Abstract
Background:
Human umbilical cord blood-derived MSCs (hUCB-MSCs) have been studied in osteoarthritis (OA) and cartilage regeneration. Our previous study demonstrated that hUCB-MSCs combined with cartilage acellular matrix injection (CAM Inj.) represent potential therapeutic agents for structural improvement and anti-inflammatory effects in a rabbit model of OA.
Methods:
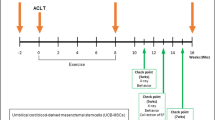
Based on a previous study, this study has evaluated the safety and efficacy of hUCB-MSCs combined with CAM Inj. in an anterior cruciate ligament transection (ACLT) with medial meniscectomy (MMx) in a goat model. In this study, 27 goats were divided into 5 groups: normal (n = 3), OA (n = 6), OA + CAM Inj. (n = 6), OA + hUCB-MSCs (n = 6), and OA + hUCB-MSCs + CAM Inj. (n = 6). Lameness and radiographic parameters were assessed 6 months after administration, and macroscopic and histological evaluations of the goat articular cartilage were performed 6 months after intervention.
Results:
The results showed significant improvement in lameness score only in the OA + hUCB-MSCs group at 5 months after treatment (*p < 0.05), whereas the K&L score showed significant improvement only in the OA + hUCB-MSCs + CAM Inj. group 6 months after intervention (*p < 0.05). In addition, the gross findings showed significance in OA + CAM Inj. and OA + hUCB-MSCs + CAM Inj. groups 6 months after treatment (*p < 0.05 and **p < 0.01).
Conclusion:
In conclusion, treatment with a combination of hUCB-MSCs and CAM Inj. reduced OA symptoms and induced effective cartilage tissue repair in a goat model. We suggest the combination of hUCB-MSCs and CAM Inj. as an alternative therapy for OA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Osteoarthritis (OA) is a common joint disease involving degenerative changes in articular cartilage and its surrounding tissues in the joint [1,2,3]. OA patients presenting with the disorder often suffer from progressive loss of function, pain, and stiffness, which reduces the quality of life [4], resulting in a significant social burden due to elevated health care costs and premature workforce retirement. However, despite decades of research, practical disease-modifying OA drugs have not yet been identified. The clinical effects of pharmacological interventions, such as non-steroidal anti-inflammatory drugs (NSAIDs) [5, 6], corticosteroids [7, 8], and analgesics or intra-articular (IA) injection of hyaluronic acid (HA) [9, 10], are transient and associated with side effects [11, 12].
The application of mesenchymal stem cells (MSCs) in regenerative medicine is facilitated by their abundance, high cell viability, immuno-regulatory function, capacity for multilineage differentiation [13,14,15,16]. MSCs derived from various tissues, including bone marrow, adipose tissue, articular synovium, and umbilical cord blood (UCB), have been studied with satisfactory results in OA and cartilage regeneration [17,18,19,20]. Among MSCs, UCB-MSCs are suitable for clinical application because of their noninvasive tissue collection [21,22,23], lack of donor-site morbidity [22, 24], young age [25], tissue abundance [23], high expansion capacity [21, 22, 24], hypo-immunogenicity [21, 22, 24, 25], and paracrine potential for accelerating tissue regeneration [24].
Meanwhile, tissue-specific extracellular matrix (ECM) has been widely used to restore the structure and function of damaged host tissues [26,27,28]. Rothrauff et al. reported that urea-extracted fractions of decellularized cartilage ECM promote chondrogenic differentiation of MSCs. Furthermore, Yin et al. demonstrated that MSCs combined with cartilage extract-derived ECM regenerated damaged cartilage in a rabbit model of articular cartilage defect (ACD), compared with cartilage derived ECM alone [29]. Therefore, it is possible to remodel cartilage using cartilage-derived ECM, co-injected with MSCs promote chondrogenic differentiation, thereby amplifying cartilage regeneration.
Our previous study has demonstrated that human umbilical cord blood-mesenchymal stem cells (hUCB-MSCs) combined with cartilage acellular matrix injection (CAM Inj.) represent potential regenerative effect and anti-inflammatory effects in the rabbit OA model [30]. However, since small animal models such as rabbits and rats have excellent self-healing ability, it is difficult to expect equivalent efficacy in actual clinical practice [31, 32]. Therefore for the clinical application, it is required to evaluate the therapeutic efficacy, stability and biological safety in large animal model that have less self-healing ability and better mimic the anatomical features of human articular joints [33,34,35]. The aim of this study was to evaluate the therapeutic efficacy in the goat OA model using IA injection of hUCB-MSCs combined with CAM Inj. in comparison with either hUCB-MSCs or CAM Inj. alone.
2 Materials and methods
2.1 Cell culture
Isolation and culturing of hUCB-MSCs were performed according to published procedures [30]. Culture medium (KSB-3; Kangstem Biotech, Seoul, Korea) supplemented with 10% fetal bovine serum (FBS; Gibco, Gaithersburg, MD, USA) was used to culture hUCB-MSCs. hUCB-MSCs used in the experiment were derived from passages 4–7 and cultured at 37 °C with 5% CO2.
2.2 Preparation of CAM Inj.
Cartilage acellular matrix (CAM) was obtained from ATEMs (Seoul, Korea) and fabricated to solution form. For the preparation of injectable solution, CAM was added to normal saline to the concentration of 4% (w/v) and stirred at 150 rpm for 18 h at 37 °C. The solubilized CAM was then purified by centrifugation at 300 × g for 5 min at 25 °C. The prepared CAM Inj. was stored at − 80 °C until further analysis.
2.3 Physicochemical characterization of CAM Inj.
Native cartilage and CAM Inj. were suspended in distilled water for sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). SDS loading buffer and reducing agent (Thermo Fisher Scientific, Waltham, MA, USA, respectively) were added to 10 µg of total protein and heated for 5 min at 98 °C. Proteins were loaded into a pre-cast gel (Bio-Rad, Hercules, CA, USA) and separated by electrophoresis using a running buffer for 90 min at 100 V. For Coomassie staining, the gel was stained with Coomassie staining buffer (Bio-Rad) for 1 h before it was washed three times in distilled water. For western blot, the gel was transferred onto PVDF membranes (BSP0161, PALL Corporation, Port Washington, NY, USA) and the membranes were blocked in Difco Skim milk (23100, BD, Sparks, MD, USA) in TBS containing 0.1% Tween-20 (TBST). Membranes were incubated with the primary antibodies of COL2 (MA1-37493, Invitrogen, Carlsbad, CA, USA) that were used at a dilution of 1:1000 for 1 h at 37 °C. The membrane was subsequently incubated with Goat anti-Mouse IgG2a secondary antibody (A-10685, Invitrogen) at 37 °C for 1 h. Rheological measurements were performed using a rotational rheometer (TA Instruments, ARES G2, New Castle, DE, USA) equipped with a 25 mm parallel-plate geometry, 1 mm gap at 25 °C. The complex viscosities were measured with a shear rate ranging from 1 to 100 s−1.
For biochemical analysis, CAM Inj. was lyophilized before collagen and sulfated glycosaminoglycan (sGAG) contents were evaluated. The collagen content was quantified using the Sircol™ soluble collagen assay kit (Biocolor, Carrick Fergus, UK) according to the manufacturer's instructions. For collagen quantification, lyophilized CAM Inj. was extracted with pepsin (Sigma, St. Louis, MO, USA) solution by incubation at 4 °C for 48 h. The sGAG content was quantified with a Blyscan assay kit (Biocolor) according to the manufacturer’s instruction. The sGAG was extracted from the lyophilized CAM Inj. by incubating it at 60 °C for 24 h in papain solution (Sigma) before being quantified.
2.4 Chondrogenic activity of CAM Inj. in the hUCB-MSCs in vitro
A pellet of hUCB-MSCs (3 × 105 cells) was distributed into each 15 mL conical tubes and centrifuged for 10 min at 500 × g. After 24 h, the pellets were transferred to a 96-well round (U) bottom plate (Nunc, Roskilde, Denmark) and cultured for 21 days in two groups: (1) chondrogenic media (Promocell, Heidelberg, Germany); (2) chondrogenic media + 10% CAM Inj. The pellets were collected on day 21 for histological analyses.
2.5 Histological analysis
Pellet samples were fixed in 10% phosphate-buffered formalin before they were serially dehydrated, then embedded in paraffin, and sectioned (4 μm). Safranin-O staining and immuno-histochemical analysis (IHC) were performed followed by de-paraffinization. For safranin-O staining, samples were stained with American MasterTech Scientific kit according to the manufacturer's instructions (KTSFO, Lodi, CA, USA). IHC was performed using 3,3’-diamino-benzidine (DAB), using anti-type 2 collagen (COL2) monoclonal antibody (Ab34712, Abcam, Cambridge, UK).
3 Effect of OA improvement in goat models
3.1 OA induction in goat
This test was approved by the Experimental Animal Ethics Committee of KPC Co., Ltd. (approval no.: P18042). One-year-old hybrid Korean black goats and Australian boer goats (about 30–50 kg in weight) were used. In this study, OA was induced by anterior cruciate ligament transection (ACLT) with medial meniscectomy (MMx) [36]. All animals that fasted for 16 h were anesthetized by injecting a mixture of ketamine 11 mg/kg intra-muscularly (Yuhan Corp., Seoul, Korea) and xylazine 0.22 mg/kg (Rompun; Bayer Korea Corp., Seoul, Korea). For each individual, the hair of the right knee joint and the surrounding femoral area was removed using an epilator and was disinfected. Subsequently, approximately a 5 cm longitudinal incision was made around the tibial tubercle using the medial parapatellar approach. After incising the exposed joint capsule, the knee joint was positioned in the outer direction. The ACL and the meniscus were exposed by bending the knee prior completely to cutting the middle of the ACL. The medial meniscus was completely removed and the joint capsule and subcutaneous tissue and skin were sutured.
3.2 IA injection of hUCB-MSCs and CAM Inj.
The experiment involved a total of five groups (Test 1; a control group, an OA group, a hUCB-MSCs group, a CAM Inj. group and a hUCB-MSCs + CAM Inj. group) or three groups (Test 2; a control group, an OA group, and a hUCB-MSCs + CAM Inj. group). For test groups, osteoarthritis was induced via ACLT and MMx after incision of the right knee joint (Table 1).
3.3 Lameness scoring
Upon visiting the farm, the tester let each individual exercise freely and evaluated the level of claudication based on indices specified in the table below through macroscopic observation. Lameness was scored via behavioral analysis a total of 7 times (Test 1) or 13 times (Test 2) before administering the test substance and once a month for 6 months or 12 months after treatment (max = 4; Table 2).
3.4 X-ray imaging and evaluation of the right knee joint
Each animal was held in the supine position and digital images were obtained by photographing the right knee joint in the ventro-dorsal position. X-ray (PXM-20 BT, Poskom, Korea) images were acquired under the same exposure conditions (kVp: 70, mAs: 10) every time. Based on the digital images obtained, the level of joint deformation was evaluated according to the modified Kellgren & Lawrence grade system (K&L score: 0–4) by performing radiography a total of 4 times: before surgery, before test substance administration (before group separation), and at 3 and 6 months (Test 1), or 3, 6, 9 and 12 months (Test 2) after intervention (max = 4; Table 3).
3.5 Autopsy and evaluation of macroscopic findings
All individuals were autopsied at 6 months (Test 1) or 12 months (Test 2) after test substance administration. Individuals were euthanized under deep anaesthesia. For each test individual, the knee joint was removed undamaged by separating the right hip and ankle joints. After incising the articular capsule, the exposed femoral cartilage was visually evaluated and fixed in 10% phosphate-buffered formalin.
Changes in the articular cartilage of the right knee joint were macroscopically examined. After obtaining a close-up photograph of the femur using a digital camera (Coolpix 5700, Nikon Corp, Japan), structural changes in the joint were scored via macroscopic examination based on four indices specified in the table below: loss of the superficial layer of articular cartilage, ulceration, osteophyte formation, and joint hypertrophy (max = 16; Table 4).
3.6 Histological evaluation of harvested specimens
Safranin-O staining was performed with Weigert's iron haematoxylin (Sigma) solution for 10 min, rinsed with water, followed by staining with 0.02% fast green solution (Sigma) for 5 min. Subsequently, the slides were treated with 1% acetic acid solution and stained with 0.1% safranin-O (Sigma) solution, followed by rinsing with water for 5 min to visualize nuclei. These slides were analysed after dehydration and clarification.
The level of articular cartilage degeneration and pathological damage in each sample were histologically evaluated. The degrees of structural, cellularity and chondrocyte cloning changes were analyzed under an optical microscope. To evaluate the progression of degenerative arthritis and identify the disease stage of cartilage, modified Osteoarthritis Research Society International scores (OARSI; max = 18) were determined based on a total of 3 indices (Table 5). Samples used in immunofluorescence staining were treated with antigen retrieval buffer (Dako, Glostrup, Denmark), followed by permeabilization and blockage of samples in Triton X-100 and bovine serum albumin (BSA) in phosphate-buffered saline (PBS). The samples were then stained with human nuclei (MAB1281, Sigma) and COL2 (MA1-37493, Invitrogen). Hoechst (62249, Invitrogen) was used to counterstain samples following nuclear staining.
3.7 Statistical analysis
All results obtained in the experiment are expressed as mean ± standard deviation and tested using SPSS version 20 (IBM SPSS Statistics, Armonk, NY, USA). The results were compared between the normal group and the induced group using Student’s t-test at p < 0.05. To determine the significance of difference between normal and induced groups, one-way analysis of variance (ANOVA) was carried out in case of equal variances. Fisher's least significant difference (LSD) test was used for post-hoc analysis. In cases of heteroscedasticity, the Dunnett’s T3 test was used for post-hoc analysis (significance level: 5% and 1% for both sides).
4 Results
4.1 Characterization of CAM Inj.
SDS-PAGE results showed that the native cartilage was enriched with collagen and diverse protein cargo. Conversely, the CAM Inj. was principally composed of collagen, with diverse small-molecule protein expression (Fig. 1A and Supplementary Fig. S1A). The CAM Inj. was translucent and was available in a convenient injectable form (Fig. 1B, C). CAM Inj. had a viscosity of around 6.2 ± 3.3 Pa.s at a shear rate of 1 s−1, 1 ± 0.4 Pa.s at a shear rate of 10 s−1 and shearing down to 0.3 ± 0.1 Pa.s at a sheer rate of 100 s−1 (Fig. 1D). The biochemical analysis revealed that the collagen content of native cartilage constituted approximately 460 ± 11.9 µg/mg and that of CAM Inj. was 174.8 ± 19.7 µg/mg, which was approximately 38.0% compared with native cartilage tissue (Fig. 1E). The sGAG of native cartilage and CAM Inj. was approximately 201.2 ± 16.3 µg/mg and 167.6 ± 8.3.9 µg/mg, respectively (Fig. 1F), which was approximately 83.3% in the CAM Inj. To determine the chondrogenic effect of CAM Inj., the hUCB-MSCs pellets were cultured for up to 21 days with or without CAM Inj.. Histological observations revealed an increase in COL2 deposition when the CAM Inj. was included in the chondrogenic differentiation media (Supplementary Fig. S1B). These data demonstrate that CAM Inj. preserved the major components of native cartilage ECM. In addition, CAM Inj. is in a convenient injectable form also inducing chondrogenic differentiation of hUCB-MSCs.
Characterization of CAM Inj. A SDS-PAGE analysis of native cartilage and CAM Inj.. B Gross image of CAM Inj. contained in tube. C The extrusion of CAM Inj. from 22-gauge syringe needle to show injectable properties. D Viscosities of CAM Inj. measured at 25 °C with a constant shear rate of 1, 10 and 100 s−1 respectively (n = 3). E Collagen contents and F sGAG contents of native cartilage and CAM Inj. (n = 3). SDS-PAGE: sodium dodecyl sulfate–polyacrylamide gel electrophoresis, sGAG: sulfated glycosaminoglycan
4.2 Efficacy of hUCB-MSCs/CAM Inj. in a goat model of OA
We tested the alleviation of OA symptoms using lameness scores at 0, 1, 2, 3, 4, 5 and 6 months with normal, OA, OA + hUCB-MSCs, OA + CAM Inj. and OA + hUCB-MSCs + CAM Inj. (Fig. 2; Test 1). As a result, the hUCB-MSCs group showed improvements in lameness scores at month 5 after injection compared with the control group (*p < 0.05; Fig. 3A). In addition, we performed a 12-month monitoring of normal, OA, and OA + hUCB-MSCs + CAM Inj. groups (Test 2; Supplementary Fig. S2). During the 12-month monitoring period, the OA group showed 2.20–3.2 of variance, with OA + hUCB-MSCs + CAM Inj. of 1.50–2.67 variance. Especially, the OA + hUCB-MSCs + CAM Inj. group showed statistically significant low scores compared with the OA group at months 11 and 12 (*p < 0.05; Supplementary Fig. S3A).
Clinical lameness score and radiographic analysis of OA symptoms in a goat model. A Main lameness score over the study period. B Representative radiological images of knee joints of experimental goats at 6 months. C Radiological score according to the K&L scale at initial, 3 and 6 months. Results represent means ± SEM, *p < 0.05. OA: osteoarthritis, K&L: Kellgren & Lawrence
The efficacy of hUCB-MSCs along with CAM Inj. in goats with OA was evaluated radiographically and quantified at 0, 3 and 6 months after administration. The radiographic images were used to evaluate the degree of joint change via modified K&L scoring (Fig. 3B; Table 3). As a result, at month 6 of the OA group, OA + hUCB-MSCs group, OA + CAM Inj. group, and OA + hUCB-MSCs + CAM Inj. group scores were 3.50, 3.00, 3.08 and 2.92*, respectively (Fig. 3C). All the test groups scored lower than the OA group under each of the criteria. The OA + hUCB-MSCs + CAM Inj. group showed a statistically significant low score compared with the OA group at month 6 (*p < 0.05). In addition, after 12 months of monitoring (Test 2; Supplementary Fig. S3B), the scores of OA group and OA + hUCB-MSCs + CAM Inj. group were 3.40 and 3.00, respectively (Supplementary Fig. S3C). Based on the evaluation every three months after treatment, the scores of OA + hUCB-MSCs + CAM Inj. group were lower than in OA group. These results indicate that hUCB-MSCs along with CAM Inj. increased the efficacy of OA symptom alleviation than hUCB-MSCs or CAM Inj. administration alone.
4.3 Regenerative effect of hUCB-MSCs/CAM Inj. administration in a goat model of OA
Goats were sacrificed to evaluate the cartilage tissue repair at month 6 (Test 1) and month 12 (Test 2) after treatment. The cartilage tissues were visually evaluated and quantified after femoral condyle retrieval (Table 4). Test 1 results indicated severe damage involving the loss of superficial layer, joint ulceration, osteophyte and hypertrophy in the OA group. Compared with the results of the OA + hUCB-MSCs or OA + CAM Inj. group, the repaired area was smooth and full in the OA + hUCB-MSCs + CAM Inj. group (Fig. 4A). Based on gross evaluation, the scores of OA group, OA + hUCB-MSCs group, OA + CAM Inj. group, and OA + hUCB-MSCs + CAM Inj. group were 12.00, 8.33, 8.33* and 6.83** respectively (*p < 0.05, **p < 0.01; Fig. 4B). Based on 12-month observation, the overall score of the OA group was 10.40 compared with OA + hUCB-MSCs + CAM Inj. group, which had a score of 7.0. The OA + hUCB-MSCs + CAM Inj. group showed less damage than the OA group (Test 2; Supplementary Fig. S3D and S3E).
Macroscopic and histological evaluation of the goat articular cartilage. A Representative gross images of the femoral condyle. The black circle indicates the defects or repaired lesion. B Comparison of the gross finding scores of the lesion in the affected articular cartilage. C Microscopic lesions of the articular cartilage in the affected knees of all experimental groups (Scale bars = 1 mm). D Comparison of the modified OARSI histopathologic scores in the affected articular cartilage. Results represent means ± SEM, *p < 0.05 and **p < 0.01. COL2: type 2 collagen
Histological analysis was conducted using H&E, safranin-O and COL2. Safranin-O deposition and COL2 showed clear differences between OA and OA + hUCB-MSCs + CAM Inj. groups (Fig. 4C and Supplementary Fig. S3F). Based on quantitative evaluation, modified OARSI score was consistent with the resulting histology (Table 5). Five grading criteria were analyzed and each was evaluated to determine the overall grade. As a result, the OA group, OA + CAM Inj. group, OA + hUCB-MSCs group, and OA + hUCB-MSCs + CAM Inj. group scores were 6.8, 5.3, 6.3 and 3.7, respectively, with improved cartilage tissue repair in the OA + hUCB-MSCs + CAM Inj. group (Test 1; Fig. 4D). Based on 12-month observation, the OA group and OA + hUCB-MSCs + CAM Inj. group scores were 9.0 and 6.5 (Test 2; Supplementary Fig. S3G). Antibodies to human-specific nuclei (HuNu) were used in immuno-staining to detect the transplanted hUCB-MSCs. The results showed the presence of HuNu-positive cells in the cartilage tissues transplanted with OA + hUCB-MSCs and in the OA + hUCB-MSCs + CAM Inj. group. Furthermore, hUCB-MSCs present in cartilage tissue were also detected in the lacuna and in COL2 expression (Fig. 5A, B).
Representative fluorescence images confirm human cells and COL2 at 6 months after administration. A Expression of type COL2 and HuNu at 6 months after transplantation. B Merged image of OA + hUCB-MSCs group (M1) and OA + hUCB-MSCs + CAM Inj. group (M2). The yellow arrows indicate the human cells (Scale bars = 100 μm). COL2: type 2 collagen, HuNu: human-specific nuclei
5 Discussion
The purpose of this study was to evaluate the therapeutic efficacy of hUCB-MSCs combined with CAM Inj. in a large animal model. The CAM Inj., injectable cell delivery solution, contained the major components of cartilage ECM including collagen and sGAG in addition to diverse small molecules compared with native cartilage. The CAM Inj. accelerated the chondrogenic differentiation of hUCB-MSCs and the synthesis of cartilaginous ECM during chondrogenic differentiation. Finally, we evaluated the therapeutic efficacy of hUCB-MSCs and CAM Inj. in OA treatment using a goat model. Co-administration of both hUCB-MSCs and CAM Inj. significantly reduced OA symptoms and promoted regeneration of damaged cartilage tissues. The regeneration of cartilage tissues was mainly attributed to hUCB-MSCs in combination with CAM Inj., which indicates that CAM Inj. induce the differentiation of the co-administered hUCB-MSCs.
It is crucial to perform efficacy and safety evaluation in large animals prior to clinical trials. Although a previous study established the efficacy of combining hUCB-MSCs with CAM Inj. in a rabbit model of OA, a large animal model of OA has yet to be clearly identified. Therefore, in this study, the evaluation in a large animal model that can be analyzed more objectively for application to clinical trials was carried out. Meanwhile, the larger joint size and thicker cartilage of large animal models are more appropriate and desirable in translational studies [37,38,39,40]. In the case of goat, the stifle joint displayed large anatomic similarities with the human knee aside from a long trochlear groove with medial and lateral ridges as well as intercondylar notch width in a comparative analysis [33,34,35]. Therefore, we investigated whether the hUCB-MSCs combined with CAM Inj. represent potential therapeutic agents in a goat ACLT + MMx model.
In our study, combination of hUCB-MSCs with CAM Inj. exhibited a synergistic regenerative effect compared to hUCB-MSCs alone or CAM Inj. alone. Results of the lameness score of OA goat model confirmed that the significance of lameness score was established only in the OA + hUCB-MSCs group at 5 months after administration (*p < 0.05), whereas the K&L score based on radiographic results showed significance only in the OA + hUCB-MSCs + CAM Inj. group 6 months after administration (*p < 0.05). In addition, based on the gross findings, the significance was observed in OA + CAM Inj. and OA + hUCB-MSCs + CAM Inj. groups 6 months after intervention (*p < 0.05 and **p < 0.01, respectively). In addition, the histology-based modified OARSI results showed that the OA + hUCB-MSCs + CAM Inj. group scored better than the OA + CAM Inj. and OA + hUCB-MSCs groups. In the histological analysis, the hUCB-MSCs combined with CAM Inj. group, hUCB-MSCs showed lacunae in the repaired cartilage lesions by 6 months. These results demonstrated that treatment with hUCB-MSCs alone was effective in alleviating OA symptoms, and that the effect was increased by co-administering CAM Inj.. In addition, the hUCB-MSCs with increased differentiation capacity following the co-administration of CAM Inj., were directly involved in tissue regeneration without any signs of inflammation or immune reaction.
Although many studies investigated the therapeutic role of MSCs in OA model, some studies suggest that the quality of cartilage regeneration induced by MSCs is not perfect [41,42,43]. Our results also confirmed that hUCB-MSCs administered alone showed improvement in lameness score, whereas cartilage regeneration was insignificant based on histological observations. The limited regenerative effect of hUCB-MSCs alone group may be due to the lack of chondrogenic signals. Previous studies have reported that co-administration of hyaluronic acid (HA) or collagen with MSCs promotes chondro-inductive potential, resulting in improved cartilage regeneration compared to MSCs alone treatment [44,45,46]. Therefore, it is important to amplify the differentiation capacity of MSCs by combination with chondro-inductive biomaterial rather than using MSCs alone when using MSCs for OA treatment.
Cartilaginous ECM have been demonstrated the chondro-inductive characteristics supporting chondrogenic differentiation of MSCs. Burnsed et al. reported that the hydrogel derived from shark and porcine cartilage ECM induced chondrogenic differentiation of MSCs without exogenous growth factors [47]. Rothrauff et al. also demonstrated that the cartilage-derived ECM extracted with urea induced the chondrogenic differentiation of MSCs [48]. Similarly, Yun et al. showed that decellularized meniscus ECM derived from inner cartilaginous zone induced chondrogenic differentiation of MSCs [49]. Our results tend to be consistent with these previous findings. Therefore, CAM Inj. derived from cartilaginous ECM may be a suitable candidate for bioactive substances that induce chondrogenic differentiation of MSCs.
ECM-based biomaterials should retain biological components from the native ECM during fabrication process [50,51,52]. Many studies have emphasized the importance of careful and reproducible processes as many manufacturing processes involve extreme physicochemical stress that cause loss of biological properties. In our study, SDS-PAGE revealed diverse protein cargo as well as collagen chains in CAM Inj.. In the biochemical analysis, CAM Inj. maintained 38% of collagen and 83.3% of sGAG contents compared with native cartilage tissue. The collagen and sGAG content decreased slightly during solubilization, and these results have also been reported in other papers [48, 53]. We plan to increase the recovery rate of collagen and sGAG through follow-up studies. In addition, the CAM Inj. was not associated with cytotoxicity or inflammatory reaction in the previous and current studies, and instead promoted the chondrogenic differentiation of hUCB-MSCs [30]. Therefore, CAM Inj. is considered a biocompatible material that adequately maintains bioactive signaling factors as well as major components of native cartilage.
We formulated CAM Inj. into an injectable solution form in an effort to disperse hUCB-MSCs evenly in the joint cavity as well as induce chondrogenic differentiation. Therefore, it is convenient to administer together with cell therapy. In addition, this study findings suggest that only a single co-administration of hUCB-MSCs and CAM Inj. in goat OA model resulted in improvement in lameness, K&L grade, gross findings and modified OARSI scores. Overall, CAM Inj. maximizes the therapeutic effect by amplifying the chondrogenic differentiation of hUCB-MSCs administered simultaneously rather than alone. Based on the findings, we suggest that the combination of hUCB-MSCs and CAM Inj. can be suitable candidate for alternative OA therapeutics. This study may serve as a reference for clinical application of chondrogenic differentiation in improving osteoarthritis.
References
Shane Anderson A, Loeser RF. Why is osteoarthritis an age-related disease? Best Pract Res Clin Rheumatol. 2010;24:15–26.
Valdes AM, Stocks J. Osteoarthritis and ageing. EMJ. 2018;3:116–23.
Chen D, Shen J, Zhao W, Wang T, Han L, Hamilton JL, et al. Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Res. 2017;5:16044.
Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–707.
Pelletier JP, Martel-Pelletier J, Rannou F, Cooper C. Efficacy and safety of oral NSAIDs and analgesics in the management of osteoarthritis: Evidence from real-life setting trials and surveys. Semin Arthritis Rheum. 2016;45:S22-7.
Cho SK, Kim H, Park HR, Choi W, Choi S, Jung SY, et al. Nonsteroidal anti-inflammatory drugs-sparing effect of symptomatic slow-acting drugs for osteoarthritis in knee osteoarthritis patients. Int J Rheum Dis. 2019;26:179–85.
Arroll B, Goodyear-Smith F. Corticosteroid injections for osteoarthritis of the knee: meta-analysis. BMJ. 2004;328:869.
Shimizu M, Higuchi H, Takagishi K, Shinozaki T, Kobayashi T. Clinical and biochemical characteristics after intra-articular injection for the treatment of osteoarthritis of the knee: prospective randomized study of sodium hyaluronate and corticosteroid. J Orthop Sci. 2010;15:51–6.
Ayhan E, Kesmezacar H, Akgun I. Intraarticular injections (corticosteroid, hyaluronic acid, platelet rich plasma) for the knee osteoarthritis. World J Orthop. 2014;5:351–61.
Altman RD, Manjoo A, Fierlinger A, Niazi F, Nicholls M. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: a systematic review. BMC Musculoskelet Disord. 2015;16:321.
Zhang W, Ouyang H, Dass CR, Xu J. Current research on pharmacologic and regenerative therapies for osteoarthritis. Bone Res. 2016;4:15040.
Grässel S, Muschter D. Recent advances in the treatment of osteoarthritis. F1000Res. 2020;9:F1000 Faculty Rev-325.
Le H, Xu W, Zhuang X, Chang F, Wang Y, Ding J. Mesenchymal stem cells for cartilage regeneration. J Tissue Eng. 2020;11:2041731420943839.
Maumus M, Guérit D, Toupet K, Jorgensen C, Noël D. Mesenchymal stem cell-based therapies in regenerative medicine: applications in rheumatology. Stem Cell Res Ther. 2011;2:14.
Han Y, Li X, Zhang Y, Han Y, Chang F, Ding J. Mesenchymal stem cells for regenerative medicine. Cell. 2019;8:886.
Harrell CR, Markovic BS, Fellabaum C, Arsenijevic A, Volarevic V. Mesenchymal stem cell-based therapy of osteoarthritis: current knowledge and future perspectives. Biomed Pharmacother. 2019;109:2318–26.
Chanda D, Kumar S, Ponnazhagan S. Therapeutic potential of adult bone marrow-derived mesenchymal stem cells in diseases of the skeleton. J Cell Biochem. 2010;111:249–57.
Zhang R, Ma J, Han J, Zhang W, Ma J. Mesenchymal stem cell related therapies for cartilage lesions and osteoarthritis. Am J Transl Res. 2019;11:6275–89.
Huang YZ, Xie HQ, Silini A, Parolini O, Zhang Y, Deng L, et al. Mesenchymal stem/progenitor cells derived from articular cartilage, synovial membrane and synovial fluid for cartilage regeneration: current status and future perspectives. Stem Cell Rev. 2017;13:575–86.
Park YB, Ha CW, Lee CH, Yoon YC, Park YG. Cartilage regeneration in osteoarthritic patients by a composite of allogeneic umbilical cord blood-derived mesenchymal stem cells and hyaluronate hydrogel: results from a clinical trial for safety and proof-of-concept with 7 years of extended follow-up. Stem Cells Transl Med. 2017;6:613–21.
Song JS, Hong KT, Kim NM, Jung JY, Park HS, Lee SH, et al. Implantation of allogenic umbilical cord blood-derived mesenchymal stem cells improves knee osteoarthritis outcomes: two-year follow-up. Regen Ther. 2020;14:32–9.
Cui B, Li E, Yang B, Wang B. Human umbilical cord bloodderived mesenchymal stem cell transplantation for the treatment of spinal cord injury. Exp Ther Med. 2014;7:1233–6.
Pham PV, Vu NB, Pham VM, Truong NH, Pham TL, Dang LT, et al. Good manufacturing practice-compliant isolation and culture of human umbilical cord blood-derived mesenchymal stem cells. J Transl Med. 2014;12:56.
Kim JY, Jeon HB, Yang YS, Oh W, Chang JW. Application of human umbilical cord blood-derived mesenchymal stem cells in disease models. World J Stem Cells. 2010;2:34–8.
Wang M, Yang Y, Yang D, Luo F, Liang W, Guo S, et al. The immunomodulatory activity of human umbilical cord blood-derived mesenchymal stem cells in vitro. Immunology. 2009;126:220–32.
Howard D, Buttery LD, Shakesheff KM, Roberts SJ. Tissue engineering: strategies, stem cells and scaffolds. J Anat. 2008;213:66–72.
Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3:a005058.
Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786–801.
Yin H, Wang Y, Sun Z, Sun X, Xu Y, Li P, et al. Induction of mesenchymal stem cell chondrogenic differentiation and functional cartilage microtissue formation for in vivo cartilage regeneration by cartilage extracellular matrix-derived particles. Acta Biomater. 2016;33:96–109.
Jeon HJ, Yoon KA, An ES, Kang TW, Sim YB, Ahn J, et al. Therapeutic effects of human umbilical cord blood-derived mesenchymal stem cells combined with cartilage acellular matrix mediated via bone morphogenic protein 6 in a rabbit model of articular cruciate ligament transection. Stem Cell Rev Rep. 2020;16:596–611.
Cope P, Ourradi K, Li Y, Sharif M. Models of osteoarthritis: the good, the bad and the promising. Osteoarthritis Cartilage. 2019;27:230–9.
Bendele AM. Animal models of osteoarthritis. J Musculoskelet Neuronal Interact. 2001;1:363–76.
Moran CJ, Ramesh A, Brama PA, O’Byrne JM, O’Brien FJ, Levingstone TJ. The benefits and limitations of animal models for translational research in cartilage repair. J Exp Orthop. 2016;3:1.
Vandeweerd JM, Kirschvink N, Muylkens B, Depiereux E, Clegg P, Herteman N, et al. A study of the anatomy and injection techniques of the ovine stifle by positive contrast arthrography, computed tomography arthrography and gross anatomical dissection. Vet J. 2012;193:426–32.
Proffen BL, McElfresh M, Fleming BC, Murray MM. A comparative anatomical study of the human knee and six animal species. Knee. 2012;19:493–9.
Murphy JM, Fink DJ, Hunziker EB, Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48:3464–74.
Kazemi D, Shams Asenjan K, Dehdilani N, Parsa H. Canine articular cartilage regeneration using mesenchymal stem cells seeded on platelet rich fibrin: macroscopic and histological assessments. Bone Joint Res. 2017;6:98–107.
Hoemann CD, Hurtig M, Rossomacha E, Sun J, Chevrier A, Shive MS, et al. Chitosan-glycerol phosphate/blood implants improve hyaline cartilage repair in ovine microfracture defects. J Bone Joint Surg Am. 2005;87:2671–86.
McIlwraith CW, Frisbie DD, Rodkey WG, Kisiday JD, Werpy NM, Kawcak CE, et al. Evaluation of intra-articular mesenchymal stem cells to augment healing of microfractured chondral defects. Arthroscopy. 2011;27:1552–61.
Levingstone TJ, Ramesh A, Brady RT, Brama PA, Kearney C, Gleeson JP, et al. Cell-free multi-layered collagen-based scaffolds demonstrate layer specific regeneration of functional osteochondral tissue in caprine joints. Biomaterials. 2016;87:69–81.
Xu X, Liang Y, Li X, Ouyang K, Wang M, Cao T, et al. Exosome-mediated delivery of kartogenin for chondrogenesis of synovial fluid-derived mesenchymal stem cells and cartilage regeneration. Biomaterials. 2021;269:120539.
Xia H, Liang C, Luo P, Huang J, He J, Wang Z, et al. Pericellular collagen I coating for enhanced homing and chondrogenic differentiation of mesenchymal stem cells in direct intra-articular injection. Stem Cell Res Ther. 2018;9:174.
Liu Y, Shu XZ, Prestwich GD. Osteochondral defect repair with autologous bone marrow-derived mesenchymal stem cells in an injectable, in situ, cross-linked synthetic extracellular matrix. Tissue Eng. 2006;12:3405–16.
Park YB, Ha CW, Kim JA, Han WJ, Rhim JH, Lee HJ, et al. Single-stage cell-based cartilage repair in a rabbit model: cell tracking and in vivo chondrogenesis of human umbilical cord blood-derived mesenchymal stem cells and hyaluronic acid hydrogel composite. Osteoarthritis Cartilage. 2017;25:570–80.
Park YB, Song M, Lee CH, Kim JA, Ha CW. Cartilage repair by human umbilical cord blood-derived mesenchymal stem cells with different hydrogels in a rat model. J Orthop Res. 2015;33:1580–6.
Kilmer CE, Battistoni CM, Cox A, Breur GJ, Panitch A, Liu JC. Collagen type I and II blend hydrogel with autologous mesenchymal stem cells as a scaffold for articular cartilage defect repair. ACS Biomater Sci Eng. 2020;6:3464–76.
Burnsed OA, Schwartz Z, Marchand KO, Hyzy SL, Olivares-Navarrete R, Boyan BD. Hydrogels derived from cartilage matrices promote induction of human mesenchymal stem cell chondrogenic differentiation. Acta Biomater. 2016;43:139–49.
Rothrauff BB, Yang G, Tuan RS. Tissue-specific bioactivity of soluble tendon-derived and cartilage-derived extracellular matrices on adult mesenchymal stem cells. Stem Cell Res Ther. 2017;8:133.
Yun HW, Song BR, Shin DI, Yin XY, Truong MD, Noh S, et al. Fabrication of decellularized meniscus extracellular matrix according to inner cartilaginous, middle transitional, and outer fibrous zones result in zone-specific protein expression useful for precise replication of meniscus zones. Mater Sci Eng C Mater Biol Appl. 2021;128:112312.
Keane TJ, Swinehart IT, Badylak SF. Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods. 2015;84:25–34.
Zhong Y, Jiang A, Sun F, Xiao Y, Gu Y, Wu L, et al. A comparative study of the effects of different decellularization methods and genipin-cross-linking on the properties of tracheal matrices. Tissue Eng Regen Med. 2019;16:39–50.
Singh S, Afara IO, Tehrani AH, Oloyede A. Effect of decellularization on the load-bearing characteristics of articular cartilage matrix. Tissue Eng Regen Med. 2015;12:294–305.
Khajavi M, Hajimoradloo A, Zandi M, Pezeshki-Modaress M, Bonakdar S, Zamani A. Fish cartilage: a promising source of biomaterial for biological scaffold fabrication in cartilage tissue engineering. J Biomed Mater Res A. 2021;109:1737–50.
Acknowledgements
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: H I20C0233).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Authors declare there is no conflict of interest with the manuscript.
Ethics statement
Animal experiments were approved by the Experimental Animal Ethics Committee of KPC Co., Ltd. (approval no.: P18042).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
13770_2021_407_MOESM2_ESM.tif
Supplementary Figure S2. Clinical analysis of OA symptoms in a goat model (Test 2). A: Main lameness score over the study period. B: Representative radiological images of knee joints of experimental goats at 12 months. C: Radiological score according to the K&L scale at initial, 3, 6, 9 and 12 months. D: Representative gross images of the femoral condyle. The black circle indicates the defects or repaired lesion. E: Comparison of the gross finding scores of the lesion in the affected articular cartilage. F: Microscopic lesions of the articular cartilage in the affected knees of all experimental groups (Scale bars = 1 mm). G: Comparison of the modified OARSI histopathologic scores in the affected articular cartilage. Results represent means ± SEM, *p < 0.05. OA: osteoarthritis, K&L: Kellgren & Lawrence, COL2: Type 2 collagen. (TIF 19941 kb)
13770_2021_407_MOESM3_ESM.tif
Supplementary Figure S3. Histological evaluation of the synthesis of cartilaginous ECM on hUCB-MSCs pellet. A: Protein expression levels of COL2 in CAM Inj.. B: COL2 staining of hUCB-MSCs pellets (Scale bars = 200 μm). ECM; extracellular matrix, hUCB-MSCs: Human Umbilical cord blood-mesenchymal stem cell, COL2: Type 2 collagen. (TIF 27699 kb)
Rights and permissions
About this article
Cite this article
Kim, M., Ahn, J., Lee, J. et al. Combined Mesenchymal Stem Cells and Cartilage Acellular Matrix Injection Therapy for Osteoarthritis in Goats. Tissue Eng Regen Med 19, 177–187 (2022). https://doi.org/10.1007/s13770-021-00407-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-021-00407-3