Abstract
Purpose
Mesenchymal stem cells (MSCs) express markers of hypertrophic chondrocytes during chondrogenic differentiation. We tested the suitability of parathyroid hormone-related protein (PTHrP), a regulator of chondrocyte hypertrophy in embryonic cartilage development, for the suppression of hypertrophy in an in vitro hypertrophy model of chondrifying MSCs.
Methods
Chondrogenesis was induced in human MSCs in pellet culture for two weeks and for an additional two weeks cultures were either maintained in standard chondrogenic medium or transferred to a hypertrophy-enhancing medium. PTHrP(1–40) was added to the medium throughout the culture period at concentrations from 1 to 1,000 pM. Pellets were harvested on days one, 14 and 28 for biochemical and histological analysis.
Results
Hypertrophic medium clearly enhanced the hypertrophic phenotype, with increased cell size, and strong alkaline phosphatase (ALP) and type X collagen staining. In chondrogenic medium, 1–100 pM PTHrP(1–40) did not inhibit chondrogenic differentiation, whereas 1,000 pM PTHrP(1–40) significantly reduced chondrogenesis. ALP activity was dose-dependently reduced by PTHrP(1–40) at 10–1,000 pM in chondrogenic conditions. Under hypertrophy-enhancing conditions, PTHrP(1–40) did not inhibit the induction of the hypertrophy. At the highest concentration (1,000 pM) in the hypertrophic group, aggregates were partially dedifferentiated and differentiated areas of these aggregates maintained their hypertrophic appearance.
Conclusions
PTHrP(1–40) treatment dose-dependently reduced ALP expression in MSC pellets cultured under standard chondrogenic conditions and is thus beneficial for the maintenance of the chondrogenic phenotype in this medium condition. When cultured under hypertrophy-enhancing conditions, PTHrP(1–40) could not diminish the induced enhancement of hypertrophy in the MSC pellets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mesenchymal stem cells (MSCs) can be chondrogenically differentiated in high-density aggregate cultures by a strictly defined medium containing transforming growth factor beta (TGF-β) [1–3]. During chondrogenesis, MSCs express markers of hypertrophic chondrocytes like type X collagen, alkaline phosphatase (ALP) and others [4–6]. A wide array of additional genes is regulated, stage specifically, in a manner very similar to the profiles seen during embryonic limb development [5]. Exposure of chondrogenically differentiating MSCs to culture conditions that influence hypertrophy in chondrocytes during endochondral ossification triggers the same response in chondrifying MSCs as in chondrocytes. Thus, thyroid hormone induces hypertrophy, while TGF-β and dexamethasone inhibit hypertrophy [5, 7]. This indicates that in MSCs that are chondrogenically induced with currently used culture conditions a developmental programme similar to limb development is initiated. In limb development, mesenchymal progenitor cells undergo chondrogenesis and subsequently mature, become hypertrophic and undergo apoptosis. The hypertrophic cartilage calcifies, is invaded by blood vessels and osteoprogenitor cells, and bone is formed [8, 9]. Vascular invasion and mineralisation have also been reported for chondrogenic pellet cultures of MSCs after ectopic implantation in vivo [10]. For MSC-based articular cartilage tissue engineering, vascular invasion and ossification of the engineered neocartilage are of major concern. Strategies to prevent or diminish the expression of hypertrophy-associated markers are thus highly desirable.
Parathyroid hormone-related protein (PTHrP) controls chondrocyte maturation during endochondral bone development [11, 12]. It is expressed in perichondrial cells and peri-articular chondrocytes, binds to its receptor on proliferating and pre-hypertrophic chondrocytes and slows down maturation. The PTHrP receptor is not expressed in undifferentiated MSCs and is up-regulated during chondrogenic differentiation of MSCs [4, 5]. The N-terminal fragment PTHrP(1–40) is sufficient to suppress hypertrophy in chondrocytes[13]. Therefore, we hypothesise that PTHrP(1–40) can suppress hypertrophy in MSC chondrogenesis.
In this study, we used an established in vitro hypertrophy model of chondrogenic differentiating MSCs in which hypertrophic phenotype can reproducibly be enhanced. Cultures were exposed to PTHrP(1–40) in a dose–response experiment and the effect was analysed by phenotypical methods such as histology and biochemical analyses.
Methods
Isolation of MSCs
Bone marrow was aspirated from the iliac crest of three patients (age 27–38) with Intramural Research Board approval and informed consent. MSCs were isolated by Ficoll (Biochrom, Berlin, Germany) gradient centrifugation followed by plastic adhesion. Cells were expanded in monolayer culture and used at passage 2 or 3 for the experiments.
Chondrogenic differentiation
A total of 200,000 cells per well were seeded in V-bottom 96-well polypropylene plates. After centrifugation at 250 g for five minutes, pellets were chondrogenically differentiated in DMEM (Invitrogen, Karlsruhe, Germany), 1 % ITS+3, 50 μg/ml ascorbate-2-phosphate, 40 μg/ml L-proline, 100 nM dexamethasone (Sigma, Steinheim, Germany) and 10 ng/ml TGF-β1 (R&D, Wiesbaden, Germany). After 14 days, cultures were transferred to hypertrophy-enhancing medium consisting of DMEM, 1 % ITS+3, 50 μg/ml ascorbate-2-phosphate, 40 μg/ml L-proline and 1 nM triiodothyronine (T3) (Sigma) or kept in chondrogenic medium. PTHrP(1–40) (Sigma) was added throughout the culture time at 0 (control), 1, 10, 100 or 1,000 pM. The medium was changed three times per week. Pellets were harvested on day 28 for histological and biochemical analysis. Three independent experiments with cells derived from three different donors were carried out.
Histology and immunohistochemistry
Frozen sections were stained with dimethylmethylene blue (DMMB) (Sigma). ALP histochemistry was carried out with a kit (Sigma). For immunohistochemistry, we used antibodies for type II collagen (Merck, Darmstadt, Germany) at 1:100 dilution and type X collagen (Quartett GmbH, Berlin, Germany) at 1:10 dilution. After antigen retrieval with pepsin digestion (0.1 %, pH 3, room temperature, 15 minutes) for type II collagen and additional hyaluronidase digestion (0.2 %, pH 6, 37 °C, 60 min) for type X collagen, incubation with the primary antibody was carried out at 4 °C overnight. Immunolabelling was detected with a biotinylated secondary antibody (Dianova, Hamburg, Germany), horseradish peroxidase-conjugated streptavidin (Vector Laboratories, Burlingame, CA, USA) and metal-enhanced diaminobenzidine (Sigma).
Biochemical analyses
Pellets were digested in Sigma papain digestion solution [150 μg/ml in phosphate-buffered saline (PBS), 6 mM cysteine HCl, 6 mM ethylenediaminetetraacetic acid (EDTA), pH 6.0] at 60 °C overnight. Four samples per condition were prepared. DNA content was determined with a PicoGreen kit (Invitrogen) using calf thymus DNA as standard. Glycosaminoglycan (GAG) content was determined with the DMMB method with chondroitin sulphate (Sigma) as standard; 25 μl of sample was mixed with 250 μl of DMMB solution [4 μM DMMB (Sigma) in formate buffer, pH 3.0] in 96-well plates and optical density was measured at 540 nm. For determination of ALP activity, culture medium was spun down for five minutes at maximum speed and the supernatant was incubated with ALP buffer (1.5 M Tris, 1 mM ZnCl2, 1 mM MgCl2, pH 9.0) and p-nitrophenol phosphate (Sigma) at a concentration of 2 mg/ml at room temperature. Absorbance at 405 nm was continuously measured and enzymatic activity was calculated from the linear part of the reaction kinetics. The ALP assay was carried out in eight replicates.
Statistical analysis
Statistical analysis was carried out by pairwise comparisons using an unpaired, two-tailed t test using Microsoft Excel. A p value <0.05 was considered statistically significant.
Results
Representative data of one of three independent experiments are shown. The DNA content per aggregate was not statistically different between the groups on day 28 (Fig. 1a). GAG normalised to DNA was higher in the chondrogenic group than the respective hypertrophic group (Fig. 1b). The differences were not statistically significant in the minus-PTHrP(1–40) control and at 1 and 10 pM PTHrP(1–40), but significant at 100 and 1,000 pM PTHrP(1–40). Comparison of GAG/DNA between the minus-PTHrP conditions and those exposed to PTHrP(1–40) at various concentrations showed significantly less GAG/DNA at 1,000 pM PTHrP(1–40) in the hypertrophic group. In the chondrogenic group, differences were not significant (Fig. 1b).
Biochemical analysis. Effect of PTHrP(1–40) treatment on DNA (a) and GAG (b) content and ALP secretion (c) in standard chondrogenic medium (CM) and hypertrophy-enhancing medium (Hyp) on day 28. GAG contents are expressed normalised to DNA contents. Values shown are mean ± SD (n = 4); *significantly different from control without PTHrP(1–40); +significant difference between chondrogenic and hypertrophic group at the respective PTHrP(1–40) concentration (p < 0.05); a and b: n = 4; c: n = 8
ALP activity in the medium was significantly higher in the hypertrophic groups than in the chondrogenic groups at 0–100 pM PTHrP(1–40) (Fig. 1c). ALP secretion decreased dose-dependently under standard chondrogenic conditions with significantly lower ALP activity detected at 10–1,000 pM PTHrP(1–40). Under hypertrophy-enhancing culture conditions, there was significantly less ALP production only at 1,000 pM PTHrP(1–40).
DMMB staining (Fig. 2) showed chondrogenic differentiation in standard chondrogenic medium at all PTHrP(1–40). Pellets did not differ histologically and in size between the control without PTHrP(1–40) (Fig. 2a) and those at 1–100 pM PTHrP(1–40) (Fig. 2b–d). At 1,000 pM PTHrP(1–40), pellets were smaller (Fig. 2e) but retained similar cell morphology. Under hypertrophic conditions (Fig. 2f–j), a different cell morphology compared to chondrogenic aggregates with large lacunae was seen in all cultures. Histologically, there was no difference between the hypertrophic control without PTHrP(1–40) and PTHrP(1–40) at 1–100 pM (Fig. 2f–i). At 1,000 pM PTHrP(1–40), the aggregates were much reduced in size with both hypertrophic and dedifferentiated areas within the pellets (Fig. 2j). Aggregates in this group showed variable relative abundance of hypertrophic to undifferentiated areas within the pellets. In Fig. 2j, the bottom third of the aggregate shows weak DMMB staining and there is a relatively broad peripheral ring that appears to be blue and does not have the metachromasia that is typical for cartilage. Some of the aggregates cultured under hypertrophic conditions appeared completely dedifferentiated on day 28 upon treatment with 1,000 pM PTHrP(1–40).
DMMB staining on day 28 of chondrogenic (a–e) and hypertrophic (f–j) aggregates without (a, f) and with 1 pM (b, g), 10 pM (c, h), 100 pM (d, i) and 1,000 pM PTHrP(1–40) (e, j). j Black arrows dedifferentiated peripheral ring without metachromasia, white arrow low DMMB staining in the bottom third of the aggregate. Magnification ×100, bar 100 μm
The immunohistochemical staining pattern for type II collagen (Fig. 3) was consistent with that observed with DMMB staining. Type II collagen staining was positive at all PTHrP(1–40) concentrations under standard chondrogenic conditions (Fig. 3a–e). Upon culture in hypertrophy-enhancing medium (Fig. 3f–j), a different morphology with large lacunae was evident. There was no apparent difference between the control without PTHrP(1–40) (Fig. 3f) and PTHrP(1–40) at 1–100 pM (Fig. 3g–i). At 1,000 pM PTHrP(1–40) there were hypertrophic, type II collagen-positive areas (Fig. 3j) as well as dedifferentiated, type II collagen-negative areas. Consistent with DMMB staining, type II collagen was not detectable at 1,000 pM PTHrP(1–40) in hypertrophic medium on day 28 in dedifferentiated aggregates.
Type II collagen immunohistochemistry. Immunohistochemical staining for type II collagen on day 28 of chondrogenic (a–e) and hypertrophic (f–j) aggregates without (a, f) and with 1 pM (b, g), 10 pM (c, h), 100 pM (d, i) and 1,000 pM PTHrP(1–40) (e, j). Arrows indicate dedifferentiated type II collagen-negative areas in f. Magnification ×100, bar 100 μm
Under standard chondrogenic conditions (Fig. 4a–e), type X collagen was positive on day 28 in the central areas with no obvious difference between the minus-PTHrP(1–40) control and the 1–1,000 pM PTHrP cultures. Type X collagen stained strongly in hypertrophic cultures on day 28 at PTHrP(1–40) concentrations from 0 to 1,000 pM (Fig. 4f–j) in hypertrophic areas. Dedifferentiated aggregates in the 1,000 pM PTHrP(1–40) group did not express type X collagen.
Type X collagen immunohistochemistry. Immunohistochemical staining for type X collagen on day 28 of chondrogenic (a–e) and hypertrophic (f–j) aggregates without (a, f) and with 1 pM (b, g), 10 pM (c, h), 100 pM (d, i) and 1,000 pM PTHrP(1–40) (e, j). Arrows indicate dedifferentiated type X collagen-negative areas in f. Magnification ×100, bar 100 μm
Histochemical ALP staining on day 28 (Fig. 5) showed ALP-positive cells restricted to the periphery in standard chondrogenic medium without PTHrP(1–40) (Fig. 5a). Staining in the periphery of the pellets became less intense with increasing PTHrP(1–40) concentration (Fig. 5b–e). At 1,000 pM PTHrP(1–40), almost no ALP-positive cells were detectable (Fig. 5e) in chondrogenic medium. In hypertrophy medium without PTHrP(1–40), hypertrophic cells in the central areas as well as peripheral cells were ALP positive (Fig. 5f). This pattern did not change with increasing PTHrP(1–40) concentration up to 100 pM (Fig. 5g–i). At 1,000 pM PTHrP(1–40), hypertrophic cells were ALP positive and cells in the periphery of these aggregates were also ALP positive (Fig. 5j). These peripheral ALP-positive cells are indicated by arrows in Fig. 5f and their morphology differs from the typical large size and round shape of hypertrophic chondrocytes. Also, these small ALP-positive cells are located in type II and type X collagen-negative areas. In dedifferentiated pellets in the 1,000 pM PTHrP(1–40) group, ALP staining was hardly detectable.
ALP histochemistry. Histochemical ALP staining on day 28 of chondrogenic (a–e) and hypertrophic (f–j) aggregates without (a, f) and with 1 pM (b, g), 10 pM (c, h), 100 pM (d, i) and 1,000 pM PTHrP(1–40) (e, j). Blue ALP-positive areas, red neutral red counterstaining. Arrows in f indicate areas that represent dedifferentiated regions without the typical cartilage-like morphology. Magnification ×100, bar 100 μm
Discussion
In this study, the hypertrophic phenotype was significantly enhanced by the hypertrophic medium compared to the chondrogenic standard medium. PTHrP(1–40) did not significantly inhibit chondrogenesis at concentrations up to 100 pM in this experiment. At 1,000 pM PTHrP(1–40) appears to inhibit chondrogenesis and is therefore out of therapeutic considerations if applied throughout the four weeks of differentiation as described here. Similar to our results, Weiss et al. [14] could show inhibition of early and late stages of MSC chondrogenesis by PTHrP(1–34) without selective inhibition of hypertrophy markers. Both the expression of chondrogenic markers (type II collagen) and hypertrophic markers (type X collagen and ALP) were suppressed by PTHrP.
Under standard chondrogenic conditions, PTHrP(1–40) seems to be of some benefit in terms of inhibition of hypertrophy markers. A dose-dependent reduction of ALP level with significant differences at 10–1,000 pM PTHrP(1–40) was observed on day 28 without significant reduction of chondrogenic differentiation at 10 and 100 pM, suggesting this range as a potentially applicable therapeutic range for inhibition of hypertrophy in cartilage tissue engineering. However, the staining intensity for type X collagen was not influenced by PTHrP(1–40). The histochemical correlate of the dose-dependent reduction of ALP secretion in chondrogenic medium by PTHrP(1–40) is the gradual reduction of ALP staining in the periphery of the pellets. These cells are not hypertrophic chondrogenic cells because they do not express type II or type X collagen. The extracellular matrix in this area of the aggregates is known to contain type I collagen [2] and we have seen this in previous experiments [6]. We speculate that these cells express a more bone-like than a hypertrophic chondrocyte-like phenotype. The central chondrogenic cells express little or no ALP and contribute little if any to the ALP activity in the medium. An open question is how PTHrP(1–40) elicits this dose-dependent reduction of ALP in the cells located in the peripheral zone of the pellets. Li et al. [15] have shown in proximal chick sternum chondrocytes that PTHrP slows down maturation by inhibition of Runx2 expression. Runx2 is a positive regulator of both chondrocyte maturation and osteogenic differentiation [16–18] and direct suppression of Runx2 in the peripheral cells is thus possible.
There is a heterogeneous response to 1,000 pM PTHrP(1–40) under hypertrophy-enhancing culture conditions in our experiment. For this we suggest the following explanation: MSCs are a heterogeneous cell population and even within one aggregate, cells with varying chondrogenic differentiation potential are present. For enhancement of the hypertrophic phenotype by switching to hypertrophy-enhancing medium on day 14, we think that cells must have reached a certain level of chondrogenic differentiation. If the progression of chondrogenesis is not sufficient by day 14, when TGF-β1, the major pro-chondrogenic stimulus, is removed, we think that the cells dedifferentiate. PTHrP(1–40) inhibits chondrogenesis at 1,000 pM and may prevent cells with lower chondrogenic potential to reach the threshold that allows them to undergo hypertrophy, while cells with higher chondrogenic potential are capable of reaching a sufficient differentiation level in the presence of 1,000 pM PTHrP(1–40). In preliminary experiments, higher concentrations (10 nM) caused a clear reduction of chondrogenesis and no induction of hypertrophy could be detected at all (data not shown).
Under the hypertrophy-enhancing culture conditions, PTHrP(1–40) did not have a clear inhibitory effect on hypertrophy. On day 28, ALP secretion was not significantly reduced at concentrations up to 100 pM. The significant reduction of ALP activity at 1,000 pM PTHrP(1–40) is probably due to dedifferentiation of cells and not to direct inhibition of hypertrophy, as the number of hypertrophic cells is reduced and hypertrophic cells still stain positive for ALP (Fig. 5). In this group, the lower number of ALP-positive cells probably contributes more to the lower ALP activity in the medium than the eventually lower ALP activity per hypertrophic cell that theoretically could be induced by PTHrP(1–40). Also, the intensity of type X collagen staining and the development of the typical larger lacunae morphologically associated with hypertrophy were inhibited by PTHrP(1–40) treatment.
Based on histochemical results, the hypertrophic group treated with 1,000 pM PTHrP(1–40) shows variable effects on ALP expression. If hypertrophic cells are detectable, they stain for ALP and also type II and type X collagen-negative cells in the periphery are ALP positive. If the aggregates are dedifferentiated, there is very weak or no ALP activity detectable. This suggests that there may be crosstalk between the central hypertrophic cells that act to maintain the peripheral cells in an ALP-positive state. In skeletal development, hypertrophic chondrocytes express growth factors like bone morphogenetic protein (BMP)-2 and BMP-6 that can induce osteoblastic differentiation [19, 20].
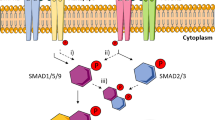
The different response to PTHrP(1–40) in the two culture conditions, standard chondrogenic medium and hypertrophic medium, is likely to be a function of differences in medium composition. Upon switching on day 14 from standard chondrogenic to hypertrophic medium, TGF-β1 and dexamethasone are withdrawn and thyroid hormone is added. The latter has been shown to enhance hypertrophy both in embryonic mesenchymal cells and growth plate chondrocytes [21–23]. Thyroid hormone induces terminal differentiation in growth plate chondrocytes through induction of BMP-2, and BMP-2 can induce chondrocytes to undergo maturation [24]. This can possibly counteract the inhibitory effect of PTHrP(1–40) under hypertrophy-enhancing culture conditions. On the other hand, continuous application of dexamethasone and TGF-β1 may be necessary to facilitate PTHrP action or act synergistically in standard chondrogenic medium. Dexamethasone has been shown to suppress the increase of ALP activity in chick sternal chondrocytes [25] and TGF-β prevents hypertrophy in epiphyseal chondrocytes [26]. Possible effects of PTHrP in our model that are discussed here are summarised in Fig. 6.
Possible effects of PTHrP in the model used. PTHrP inhibits chondrogenic differentiation of MSCs at high concentrations (1). After chondrogenic conditioning when hypertrophy is induced by switching the medium conditions, PTHrP seems to promote dedifferentiation (2) and may inhibit terminal differentiation towards a hypertrophic phenotype (3). ALP activity in the periphery of the aggregates is possibly induced by chondrocytes and hypertrophic chondrocytes in the centre of the aggregates and PTHrP may indirectly inhibit ALP induction in the periphery by inhibiting chondrogenesis and maturation (4). PTHrP may directly suppress ALP expression in the peripheral non-chondrogenic cells (5)
In conclusion, PTHrP(1–40) has some inhibitory effect on the expression of ALP in MSCs under standard chondrogenic conditions. Under pro-hypertrophic conditions, PTHrP(1–40) failed to counteract the induction of a hypertrophic cell morphology and did not clearly reduce ALP activity and type X collagen deposition in hypertrophic cells.
References
Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU (1998) In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res 238(1):265–272
Yoo JU, Barthel TS, Nishimura K, Solchaga L, Caplan AI, Goldberg VM, Johnstone B (1998) The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J Bone Joint Surg Am 80(12):1745–1757
Mueller MB, Blunk T, Appel B, Maschke A, Goepferich A, Zellner J, Englert C, Prantl L, Kujat R, Nerlich M, Angele P (2013) Insulin is essential for in vitro chondrogenesis of mesenchymal progenitor cells and influences chondrogenesis in a dose-dependent manner. Int Orthop 37(1):153–158
Sekiya I, Vuoristo JT, Larson BL, Prockop DJ (2002) In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis. Proc Natl Acad Sci U S A 99(7):4397–4402
Mueller MB, Tuan RS (2008) Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheum 58(5):1377–1388
Mueller MB, Fischer M, Zellner J, Berner A, Dienstknecht T, Prantl L, Kujat R, Nerlich M, Tuan RS, Angele P (2010) Hypertrophy in mesenchymal stem cell chondrogenesis: effect of TGF-beta isoforms and chondrogenic conditioning. Cells Tissues Organs 192(3):158–166
Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, Pittenger MF (1998) Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng 4(4):415–428
Goldring MB, Tsuchimochi K, Ijiri K (2006) The control of chondrogenesis. J Cell Biochem 97(1):33–44
Shimizu H, Yokoyama S, Asahara H (2007) Growth and differentiation of the developing limb bud from the perspective of chondrogenesis. Dev Growth Differ 49(6):449–454
Pelttari K, Winter A, Steck E, Goetzke K, Hennig T, Ochs BG, Aigner T, Richter W (2006) Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum 54(10):3254–3266
Guo J, Chung UI, Yang D, Karsenty G, Bringhurst FR, Kronenberg HM (2006) PTH/PTHrP receptor delays chondrocyte hypertrophy via both Runx2-dependent and -independent pathways. Dev Biol 292(1):116–128
Kronenberg HM (2006) PTHrP and skeletal development. Ann N Y Acad Sci 1068:1–13
Weisser J, Riemer S, Schmidl M, Suva LJ, Pöschl E, Bräuer R, von der Mark K (2002) Four distinct chondrocyte populations in the fetal bovine growth plate: highest expression levels of PTH/PTHrP receptor, Indian hedgehog, and MMP-13 in hypertrophic chondrocytes and their suppression by PTH (1–34) and PTHrP (1–40). Exp Cell Res 279(1):1–13
Weiss S, Hennig T, Bock R, Steck E, Richter W (2010) Impact of growth factors and PTHrP on early and late chondrogenic differentiation of human mesenchymal stem cells. J Cell Physiol 223(1):84–93
Li TF, Dong Y, Ionescu AM, Rosier RN, Zuscik MJ, Schwarz EM, O’Keefe RJ, Drissi H (2004) Parathyroid hormone-related peptide (PTHrP) inhibits Runx2 expression through the PKA signaling pathway. Exp Cell Res 299(1):128–136
Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G (1997) Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89(5):747–754
Enomoto H, Enomoto-Iwamoto M, Iwamoto M, Nomura S, Himeno M, Kitamura Y, Kishimoto T, Komori T (2000) Cbfa1 is a positive regulatory factor in chondrocyte maturation. J Biol Chem 275(12):8695–8702
Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T (1997) Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89(5):755–764
Canalis E, Economides AN, Gazzerro E (2003) Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr Rev 24(2):218–235
Kronenberg HM (2003) Developmental regulation of the growth plate. Nature 423(6937):332–336
Mello MA, Tuan RS (2006) Effects of TGF-beta1 and triiodothyronine on cartilage maturation: in vitro analysis using long-term high-density micromass cultures of chick embryonic limb mesenchymal cells. J Orthop Res 24(11):2095–2105
Okubo Y, Reddi AH (2003) Thyroxine downregulates Sox9 and promotes chondrocyte hypertrophy. Biochem Biophys Res Commun 306(1):186–190
Quarto R, Campanile G, Cancedda R, Dozin B (1997) Modulation of commitment, proliferation, and differentiation of chondrogenic cells in defined culture medium. Endocrinology 138(11):4966–4976
Ballock RT, O’Keefe RJ (2003) The biology of the growth plate. J Bone Joint Surg Am 85-A(4):715–726
Leboy PS, Sullivan TA, Nooreyazdan M, Venezian RA (1997) Rapid chondrocyte maturation by serum-free culture with BMP-2 and ascorbic acid. J Cell Biochem 66(3):394–403
Ballock RT, Heydemann A, Wakefield LM, Flanders KC, Roberts AB, Sporn MB (1993) TGF-beta 1 prevents hypertrophy of epiphyseal chondrocytes: regulation of gene expression for cartilage matrix proteins and metalloproteases. Dev Biol 158(2):414–429
Acknowledgments
Supported by AO Research Fund (S-07-3M), DFG (MU-2318/1) and the Intramural Research Program of NIAMS, NIH (AR Z01 41131).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mueller, M.B., Fischer, M., Zellner, J. et al. Effect of parathyroid hormone-related protein in an in vitro hypertrophy model for mesenchymal stem cell chondrogenesis. International Orthopaedics (SICOT) 37, 945–951 (2013). https://doi.org/10.1007/s00264-013-1800-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-013-1800-1