for controlled and stable chondrogenesis. Although cartilaginous tissue structure is well established, its simulation in vitro has proven very challenging, yet novel technologies and increasing acquisition of comprehensive knowledge in regenerative medicine and tissue engineering are encouraging for future cartilage treatment options in both veterinary and human medicine.
6 Summary
Cartilage’s unique characteristics encourage scientist to develop methods to overcome its inability to heal. So far, medication-mediated treatment is often the first choice of therapy; however, the therapy is focused on relieving the symptoms but cannot induce repair or regeneration and is often associated with severe side effects. Due to cartilage avascularity, bone marrow stimulation techniques were developed, which have shown some short-term beneficial effects but resulted in a formation of fibrocartilage, which is mechanically insufficient to bear loading stress. Further attempts at repairing cartilage were focused on using native tissue to produce osteochondral grafts. The main disadvantages of this method are the limited amount of donor cartilage availability, donor site morbidity, and the lack of osteochondral graft integration. To overcome the lack of significant cellular activity with osteochondral grafts, ACI was proposed. ACI seemed to be advantageous over other techniques in that the cartilage that formed was predominantly hyaline-like, containing collagen type II. However, there was an issue with the non-homogenous distribution of chondrocytes and the consequential need for periosteal coverage, resulting in damage associated with periosteal harvest. The latter was overcome with the use of MACI. Although MACI treatment improved cartilage healing, the tissue formed was still inferior to the native hyaline cartilage. Moreover, cultivating chondrocytes is associated with chondrocyte dedifferentiation and thus potentially variable treatment results. Although this was shown as possible to overcome with one-step surgery where minced cartilage instead of isolated chondrocytes were used, novel methods to substitute the use of chondrocytes are being developed. MSCs’ immunomodulatory properties and multilineage differentiation ability make them attractive candidates as an alternative to chondrocytes. However, the generation of cartilage tissue from MSC is challenging as in vitro chondrogenic differentiation of MSC reflects endochondral ossification unable to maintain a stable hyaline stage. Hypertrophic development of MSCs leads to the bone formation on ectopic sites and is thus unsuitable for cartilage therapy in vivo. Other approaches in the induction of stable chondrogenic phenotype of MSCs are being investigated, with the focus on recapitulating MSCs native environment and providing MSCs the best options to express their biological function. Many novel biomaterials are thus at the forefront of cartilage regeneration research, from standard collagen-based matrices to novel decellularized ECM cell carriers. Recapitulating the exact architecture of cartilage tissue has proven challenging yet of great importance for cartilage tissue engineering. Despite advances made in biomaterial-based stem cells therapies, each scaffold material currently used in tissue engineering approaches is still limited in possessing all the requirements needed for cartilage regeneration. Moreover, the knowledge of stem cell mechanisms of action is still elusive. A more detailed comprehensive understanding of the MSC mechanisms of action and their responses to complex structural, architectural, and geometrical properties of biomaterials is therefore needed to find the most appropriate way of delivering stable cartilage tissue formation. Combining technologies and knowledge of different scientific fields is essential for engineering a biomaterial that would fundamentally contribute to cartilage regeneration. The collaboration of scientists from interdisciplinary fields is thus of key importance for the further development of advanced cartilage therapies. Looking forward, one can be hopeful that, based on the novel cutting-edge technologies being available and progressive knowledge acquisition, we are on the verge of future developmental breakthroughs in the field of cartilage regeneration.
Abbreviations
- ACI:
-
Autologous chondrocyte implantation
- Anti-NGF mAB:
-
Anti-nerve growth factor monoclonal antibodies
- BMP2:
-
Bone morphogenetic protein-2
- CD105:
-
Cluster of differentiation 105
- CD73:
-
Cluster of differentiation 73
- CD90:
-
Cluster of differentiation 90
- CD45:
-
Cluster of differentiation 45
- CD34:
-
Cluster of differentiation 34
- CD14:
-
Cluster of differentiation 14
- CD11b:
-
Cluster of differentiation 11b
- CD79a:
-
Cluster of differentiation 79a
- CD19:
-
Cluster of differentiation 19
- COMP:
-
Cartilage oligomeric matrix protein
- ECM:
-
Extracellular matrix
- EGF:
-
Epidermal growth factor
- FGF:
-
Fibroblast growth factor
- GAGs:
-
Glycosaminoglycans
- HA:
-
Hyaluronic acid
- HIF-1α:
-
Hypoxia-inducible factor-1alpha
- HLA:
-
Human leukocyte antigen
- IGF:
-
Insulin-like growth factor
- MACI:
-
Matrix-induced autologous chondrocyte implantation
- MAT-3:
-
Matrilin-3 protein
- MMP13:
-
Matrix metalloproteinase 13
- MMP:
-
Modified Maquet procedure
- MSCs:
-
Mesenchymal stem cells/medicinal signaling cells
- NGF:
-
Nerve growth factor
- NSAIDs:
-
Nonsteroidal anti-inflammatory drugs
- OA:
-
Osteoarthritis
- OCD:
-
Osteochondritis dissecans
- PDGF:
-
Platelet-derived growth factor
- PRP:
-
Platelet-rich plasma
- PCL:
-
Polycaprolactone
- PEG:
-
Polyethylene glycol
- PGA:
-
Polyglycolic acid
- PLA:
-
Polylactic acid
- PLGA:
-
Polylactic-co-glycolic acid
- PTHrP:
-
Parathyroid hormone-related protein
- ROS:
-
Reactive oxygen species
- RUNX2:
-
Runt-related transcription factor 2
- SOX9:
-
SRY-box transcription factor 9
- TGF-β:
-
Transforming growth factor beta
- TPLO:
-
Tibial plateau leveling osteotomy
- TSP-1:
-
Thrombospondin-1
- TTA:
-
Tibial tuberosity advancement
- VEGF:
-
Vascular endothelial growth factor
- 2D:
-
Two-dimensional
- 3D:
-
Three-dimensional
References
Acosta CA et al (2006) Gene expression and proliferation analysis in young, aged, and osteoarthritic sheep chondrocytes effect of growth factor treatment. J Orthop Res 24(11):2087–2094
Ahmed TA, Hincke MT (2010) Strategies for articular cartilage lesion repair and functional restoration. Tissue Eng Part B Rev 16(3):305–329
Ahmed MR et al (2014) Combination of ADMSCs and chondrocytes reduces hypertrophy and improves the functional properties of osteoarthritic cartilage. Osteoarthr Cartil 22(11):1894–1901
Altman RD et al (1992) Preliminary observations of chondral abrasion in a canine model. Ann Rheum Dis 51(9):1056–1062
Alves JC et al (2020) A pilot study on the efficacy of a single intra-articular administration of triamcinolone acetonide, hyaluronan, and a combination of both for clinical management of osteoarthritis in police working dogs. Front Vet Sci 7:512523
Amann E et al (2017) Hyaluronic acid facilitates chondrogenesis and matrix deposition of human adipose derived mesenchymal stem cells and human chondrocytes co-cultures. Acta Biomater 52:130–144
An C et al (2010) IGF-1 and BMP-2 induces differentiation of adipose-derived mesenchymal stem cells into chondrocytes-like cells. Ann Biomed Eng 38(4):1647–1654
Anderson KL et al (2020) Risk factors for canine osteoarthritis and its predisposing arthropathies: a systematic review. Front Vet Sci 7:220
Anderson-Baron M et al (2020) Suppression of hypertrophy during in vitro chondrogenesis of cocultures of human mesenchymal stem cells and nasal chondrocytes correlates with lack of in vivo calcification and vascular invasion. Front Bioeng Biotechnol 8:572356
Ando I et al (2016) Changes in serum NGF levels after the exercise load in dogs: a pilot study. J Vet Med Sci 78(11):1709–1712
Ando I et al (2020) Evaluation of stress status using the stress map for guide dog candidates in the training stage using variations in the serum cortisol with nerve growth factor and magnesium ions. Vet Anim Sci 10:100129
Antoni D et al (2015) Three-dimensional cell culture: a breakthrough in vivo. Int J Mol Sci 16(3):5517–5527
Aragon CL, Hofmeister EH, Budsberg SC (2007) Systematic review of clinical trials of treatments for osteoarthritis in dogs. J Am Vet Med Assoc 230(4):514–521
Arevalo-Turrubiarte M et al (2019) Analysis of mesenchymal cells (MSCs) from bone marrow, synovial fluid and mesenteric, neck and tail adipose tissue sources from equines. Stem Cell Res 37:101442
Armiento AR, Alini M, Stoddart MJ (2019) Articular fibrocartilage – why does hyaline cartilage fail to repair? Adv Drug Deliv Rev 146:289–305
Barlian A et al (2018) Chondrogenic differentiation of adipose-derived mesenchymal stem cells induced by L-ascorbic acid and platelet rich plasma on silk fibroin scaffold. Peer J 6:e5809
Barnewitz D et al (2003) Tissue engineering: new treatment of cartilage alterations in degenerative joint diseases in horses – preliminary results of a long term study. Berl Munch Tierarztl Wochenschr 116(3-4):157–161
Bedingfield SK et al (2020) Matrix-targeted nanoparticles for MMP13 RNA interference blocks post-traumatic osteoarthritis. bioRxiv:2020.01.30.925321
Bekkers JE et al (2013a) Single-stage cell-based cartilage regeneration using a combination of chondrons and mesenchymal stromal cells: comparison with microfracture. Am J Sports Med 41(9):2158–2166
Bekkers JE et al (2013b) One-stage focal cartilage defect treatment with bone marrow mononuclear cells and chondrocytes leads to better macroscopic cartilage regeneration compared to microfracture in goats. Osteoarthr Cartil 21(7):950–956
Benninghoff A (1925) Form und Bau der Gelenkknorpel in ihren Beziehungen zur Funktion. Z Zellforsch Mikrosk Anat 2(5):783–862
Benoit DS et al (2008) Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells. Nat Mater 7(10):816–823
Bertoni L et al (2021) Evaluation of allogeneic bone-marrow-derived and umbilical cord blood-derived mesenchymal stem cells to prevent the development of osteoarthritis in an equine model. Int J Mol Sci 22(5)
Bian L et al (2012) Dynamic compressive loading enhances cartilage matrix synthesis and distribution and suppresses hypertrophy in hMSC-laden hyaluronic acid hydrogels. Tissue Eng Part A 18(7-8):715–724
Black LL et al (2007) Effect of adipose-derived mesenchymal stem and regenerative cells on lameness in dogs with chronic osteoarthritis of the coxofemoral joints: a randomized, double-blinded, multicenter, controlled trial. Vet Ther 8(4):272–284
Bodo G et al (2004) Autologous osteochondral grafting (mosaic arthroplasty) for treatment of subchondral cystic lesions in the equine stifle and fetlock joints. Vet Surg 33(6):588–596
Bora FW Jr, Miller G (1987) Joint physiology, cartilage metabolism, and the etiology of osteoarthritis. Hand Clin 3(3):325–336
Borochowitz ZU et al (2004) Spondylo-epi-metaphyseal dysplasia (SEMD) matrilin 3 type: homozygote matrilin 3 mutation in a novel form of SEMD. J Med Genet 41(5):366–372
Bosnakovski D et al (2004) Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells in pellet cultural system. Exp Hematol 32(5):502–509
Bouck GR, Miller CW, Taves CL (1995) A comparison of surgical and medical treatment of fragmented coronoid process and osteochondritis dissecans of the canine elbow. Vet Comp Orthop Traumatol 08(04):177–183
Branly T et al (2018) Improvement of the chondrocyte-specific phenotype upon equine bone marrow mesenchymal stem cell differentiation: influence of culture time, transforming growth factors and type I collagen siRNAs on the differentiation index. Int J Mol Sci 19(2)
Breinan HA et al (1997) Effect of cultured autologous chondrocytes on repair of chondral defects in a canine model. J Bone Joint Surg Am 79(10):1439–1451
Breinan HA et al (2000) Healing of canine articular cartilage defects treated with microfracture, a type-II collagen matrix, or cultured autologous chondrocytes. J Orthop Res 18(5):781–789
Brighton CT, Heppenstall RB (1971) Oxygen tension in zones of the epiphyseal plate, the metaphysis and diaphysis. An in vitro and in vivo study in rats and rabbits. J Bone Joint Surg Am 53(4):719–728
Brittberg M et al (1994) Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 331(14):889–895
Brondeel C et al (2021) Review: mesenchymal stem cell therapy in canine osteoarthritis research: “Experientia Docet” (Experience will teach us). Front Vet Sci 8:668881
Browe DC et al (2019) Hypoxia activates the PTHrP -MEF2C pathway to attenuate hypertrophy in mesenchymal stem cell derived cartilage. Sci Rep 9(1):13274
Bruderer M et al (2014) Role and regulation of RUNX2 in osteogenesis. Eur Cell Mater 28:269–286
Burks RT et al (2006) The use of a single osteochondral autograft plug in the treatment of a large osteochondral lesion in the femoral condyle: an experimental study in sheep. Am J Sports Med 34(2):247–255
Cao Z et al (2017) Hypertrophic differentiation of mesenchymal stem cells is suppressed by xanthotoxin via the p38MAPK/HDAC4 pathway. Mol Med Rep 16(3):2740–2746
Caplan AI (1991) Mesenchymal stem cells. J Orthop Res 9(5):641–650
Caplan AI (2017) Mesenchymal stem cells: time to change the name! Stem Cells Transl Med 6(6):1445–1451
Carrade DD et al (2011) Clinicopathologic findings following intra-articular injection of autologous and allogeneic placentally derived equine mesenchymal stem cells in horses. Cytotherapy 13(4):419–430
Catarino J et al (2020) Treatment of canine osteoarthritis with allogeneic platelet-rich plasma: review of five cases. Open Vet J 10(2):226–231
Chen S et al (2019) MicroRNA-218 promotes early chondrogenesis of mesenchymal stem cells and inhibits later chondrocyte maturation. BMC Biotechnol 19(1):6
Cherubino P et al (2003) Autologous chondrocyte implantation using a bilayer collagen membrane: a preliminary report. J Orthop Surg (Hong Kong) 11(1):10–15
Chiara G, Ranieri C (2009) Cartilage and bone extracellular matrix. Curr Pharm Des 15(12):1334–1348
Cho H, Lee A, Kim K (2018) The effect of serum types on chondrogenic differentiation of adipose-derived stem cells. Biomater Res 22:6
Choi KH et al (2010) The chondrogenic differentiation of mesenchymal stem cells on an extracellular matrix scaffold derived from porcine chondrocytes. Biomaterials 31(20):5355–5365
Clarke SP et al (2005) Prevalence of radiographic signs of degenerative joint disease in a hospital population of cats. Vet Rec 157(25):793–799
Cohen JM et al (2009) Long-term outcome in 44 horses with stifle lameness after arthroscopic exploration and debridement. Vet Surg 38(4):543–551
Cook JL, Payne JT (1997) Surgical treatment of osteoarthritis. Vet Clin North Am Small Anim Pract 27(4):931–944
Cook JL, Hudson CC, Kuroki K (2008) Autogenous osteochondral grafting for treatment of stifle osteochondrosis in dogs. Vet Surg 37(4):311–321
Cook JL et al (2014) A novel system improves preservation of osteochondral allografts. Clin Orthop Relat Res 472(11):3404–3414
Cook JL et al (2016) Importance of donor chondrocyte viability for osteochondral allografts. Am J Sports Med 44(5):1260–1268
Cope PJ et al (2019) Models of osteoarthritis: the good, the bad and the promising. Osteoarthr Cartil 27(2):230–239
Cortes I et al (2021) A scaffold- and serum-free method to mimic human stable cartilage validated by secretome. Tissue Eng Part A 27(5-6):311–327
Cuervo B et al (2020) Objective comparison between platelet rich plasma alone and in combination with physical therapy in dogs with osteoarthritis caused by hip dysplasia. Animals (Basel) 10(2)
Curran JM, Chen R, Hunt JA (2006) The guidance of human mesenchymal stem cell differentiation in vitro by controlled modifications to the cell substrate. Biomaterials 27(27):4783–4793
De Angelis E et al (2020) Gene expression markers in horse articular chondrocytes: chondrogenic differentiation IN VITRO depends on the proliferative potential and ageing. Implication for tissue engineering of cartilage. Res Vet Sci 128:107–117
De Armond CC et al (2021) Three-dimensional-printed custom guides for bipolar coxofemoral osteochondral allograft in dogs. PLoS One 16(2):e0244208
De Bari C, Dell’Accio F, Luyten FP (2001) Human periosteum-derived cells maintain phenotypic stability and chondrogenic potential throughout expansion regardless of donor age. Arthritis Rheum 44(1):85–95
Dell’Accio F et al (2003) Expanded phenotypically stable chondrocytes persist in the repair tissue and contribute to cartilage matrix formation and structural integration in a goat model of autologous chondrocyte implantation. J Orthop Res 21(1):123–131
Dennis JE et al (1999) A quadripotential mesenchymal progenitor cell isolated from the marrow of an adult mouse. J Bone Miner Res 14(5):700–709
Denys M et al (2020) Biosafety evaluation of equine Umbilical Cord-derived Mesenchymal Stromal Cells (UC-MSCs) by systematic pathogen screening in peripheral maternal blood and paired UC-MSCs. Biopreserv Biobank
Dew TL, Martin RA (1992) Functional, radiographic, and histologic assessment of healing of autogenous osteochondral grafts and full-thickness cartilage defects in the talus of dogs. Am J Vet Res 53(11):2141–2152
Dieppe PA, Lohmander LS (2005) Pathogenesis and management of pain in osteoarthritis. Lancet 365(9463):965–973
Dominici M et al (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy 8(4):315–317
Dou C et al (2016) Xanthotoxin prevents bone loss in ovariectomized mice through the inhibition of RANKL-induced osteoclastogenesis. Osteoporos Int 27(7):2335–2344
Dreher SI et al (2020) Significance of MEF2C and RUNX3 regulation for endochondral differentiation of human mesenchymal progenitor cells. Front Cell Dev Biol 8:81
Dudakovic A et al (2014) High-resolution molecular validation of self-renewal and spontaneous differentiation in clinical-grade adipose-tissue derived human mesenchymal stem cells. J Cell Biochem 115(10):1816–1828
Dyson SJ (2004) Medical management of superficial digital flexor tendonitis: a comparative study in 219 horses (1992–2000). Equine Vet J 36(5):415–419
Egger D et al (2019) From 3D to 3D: isolation of mesenchymal stem/stromal cells into a three-dimensional human platelet lysate matrix. Stem Cell Res Ther 10(1):248
Enomoto M et al (2019) Anti-nerve growth factor monoclonal antibodies for the control of pain in dogs and cats. Vet Rec 184(1):23
Familiari F et al (2018) Clinical outcomes and failure rates of osteochondral allograft transplantation in the knee: a systematic review. Am J Sports Med 46(14):3541–3549
Fan L et al (2012) Vitamin C-reinforcing silk fibroin nanofibrous matrices for skin care application. RCS Adv 2:4110–4119
Feng Q et al (2017) Sulfated hyaluronic acid hydrogels with retarded degradation and enhanced growth factor retention promote hMSC chondrogenesis and articular cartilage integrity with reduced hypertrophy. Acta Biomater 53:329–342
Ferris DJ et al (2014) Clinical outcome after intra-articular administration of bone marrow derived mesenchymal stem cells in 33 horses with stifle injury. Vet Surg 43(3):255–265
Fischer J et al (2010) Human articular chondrocytes secrete parathyroid hormone-related protein and inhibit hypertrophy of mesenchymal stem cells in coculture during chondrogenesis. Arthritis Rheum 62(9):2696–2706
Fischer J et al (2014) Intermittent PTHrP(1-34) exposure augments chondrogenesis and reduces hypertrophy of mesenchymal stromal cells. Stem Cells Dev 23(20):2513–2523
Fitzpatrick N, Yeadon R, Smith TJ (2009) Early clinical experience with osteochondral autograft transfer for treatment of osteochondritis dissecans of the medial humeral condyle in dogs. Vet Surg 38(2):246–260
Fitzpatrick N et al (2010) Osteochondral autograft transfer for treatment of osteochondritis dissecans of the caudocentral humeral head in dogs. Vet Surg 39(8):925–935
Fitzpatrick N et al (2012) Osteochondral autograft transfer for the treatment of osteochondritis dissecans of the medial femoral condyle in dogs. Vet Comp Orthop Traumatol 25(2):135–143
Fortier LA et al (1998) Isolation and chondrocytic differentiation of equine bone marrow-derived mesenchymal stem cells. Am J Vet Res 59(9):1182–1187
Foster TE et al (2009) Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med 37(11):2259–2272
Franklin SP, Cook JL (2013) Prospective trial of autologous conditioned plasma versus hyaluronan plus corticosteroid for elbow osteoarthritis in dogs. Can Vet J 54(9):881–884
Franklin SP, Garner BC, Cook JL (2015) Characteristics of canine platelet-rich plasma prepared with five commercially available systems. Am J Vet Res 76(9):822–827
Frisbie DD et al (1999) Arthroscopic subchondral bone plate microfracture technique augments healing of large chondral defects in the radial carpal bone and medial femoral condyle of horses. Vet Surg 28(4):242–255
Frisbie DD, Cross MW, McIlwraith CW (2006) A comparative study of articular cartilage thickness in the stifle of animal species used in human pre-clinical studies compared to articular cartilage thickness in the human knee. Vet Comp Orthop Traumatol 19(3):142–146
Frisbie DD et al (2008) Evaluation of autologous chondrocyte transplantation via a collagen membrane in equine articular defects: results at 12 and 18 months. Osteoarthr Cartil 16(6):667–679
Frisbie DD et al (2009) In vivo evaluation of autologous cartilage fragment-loaded scaffolds implanted into equine articular defects and compared with autologous chondrocyte implantation. Am J Sports Med 37(Suppl 1):71S–80S
Fujiki M et al (2007) Effects of treatment with polysulfated glycosaminoglycan on serum cartilage oligomeric matrix protein and C-reactive protein concentrations, serum matrix metalloproteinase-2 and -9 activities, and lameness in dogs with osteoarthritis. Am J Vet Res 68(8):827–833
Gale AL et al (2019) The effect of hypoxia on chondrogenesis of equine synovial membrane-derived and bone marrow-derived mesenchymal stem cells. BMC Vet Res 15(1):201
Gandhimathi C (2015) Controlled release of dexamethasone in PCL/silk fibroin/ascorbic acid nanoparticles for the initiation of adipose derived stem cells into osteogenesis. J Drug Metab Toxicol 6(1):2
Ge Z et al (2012) Functional biomaterials for cartilage regeneration. J Biomed Mater Res A 100(9):2526–2536
Gearing DP et al (2013) A fully caninised anti-NGF monoclonal antibody for pain relief in dogs. BMC Vet Res 9:226
Gearing DP et al (2016) In vitro and in vivo characterization of a fully felinized therapeutic anti-nerve growth factor monoclonal antibody for the treatment of pain in cats. J Vet Intern Med 30(4):1129–1137
Gelse K et al (2011) Thrombospondin-1 prevents excessive ossification in cartilage repair tissue induced by osteogenic protein-1. Tissue Eng Part A 17(15-16):2101–2112
Gencoglu H et al (2020) Undenatured type II Collagen (UC-II) in Joint health and disease: a review on the current knowledge of companion animals. Animals (Basel) 10(4)
Giannoni P et al (2005) Species variability in the differentiation potential of in vitro-expanded articular chondrocytes restricts predictive studies on cartilage repair using animal models. Tissue Eng 11(1-2):237–248
Gill TJ, Steadman JR (2004) Bone marrow stimulation techniques: microfracture, drilling, and abrasion. In: Cole BJ, Malek MM (eds) Articular cartilage lesions: a practical guide to assessment and treatment. Springer, New York, pp 63–72
Giovannini S et al (2010) Micromass co-culture of human articular chondrocytes and human bone marrow mesenchymal stem cells to investigate stable neocartilage tissue formation in vitro. Eur Cell Mater 20:245–259
Glenn RE Jr et al (2006) Comparison of fresh osteochondral autografts and allografts: a canine model. Am J Sports Med 34(7):1084–1093
Godwin EE et al (2012) Implantation of bone marrow-derived mesenchymal stem cells demonstrates improved outcome in horses with overstrain injury of the superficial digital flexor tendon. Equine Vet J 44(1):25–32
Goldman SM, Barabino GA (2016) Hydrodynamic loading in concomitance with exogenous cytokine stimulation modulates differentiation of bovine mesenchymal stem cells towards osteochondral lineages. BMC Biotechnol 16:10
Grassel S, Ahmed N (2007) Influence of cellular microenvironment and paracrine signals on chondrogenic differentiation. Front Biosci 12:4946–4956
Griffin DJ et al (2015) Mechanical characterization of matrix-induced autologous chondrocyte implantation (MACI(R)) grafts in an equine model at 53 weeks. J Biomech 48(10):1944–1949
Gruen ME et al (2016) A feline-specific anti-nerve growth factor antibody improves mobility in cats with degenerative joint disease-associated pain: a pilot proof of concept study. J Vet Intern Med 30(4):1138–1148
Guillen-Garcia P et al (2014) Increasing the dose of autologous chondrocytes improves articular cartilage repair: histological and molecular study in the sheep animal model. Cartilage 5(2):114–122
Guimaraes-Camboa N et al (2017) Pericytes of multiple organs do not behave as mesenchymal stem cells in vivo. Cell Stem Cell 20(3):345–359 e5
Haddo O et al (2004) The use of chondrogide membrane in autologous chondrocyte implantation. Knee 11(1):51–55
Handorf AM, Li WJ (2011) Fibroblast growth factor-2 primes human mesenchymal stem cells for enhanced chondrogenesis. PLoS One 6(7):e22887
Harman R et al (2016) A prospective, randomized, masked, and placebo-controlled efficacy study of intraarticular allogeneic adipose stem cells for the treatment of osteoarthritis in dogs. Front Vet Sci 3:81
Harper TAM (2017a) Femoral head and neck excision. Vet Clin North Am Small Anim Pract 47(4):885–897
Harper TAM (2017b) INNOPLANT total hip replacement system. Vet Clin North Am Small Anim Pract 47(4):935–944
Haugh MG et al (2011) Temporal and spatial changes in cartilage-matrix-specific gene expression in mesenchymal stem cells in response to dynamic compression. Tissue Eng Part A 17(23-24):3085–3093
Hayes DW Jr, Brower RL, John KJ (2001) Articular cartilage. Anatomy, injury, and repair. Clin Podiatr Med Surg 18(1):35–53
Hirao M et al (2006) Oxygen tension regulates chondrocyte differentiation and function during endochondral ossification. J Biol Chem 281(41):31079–31092
Hoshi K et al (2018) Biological aspects of tissue-engineered cartilage. Histochem Cell Biol 149(4):375–381
Hu Y et al (2020) A lithium-containing biomaterial promotes chondrogenic differentiation of induced pluripotent stem cells with reducing hypertrophy. Stem Cell Res Ther 11(1):77
Huang FS et al (2004) Effects of small incongruities in a sheep model of osteochondral autografting. Am J Sports Med 32(8):1842–1848
Huang AH et al (2010) Long-term dynamic loading improves the mechanical properties of chondrogenic mesenchymal stem cell-laden hydrogel. Eur Cell Mater 19:72–85
Huang J et al (2021) 3D printed gelatin/hydroxyapatite scaffolds for stem cell chondrogenic differentiation and articular cartilage repair. Biomater Sci 9(7):2620–2630
Hunziker EB, Stahli A (2008) Surgical suturing of articular cartilage induces osteoarthritis-like changes. Osteoarthr Cartil 16(9):1067–1073
Hurtig M et al (2001) Arthroscopic mosaic arthroplasty in the equine third carpal bone. Vet Surg 30(3):228–239
Ibarra C et al (2006) Tissue engineered arthroscopic repair of experimental cartilage lesions in horses (SS-47). Arthroscop J Arthroscop Relat Surg 22(6, Supplement):e24
Isola M et al (2011) Nerve growth factor concentrations in the synovial fluid from healthy dogs and dogs with secondary osteoarthritis. Vet Comp Orthop Traumatol 24(4):279–284
Janakiramanan N et al (2006) Osteoarthritis cartilage defects: does size matter? Curr Rheumatol Rev 2(4):311–317
Jayasuriya CT et al (2012) Matrilin-3 induction of IL-1 receptor antagonist is required for up-regulating collagen II and aggrecan and down-regulating ADAMTS-5 gene expression. Arthritis Res Ther 14(5):R197
Jensen C, Teng Y (2020) Is it time to start transitioning from 2D to 3D cell culture? Front Mol Biosci 7:33
Johnstone B et al (1998) In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res 238(1):265–272
Juhasz T et al (2014) Mechanical loading stimulates chondrogenesis via the PKA/CREB-Sox9 and PP2A pathways in chicken micromass cultures. Cell Signal 26(3):468–482
Kang BJ et al (2012) Comparing the osteogenic potential of canine mesenchymal stem cells derived from adipose tissues, bone marrow, umbilical cord blood, and Wharton’s jelly for treating bone defects. J Vet Sci 13(3):299–310
Kanichai M et al (2008) Hypoxia promotes chondrogenesis in rat mesenchymal stem cells: a role for AKT and hypoxia-inducible factor (HIF)-1alpha. J Cell Physiol 216(3):708–715
Kapusetti G, More N, Choppadandi M (2019) Introduction to ideal characteristics and advanced biomedical applications of biomaterials. In: Paul S (ed) Biomedical engineering and its applications in healthcare. Springer, Singapore, pp 171–204
Karl A et al (2014) Thyroid hormone-induced hypertrophy in mesenchymal stem cell chondrogenesis is mediated by bone morphogenetic protein-4. Tissue Eng Part A 20(1-2):178–188
Kawamoto K et al (1996) Nerve growth factor activity detected in equine peripheral blood of horses with fever after truck transportation. J Equin Sci 7(2):43–46
Kendall A et al (2021) Nerve growth factor in the equine joint. Vet J 267:105579
Kim SJ et al (2020a) Articular cartilage repair using autologous collagen-induced chondrogenesis (ACIC): a pragmatic and cost-effective enhancement of a traditional technique. Knee Surg Sports Traumatol Arthrosc 28(8):2598–2603
Kim SA et al (2020b) Atelocollagen promotes chondrogenic differentiation of human adipose-derived mesenchymal stem cells. Sci Rep 10(1):10678
Kisiday JD et al (2009) Dynamic compression stimulates proteoglycan synthesis by mesenchymal stem cells in the absence of chondrogenic cytokines. Tissue Eng Part A 15(10):2817–2824
Kisiel AH et al (2012) Isolation, characterization, and in vitro proliferation of canine mesenchymal stem cells derived from bone marrow, adipose tissue, muscle, and periosteum. Am J Vet Res 73(8):1305–1317
Koch TG et al (2007) Isolation of mesenchymal stem cells from equine umbilical cord blood. BMC Biotechnol 7:26
Kriston-Pal E et al (2020) A regenerative approach to canine osteoarthritis using allogeneic, adipose-derived mesenchymal stem cells. Safety results of a long-term follow-up. Front Vet Sci 7:510
Kronenberg HM (2003) Developmental regulation of the growth plate. Nature 423(6937):332–336
Kundu B et al (2013) Silk fibroin biomaterials for tissue regenerations. Adv Drug Deliv Rev 65(4):457–470
Lafaver S et al (2007) Tibial tuberosity advancement for stabilization of the canine cranial cruciate ligament-deficient stifle joint: surgical technique, early results, and complications in 101 dogs. Vet Surg 36(6):573–586
Lane JG et al (2004) Follow-up of osteochondral plug transfers in a goat model: a 6-month study. Am J Sports Med 32(6):1440–1450
Lascelles BD et al (2015) A canine-specific anti-nerve growth factor antibody alleviates pain and improves mobility and function in dogs with degenerative joint disease-associated pain. BMC Vet Res 11:101
Lee CR et al (2000) Effects of harvest and selected cartilage repair procedures on the physical and biochemical properties of articular cartilage in the canine knee. J Orthop Res 18(5):790–799
Lee CR et al (2003) Effects of a cultured autologous chondrocyte-seeded type II collagen scaffold on the healing of a chondral defect in a canine model. J Orthop Res 21(2):272–281
Lee J et al (2016) Chondrogenic potential and anti-senescence effect of hypoxia on canine adipose mesenchymal stem cells. Vet Res Commun 40(1):1–10
Levorson EJ et al (2014) Cell-derived polymer/extracellular matrix composite scaffolds for cartilage regeneration, Part 2: construct devitalization and determination of chondroinductive capacity. Tissue Eng Part C Methods 20(4):358–372
Li J, Pei M (2018) A protocol to prepare decellularized stem cell matrix for rejuvenation of cell expansion and cartilage regeneration. Methods Mol Biol 1577:147–154
Li WJ, Jiang YJ, Tuan RS (2006) Chondrocyte phenotype in engineered fibrous matrix is regulated by fiber size. Tissue Eng 12(7):1775–1785
Li Z et al (2010) Chondrogenesis of human bone marrow mesenchymal stem cells in fibrin-polyurethane composites is modulated by frequency and amplitude of dynamic compression and shear stress. Tissue Eng Part A 16(2):575–584
Liu X et al (2010) In vivo ectopic chondrogenesis of BMSCs directed by mature chondrocytes. Biomaterials 31(36):9406–9414
Liu Q et al (2018) Suppressing mesenchymal stem cell hypertrophy and endochondral ossification in 3D cartilage regeneration with nanofibrous poly(l-lactic acid) scaffold and matrilin-3. Acta Biomater 76:29–38
Longhini ALF et al (2019) Peripheral blood-derived mesenchymal stem cells demonstrate immunomodulatory potential for therapeutic use in horses. PLoS One 14(3):e0212642
Lu Y et al (2006) Minced cartilage without cell culture serves as an effective intraoperative cell source for cartilage repair. J Orthop Res 24(6):1261–1270
Magri C et al (2019) Comparison of efficacy and safety of single versus repeated intra-articular injection of allogeneic neonatal mesenchymal stem cells for treatment of osteoarthritis of the metacarpophalangeal/metatarsophalangeal joint in horses: A clinical pilot study. PLoS One 14(8):e0221317
Maki CB et al (2020) Intra-articular administration of allogeneic adipose derived MSCs reduces pain and lameness in dogs with hip osteoarthritis: a double blinded, randomized, placebo controlled pilot study. Front Vet Sci 7:570
Mantyh PW et al (2011) Antagonism of nerve growth factor-TrkA signaling and the relief of pain. Anesthesiology 115(1):189–204
Marinas-Pardo L et al (2018) Allogeneic adipose-derived mesenchymal stem cells (Horse Allo 20) for the treatment of osteoarthritis-associated lameness in horses: characterization, safety, and efficacy of intra-articular treatment. Stem Cells Dev 27(17):1147–1160
Martino S et al (2012) Stem cell-biomaterial interactions for regenerative medicine. Biotechnol Adv 30(1):338–351
Masri M et al (2007) Matrix-encapsulation cell-seeding technique to prevent cell detachment during arthroscopic implantation of matrix-induced autologous chondrocytes. Arthroscopy 23(8):877–883
Matsuda H et al (1991) Nerve growth factor-like activity detected in equine peripheral blood after running exercise. Zentralbl Veterinarmed A 38(7):557–559
Maumus M et al (2013) Adipose mesenchymal stem cells protect chondrocytes from degeneration associated with osteoarthritis. Stem Cell Res 11(2):834–844
McCarty EC et al (2016) Fresh osteochondral allograft versus autograft: twelve-month results in isolated canine knee defects. Am J Sports Med 44(9):2354–2365
McCarthy J et al (2020) Elbow arthrodesis using a medially positioned plate in 6 dogs. Vet Comp Orthop Traumatol 33(1):51–58
McGonagle D, Baboolal TG, Jones E (2017) Native joint-resident mesenchymal stem cells for cartilage repair in osteoarthritis. Nat Rev Rheumatol 13(12):719–730
Mehana EE, Khafaga AF, El-Blehi SS (2019) The role of matrix metalloproteinases in osteoarthritis pathogenesis: an updated review. Life Sci 234:116786
Meirelles Lda S et al (2009) Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev 20(5-6):419–427
Mello MA, Tuan RS (1999) High density micromass cultures of embryonic limb bud mesenchymal cells: an in vitro model of endochondral skeletal development. In Vitro Cell Dev Biol Anim 35(5):262–269
Mensing N et al (2011) Isolation and characterization of multipotent mesenchymal stromal cells from the gingiva and the periodontal ligament of the horse. BMC Vet Res 7:42
Meretoja VV et al (2013) The effect of hypoxia on the chondrogenic differentiation of co-cultured articular chondrocytes and mesenchymal stem cells in scaffolds. Biomaterials 34(17):4266–4273
Min BH et al (2007) The fate of implanted autologous chondrocytes in regenerated articular cartilage. Proc Inst Mech Eng H 221(5):461–465
Mirza MH et al (2016) Gait changes vary among horses with naturally occurring osteoarthritis following intra-articular administration of autologous platelet-rich plasma. Front Vet Sci 3:29
Mobasheri A et al (2017) The role of metabolism in the pathogenesis of osteoarthritis. Nat Rev Rheumatol 13(5):302–311
Mohoric L et al (2016) Blinded placebo study of bilateral osteoarthritis treatment using adipose derived mesenchymal stem cells. Slov Vet Res 53(3):167–174
Morrison SJ et al (1997) Identification of a lineage of multipotent hematopoietic progenitors. Development 124(10):1929–1939
Mueller MB, Tuan RS (2008) Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheum 58(5):1377–1388
Mueller MB et al (2013) Effect of parathyroid hormone-related protein in an in vitro hypertrophy model for mesenchymal stem cell chondrogenesis. Int Orthop 37(5):945–951
Muir P et al (2016) Autologous bone marrow-derived mesenchymal stem cells modulate molecular markers of inflammation in dogs with cruciate ligament rupture. PLoS One 11(8):e0159095
Muttigi MS et al (2020) Matrilin-3-primed adipose-derived mesenchymal stromal cell spheroids prevent mesenchymal stromal-cell-derived chondrocyte hypertrophy. Int J Mol Sci 21(23)
Mwale F et al (2006) Limitations of using aggrecan and type X collagen as markers of chondrogenesis in mesenchymal stem cell differentiation. J Orthop Res 24(8):1791–1798
Mwale F et al (2010) Effect of parathyroid hormone on type X and type II collagen expression in mesenchymal stem cells from osteoarthritic patients. Tissue Eng Part A 16(11):3449–3455
Nakase T et al (2002) Distribution of genes for parathyroid hormone (PTH)-related peptide, Indian hedgehog, PTH receptor and patched in the process of experimental spondylosis in mice. J Neurosurg 97(1 Suppl):82–87
Naruse K et al (2004) Spontaneous differentiation of mesenchymal stem cells obtained from fetal rat circulation. Bone 35(4):850–858
Ness MG (2016) The Modified Maquet Procedure (MMP) in dogs: technical development and initial clinical experience. J Am Anim Hosp Assoc 52(4):242–250
Nganvongpanit K et al (2009) Prospective evaluation of serum biomarker levels and cartilage repair by autologous chondrocyte transplantation and subchondral drilling in a canine model. Arthritis Res Ther 11(3):R78
Nicpon J, Marycz K, Grzesiak J (2013) Therapeutic effect of adipose-derived mesenchymal stem cell injection in horses suffering from bone spavin. Pol J Vet Sci 16(4):753–754
Nishiwaki H et al (2017) A novel method to induce cartilage regeneration with cubic microcartilage. Cells Tissues Organs 204(5-6):251–260
Nixon AJ et al (2011) Autologous chondrocyte implantation drives early chondrogenesis and organized repair in extensive full- and partial-thickness cartilage defects in an equine model. J Orthop Res 29(7):1121–1130
Nixon AJ et al (2015) A chondrocyte infiltrated collagen type I/III membrane (MACI(R) implant) improves cartilage healing in the equine patellofemoral joint model. Osteoarthr Cartil 23(4):648–660
Nixon AJ et al (2017) Matrix-induced Autologous Chondrocyte Implantation (MACI) using a cell-seeded collagen membrane improves cartilage healing in the equine model. J Bone Joint Surg Am 99(23):1987–1998
Nürnberger S et al (2006) Ultrastructural insights into the world of cartilage: electron microscopy of articular cartilage. Osteosynth Trauma Care 14(03):168–180
Nuernberger S et al (2011) The influence of scaffold architecture on chondrocyte distribution and behavior in matrix-associated chondrocyte transplantation grafts. Biomaterials 32(4):1032–1040
Nurnberger S et al (2021) Repopulation of decellularised articular cartilage by laser-based matrix engraving. EBioMedicine 64:103196
O’Driscoll SW, Fitzsimmons JS (2001) The role of periosteum in cartilage repair. Clin Orthop Relat Res 391:S190–S207
O’Conor CJ, Case N, Guilak F (2013) Mechanical regulation of chondrogenesis. Stem Cell Res Ther 4(4):61
Olvera D et al (2017) Modulating microfibrillar alignment and growth factor stimulation to regulate mesenchymal stem cell differentiation. Acta Biomater 64:148–160
Pacini S et al (2007) Suspension of bone marrow-derived undifferentiated mesenchymal stromal cells for repair of superficial digital flexor tendon in race horses. Tissue Eng 13(12):2949–2955
Patil AS, Sable RB, Kothari RM (2012) Role of insulin-like growth factors (IGFs), their receptors and genetic regulation in the chondrogenesis and growth of the mandibular condylar cartilage. J Cell Physiol 227(5):1796–1804
Pei M et al (2009) Histone deacetylase 4 promotes TGF-beta1-induced synovium-derived stem cell chondrogenesis but inhibits chondrogenically differentiated stem cell hypertrophy. Differentiation 78(5):260–268
Pelosi M et al (2013) Parathyroid hormone-related protein is induced by hypoxia and promotes expression of the differentiated phenotype of human articular chondrocytes. Clin Sci (Lond) 125(10):461–470
Pelttari K et al (2006) Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum 54(10):3254–3266
Prado AA et al (2015) Characterization of mesenchymal stem cells derived from the equine synovial fluid and membrane. BMC Vet Res 11:281
Pritzker KP et al (1977) Articular cartilage transplantation. Hum Pathol 8(6):635–651
Radtke CL et al (2013) Characterization and osteogenic potential of equine muscle tissue- and periosteal tissue-derived mesenchymal stem cells in comparison with bone marrow- and adipose tissue-derived mesenchymal stem cells. Am J Vet Res 74(5):790–800
Raftery RM et al (2016) Multifunctional biomaterials from the sea: Assessing the effects of chitosan incorporation into collagen scaffolds on mechanical and biological functionality. Acta Biomater 43:160–169
Rakic R et al (2017) RNA interference and BMP-2 stimulation allows equine chondrocytes redifferentiation in 3D-hypoxia cell culture model: application for matrix-induced autologous chondrocyte implantation. Int J Mol Sci 18(9)
Ramezanifard R, Kabiri M, Hanaee Ahvaz H (2017) Effects of platelet rich plasma and chondrocyte co-culture on MSC chondrogenesis, hypertrophy and pathological responses. EXCLI J 16:1031–1045
Ranera B et al (2013) Expansion under hypoxic conditions enhances the chondrogenic potential of equine bone marrow-derived mesenchymal stem cells. Vet J 195(2):248–251
Ren YJ et al (2009) In vitro behavior of neural stem cells in response to different chemical functional groups. Biomaterials 30(6):1036–1044
Ribeiro JCV et al (2017) Versatility of chitosan-based biomaterials and their use as scaffolds for tissue regeneration. Sci World J 2017:8639898
Rink BE et al (2017) Isolation and characterization of equine endometrial mesenchymal stromal cells. Stem Cell Res Ther 8(1):166
Roach BL et al (2015) Fabrication of tissue engineered osteochondral grafts for restoring the articular surface of diarthrodial joints. Methods 84:103–108
Rockwood DN et al (2011) Materials fabrication from Bombyx mori silk fibroin. Nat Protoc 6(10):1612–1631
Rosadi I et al (2019) In vitro study of cartilage tissue engineering using human adipose-derived stem cells induced by platelet-rich plasma and cultured on silk fibroin scaffold. Stem Cell Res Ther 10(1):369
Rucinski K et al (2019) Effects of compliance with procedure-specific postoperative rehabilitation protocols on initial outcomes after osteochondral and meniscal allograft transplantation in the knee. Orthop J Sports Med 7(11):2325967119884291
Russlies M et al (2005) Periosteum stimulates subchondral bone densification in autologous chondrocyte transplantation in a sheep model. Cell Tissue Res 319(1):133–142
Ryan VH et al (2008) NGF gene expression and secretion by canine adipocytes in primary culture: upregulation by the inflammatory mediators LPS and TNFalpha. Horm Metab Res 40(12):861–868
Rychel JK (2010) Diagnosis, and treatment of osteoarthritis. Top Companion Anim Med 25(1):20–25
Salonius E et al (2020) Chondrogenic differentiation of human bone marrow-derived mesenchymal stromal cells in a three-dimensional environment. J Cell Physiol 235(4):3497–3507
Sanderson RO et al (2009) Systematic review of the management of canine osteoarthritis. Vet Rec 164(14):418–424
Sanz-Ramos P et al (2014) Improved chondrogenic capacity of collagen hydrogel-expanded chondrocytes: in vitro and in vivo analyses. J Bone Joint Surg Am 96(13):1109–1117
Sartore L et al (2021) Polysaccharides on gelatin-based hydrogels differently affect chondrogenic differentiation of human mesenchymal stromal cells. Mater Sci Eng C Mater Biol Appl 126:112175
Sasaki A et al (2018) Canine mesenchymal stem cells from synovium have a higher chondrogenic potential than those from infrapatellar fat pad, adipose tissue, and bone marrow. PLoS One 13(8):e0202922
Sato K et al (2016) Isolation and characterisation of peripheral blood-derived feline mesenchymal stem cells. Vet J 216:183–188
Schaap-Oziemlak AM et al (2014) Biomaterial–stem cell interactions and their impact on stem cell response. RSC Adv 4(95):53307–53320
Schagemann JC et al (2013) Chondrogenic differentiation of bone marrow-derived mesenchymal stromal cells via biomimetic and bioactive poly-epsilon-caprolactone scaffolds. J Biomed Mater Res A 101(6):1620–1628
Schreiner AJ et al (2020) Unicompartmental bipolar osteochondral and meniscal allograft transplantation is effective for treatment of medial compartment gonarthrosis in a canine model. J Orthop Res
Shah K et al (2018) Outcome of allogeneic adult stem cell therapy in dogs suffering from osteoarthritis and other joint defects. Stem Cells Int 2018:7309201
Shintani N, Hunziker EB (2011) Differential effects of dexamethasone on the chondrogenesis of mesenchymal stromal cells: influence of microenvironment, tissue origin and growth factor. Eur Cell Mater 22:302–319. discussion 319-20
Shortkroff S et al (1996) Healing of chondral and osteochondral defects in a canine model: the role of cultured chondrocytes in regeneration of articular cartilage. Biomaterials 17(2):147–154
Slocum B, Slocum TD (1993) Tibial plateau leveling osteotomy for repair of cranial cruciate ligament rupture in the canine. Vet Clin North Am Small Anim Pract 23(4):777–795
Smith RK et al (2013) Beneficial effects of autologous bone marrow-derived mesenchymal stem cells in naturally occurring tendinopathy. PLoS One 8(9):e75697
Spaas JH et al (2015) Chondrogenic priming at reduced cell density enhances cartilage adhesion of equine allogeneic MSCs – a loading sensitive phenomenon in an organ culture study with 180 explants. Cell Physiol Biochem 37(2):651–665
Stefansson SE et al (2003) Genomewide scan for hand osteoarthritis: a novel mutation in matrilin-3. Am J Hum Genet 72(6):1448–1459
Steinert AF et al (2012) Indian hedgehog gene transfer is a chondrogenic inducer of human mesenchymal stem cells. Arthritis Res Ther 14(4):R168
Stevenson S et al (1989) The fate of articular cartilage after transplantation of fresh and cryopreserved tissue-antigen-matched and mismatched osteochondral allografts in dogs. J Bone Joint Surg Am 71(9):1297–1307
Stevenson S, Shaffer JW, Goldberg VM (1996) The humoral response to vascular and nonvascular allografts of bone. Clin Orthop Relat Res 326:86–95
Stockwell RA (1971) The interrelationship of cell density and cartilage thickness in mammalian articular cartilage. J Anat 109(Pt 3):411–421
Stockwell RA (1978) Chondrocytes. J Clin Pathol Suppl (R Coll Pathol) 12:7–13
Stoker AM et al (2018) Bone marrow aspirate concentrate versus platelet rich plasma to enhance osseous integration potential for osteochondral allografts. J Knee Surg 31(4):314–320
Stupina TA, Stepanov MA, Teplen’kii MP (2015) Role of subchondral bone in the restoration of articular cartilage. Bull Exp Biol Med 158(6):820–823
Stupina TA, Makushin VD, Stepanov MA (2012) Experimental morphological study of the effects of subchondral tunnelization and bone marrow stimulation on articular cartilage regeneration. Bull Exp Biol Med 153(2):289–293
Sun J, Tan H (2013) Alginate-based biomaterials for regenerative medicine applications. Materials (Basel) 6(4):1285–1309
Tamada Y, Ikada Y (1993) Effect of preadsorbed proteins on cell adhesion to polymer surfaces. J Colloid Interface Sci 155(2):334–339
Tchetina EV, Squires G, Poole AR (2005) Increased type II collagen degradation and very early focal cartilage degeneration is associated with upregulation of chondrocyte differentiation related genes in early human articular cartilage lesions. J Rheumatol 32(5):876–886
Thorpe SD et al (2012) European Society of Biomechanics S.M. Perren Award 2012: the external mechanical environment can override the influence of local substrate in determining stem cell fate. J Biomech 45(15):2483–2492
Tibbitt MW, Anseth KS (2009) Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng 103(4):655–663
Toh WS et al (2012) Modulation of mesenchymal stem cell chondrogenesis in a tunable hyaluronic acid hydrogel microenvironment. Biomaterials 33(15):3835–3845
Tomlinson RE et al (2017) NGF-TrkA signaling in sensory nerves is required for skeletal adaptation to mechanical loads in mice. Proc Natl Acad Sci U S A 114(18):E3632–E3641
Ueno T et al (2001) Cellular origin of endochondral ossification from grafted periosteum. Anat Rec 264(4):348–357
Vainieri ML et al (2020) Evaluation of biomimetic hyaluronic-based hydrogels with enhanced endogenous cell recruitment and cartilage matrix formation. Acta Biomater 101:293–303
Veilleux NH, Yannas IV, Spector M (2004) Effect of passage number and collagen type on the proliferative, biosynthetic, and contractile activity of adult canine articular chondrocytes in type I and II collagen-glycosaminoglycan matrices in vitro. Tissue Eng 10(1-2):119–127
Venator KP et al (2020) Assessment of a single intra-articular stifle injection of pure platelet rich plasma on symmetry indices in dogs with unilateral or bilateral stifle osteoarthritis from long-term medically managed cranial cruciate ligament disease. Vet Med (Auckl) 11:31–38
Vilar JM et al (2013) Controlled, blinded force platform analysis of the effect of intraarticular injection of autologous adipose-derived mesenchymal stem cells associated to PRGF-Endoret in osteoarthritic dogs. BMC Vet Res 9:131
Vilar JM et al (2018) Effect of leukocyte-reduced platelet-rich plasma on osteoarthritis caused by cranial cruciate ligament rupture: a canine gait analysis model. PLoS One 13(3):e0194752
Voga M et al (2020) Stem cells in veterinary medicine-current state and treatment options. Front Vet Sci 7:278
Walker GD et al (1995) Expression of type-X collagen in osteoarthritis. J Orthop Res 13(1):4–12
Webb TL, Quimby JM, Dow SW (2012) In vitro comparison of feline bone marrow-derived and adipose tissue-derived mesenchymal stem cells. J Feline Med Surg 14(2):165–168
Webster RP, Anderson GI, Gearing DP (2014) Canine brief pain inventory scores for dogs with osteoarthritis before and after administration of a monoclonal antibody against nerve growth factor. Am J Vet Res 75(6):532–535
Weiss S et al (2010) Impact of growth factors and PTHrP on early and late chondrogenic differentiation of human mesenchymal stem cells. J Cell Physiol 223(1):84–93
Xu X et al (2013) Cultivation and spontaneous differentiation of rat bone marrow-derived mesenchymal stem cells on polymeric surfaces. Clin Hemorheol Microcirc 55(1):143–156
Yang C et al (2009) The differential in vitro and in vivo responses of bone marrow stromal cells on novel porous gelatin-alginate scaffolds. J Tissue Eng Regen Med 3(8):601–614
Yang Q et al (2008) A cartilage ECM-derived 3-D porous acellular matrix scaffold for in vivo cartilage tissue engineering with PKH26-labeled chondrogenic bone marrow-derived mesenchymal stem cells. Biomaterials 29(15):2378–2387
Yang X et al (2014) Matrilin-3 inhibits chondrocyte hypertrophy as a bone morphogenetic protein-2 antagonist. J Biol Chem 289(50):34768–34779
Yang Z et al (2021) 3D-bioprinted difunctional scaffold for in situ cartilage regeneration based on aptamer-directed cell recruitment and growth factor-enhanced cell chondrogenesis. ACS Appl Mater Interfaces 13(20):23369–23383
Yoo JU et al (1998) The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J Bone Joint Surg Am 80(12):1745–1757
Zamanlui S et al (2018) Enhanced chondrogenic differentiation of human bone marrow mesenchymal stem cells on PCL/PLGA electrospun with different alignments and compositions. Int J Polym Mater Polym Biomater 67(1):50–60
Zhang L et al (2010) Chondrogenic differentiation of human mesenchymal stem cells: a comparison between micromass and pellet culture systems. Biotechnol Lett 32(9):1339–1346
Zhang T et al (2015) Cross-talk between TGF-beta/SMAD and integrin signaling pathways in regulating hypertrophy of mesenchymal stem cell chondrogenesis under deferral dynamic compression. Biomaterials 38:72–85
Zhang BY et al (2018) Evaluation of the curative effect of umbilical cord mesenchymal stem cell therapy for knee arthritis in dogs using imaging technology. Stem Cells Int 2018:1983025
Zheng L et al (2021) The role of metabolism in chondrocyte dysfunction and the progression of osteoarthritis. Ageing Res Rev 66:101249
Zhou N et al (2015) HIF-1alpha as a regulator of BMP2-induced chondrogenic differentiation, osteogenic differentiation, and endochondral ossification in stem cells. Cell Physiol Biochem 36(1):44–60
Zylinska B et al (2018) Treatment of articular cartilage defects: focus on tissue engineering. In Vivo 32(6):1289–1300
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Voga, M., Majdic, G. (2022). Articular Cartilage Regeneration in Veterinary Medicine. In: Turksen, K. (eds) Cell Biology and Translational Medicine, Volume 17. Advances in Experimental Medicine and Biology(), vol 1401. Springer, Cham. https://doi.org/10.1007/5584_2022_717
Download citation
DOI: https://doi.org/10.1007/5584_2022_717
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-20513-2
Online ISBN: 978-3-031-20514-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)