Abstract
Organ, histotypic, and organotypic culture using mammalian three-dimensional (3D) cell culture technologies are the primary focus of this chapter. The major purpose of organ, histotypic, and organotypic culture is to create in vitro models comparable to the in vivo environment which may replace old, damaged/injured, or diseased organs. Organ culture describes the in vitro maintenance of growth of a part or whole of an organ in which the various tissue components, such as parenchyma and stroma and their anatomical relationship and function(s), are preserved. The methods utilized for organ cultures are plasma clots, agar gels, raft, and grid. The histotypic tissue culture allows cells to be grown in high densities in a 3D matrix or scaffolds, thereby creating in vitro morphologies that closely mimic the realistic in vivo tissue functioning. In organotypic culture, a component of an organ is created using cells from different lineages in proper ratio and spatial relationship under laboratory conditions. Additionally, this chapter describes tissue engineering, an in vitro process that produces entirely functional tissue (closely resembling the natural tissue) by seeding cells into a biomaterial matrix called a scaffold. The scaffolds are made up of either natural biomatrix materials or synthetic materials. The general purpose of tissue engineering is to implant the generated tissue into the body’s replacement of old, damaged/injured, or diseased tissue or organs. Besides the general process of tissue engineering, the benefits and shortcomings of tissue engineering are also included in this chapter.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Organ culture
- Histotypic culture
- Organotypic culture
- Plasma clot method/watch glass method
- Agar gel method
- Raft method
- Grid method
- Tissue engineering
- Scaffolds
- Regenerative medicine
1 Introduction
This chapter describes an overview and importance of organ, histotypic, and organotypic cultures . In a mammalian body, an organ is defined as a specialized structure in a body that is capable to fulfill specialized function(s). Basically, in an organ, various tissues are collectively involved in maintaining the overall structural and functional integrity. The culture of a whole or part of an organ is called organ culture. While in cell culture a particular type of isolated cell or a mixture of isolated cells is cultured, in organ culture, either whole or a part of an organ is cultured. Thus, in organ culture, isolation/separation or disaggregation of tissues/organs into cells is not witnessed. Moreover, cellular differentiation can be monitored in organ culture. It appears that embryonic organ culture is an easier alternative to organ culture derived from adult mammals. Greater oxygen requirement by the adult organ cells may be one of the reasons for their slow growth. The support materials used in organ culture comprise filter-well inserts made up of ceramic, collagen or nitrocellulose, stainless steel grid, semisolid agar gel, clotted plasma, micropore filter, lens paper, or strips of perspex/plexiglass. Four different organ culture methods are described here, namely, (i) Plasma clot method, (ii) Agar gel method, (iii) Raft method, and (iv) Grid method.
For the histotypic culture, (A) Gel and sponge method, (B) Hollow fiber method, (C) Spheroids method, and (D) Multicellular tumor spheroid method are important. In histotypic culture, cell lines are grown in high density in a three-dimensional (3D) matrix. On the other hand, in organotypic culture, cells of different lineages in a proper ratio are cultured in a laboratory condition to create a component of an organ.
The major purpose of organ, histotypic, and organotypic cultures is to create in vitro models comparable to the in vivo system. The words tissue engineering and regenerative medicine are complementary to each other. Scientists from various institutes of regenerative medicine as well as tissue engineering believe that, 1 day, they will be able to grow complete structures of various organs, useful for human transplantation. In summary, this chapter enhances the reader’s overall knowledge of organs, the histotypic and organotypic cultures.
2 The Concept of Organ, the Histotypic, and Organotypic Cultures
-
An organ is defined as a particular specialized structure within a mammalian body, capable of fulfilling specialized function(s).
-
Each organ serves as a separate yet integrative entity in maintaining the overall structural and functional integrity of a mammalian body.
-
The various tissues within an organ are collectively involved in maintaining its overall structural integrity alongside the characteristic function(s).
-
Differentiation at the cellular level has mostly been studied in organ, rather than cell, cultures.
-
In organ culture, the cell-to-cell interaction and formation of a structure closely resemble the in vivo structure, and therefore cells are integrated as a single unit.
-
In organ culture, due to the generation of integrated into vivo-like structure, it is capable of cell-to-cell interaction through adhesion and integrin molecules as well as bidirectional (inside out and outside in) cell signaling.
-
By definition, organ culture describes the in vitro growth maintenance of a part or whole of an organ, wherein various tissue components such as parenchyma, stroma, and their anatomical relationship and function(s) are preserved.
-
Characteristically in an in vitro organ culture, the explanted tissues closely resemble the in vivo parental tissues, retaining their parental ability to allow architecture differentiation or preservation and organ functioning.
-
Of note, an explant is a cell, organ, or piece of tissue, transferred from animals or plants to a nutrient medium for culture and growth.
-
By selecting the suitable culture conditions, the outgrowth of isolated cells from the periphery of the explant is discouraged and minimized.
-
For histotypic culture, the cell lines should be grown in three-dimensional matrices to a high density.
-
In an organotypic culture, various cells having different lineages are cultured in a proper ratio that resembles the in vivo conditions. This produces an organ component.
-
Organ, histotypic, and organotypic culture methods can be analyzed via histology, autoradiography, and immunochemistry.
-
Together organ, histotypic, and organotypic culture techniques enhance our understanding of the development/growth and functional behavior of organs.
-
The organ created may replace the diseased/injured/damaged organs and therefore may be of significant value to Institutes of Regenerative Medicines, as well as tissue engineering (Fell 1953; Borghese 1958; Kahn 1958; Merchant et al. 1964).
3 Organ Culture: Brief History of Mammalian Organ Culture
-
In 1897, Loeb first attempted the organ culture.
-
Loeb generated for the first time a plasma clot by mixing 15 drops of plasma with 05 drops of embryo extract.
-
NB: All the initial studies used chick plasma and chick embryo extract.
-
He took the plasma clot in the test tube and incubated the adult rabbit ovary, thyroid, liver, and kidney and observed that these organs retained their normal histological features for up to 3 days.
-
In the year 1910, a scientist named Burrow for the first time established the chick embryo organ culture outside the body. Burrow used a technique, famously named as “Plasma Clot Method.”
-
Later in 1919, Loeb and Fleischer reported that to prevent central necrosis of the cultured explants, it was necessary to fill the tube with O2.
-
Pioneering works of organ culture technology were reported in 1949 from Hardy during the growth of hair and hair follicles.
-
In 1963, Cleffman studied the pigment formation in the hair-follicle melanocytes of agouti mice.
-
Under organ culture, almost every organ of the mouse has been cultivated in vitro.
-
In organ culture, the whole organs or small fragments of an organ, with their special and intrinsic properties intact, are used for culturing.
-
The adult human organs require more oxygen than their embryonic counterpart, and this may be one of the reasons for the difficulty to culture adult human organs or their parts.
-
This is also one of the reasons for the preferential culture of embryonic organs, rather than adult organs.
-
The specially designed apparatus are used to culture several adult organs using a special medium. The most preferred apparatus is Towell’s II culture chamber.
-
Since serum was found as toxic, serum-free medium (e.g., T8) was used, with the special apparatus (e.g., Towell’s II culture chamber) permitting the use of 95% oxygen.
-
The most important original references in the development of organ culture include the publications from Fell (1953), Borghese (1958), Kahn (1958), and Merchant et al. (1964).
NB: Embryonic organs are easier and faster to grow in the artificial organ culture environment.
4 Factors Affecting Mammalian Organ Culture
Several factors affect the successful culture and growth of organs. These factors must be optimized and maintained for successful organ culture.
-
1.
Gas and liquid phase
-
2.
Structural integrity
-
3.
Blocked differentiation
Here is a sequential presentation of the above factors that affect the organ culture:
4.1 Gas and Liquid Phase
Diffusion of gases and nutrients into the cultured organs may be an issue in successful organ culture. If the organs are considered a solid mass, it would be difficult to exchange gases and nutrients across these solid masses. However, if the cells grow as monolayers there will be an easy transfer of gases, nutrients, etc. Under these conditions, it is necessary to transport the exact amount of gases and nutrients into the cultured cells. The liquid-gas interface incubation of the tissues/organs is necessary for the proper transfer of gases and nutrients.
While incubating in a liquid-gas interface, the following points should be kept in mind:
-
The cells occupy a special shape when growing in a liquid-gas interface at the appropriate depth.
-
The diffusion of gases does not happen if culturing is done at a deeper level of the medium/liquid.
-
As and when the tissues/organs are cultured in the shallow medium, the outgrowth of the cells would occur due to more surface tension. In this case, no accuracy of the culture can be maintained.
-
For ideal growth, O2 is supplied either as hyperbaric O2 or as 95% pure O2.
NB: The limited diffusion of extracellular molecules into thick tissues restricts organ culture to embryonic or thin organs.
4.2 Structural Integrity
Cells in an organ culture must interact in a manner so that the combined effect of cells is the same as an integrated organ, and thus intercellular communication could be sustained.
4.3 Blocking of Differentiation
In general, the induction of differentiation of the cultured tissues and organs may result in a loss of their proper regulation. This may be one of the major reasons for blocking differentiation during tissue/organ culture.
4.4 Support Materials Needed for Organ Culture
-
The organ culture mandates optimization of nutrient and gas exchanges by keeping the tissues at the gas-limited interface.
The support materials used in organ culture are as follows:
-
Semisolid agar gel
-
Clotted plasma
-
Micropore filter
-
Lens paper
-
Perspex or plexiglass strips
-
Stainless steel grid
-
Ceramic, collagen, or nitrocellulose-made filter well insert
The successful use of these inserts leads to the development of functionally integrated various epithelia such as renal epithelium, stratified epidermis, integrated thyroid epithelium, and intestinal epithelium.
5 Sources of Organ Culture
-
Developing chick or any other animal’s embryo.
-
Organs would be isolated from adult animals.
-
Human organs/tissues (e.g., skin, kidney, cornea, etc.) from a voluntary donor.
-
Surgically removed organs of a disease-affected person, intended for transplantation replacement.
-
Human organs would be isolated from an accidentally dead person.
-
Aborted human fetus.
NB: For using human subjects, the suitable ethical committee must permit the work conduct even before taking the consent of the suitable organ donor. Similarly, one must take the permission of the animal ethics committee before sacrificing or even collecting any animal.
6 General Requisition for Organ Culture
A researcher must complete the following steps before commencing the organ culture
-
Complete all the formalities and maintain all the ethical standards before sacrificing animal embryos or adult animals.
-
Similarly, all the ethical norms including the donor’s signed approval, and approval of human organ isolation protocol by a constitutional departmental or institution committee, are necessary before even collecting any organ.
-
The organs to be cultured must be fresh and cultured as soon as possible (within 1–3 h).
NB: Generally frozen or stored organs do not grow and therefore only fresh organs are recommended for culturing.
-
Organs must not be dried before culture. Drying results in loss of growth capacity.
-
Thus, immediately after collection, the organ must be stored in Hanks Balanced Salt Solution (HBSS) or in suitable other salt solution (DPBS/PBS) until being used for culture.
-
Remove the organ from the primary source (e.g., chick embryo/other animal organs, etc.) using a dissection microscope.
-
For humans, only suitable expert professional physicians or technicians are allowed for organ collection.
-
During dissection, the organs must be kept moist with HBSS containing 1–2% serum. Dissection must be performed carefully without damaging the tissues within the organ.
-
Now transfer the dissected properly sized (generally <1 mm) tissue piece from the organ in a cavity slide containing HBSS for its careful washing.
-
Now choose a particular organ culture technique and proceed with the culture procedure.
-
In general, the tissue should be cultured at the gas-medium (liquid) interface.
-
Once the tissue is placed, incubate the culture vessel in a CO2 incubator, with 95% moisture at 37 °C.
-
Change the medium (M199 or CMRL1066, for human organ culture) as frequently as desired.
NB: M199 is a general medium used for various organ cultures.
However, a specific organ or outgrowth such as cancer tissue may need a specialized medium for its growth. For example, a section of the breast cancer tissue may grow well in MEM or DMEM medium.
Four techniques are generally used to analyze the cultured organs:
-
Microscopy for live growing organs
-
Histology
-
Autoradiography
-
Immunohistochemistry
7 Methodologies of Organ Culture
The following four methods are used for organ culture:
-
1.
Organ culture by plasma clot method
-
2.
Organ culture by agar gel method
-
3.
Organ culture by raft method
-
4.
Organ culture by grid method
There are pros and cons of each of the above cultural techniques. Ahead is a brief discussion about them.
7.1 Organ Culture by Plasma Clot Method
-
Fell and Robinson introduced this method to culture the avian limb bone rudiment development.
-
Later on, this method was utilized not only for the growth and differentiation of other avian organs but also for the development of mammalian organs.
-
Before culture, the chick plasma and chick embryo extract are prepared.
-
Now, the chick plasma and chick embryo extract are placed on the surface of a clot that is put in a watch glass. Therefore, this procedure is also famously called as “Watch Glass Technique .”
-
A glass lid sealed with paraffin wax may or may not close the watch glass.
-
In a Petri dish carpeted with moist cotton wool or filter paper, one or two such watch glasses are enclosed.
This method requires chick plasma and chick embryo extract. So, before starting the procedure one must prepare the chick embryo extract.
7.1.1 Preparation of Chick Embryo Extracts
Chick embryo extracts are one of the common materials needed for various organ culture methods.
The materials and methods needed to prepare chick embryo extracts are as follows:
7.1.1.1 Materials for Chick Embryo Extract Preparation
-
Fertilized hen eggs incubated for 7–8 days
-
Petri dishes
-
Straight forceps
-
Curved forceps
-
Curved scissors
-
Homogenizer
-
Glass with teflon pestle
-
Centrifuge tubes (15 and 50 ml)
-
Pasteur pipettes
-
Wide-mouthed pipettes, made via cutting the tops of ordinary pipettes
-
Storage vials
-
Hanks balanced salt solution (HBSS)
-
70% Alcohol
NB: All reagents and instruments must be sterile.
7.1.1.2 Method to Prepare Chick Embryo Extract
The following lines describe the preparation of chick embryo extracts:
-
Soak cotton wool with 70% ethanol.
-
Wipe the blunt end of the egg and sterilize the surface.
-
Tapping the upper end of the shell, crack it and peel inward.
-
Then tear open the inner shell membrane covering the embryo with a pair of straight forceps.
-
Use a pair of curved forceps, lift the embryos out by their heads, and then gently drop the embryos into a Petri dish.
-
Wash the embryo with HBSS.
-
Remove the eyes.
-
Use a pair of curved scissors to mince the embryos by hand.
-
Transfer the minced tissues to a homogenizer.
-
Hand grind the minced tissue for a few minutes.
-
Add an equal amount of HBSS to homogenate and mix it.
-
Centrifuge the mixture at 20 g for 10 min at 4 °C.
-
Remove the supernatant.
-
The final supernatant should be free of cells and appear opalescent.
-
The extract can be used immediately or transferred to vials in 1 ml aliquots for storage at −20 °C.
-
Use within 3 months. Figure 1 summarizes the steps for an easy follow-up.
7.1.2 The Plasma Clot Method of Organ Culture
The plasma clot method has the following steps:
-
Take a watch glass and add 15 drops of chick plasma and 5 drops of chick embryo extract and mix them.
-
Take a Petri dish, put a cotton wool pad, and moisten it.
-
On the top of the moist wool, put the watch glass.
-
The cotton wool would wait as long as the culture continues.
-
Now carefully transfer the washed dissected tissue pieces (1 mm or less, thin slices grow better because of easy nutrients and oxygen transfer) on the top of plasma clots in the watch glass.
-
Place the watch glass in a CO2 incubator at 37 °C (Although, in general, incubation time is up to 4 weeks, it may vary).
-
Allow the organ to grow (Fig. 2).
7.1.3 Advantages of Plasma Clot Method
-
Inexpensive
-
Permits light microscope, hence suitable to study hair growth, fetal mouse skin differentiation, etc.
-
Easy to follow technique
7.1.4 Disadvantages of Plasma Clot Method
-
Clot liquefies in the vicinity of explants, consequently immersing them in the medium.
-
Short culture duration (<4 weeks).
-
The complexity of the medium forbids biochemical inspection.
7.1.5 Application of Plasma Clot Method
-
The major use of the plasma clot method is to study embryonic morphogenesis.
-
The other use of this method is to study the effects of carcinogens, vitamins, hormones, growth factors, etc. on cultured tissues isolated from adult organs.
7.2 Organ Culture by Using the Agar Gel Method
-
Spratt introduced the agar gel method.
-
Later on, Gaillard’s technique was modified by Wolff and Haffen and used agar gel contained in an embryological watch glass.
-
The major use of this technique is morphogenetic and in developmental studies.
7.2.1 Organ Culture by Agar Gel Method Has the Following Steps
-
Make an organ culture medium by mixing three parts of chick embryo extracts with three parts of horse serum.
-
Add seven parts of 1% agar in HBSS into the above medium to assist in solidification.
-
So, the agar gel is prepared by mixing chick embryo extracts: horse serum with 1% agar in HBSS at 3:3:7 stoichiometries.
-
The agar-containing medium is a semisolid material; it does not liquefy. Therefore, it provides mechanical support for the cultured organ(s).
-
Take two cataract knives, and with the help of them transfer the organ to the agar gel. Alternatively, transfer via sucking them up in a wide-mouthed pipette with HBSS, and finally depositing them on the agar.
-
Use two needles to orient the explants on the agar.
-
Use a fine pipette to suck off the excess fluid.
-
Usually, each gel accommodates one explant.
-
“The optimum size of the explant is (1.5 × 1.5 × 1) mm, with the maximum size being (2 × 2 × 1.5) mm. However, if the tissue is very thin (<0.5 rams thick), the other dimensions can be larger.”
-
To seal the glass lids onto the chambers, take a small paint brush and apply at least two warm paraffin wax (60 °C) coats.
-
Now transfer the chamber to the CO2 incubator and incubate at 37 °C.
-
NB: Instead of putting the chamber directly on the metal shelves of the incubator, it should be put using polystyrene foam tile. This will insulate the chamber from the metal tiles.
-
Figure 3 summarizes the steps for an easier follow-up.
7.2.2 Note the Following
-
While embryonic tissues/organs grow very well using agar, the adult organs do not survive in this medium. This may be related to the more oxygen requirement of the adult tissues.
-
Some of the experimental observations such as morphogenetic changes and cytodifferentiation require only 3–7 days of culture. These experiments are completed within 3–7 days and do not require any changes in the cell culture medium.
-
In case of longer duration experiments, the explants mandate a relocation to a fresh agar gel every 5–7 days. In this case, fresh chambers are used to make a fresh gel with a defined medium and the same proportion of agar as used before.
-
To transfer the explants from the old gel to the new gel, first remove the lids from the old watch glass using a warm razor. Now take two cataract knives or one cataract knife and a dissecting needle and lift off the explants from the old gel. Wash the explants in HBSS containing a cavity slide. Transfer the washed explants to fresh gel. Finally, using a small paint brush and paraffin wax seal the watch glasses with a fresh sterile glass lid (Fig. 4).
-
For examining the viability of the cultured explants, they can be viewed using daylight or via a light source from the dissecting binocular. While the translucent and shiny surface tissues indicate good health of the cultured explanted tissues, opacity suggests loss of viability or the necrosis initiation of the explanted tissue.
7.3 Organ Culture by Raft Method
-
The initial works were carried out by Chen and associates. They used cleaning microscope lenses (Gurr) that are nonwettable and float on the fluid medium. A 25 × 25 mm raft of lens paper which was floated on serum in a watch glass was used by Chen et al. to put four to five explant cultures.
-
It was Richter who improved the floating properties of this methodology via lens paper treatment with silicone.
-
Further modification of the lens paper was done by Lash and accomplices, who combined the lens paper with the Millipore filters.
-
In the process, the Millipore filter covers a small hole that was punched in the center of the lens paper.
-
It was claimed that either side of the filters is suitable for the culture of different cells.
-
It was Shaffer who replaced the lens paper with rayon acetate.
-
Further modifications of the rayon acetate strips were done by several scientists to make them float on the cell culture medium.
-
For this process, the four corners of the rayon acetate strips were treated with silicone.
-
Since the rayon acetate is easily soluble in acetone, it can be dissolved during the histological procedures via acetone immersion.
7.3.1 Organ Culture by Raft Method Has the Following Steps
-
Take a watch glass.
-
Put serum into the watch glass.
-
Float a raft of lens paper or rayon acetate on the serum. Rayon acetate rafts are made to float on the serum via silicone treatment of their corners.
-
Similarly, the flowability of lens paper gets enhanced on silicone treatment. On each raft, usually, four or more explants are placed.
-
A combination of clot and raft techniques can be utilized for explants culture. To offer the combination, the explants are first placed on a suitable raft before their placement on a plasma clot.
-
The medium changes will be easy because of this modification, and it also prevents the explants from sinking into liquefied plasma.
7.3.2 Major Drawback of the Raft Method
The floating of a raft on a fluid medium does not provide an ideal condition since the raft often sank with the tissue in varying medium depths. Thus, the main objective of the raft method, i.e., floating on the medium with the tissue cannot be fulfilled (Fig. 5).
7.4 Organ Culture by Grid Method
-
In 1954, the grid method was introduced by Trowell.
-
The name indicates the use of metal grids, instead of the raft which overcomes the difficulties faced by the raft method.
-
Initially, tantalum wire gauze was used to make the grid.
-
Later on, stainless steel or titanium was used that made a continuous, expanded, rigid raft.
-
In the original culture method, Trowell used adult mammalian tissues/organs and perfused the tissue with carbon dioxide and oxygen.
-
He was able to preserve the histological structure as well as the viability of various adult human tissues such as kidneys, prostate glands, pituitary gland, and thyroid gland.
-
At present, the technique has been simplified (particularly the gas phase) and is still in use.
7.4.1 Steps in Organ Culture Using Grid Method
-
To prepare a grid, 25 × 25 sq.mm pieces of a suitable wire mesh or perforated stainless steel sheet are used. Further, the edges of this sheet are bent to form four legs of about 4 mm height (Fig. 6).
-
The placing of the tissues on the grid is completed either by directly keeping the tissues on the grid or if the tissues are soft, first they are put on a raft, and then the raft is kept on the grid.
-
Now the culture chambers are used to put the gird into them.
-
In the next step, the culture chambers are filled with the medium up to the GRID level.
-
The chambers are infused with both oxygen and carbon dioxide to meet the demand of the adult tissues (the embryonic tissues do not need the infusion of oxygen and carbon dioxide).
7.4.2 The Advantages and Disadvantages of the Grid Method
NB: It is of fundamental interest to understand how cells build tissues and organs. However, the long timescale of mammalian development and its location deep within an opaque animal restrict most organogenesis investigations to comparisons of fixed sections from different animals.
-
Most of the three-dimensional (3D) cultures were initially developed for direct observation of the development process.
-
The mechanism of organ formation is difficult to understand due to the use of a large number of heterogenic cells with diversified morphology and functions.
-
Due to a lack of understanding of the development trajectory, it is difficult to understand the mechanistic implications of mutant phenotypes.
7.4.2.1 Advantages of Grid Method
-
The method allows separate handling of skeletal and soft tissues, wherein the former are directly placed on the grid but softer tissues like glands or skin are initially put on rafts and thereafter transferred to the grids. This provision preserves the tissue-specific viability for a long duration and helps to systemize the monitored response.
-
To meet the high oxygen requirement of adult mammalian organs, the grids are placed in a culture chamber to which O2 and CO2 are provided.
-
Several modifications of the standardized grid method are primed for studying the development and differentiation of adult and embryonic tissues.
7.4.2.2 Disadvantages of Grid Method for Organ Culture
-
Mandates handling by skilled manpower.
-
Costly method due to high dependence on culturing conditions.
-
There is no provision to simultaneously culture the organs from two distinct tissues, often encountered with contrasting growth and development prospects.
8 Histotypic Cultures of Cells/Tissues
-
The histotypic tissue culture allows the cells to be grown in high densities in 3D matrix or scaffolds, thereby creating in vitro morphologies closely mimicking realistic tissue functions.
-
In this procedure, only single cells are grown over 3D scaffolds.
-
Using the histotypic culture, it is possible to use dispersed monolayers to regenerate tissue-like structures.
-
The most important aspect of the histotypic culture is the growth and propagation of cell lines in the 3D matrix to yield a high cell density (Maslow and Mayhew 1974; Caffe et al. 1989).
8.1 Method of the Histotypic Culture
The basic material needed for the histotypic cell culture is the primary cells, directly isolated from a fresh organ. The culture should enable the creation of a matrix or scaffold conferring the cells a 3D architecture, resembling the natural extracellular matrix (ECM). Other materials needed for the histotypic culture are general requirements such as medium, CO2 incubator, etc.
The chronological steps in the histotypic culture are as follows:
-
1.
Isolation of cells from organs
-
2.
Creation of a 3D scaffold
-
3.
Droplet/cell suspension technique to seed the cells into a 3D scaffold
-
4.
Incubation and culture of cells in scaffold
-
5.
Use of the cultured scaffolds
-
6.
Cautions and assets of the histotypic culture practices
Here is a brief discussion of the above steps:
8.1.1 Isolation of Cells from Organs
-
Sterilization of the work area with 70% ethanol.
-
Autoclave/sterilize the dissecting instruments.
-
UV sterilize and clean the biosafety cabinet with 70% ethanol.
-
Place a sterile drape on the work surface.
-
Then put the sterile surgical instruments on the sterile drape.
-
Open a sterile number 20 scalpel blade.
-
Euthanize the specimen.
-
When ready, put the specimen in a surgical tray.
-
Aseptically isolate the specific organ(s) from the specimen.
-
Organs can be collected from the experimental animals or discarded lots from the transplanted surgery or accidental death-meeting persons after fulfilling all the ethical rules and regulations.
-
Once the fresh organ is collected, it should be soon subjected to cell isolation either via proteolytic digestion or mechanical disintegration.
-
However, proteolytic digestion is the most widely used procedure, the details of which have already been discussed in various chapters including chapter “Isolation and Primary Culture of Various Mammalian Cells,” and therefore are not described here.
8.1.2 Creation of a Three-Dimensional Scaffold
For 3D culture, designing a scaffold is necessary on which cells are seeded to grow. The growing cells closely mimic the natural native morphology and facilitate the development of intercellular networks and communication pathways.
-
A variety of 3D polymer networks, including gel and sponge methods such as hydrogels, matrigel, and electrospun silk mats, offer convenient methods for 3D cultures of tissue-specific cells.
-
Leighton was the first to demonstrate that both normal and malignant cells penetrate cellulose sponge. The used gel or sponge provides the matrix for morphogenesis and cell growth. The cells penetrate these gels and sponges while growing.
-
One of the highly used 3D scaffold materials is collagen gel. This collagen gel provides the matrix for the culture of various mammalian cells such as epithelial cells. The morphogenesis of the cells and establishment of tissue-like morphology are possible using this type of scaffold. It was observed that the kidney-epithelial cells (MDCK), if grown in collagen gel, respond to paracrine stimulation from fibroblasts via forming tubular structures.
-
Since various 3D scaffolds are already discussed in the previous chapter (chapter “Mammalian Cell Culture in Three Dimensions: Basic Guidelines”), they are not discussed here.
8.1.3 Droplet/Cell Suspension Technique to Seed the Cells into a Three-Dimensional Scaffold
-
Once the cells are isolated and a scaffold is ready, the cells are seeded using the droplet technique or cell suspension technique.
-
The droplet technique involves pipetting a cell solution onto the scaffold at a slow and constant rate.
-
In the cell suspension technique, the scaffolds are submerged in a cell suspension. The shaking of the scaffolds and the cell suspension may encourage cell migration into the matrix.
-
The bioengineered constructs with high cell densities are the products of both techniques.
8.1.4 Incubation and Culture of Cells into Scaffold
-
One can prepare a scaffold using any of the above materials. After the scaffold preparation, the cell seeding is optimally initiated.
-
Take one 96-well mammalian cell culture plate.
-
Aseptically transfer one sterile scaffold per well.
-
Immediately add 400 μl cell culture medium to each well.
-
Incubate in a CO2 incubator for 30 min to equilibrate the spheroid with the medium.
-
Aspirate the excess medium.
-
Add the cell suspension to be cultured on the spheroids.
-
Incubate the plate in a CO2 incubator overnight to attach the cell to the scaffold.
-
Next day, carefully remove the nonattached cells and 200 μl fresh culture medium per well.
-
Allow the porous scaffold to be grown at a high cell density.
8.1.5 Use of the Cultured Scaffolds
-
The purpose of the histotypic culture is to create a model system that closely resembles the in vivo physiological system and therefore cellular microenvironment. This 3D model system is utilized to study the behavior of the cells under various conditions. For example, to understand the process and mechanism of differentiation as well as regeneration of the stem cells, 3D scaffolds can be used. The stem cells grown in 3D scaffolds would give significant information about the various aspects of stem cell biology.
-
The 3D scaffolds may be experimental subjects to various mechanical loads equivalent to that of natural tissues in the in vivo environment.
-
The compression and biaxial loads applied during the cell growth may provide a preconditioned bioengineered tissue scaffold with a cellular structure resembling the native tissue.
-
The major use of the scaffolds with engineered tissues is to replace or repair the damaged or defected tissues. The scaffolds (with engineered tissues with developed vasculature) are implanted into the defective region of the body and allow that region to grow.
-
Gradually the scaffolds would be degraded and replaced by the newly formed natural matrix by the implanted, engineered tissues (Eberly et al. 2009).
8.1.6 Assets and Cautions of the Histotypic Culture
-
Development of a cell line over several generations.
-
Scale-up feasibility.
-
Absolute control of the physical environment.
-
Sample homogeneity.
-
The low nutrient content is needed compared to that of animal models.
8.1.6.1 Cautions
-
Cells may lose some differentiated characteristics.
-
Cells are hard to maintain in the histotypic culture.
-
Provides small tissue growth at a high cost.
-
De-differentiation possibility.
-
Instability/aneuploidy.
9 Organotypic Culture of Cells/Tissues
-
In an organotypic culture, various cells having different lineages are cultured in a proper ratio and spatial relationship that resemble the in vivo conditions. This produces an organ component under laboratory conditions.
-
So, the difference between organ and organotypic culture is that the former involves a part or section of the original organ for culturing in in vitro conditions without separating any cells from the organs. On the contrary, organoid culture involves culturing of the entire organ.
-
Whereas in organotypic culture, the various cells isolated from the organs are cultured separately under the in vitro conditions before being mixed in a proper ratio and cultured over a 3D scaffold to grow and form an original organ-like structure.
-
Additionally, the organ culture endows survival till 3 weeks as usual, but it does not allow the feasibility of further propagation.
So, for organotypic culture the following knowledge is highly essential:
-
1.
Proper complex architecture of an organ.
-
2.
Various constituent cells in an organ with their specific functions.
-
3.
The ratio at which all the cells are present in an organ.
-
4.
Type of common matrix preferred by all constituent cells of an organ.
-
5.
Ability to culture the cells separately to get sufficient cell population in proportions having correct matrix or scaffolds.
-
The basic purpose of the organotypic culture is to synthesize/construct a tissue/organ equivalent and to understand cell-to-cell crosstalk and cell-matrix crosstalk under both physiology and pathophysiology.
-
To simulate a tissue equivalent or multilayered cell culture, various methods can be used such as via monolayer perfusion (Kruse et al. 1970) to a complex perfused membrane (Klement et al. 1987) or capillary beds (Knazek et al. 1972).
-
For organotypic culture, cells have to be separated/disaggregated and purified from organs, then mixed in a proper ratio to culture in 3D scaffolds.
-
The best success in organotypic culture came from synthesizing the skin equivalent (Michel et al. 1999; Schaller et al. 2002; Regnier et al. 1997; Laning et al. 1999).
9.1 Organotypic Cultures in Epidermal Research
-
Skin tissues are one of the most exposed tissues of the human body. For various reasons, human skin can damage and needs to be replaced, as in burn patients. Additionally, epidermal disorders including melanoma and psoriasis are among the most prevalent, often life-threatening, alongside having mechanistically complex pathologies.
-
In its very basic form, the skin consists of a collagen-rich stroma dominated by fibroblasts and topped with a stratified epidermis. So, the major cells present in the skin are fibroblasts impregnated into stratified epidermis and keratinocytes. Other cells like melanocytes, Merkel cells, and Langerhans cells are also present.
-
The key step in the skin reconstruction by the self-assembly approach is to use fibroblasts capable of secreting a mature ECM and keratinocytes being associated with one another to form a stratified, differentiated epidermis.
-
Determining the most efficient way for extracting multiple cell types from a single cutaneous biopsy is to incubate the biopsy sample with different proteolytic enzymes at varying temperatures and time durations.
-
Organotypic culture of skin cells can efficiently replicate the 3D region within which the dermatological cells can survive and function normally, proportionate to in vivo environment. The usual commencement of epidermal organotypic culture happens with the addition of fibroblast support cells to the vacant scaffolds. These scaffolds could vary from the freshly fabricated collagen gel in the laboratory to the manifold commercial-grade matrices, and even the skin dermis priorly eliminated off its native cells. The transferred fibroblasts are provided an optimum time to enrich the scaffold, after which the epidermal keratinocytes are seeded over as the topmost layer. Henceforth, stratified differentiation of keratinocytes is triggered via upfronting the scaffold above the air-liquid interface (Oh 2013).
-
As soon as a culture technique is activated, the behavior of the human epidermis can be analyzed and assessed under diversified diseased conditions. For example, Ridky and colleagues (2010) engineered the oncogene-powered epidermal neoplasia by replacing the normal epidermal cells with virally transduced cells (amid an organotypic culture) for overexpression of mutant cell cycle proteins.
-
This created a way for a bypassing of normal cell checkpoint mechanisms by the transduced epidermal cells, resulting in mimicking of genetic manipulations frequently witnessed in the spontaneous in vivo malignant transformation of human epidermal cells. Such an in vitro epidermal neoplasia model made way for reproducing the basement membrane invasion, a prominent step in the in vivo metastasis of epidermal cancer cells.
-
The rapidity and ease of understanding the establishment of an organotypic culture under the standard laboratory conditions propel them as the optimum system to study in the research projects aimed toward understanding the signaling mechanisms and monitoring the therapeutic efficacy of multiple drugs for human epidermal disorders.
-
In their analysis, Ridky and associates (2010) also demonstrated the capability of epidermal neoplasia organotypic culture for a discrete validation of cancer inhibitors based on their potential to construct the basement membrane invasion. The same conditions were also found suitable to screen the anticancer potential of soluble peptides, neutralizing antibodies, or small hairpin RNAs.
-
Several other cells (apart from fibroblasts and keratinocytes) could also be screened and evaluated concerning the skin organotypic culture. For instance, adding normal or malignant melanocytes can provide knowledge about the mechanisms of epidermal pigmentation or melanoma progression (Eves et al. 2000). Furthermore, the immune cells such as macrophages can be supplemented to the genotypically cultured scaffolds to analyze the more complex in vivo epidermal-dermal-immune signaling in the in vitro conditions (Bechetoille et al. 2011).
-
Besides the skin, some other organs have also been modeled (grown) in organotypic cultures, such as prostate and prostate cancer, breast and breast cancer, and lung and lung cancer.
-
Some important research attempts in the field of organotypic culture include human esophageal epithelial cell 3D culture (Kalabis et al. 2012), tubular organotypic culture human kidney model (Jun et al. 2018), and organotypic brain slices culture (Humpel 2015).
10 Organotypic Culture Techniques and Their Merging
To overcome the various methods used in organotypic culture, a combination of different methods and the development of “organ on a chip” have been made. In this method, the microfluidic technologies on a chip approaches deliver a constant steady-state culture medium flow to the tissues.
At present, reconstituted endothelial cells with microvascular development by bioengineering create tremendous hope to understand microvascular biology.
As and when these networks are allowed to anatomize the pre-engineered microfluidic channels, this approach can support in vivo perfusion and physical scaling of OCS size, presently significantly limited owing to the passive diffusion inefficiency. (Sakaguchi et al. 2013)
10.1 Benefits of Organotypic Culture
-
Human cells can be utilized for organotypic culture. Otherwise, the direct use of human subjects for experimental purposes is not only unsafe but also unethical.
-
Organotypic culture helps to understand cell-to-cell crosstalk, cell-to matrix interaction, the complex behavior of various cells within a tissue, and cell signaling including bidirectional signaling (inside out and outside in the cells).
-
It may be useful for understanding the developmental mechanism of various disease conditions.
-
The efficacy and therapeutic potential of various experiments can be enhanced using the organotypic culture (Shamir and Ewald 2014).
10.2 Limitations of Organotypic Culture
-
It is yet to develop a complete organ using organotypic culture. Therefore, the recapitulation of only a part of a normal organ may not reflect the overall structure and functions of an organ.
-
The inefficient nutrient supply to the organotypic culture limits the size of an organotypic culture.
-
In vitro study of diseased states requires analyzing tissues from disease-affected donors.
-
In the present scenario, it is very challenging in terms of integration, analysis, modeling, and ultimately application of the data generated in such a complex three-dimensional culture model.
-
It is essential to receive approval from specific patients to isolate induced pluripotent stem cells for the efficacy and testing of human-relevant drugs and other molecules (Esch 2015).
-
At present, computational approaches are employed to reliably extrapolate the tissue model responses to the whole organism and provide testable predictions (Mortensen et al. 2016).
11 Benefits of Organ, the Histotypic and Organotypic Culture
The followings are the benefits of organ, histotypic, and organotypic cultures:
-
1.
Organ culture provides a better and more accurate reflection of an organism’s physiology.
-
2.
Organ, histotypic, and organotypic cultures help to develop the behavioral interactions between cells within an organ, their growth characteristics in three-dimensional matrixes, or the process of organ development from simple cells. The aim of these culturing configurations involves the creation of in vitro models comparable to the in vivo system.
-
3.
Organ culture is necessary because it is impossible to study various in vivo cellular interactions occurring in isolated cells or cultures.
-
4.
Metabolism of the cells within an organ can also be studied using organ culture.
-
5.
Organ cultures are also used to examine the effects of various drugs, cytokines, chemokines, toxins, and other experimental agents that limit the use of animal sacrifice.
-
6.
This type of culture helps to understand the development of the human tissues and organs including growth patterns, differentiation, and morphogenesis. In this case, the various biochemical factors that influence proliferation, differentiation, and morphogenesis can be characterized.
-
7.
The functions of these cultured tissues/organs under both physiology and pathophysiology can be examined.
-
8.
It may give some clues about the developmental anomalies of various organs.
-
9.
These cultures considerably reduce the number of experiments necessary for culturing whole animals to investigate a given problem.
-
10.
Scientists from various Regenerative Medicine Institutes anticipate the growth of the complete structure of various organs, useful for human transplantation.
The most important organs on the verge of experimental development are as follows:
-
A jaw bone by Columbia University.
-
A lung by Yale University.
-
Beating rat heart by Minnesota University.
-
A kidney by Michigan University.
-
Heart perfusion w/stem cells created a “new heart” by the University of Minnesota in 2007.
12 Limitations of Organ, Histotypic, and Organotypic Culture
The success rate of developing a complete organ by organ culture is very much limited due to the following reasons:
-
1.
The major and most important limitation of organ culture is the lack of proper vasculature in the growing organ. Since nutrients and oxygen do not diffuse properly to the core or center of the organ, these cells eventually die via necrosis.
-
2.
Another major limitation of organ culture is its nonpropagated property. Every time experimenting, one needs to start with a fresh sample; therefore, reproducibility of the data is difficult and expenditure cost is high.
-
3.
Finally, experimental results of in vitro cultured organs with drugs, toxins, and other agents are often not comparable to those from in vivo studies (e.g., studies on drug action) since the drugs are metabolized in vivo.
-
4.
Despite all these limitations, organ culture is one of the most important research domains, notably for basic developmental biology, regenerative medicine, transplantation immunology, and pharmaceutical biochemistry.
-
5.
Newer culture approaches, such as 3D bioprinting, organoids, or organs-on-a-chip attempt to improve the replication of tissue microenvironment, although limited success at present promises a big hope for the future.
13 The Concept of Tissue Engineering and Regenerative Medicine
-
A commonly applied definition of tissue engineering as stated by Langer and Vacanti is as follows:
“An interdisciplinary field that applies the principles of engineering and life sciences towards the development of biological substitutes capable of restoring, maintaining or improving functions of biological tissues or an organ, as a whole.”
-
Tissue engineering is an in vitro process that produces functional tissue (closely resembling the natural tissue) by seeding cells into a biomaterial matrix, called scaffolds before subsequent implanting of generated tissue in the body.
-
For incorporation into scaffolds, remodeling is necessary but stress-induced architecture cannot be remodeled. In this process, since the tissue is generated in vitro, there is every possibility of checking its viability under the in vitro conditions. As a multidisciplinary field, tissue engineering combines biology, biochemistry, clinical medicine, and materials science to achieve clinically targeted results (Fig. 7).
-
In contrast, regenerative technology generally denotes implantation of the biomaterial matrix with or without seeded cells into scaffolds, directly into the body to facilitate in vivo regeneration of the damaged/injured/diseased tissue.
-
In this process, the incorporation and formation of tissues are under the influence of endogenous regulators (including mechanical strain).
-
Additionally, in this process, since the cells are directly seeded into the body, there are no chances of a prior experiment regarding the feasible or infeasible tissue effectiveness under the in vivo conditions.
-
Moreover, enough chances are there that the implanted tissue may be dislodged or degraded by mechanical stresses under the in vivo conditions.
-
For repairing the damaged tissues, the complications associated with the conventional organ donation procedure can be compensated by tissue engineering and regenerative medicine.
-
This technology is used to create cells that aid in the healing of diseased or damaged organs.
-
At present, tissue engineering becomes an alternative in clinical medicine.
-
While at present, through tissue engineering it is not possible to generate a complete organ, the ultimate target is to synthesize various organs completely that could be utilized to better the survivability chances (Langer and Vacanty 1993; Meyer et al. 2009; Lanza et al. 2011; Li et al. 2016).
The section on tissue engineering has been discussed in the following parts:
-
1.
The usefulness of tissue engineering and its applications
-
2.
History of tissue engineering
-
3.
Elements of tissue engineering
-
4.
The process of tissue engineering
-
5.
Current status of tissue engineering
The above points are discussed in the subsequent paragraphs:
13.1 The Usefulness of Tissue Engineering and Its Applications
In today’s world, tissue engineering is a necessity because of the following reasons:
-
In today’s world, there is a large demand for the supply of donor organs. However, the supply of donated organs seldom matches that of the requirement. Under this condition, tissue engineering may play a very important role in the supply of engineered organs.
-
Since other available therapies such as synthetic prostheses, drug therapy, surgical reconstruction, and medical devices are not always successful, tissue engineering may be very useful.
-
Congenital abnormalities require tissue engineering.
-
Most tissues cannot regenerate following a disease or injury.
-
Even tissues that regenerate spontaneously may not be entirely efficient.
-
Permanent implants have a lot of success but also carry numerous problems.
-
Tissue engineering holds promise for the generation of better transplantable organs.
-
It is claimed that, shortly, tissue engineering may be useful against various genetic defects.
-
The major goal of tissue engineering is the in vitro construction of various tissues and organ parts.
13.1.1 Tissue Engineering May Have the Following Additional Applications
-
Tissue engineering has diagnostic applications and is utilized as a biosensor.
-
At present, tissue engineering is used to repair or replace tissues such as urinary bladder, heart valve, blood vessels, bone, cartilage, etc.).
-
The subjects of tissue engineering and regenerative medicine are closely interlinked and aim collectively at the development of functional cells, tissues, and organ substitutes to repair, replace, or enhance the lost biological function(s) to congenital abnormalities, injury, disease, or aging. Thus, the regeneration of tissues is one of the most important applications of tissue engineering.
-
The engineered tissues can be used as a biosensor.
-
Drug development is a complex process, comprising screening of novel molecules, identification of genes as potential targets, evaluation of the drug metabolism, uptake, and possible toxicity risk.
-
Tissue engineering employs living cells as potential engineering entities. For example, artificial skin that comprises viable fibroblasts, cartilage repaired with living chondrocytes, or several other cells that could be engineered via specific routes.
-
A befitting case pertains to engineered heart valves made using tissue engineering for an amicable replacement of their nonfunctional states. This is mediated by bypassing the limitations confronted with currently used bioprosthetic and mechanical heart valves. Tissue-engineered skin enables noted improvements in wound healing, taking care of the limitations of autograft usage.
The general usefulness of tissue engineering is as follows:
-
Helps a person conquer a disease or illness.
-
Fewer surgeries are needed by the person being treated.
-
No or fewer rejection chances.
-
Organ failure sufferers do not have to wait for an organ donor.
-
The need for organ donation after death could be eliminated.
-
This technology could lead to even greater and better future technologies.
-
The technique provides a permanent solution for tissue problems.
13.1.2 Major Drawbacks and Hurdles of Tissue Engineering
-
A lot of research work is needed to contract a tissue because it is very difficult to construct tissues. Cells have to stay alive inside the body and should continue to function, a criterion that is optimized with significant difficulty for complex organs.
Some limitations of successful tissue engineering are as follows:
-
Size of defect, e.g., the bone does not regenerate in large defects.
-
The collapse of surrounding tissues to the defect is often visualized. For example, periodontal defects.
-
Excessive strains in the reparative tissue are often noticed. For instance, the unstable nature of fractures (Trowell 1961).
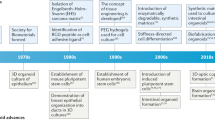
13.2 History of Tissue Engineering
-
In 1970, the early experiments of tissue engineering were demonstrated by W. T. Green amid an attempt to generate cartilage. For his experiment, Dr. Green used chondrocytes and seeded these cells onto spicules of bone before finally implanting them into nude mice. Although unsuccessful, Dr. Green’s experiment paved the way to generate new biomaterials for seeding the cells in tissue engineering.
-
In the 1980s, doctors Burke and Yannas of the Massachusetts General Hospital and M.I.T. used collagen matrix for the growth of human dermal fibroblasts both in a laboratory experiment and in human model studies.
-
Dr. Howard Green later transferred keratinocyte sheets onto burn patients.
-
Additionally, Dr. Eugene Bell seeded collagen gels with fibroblasts, referring to them as contracted collagen gels.
-
All these examples represent seeds of the new discipline, now known as “Tissue Engineering.”
-
In 1984, Wolter and Meyer first used the term tissue engineering while working on an endothelium-like layer on PMMA in the eye.
-
Additionally, in 1988 Prof. Robert Nerem at a symposium at the University of California Los Angeles (UCLA) used the term tissue engineering for the first time.
-
In the mid-1980, the research works of Dr. Joseph Vacanti and Dr. Robert Langer significantly progressed the field of tissue engineering by publishing some important papers.
-
At this juncture, the major domains in which Dr. Vacanty worked include the design and implementation of functional tissue equivalents. For this purpose, he used synthetic biocompatible/biodegradable polymer comprising branching network, configured as scaffolds seeded with viable cells. Interestingly, in 1988 at a meeting of the American College of Surgeons, Vacanti and colleagues published significant findings in the area of tissue engineering, which was published in Surgery.
-
In 1991, Cima, Vacanti, and Langer cultured chondrocytes on a PGA scaffold, in the ears of the nude mouse.
-
Henceforth, in 1993, the publication of Vacanti and associates in Science provided comprehensive knowledge about tissue engineering.
-
In the field of tissue engineering, several specialized centers developed in the USA and Europe. In the early 1990s, Peter Johnson established Pittsburgh Tissue Engineering Initiative (PTEI).
-
Similarly, Robert Nerem at the Georgia Tech laboratories developed cardiovascular tissue engineering.
-
Erstwhile in the USA, the other tissue-engineering laboratories were developed at Rice University in Houston and the UMass Medical School.
-
In the UK, Dr. Julia Polak at the Imperial College of London organized a British-origin society that formed a loose association with the Tissue Engineering Society (TES), being previously incorporated in Boston.
-
In the same 1990 period at Giesen, Germany, Dr. Una Chen started tissue engineering and stem cell research.
-
Dr. Clemente Ibarra at the National Institute for Rehabilitative Medicine in Mexico formed the Mexican Tissue Engineering Society and founded laboratories for tissue engineering.
-
In 1994, Brittberg and Peterson published a paper in the New England Journal of Medicine (NEJM) on the implantation of human autologous chondrocytes.
-
A tissue-engineering laboratory was established by Dr. Wolfgang Pulacher in Innsbruck at the Leopold Institute. At the same time, the establishment of laboratories by the combined efforts of Germany, Switzerland, and Southern France (Dr. Raymund E. Horch and Dr. G. B. Stark) gained swift momentum at the University of Freiburg. The combined efforts resulted in the formation of the German Tissue Engineering Society in Western Europe.
-
It was by the late 1990s, that Dr. R. Hetzer (a cardiovascular surgeon at the University of Berlin) and Dr. Christof Brelsch (a liver transplant specialist in Hamburg) collaborated with the Children’s Hospital in Berlin as also with Dr. Koichi Tanaka’s group from the University of Kyoto.
-
As far as Asian participation is concerned, Dr. Minora Ueda of Nagoya University, Japan, set up a large Tissue Engineering facility. This happened during the first meeting of the Japanese Tissue Engineering Society (1997) in Nagoya.
-
Inceptive efforts from China for Tissue Engineering were also encouraged by their Governmental grant, founded by Dr. Yi Lin Cao in Shanghai (Vacanti 2006; Vacanti and Vacanti 2007; Berthiaume et al. 2011).
13.3 Elements of Tissue Engineering
Mammalian tissues are composed of the following materials:
13.3.1 Cells for Tissue Engineering
Implanted and cultured cells are capable of creating new tissues.
13.3.2 Insoluble Extracellular Matrix
Various biomaterials act as a scaffold or matrix, holding the cells and supporting growth.
13.3.3 Soluble Molecules That Serve as Regulators of Cell Functions
Biological signaling molecules instruct cells to form desired tissue types (Fig. 8).
Ahead is the brief discussion of the above three components:
13.3.3.1 Cells for Tissue Engineering
Cells are the major components of tissue engineering. Cells may be originated from various sources (Naderi et al. 2011; Caplan 2007).
Based on their sources, cells are divided into the following types:
-
A.
Autologous cells
-
B.
Allogeneic cells
-
C.
Xenogenic cells
-
D.
Isogenic/syngeneic cells
-
E.
Primary cells
-
F.
Secondary cells
Here is a brief discussion of the above cell types:
13.3.3.1.1 Autologous Cells
These cells are obtained from the same individual to which they are reimplanted. In the use of these cells, there is no issue of rejection. The availability of cells is a major concern here.
The main advantages of autologous cells in tissue engineering are as follows:
-
Avoiding immune complications
-
Reduction in the possible transfer of inherent infections
There are certain disadvantages associated with autologous cells :
-
It is not always possible to obtain sufficient biopsy material from the patient.
-
Diseased state and patient age are the often encountered limiting factors.
13.3.3.1.2 Allogeneic Cells
These cells originate from a donor body within the same species. This infers that if the cells are taken from a person other than the patient, the source is often allogeneic. While there are some ethical constraints about in vitro utility of human cells, the employment of dermal fibroblasts from the human foreskin has been demonstrated as immunologically safer (Wystrychowski et al. 2014).
The main advantages of allogenic cell source are as follows:
-
Obtained in good quantity from a healthy donor.
-
Can be cultured on a large scale.
-
Cost-effective with consistent quality.
-
Available, as and when required by a patient.
The major problem of allogeneic cell sources pertains to immunological complications that may ultimately lead to graft rejection. The immune responses, however, are variable depending on the used cells. For instance, endothelial cells are more immunogenic than fibroblasts and smooth muscle cells. The age of the donor is another important factor that contributes to immunological complications. The cells from adult donors are highly immunogenic, while fetal or neonatal cells elicit little or no immune response.
13.3.3.1.3 Xenogenic Cells
These cells are isolated from individuals of another species. In particular, animal cells are quite extensively used in experiments aimed at the design of cardiovascular implants.
13.3.3.1.4 Isogenic/Syngeneic Cells
These cells are isolated from genetically identical organisms, such as twins, clones, or highly inbreed research animal species. Cells from immunologically compromised syngeneic mice could also be used experimentally.
13.3.3.1.5 Primary Cells
Any kind of cells originated from whatever source on being cultured for the first time.
13.3.3.1.6 Secondary Cells
These cells come from a cell bank or subsequent culture of the primary cultured cells.
Based on potency, the cells are divided into the following types :
-
Stem cells
-
Totipotent cells
-
Pluripotent cells
-
Multipotent cells
-
Oligopotent cells
-
Unipotent cells
Here is a brief discussion of the above cell types:
Stem Cells
-
These cells are undifferentiated with an ability to divide in culture and give rise to different specialized cells. According to their source, stem cells are divided into the following types.
Totipotent Cells
-
These cells can proliferate and differentiate into a complete body. A totipotent cell can give rise to all extraembryonic tissues, along with all body tissues including those of the germline.
Pluripotent Cells
-
Pluripotent stem cells are cells having the self-renewal capacity by dividing and developing into the three primary germ cell layers of the early embryo and therefore into all cells of the adult body, but not extraembryonic tissues, like the placenta.
Multipotent Cells
-
Multipotent stem cells are cells possessing the self-renewal capacity by dividing and developing into multiple specialized cells present in a specific tissue or organ. Most adult stem cells are multipotent stem cells.
Oligopotent Cells
-
The oligopotent cells are the progenitor cells that have the limited ability or potency to differentiate into only a few kinds of cells, for example, lymphoid or myeloid stem cells.
Unipotent Cells
-
“The word ‘uni,’ meaning itself, is derived from the Latin word ‘unus,’ meaning one.” Thus, this kind of progenitor cell has a very limited capacity to differentiate into only one cell type, i.e., lowest differentiation potential.
13.3.4 Insoluble Extracellular Matrix
-
The extracellular components of ECM support the attachments or adhesions and stabilize the cells in tissue architecture, thereby helping in their growth.
-
An ECM consists of various proteins including collagen, laminin, fibronectin glycoprotein, and proteoglycan.
-
The prominent receptor molecules of the intracellular membrane receptors that interact with the ECM and send bidirectional signals between cells and ECM are integrins. These are the proteins that function mechanically, by attaching with cell cytoskeleton to the ECM and biochemically, by sensing the adhesion. The integrin family of proteins consists of α and β subtypes, collectively forming transmembrane heterodimers (Table 1) (Williams 2019).
13.3.5 General Characteristics of the Extracellular Matrix
The ECM has the following general characteristics:
-
In the ECM, various molecules (including cell-adhesive/cell-signaling molecules) are interconnected to form complex large structures and therefore possess low diffusion ability.
-
The ECM molecules are capable of bidirectional signaling (inside out and outside in) across the cells.
-
These molecules influence the various physiological events of the cells that include differentiation, apoptosis, migration, etc.
13.3.6 Scaffolds
-
Biochemically, the scaffolds comprise either natural biomaterials such as collagen or synthetic materials such as polyglycolic acid. Both these materials are porous and absorbable.
-
Technically, scaffolds are artificial structures suitable to support the three-dimensional culture and mammalian cell growth.
-
Scaffolds are used to guide, organize, grow, and differentiate the cells amid the formation of functional tissue. Scaffolds also facilitate the delivery of both physical and chemical signals to the growing cells (Sultana 2003).
-
The details about the scaffolds are already discussed in the previous chapter (chapter “Mammalian Cell Culture in Three Dimensions: Basic Guidelines”).
13.3.7 Soluble Molecules That Serve as Regulators of Cell Functions
-
Signaling molecules that help in the growth of cells in a scaffold are divided into three distinct groups, as ahead.
-
Growth and differentiation factors such as PDGF, IGF, TGBF, fibroblasts growth factors (FGFs), Bone Morphogenetic Factors (BMFs), etc.
-
ECM proteins and attachment factors.
-
Mediators of bone metabolism.
13.4 The Process of Tissue Engineering
The purpose of tissue engineering is to generate a 3D construct that is structurally, mechanically, and functionally similar or even may be better than its normal/natural tissue counterparts. The process involves the creation of natural/synthetic cell culture support or scaffolds with a biodegradable essence to grow mammalian cells in a 3D configuration.
The process of tissue engineering involves the following steps:
-
A.
Culture of isolated cells.
-
B.
Preparation of scaffolds from various biomaterials: scaffold fabrication.
-
C.
Seeding of scaffold with living cells.
-
D.
Bathe scaffold with growth factors.
-
E.
Cell multiplication, scaffold filling, and growth into three-dimensional tissue.
-
F.
Implant the generated tissue inside the body.
-
G.
The intended natural functions of tissue would be recreated by the constituent cells.
-
H.
The tissues will be attached and connected with the new blood vessels.
-
I.
Gradually and slowly, the scaffolds would be degraded.
-
J.
Eventually, the newly grown tissue blends with the surrounding tissues.
-
Very briefly, specific mammalian cells are isolated and cultured. Simultaneously, scaffolds are synthesized. Thereafter, the scaffolds are seeded with the desired cells before being bathed with nutrients, growth factors, etc. for adequate cell growth. Cells are, thereafter, allowed to grow in scaffolds, simultaneously acting as 3D tissues. Finally, the generated tissue would be implanted into the mammalian body.
-
The methods adopted for culturing the cells in tissue engineering depend on the cell type and functions. For most cells, the conventional monolayer cultures either in Petri dishes or T flasks serve the purpose.
-
The major drawback of monolayer cultures is that the cells lose their morphology, functions, and proliferative capacity after several generations.
-
Some workers prefer 3D cultures for the cells in tissue engineering using large-scale culture containers such as bioreactors.
-
The nutrient and gaseous exchanges may be limiting factors in 3D cultures. Therefore, proper standardization of the cell culture medium is necessary.
13.4.1 Cell Orientation
The orientation of cells about specific shape and spatial arrangement is influenced by the following environmental factors:
-
Substrate or contact guidance
-
Chemical gradients
-
Mechanical cues
13.4.2 Substrate or Contact Guidance
The topographical features of the substrate determine the contact guidance. These features may be in the form of ridges, aligned fibers, etc. It is possible to use differential attachment mode to substrates, as the means of producing different cell alignments. In recent years, synthetic polymer substrate, collagen fibrils, and fibronectin have been used as bioresorbable templates for tissue engineering.
13.4.3 Chemical Gradients
The development of chemical gradients is required for cellular orientation and the stimulation of cellular functions. Certain growth factors and extracellular macromolecules are capable of creating chemical gradients, e.g., vascular endothelial growth factor (VEGF), oligosaccharide fragments of hyaluronan, fibronectin, and collagen. There are certain practical difficulties in maintaining the optimum chemical gradients for the cells in 3D cultures. This is particularly the limiting factor when the cells become dense.
13.4.4 Mechanical Cues
The response of cells to mechanical signals is complex and may result in any one or more of the following:
-
Changes in the cell alignment.
-
Deformation of the cytoskeleton.
-
Altered matrix formation.
-
Synthesis of regulatory molecules (e.g., growth factors, hormones).
There are mainly three mechanical cues governing cell populations
-
Tensional forces
-
Compression forces
-
Shear forces
13.4.5 Design and Engineering of Tissues
The following surgical criteria are taken into consideration while dealing with tissue engineering:
-
Rapid restoration of the desired function
-
Ease of fixing the tissue
-
Minimal patient discomfort
For the proper conduct of tissue engineering, the source of donor cells plays a decisive role. The use of patients’ cells (autologous cells) eliminates multiple immunological complications. Allogeneic cells are also used, particularly when the tissue engineering construct is designed for a temporary repair. It is observed that when the cells are cultured and/or preserved (i.e., cryopreservation), the antigenicity of allogeneic cells is reduced. Another important criterion in tissue engineering is the support material, its degradation products, cell adhesion characteristics, and mechanical cues (Caddeo et al. 2017).
The design and tissue engineering concerning skin, urothelial, and peripheral nerve are briefly described hereunder.
13.4.6 Tissue-Engineered Organ Types
13.4.6.1 Tissue-Engineered Skin
It was first demonstrated in 1975 that human keratinocytes could be grown in the laboratory in a form suitable for grafting. Many improvements have since been made, enabling the growth of epithelial cells and producing a continuous sheet that progressed to form carnified layers.
The major difficulty encountered while tissue engineering skin is the isolation of the dermal layer possessing blood capillaries, nerves, sweat glands, and other accessory organs. The recent past has witnessed some developments for generating implantable skin substitutes, regarded as tissue-engineered skin constructs (Bell et al. 1981).
13.4.6.2 Integra
This is a bioartificial material composed of collagen-glycosaminoglycan. Integra is not a true tissue-engineering construct. It is mainly used to carry the seeded cells.
13.4.6.3 Dermograft
This is composed of poly (glycolic acid) polymer mesh seeded with human dermal fibroblasts derived from neonatal foreskins.
13.4.6.4 Apigraft
This has human dermal fibroblasts seeded into the collagen gel. A layer of human keratinocytes is then placed on the upper surface. The tissue constructs described above have a limited shelf life (about 5 days). However, they can integrate into the surrounding normal tissue and form a good skin cover. To date, there is no evidence of immunological complications with tissue-engineering constructs.
13.4.7 Tissue-Engineered Urothelium
It is now possible to culture urothelial cells and bladder smooth muscle cells. This raises the hope for the feasibility of tissue-engineering urothelium construction. Considerable success has been reported in the development of a functional bladder in dogs.
For this purpose, a poly (glycolic acid) polymer base was shaped into a bladder with an outer surface coating of muscle cells. The luminal surface (i.e., inner surface) is coated with precultured urothelial cells. The bladder constructed in this way functioned almost like a normal one and worked successfully even after being maintained for a year.
13.4.8 Tissue-Engineered Peripheral Nerve Implants
Peripheral nerve injury is a common hallmark of trauma and tumor resection surgery, lending significant feasibility to irreversible muscle atrophy. Therefore, the repair of injured peripheral nerves assumes significance.
A diagrammatic representation of the basic design of a peripheral nerve implant is depicted in Fig. 9 .
The regeneration of the injured nerve occurs from the proximal stump to rejoin at the distal stump. The regeneration is guided by three distinct substances.
Conduct Materials
-
This is the outer layer and is the primary guidance source. Conduct material is composed of collagen-glycosaminoglycan, PLGA (poly lactic-co-glycolic acid), hyaluronan, and fibronectin. All these moieties are bioresorbable.
Filling Materials
-
This supports the neural cells for regeneration, besides guiding the regeneration process. Filling material comprises collagen, fibrin, fibronectin, and agarose.
Additives
-
Additives include a large number of growth factors, neurotrophic factors (in different forms, varying combinations), e.g., fibroblast growth factor (FGF), and nerve growth factor (NGF). Additions of Schwann cells or transfected fibroblasts promote the nerve generation process.
Tissue Modeling
-
Research is in progress to create tissue models as artificial organs. Some recent developments in experimental tissue modeling are briefly outlined.
Artificial Liver
-
Hepatocytes, cultured as spheroids or hepatocytes, and fibroblasts, cultured as heterospheroids, can be used. They are held in artificial support systems such as porous gelatin sponges, agarose, or collagen. The addition of exogenous molecules is useful for the long-term liver cell culture. Considerable progress has been reported in creating artificial liver as evident from the three-dimensional hepatocyte structure and metabolic functions.
Artificial Pancreas
-
Spheroids of insulin-secreting cells are being developed from mouse insulinoma beta cells. Some investigators implanted fetal islet-like cell clusters under the mice’s kidneys, although the functions were not encouraging due to limited oxygen supply.
Pituitary Gland
-
Multicellular spheroids were created to study certain hormonal releases, e.g., luteinizing hormone (LH), following stimulation by luteinizing hormone-releasing hormone (LHRH). Some success has also been achieved to create spheroids for melatonin generation.
Thyroid Gland
-
Thyroid cell spheroids can be used for studying cell adhesion, motility, and thyroid follicle biogenesis.
Brain Cell Culture
-
Three-dimensional brain cell cultures are in use for the study of neural myelination and demyelination, neuronal regeneration, and lead neurotoxicity. Aggregated brain cells are also used for the study of Alzheimer’s and Parkinson’s diseases.
Heart Cell Culture
-
Aggregated heart cells are in use for the study of cardiac development and physiology. Important research publications in the development of various mammalian organs, through the process of tissue engineering, include the mammalian urinary bladder (Oberpenning et al. 1999), vagina (De Filippo et al. 2003), intestinal tissues (Chen and Beierle 2004), airway (Macchiarini et al. 2008), bone (Porter et al. 2009), musculoskeletal (Nie et al. 2012), myocardial tissue (Vunjak Novakovic et al. 2014), liver (Lin et al. 2015), and many more tissues and organs.
13.4.9 Current Status of Tissue Engineering
Currently, the success rate of tissue engineering is limited.
The following limitations of tissue engineering are presently observed:
-
Full regeneration of tissues that do not regenerate spontaneously has not yet been achieved.
-
Lots of successes in bone tissue engineering.
-
Tissue-engineered skin has no glands and hair.
-
Engineered cartilage is not articulate.
13.4.10 Current Technology Review
The success of some of the companies in the tissue-engineering section is discussed in the following paragraphs:
13.4.10.1 The Mattek Corporation
-
In 1985, two scientists from the Massachusetts Institute of Technology (MIT), USA, founded the MatTek Corporation , a tissue-engineering company.
-
This company produced a human tissue model of the skin, ocular and respiratory systems.
-
Soon, these tissue systems became popular throughout the pharmaceutical and cosmetic industries.
-
Until recently, MatTek Corporation produces more than 13 products of which the most successful is the EpiDerm system, an in vitro cell-based skin tissue system.
-
Now 80% of skin transplantation-based products employ EpiDerm.
13.4.10.2 The Hurel Corporation
Hurel Corporation, North Brunswick, is a New Jersey, USA-based company. The most promising product of Hurel is its hepatic cell coculture which is already in use by Merck company. These engineered cells are specifically produced for rigorous industrial use relying on the cells’ manufacturability, ruggedness, convenience, and replicability. This coculture model is now useful for industrial testing of drugs and other molecules, their metabolism along with toxic effects on the hepatic cells. In addition, hepatic cocultures are utilized in the areas such as time-based dynamics of metabolites, clearance rates of compounds, and timed, repeat dosing for monitoring acute and chronic toxicities. Hopefully, with the advancement of this coculture technology, animal sacrifice for drug testing would be significantly reduced.
13.4.10.3 The Modern Meadow
This company was founded in 2011. This company proposed a new approach “to culture meat based on bio-printing, a computer-controlled assembly method for making meat tissue” (Mauney et al. 2005; Sharma et al. 2019).
14 Conclusion
Organ, the histotypic and organotypic cultures, and tissue engineering principles and techniques are briefly described in this chapter. Collectively, the basic purpose of these in vitro techniques is to produce various mammalian tissues or part of an organ or a complete organ, comparable to in vivo organs of the mammalian body. These tissues or organs could be used to replace old, damaged or injured, and diseased mammalian organs. While tremendous progress has been achieved in tissue engineering and the development of various organs using 3D scaffold procedures, there is still a long way to go for developing a complete mammalian organ. Intensive efforts in the field, 1 day, could enable the development of various mammalian organs, offering immense help to mankind and society.
References
Bechetoille N, Vachon H, Gaydon A, Boher A, Fontaine T, Schaeffer E, et al. A new organotypic model containing dermal-type macrophages. Exp Dermatol. 2011;20:1035–7.
Bell E, Ehrlich H, Buttle D, Nakatsuji T. Living tissue formed in vitro and accepted as skin-equivalent tissue of full thickness. Science. 1981;211:1052–4.
Berthiaume F, Maguire TJ, Yarmush ML. Tissue engineering and regenerative medicine: history, progress, and challenges. Annu Rev Chem Biomol Eng. 2011;2:403–30.
Borghese E. Organ differentiation in culture. In: McElroy WD, Glass B, editors. A symposium on the chemical basis of development. Baltimore: Johns Hopkins Press; 1958. p. 704–73.
Caddeo S, Boffito M, Sartori S. Tissue engineering approaches in the design of healthy and pathological in vitro tissue models. Front Bioeng Biotechnol. 2017;5:40.
Caffe AR, Visser H, Jansen HG, Sanyal S. Histotypic differentiation of neonatal mouse retina in organ culture. Curr Eye Res. 1989;8:1083–92.
Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341–7.
Chen MK, Beierle EA. Animal models for intestinal tissue engineering. Biomaterials. 2004;25:1675–81.
De Filippo RE, Yoo JJ, Atala A. Engineering of vaginal tissue in vivo. Tissue Eng. 2003;9:301–6.
Eberly D, Filho LF, Atala A, Yoo JJ. Composite scaffolds for the engineering of hollow organs and tissues. Methods. 2009;47:109–15.
Esch EW. Organs-on-chips at the frontiers of drug discovery. Nat Rev Drug Discov. 2015;14:248–60.
Eves P, Layton C, Hedley S, Dawson RA, Wagner M, Morandini R, et al. Characterization of an in vitro model of human melanoma invasion based on reconstructed human skin. Br J Dermatol. 2000;142:210–22.
Fell HB. Recent advances in organ culture. Sci Progr. 1953;162:212–31.
Humpel C. Organotypic brain slice cultures: a review. Neuroscience. 2015;1:86–98.
Jun DY, Kim SY, Na JC, Lee HH, Kim J, Yoon YE, et al. Tubular organotypic culture model of human kidney. PLoS One. 2018;13:e0206447.
Kahn RH. Organ culture in experimental biology. Univ Michigan Med Bull. 1958;24:242–52.
Kalabis J, Wong GS, Vega ME, Natsuizaka M, Robertson ES, Herlyn M, et al. Isolation and characterization of mouse and human esophageal epithelial cells in 3D organotypic culture. Nat Protoc. 2012;7:235–46.
Klement G, Scheirer W, Katinger HW. Construction of a large scale membrane reactor system with different compartments for cells, medium and product. Dev Biol Stand. 1987;66:221–6.
Knazek RA, Gullino P, Kohler PO, Dedrick R. Cell culture on artificial capillaries. An approach to tissue growth in vitro. Science. 1972;178:65–7.
Kruse PF Jr, Keen LN, Whittle WL. Some distinctive characteristics of high density perfusion cultures of diverse cell types. In Vitro. 1970;6:75–8.
Langer R, Vacanty JP. Tissue engineering. Science. 1993;260:920–6.
Laning JC, DeLuca JE, Hardin-Young J. Effects of immunoregulatory cytokines on the immunogenic potential of the cellular components of a bilayered living skin equivalent. Tissue Eng. 1999;5:171–81.
Lanza R, Langer R, Vacanti JP. Principles of tissue engineering. Cambridge, MA: Academic Press; 2011.
Li Z, Xie MB, Li Y, Ma Y, Li JS, Dai FY. Recent progress in tissue engineering and regenerative medicine. J Biomater Tissue Eng. 2016;6:755–66.
Lin C, Ballinger KR, Khetani SR. The application of engineered liver tissues for novel drug discovery. Expert Opin Drug Discovery. 2015;10:519–40.
Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023–30.
Maslow DE, Mayhew E. Histotypic cell aggregation in the presence of cytochalasin B. J Cell Sci. 1974;6:651–63.
Mauney JR, Volloch V, Kaplan DL. Role of adult mesenchymal stem cells in bone tissue engineering applications: current status and future prospects. Tissue Eng. 2005;11:5–6.
Merchant DJ, Kahn RH, Murphy WH. Handbook of cell and organ culture. 2nd ed. Minneapolis: Burgess; 1964. p. 263.
Meyer U, Meyer T, Handschel J, Wiesmann HP. Fundamentals of tissue engineering and regenerative medicine. Berlin: Springer; 2009.
Michel M, L’Heureux N, Pouliot R, Xu W, Auger FA, Lucie G. Characterization of a new tissue-engineered human skin equivalent with hair. In Vitro Cell Dev Biol Anim. 1999;35:318–26.
Mortensen NP, Mercier KA, McRitchie S, Cavallo TB, Pathmasiri W, Stewart D, et al. Microfluidics meets metabolomics to reveal the impact of Campylobacter jejuni infection on biochemical pathways. Biomed Microdevices. 2016;18:51.
Naderi H, Matin MM, Bahrami AR. Review paper. Critical issues in tissue engineering: biomaterials, cell sources, angiogenesis and drug delivery systems. J Biomat Appl. 2011;26:383–417.
Nie H, Lee C, Tan J, Lu C, Mendelson A, Chen M, et al. Musculoskeletal tissue engineering by endogenous stem/progenitor cells. Cell Tissue Res. 2012;347:665–76.
Oberpenning F, Meng J, Yoo JJ, Atala A. De novo reconstitution of a functional mammalian urinary bladder by tissue engineering. Nat Biotechnol. 1999;17:149–55.
Oh JW. Organotypic skin culture. J Invest Dermatol. 2013;133:e14.
Porter JR, Ruckh TT, Popat KC. Bone tissue engineering: a review in bone biomimetics and drug delivery strategies. Biotechnol Prog. 2009;25:1539–60.
Regnier M, Staquet MJ, Schmitt D, Schmidt R. Integration of Langerhans cells into a pigmented reconstructed human epidermis. J Invest Dermatol. 1997;109:510–2.
Ridky TW, Chow JM, Wong DJ, Khavari PA. Invasive three-dimensional organotypic neoplasia from multiple normal human epithelia. Nat Med. 2010;16:1450–5.
Sakaguchi K, Shimizu T, Horaguchi S, Sekine H, Yamato M, Umezu M, et al. In vitro engineering of vascularized tissue surrogates. Sci Rep. 2013;3:1316.
Schaller M, Mailhammer R, Grassl G, Sander CA, Hube B, Korting HC. Infection of human oral epithelia with Candida species induces cytokine expression correlated to the degree of virulence. J Invest Dermatol. 2002;118:652–7.
Shamir ER, Ewald AJ. Three-dimensional organotypic culture: experimental models of mammalian biology and disease. Nat Rev Mol Cell Biol. 2014;15:647–64.
Sharma P, Kumar P, Sharma R, Bhatt VD, Dhot PS. Tissue engineering: current status & futuristic scope. J Med Life. 2019;12:225–9.
Sultana N. Scaffolds for tissue engineering. MRS Bull. 2003;28:301–6.
Trowell OA. Problems in the maintenance of mature organs in vitro. Colloq Int Centre Nat Res Sci. 1961;101:237–54.
Vacanti CA. The history of tissue engineering. J Cell Mol Med. 2006;10:569–76.
Vacanti JP, Vacanti CA. The history and scope of tissue engineering. In: Principles of tissue engineering, vol. 1. Cambridge, MA: Academic Press; 2007. p. 3–6.
Vunjak Novakovic G, Eschenhagen T, Mummery C. Myocardial tissue engineering: in vitro models. Cold Spring Harbor Perspect Med. 2014;4:1–15.
Williams DF. Challenges with the development of biomaterials for sustainable tissue engineering. Front Bioeng Biotechnol. 2019;7:127.
Wystrychowski W, McAllister TN, Zagalski K, Dusserre N, Cierpka L, Heureux L. First human use of an allogeneic tissue-engineered vascular graft for hemodialysis access. J Vasc Surg. 2014;60:1353–7.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 Springer Nature Singapore Pte Ltd.
About this entry
Cite this entry
Malik, P., Mukherjee, T.K. (2023). Organ, Histotypic and Organotypic Culture, and Tissue Engineering. In: Mukherjee, T.K., Malik, P., Mukherjee, S. (eds) Practical Approach to Mammalian Cell and Organ Culture. Springer, Singapore. https://doi.org/10.1007/978-981-19-1731-8_14-2
Download citation
DOI: https://doi.org/10.1007/978-981-19-1731-8_14-2
Received:
Accepted:
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-1730-1
Online ISBN: 978-981-19-1731-8
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences
Publish with us
Chapter history
-
Latest
Organ, Histotypic and Organotypic Culture, and Tissue Engineering- Published:
- 20 January 2023
DOI: https://doi.org/10.1007/978-981-19-1731-8_14-2
-
Original
Organ, Histotypic and Organotypic Culture, and Tissue Engineering- Published:
- 21 December 2022
DOI: https://doi.org/10.1007/978-981-19-1731-8_14-1