Abstract
Angiogenesis represents one aspect in the complex process that leads to the generation of the vascular tumor stroma. The related functional constituents include responses of endothelial, mural, bone marrow-derived, and resident inflammatory cells as well as activation of coagulation and fibrinolytic systems in blood. Multiple molecular and cellular effectors participate in these events, often in a tumor-specific manner and with changes enforced through the microenvironment, genetic evolution, and responses to anticancer therapies. To capture various elements of these interactions several surrogate assays have been devised, which can be mechanistically useful and are amenable to quantification, but are individually insufficient to describe the underlying complexity and are best used in a targeted and combinatorial manner. Below, we present a survey of angiogenesis assays and experimental approaches to analyze vascular events in cancer. We also provided specific examples of validated protocols, which are less described, but enable the straightforward analysis of vascular structures and coagulant properties of cancer cells in vivo and in vitro.
Access provided by CONRICYT – Journals CONACYT. Download protocol PDF
Similar content being viewed by others
Key words
- Angiogenesis
- Tumor microenvironment
- Coagulation
- Endothelial cells
- Endoglin
- Pericytes
- Alpha smooth muscle actin
- Proliferation
- Tissue factor
1 Introduction
The vascular microenvironment defines many essential features of the neoplastic process including metabolic exchange, cellular trafficking, hormonal communication, angiocrine niche effects, metastasis , and paraneoplastic syndromes such as thrombosis and cachexia [1–3]. It is important to consider that vascular responses to tumor growth and dissemination are complex and form a continuum comprising hemostatic, inflammatory, and vessel wall-related mechanisms. While the term “angiogenesis ” is often used to describe these events globally, the specific meaning is restricted to vessel formation from pre-existing blood vessels , which is but a fragment of the vascular milieu [3].
The multistep nature of angiogenesis is now understood in considerable molecular detail including the key regulators and cellula r events involved [3]. Thus, pre-existing quiescent capillary endothelial cells (phalanx cells) receive stimulatory signals exemplified by the gradient of vascular endothelial growth factor (VEGF) surrounding hypoxic or transformed cells. To this stimulus, endothelial cells respond by activation of key signaling pathways involving VEGF receptor 2 (VEGFR2) , NOTCH/DLL4, ephrins, integrins, and other effectors resulting in the formation of structures known as angiogenic sprouts . These events begin with structural rearrangements in the capillary vessel wall including enlargement of the vascular diameter resulting in the formation of “mother vessels,” followed by dropout of pericytes surrounding the endothelial tube, focal proteolysis of the endothelial basement membrane, and directional invasion of endothelial cell cohorts (sprouts) in the direction of the stimulus [3, 4]. Each of these formations consists of a leading single endothelial tip cell (high expressor of VEGFR2 and DLL4), followed by migrating columns of stalk cells, some of which exhibit mitotic activity. The sprout extension eventually leads to anasthomosis with other sprouts/vessels to form a complete loop, a transition mediated by myeloid cell populations, and followed by the establishment of the new lumen, resumption of blood flow, and ultimately an increase in vascular density. Thus endothelial cell division, survival, migration, and morphogenesis involved in different steps of these processes could serve as surrogates of angiogenic activity and frequently represent the basis of the respective assays (Tables 1, 2, and 3) [5–7].
However, several key elements of tumor neovascularisation are not captured by these measurements. For example, tumor cell access to blood vessels may occur through non-angiogenic processes such as vasculogenesis , the recruitment of endothelial progenitor cells to form elements of the endothelial or perivascular milieu. In addition, processes of vessel splitting, vascular cooption, vasculogenic mimicry, endothelial transdifferentiation of cancer cells, and vessel invasion may contribute to the vascular networks in cancer [8]. Components of the circulating blood such as plate lets, fibrin, and coagulation factors as well as their receptors such as tissue factor (TF) in perivascular and cancer cells also contribute in various ways to blood vessel formation, and functionality. For example, in human glioblastoma, the high expression of TF coincides with exuberant neovascularisation, intraluminal thrombotic vaso-occlusion, and regions of hypoxia [9–11]. Thus, coagulant properties of cancer and stromal cells may profoundly alter the vascular microenvironment. Moreover, larger vessels, external to the tumor mass, may adapt to and facilitate the demands of the intra-tumoral vascular networks through processes of arteriogenesis and arterio-venogenesis [4, 12, 13]. Therefore, measurement of angiogenic responses is often insufficient to understand the salient features of the vascular milieu associated with cancer.
While providing detailed experimental protocols for diverse processes involved in tumor neovascularization exceeds the scope of this chapter, we include the synopsis of the relevant literature and methods in Tables 1, 2, 3, and 4, for reference. In this regard, several assays have been generated for in vitro (Table 1), in vivo (Table 2), and ex vivo (Table 3) studies of angiogenesis and of related vascular processes (Table 4), in response to test substances and conditions (e.g., anti-angiogenic and pro-angiogenic factors, or tumor cells). The simplicity of these assays must, however, be balanced with their combinatorial use and careful interpretation, as they capture only small fragments of the underlying biology, and they often do not reflect the distinctive and heterogeneous properties of angiogenic circuits and regulators in different molecular tumor subtypes [14]. The protocols included in this chapter describe immuno-localization of endothelial cells (Subheading 3.1), mural cells /pericytes (Subheading 3.1), and coagulant/tissue factor-expressing cells in tissue sections in situ, as well as quantitative characterization of tissue factor activity of cultured cells.
Immuno-localization of endothelial cells in paraffin sections of tumor tissue can be accomplished using several lineage markers, such as CD31 , CD34, vWF, lectin binding, and tumor endothelial markers (TEMs) [15, 16]. Here, we describe the use of anti-CD105 (endoglin ) staining of mouse tumor tissue sections, as a robust and effective strategy to highlight tumor blood vessels [17]. In this protocol, CD105 is combined with staining for Ki-67 to highlight proliferating endothelial cells specifically [13, 15, 18].
The dynamics of the tumor neovascularization process and responses to antiangiogenic therapies can be inferred from staining for markers of endothelial cells (above), basement membrane, and mural cells such as pericytes and smooth muscle cells [19, 20]. In particular, the tight pericyte coverage of endothelial tubes along with structural changes in the vascular pattern often signifies vessel maturation or normalization [3]. Here, we describe the combined use of anti-CD105 (endoglin) staining of endothelial cells and anti-alpha smooth muscle actin (aSMA) staining in mouse tumor tissue sections to reveal the changes in vessel maturation [17].
Tissue factor (TF) is a transmembrane receptor for the coagulation factor VII/VIIa, which is expressed on perivascular cells and is often upregulated by cancer cells, activated endothelium, inflammatory cells, or stroma [21]. TF renders cancer cells pro-coagulant and mediates their angiogenic and metastatic responses through fibrin deposition, or intracellular signaling transduced by protease-activated receptors and integrins [22, 23]. The expression of TF by cancer cells is often reflective of the degree and pathway of malignant transformation as documented in glioblastoma, medulloblastoma, and other malignancies [10, 14, 24]. Here, we describe an effective protocol to stain tumor sections for TF antigen.
The expression of TF by cancer cells is often indicative of their malignant transformation [22] and their ability to respond to stimulation upon exposure to TF ligands such as coagulation factor VII/VIIa. These interactions play a role in the regulation of gene expression [25], as well as pro-angiogenic, pro-inflammatory, invasive, and growth responses of cancer cells upon exposure to effectors of the coagulation system [14, 26]. Detection of tissue factor pro-coagulant activity (TF-PCA) represents a sensitive method to measure the level of biologically active TF on the surface of cancer cells. In this assay, TF is allowed to bind to activated VIIa and convert inactive coagulation factor X to an active form (Xa), which can be measured by conversion of the S-2765 chromogenic substrate . Here, we describe a simple and effective protocol to measure TF PCA using cultured cancer cells.
2 Materials
Reagents may be obtained from a number of suppliers with the exception of specific validated antibodies or products, as indicated. The compatibility with formalin -fixed tissues may differ between different antibodies which may result in high background and low specificity. High-quality deionized water should be used to prepare all the indicated buffers and solutions. The procedures require access to general wet lab space, basic histology equipment (tissue processing and embedding stations, slides) cell culture and microscopy facilities.
-
1.
Slide dewaxing reagents: 100 % Xylene and 99 % ethanol should be diluted as described for the intended use in immunostaining protocols.
-
2.
Buffers: Dulbecco’s phosphate-buffered saline (DPBS, used as supplied) and Tris-buffered saline (TBS , pH 7.6) should contain the final concentrations of 20 mM Tris and 150 mM NaCl . For Tris-buffered saline (TBS, pH 7.4), prepare the solution containing 50 mM Tris, 120 mM NaCl, 2.7 mM KCl, and 3 mg/mL BSA.
-
3.
Tissue permeabilization, antigen unmasking, and blocking reagents: For permeabilization 0.3 % Triton X-100 is prepared in DPBS. Vector Antigen Unmasking Solution (Vector Labs Burlington, ON) containing 1 % citrate is prepared by mixing 2.5 mL of the supplied stock with 247.5 mL of distilled water. Hydrogen peroxide (H2O2) should be diluted to 1.5 % final concentration in TBS. Bovine serum albumin (BSA) for tissue blocking is prepared at 1 % concentration by dissolving 0.1 g of BSA powder in a total final volume of 10 mL DPBS. Blocking rabbit serum is prepared at 10 % concentration in DPBS. For blocking tissue normal donkey serum (NDS) is used at the final concentration of 5 %, prepared by diluting 200 μL of NDS stock in 4 mL of DPBS.
-
4.
Primary antibodies : Immunostaining procedures described herein are sensitive to the antibodies used and we have used the following products: Anti-mouse Endoglin /CD-105 Affinity Purified Goat IgG (AF1320; R&D Systems, Minneapolis, MN, USA; used at 1:100 dilution); Anti-Ki-67 rabbit polyclonal antibody (RM-9106, ThermoFisher, Ottawa, ON, Canada; used at 1:200 dilution); Rabbit Anti-Alpha Smooth Muscle Actin Antibody (ab5694; ABCAM, Toronto, ON, Canada; used at 1:100 dilution); Sheep Anti-human TF Antibody (SATF-IG; Affinity Biologicals, Ancaster, ON, Canada; used at 1:100 to 1:10,000 dilution).
-
5.
Secondary antibodie s: Chicken Anti-goat, Alexa Flour 488, IgG, H + L (A21467; Life Technologies Inc., Burlington, ON, Canada; dilution 1:200) and Donkey Anti-rabbit Alexa Fluor 594 IgG, H + L (A21207; Life Technologies, Eugene, OR, USA; dilution 1:200).
-
6.
Immunohistochemistry reagents, and slide-mounting media: Vectastain Elite kit (PK-4006) and ImPACT DAB (SK-4105), VectaMount Mounting Medium (H-5000) from Vector Labs, Burlington, ON, Canada.
-
7.
TF-PCA reagents and supplies: The assay requires cell culture supplies (cell culture media, cell culture-grade trypsin/ethylenediaminetetraacetic acid (EDTA ) solution, plasticware including 24-well culture plates, pipettes, biosafety cabinet), and a timer. The key assay reagents include Factor VIIa (FVIIa ; ADG407act), Factor X (FX; 527), both from Sekisui Diagnostics (Lexington, MA, USA), 2 mM solution of Chromogenic Substrate (S-2765; Chromogenix, Mississauga, ON, Canada), and Rabbit Brain Thromboplastin (RBT) TF activity standard (Sigma, Oakville, ON, Canada; 2 mM/mL solution). In addition, the procedure requires preparation of the anhydrous CaCl2 (100 mM), acetic acid (50 %), and protein lysis buffer solutions (0.7 mL basic lysis buffer: 2 mL 10 % Triton-X-100 ; 400 μL 0.5 M EDTA, pH 8.0; 200 μL 1 M Tris–HCl, pH 7.0; 0.175 g NaCl; 6 mL glycerol ; 11.4 mL H2O, supplemented with 170 μL of 7× Mini protease inhibitor , 10 μL of 100 mM phenylmethanesulfonyl fluoride (PMSF ), 100 μL of 0.2 M sodium orthovanadate, and 50 μL 1 M sodium fluoride) as well as protein quantification reagents.
3 Methods
3.1 Fluorescent Multicolor Tissue Immunostaining (IF)
Multicolor immunofluorescent staining protocols can be challenging; yet they are increasingly used to reveal complex features of cells in their natural milieu. One practical approach to this question is to employ a three-color procedure, including staining for two different antigens (red and green fluorescence , respectively) and a nuclear counterstain (DAPI-blue ). Examples of such protocols compiled in this section are relatively straightforward and amenable to modifications and to imaging by standard fluorescent microscopy (see Note 1 ). For practical reasons, a double immunostaining is optimized for a 3-day cycle:
-
1.
(Day 1) Dewaxing and rehydration of tissues: Processing tissue samples, embedding, and sectioning are described in other sources easily accessible in the literature [27]. Once the sections are cut and mounted on slides the staining begins with the following steps: Place the 5 μm thick paraffin-embedded tissue sections in 100 % xylene (histological xylene ) in the fume hood for 10 min at room temperature followed by two xylene washes for 5 min each. Remove slides and place in three consecutive washes of 99 % ethanol for 5 min each. Sequentially wash the slides in dilutions of ethanol of 95 % , 80 %, 70 %, and 50 % and then running tap water to remove any trace of ethanol. For anti-TF staining, the slides are deparaffinized and rehydrated according to a protocol provided by Abcam with slight modification, including the following sequential washes: xylene (3 × 7 min), xylene 1:1 with 100 % ethanol (3 min), 100 % ethanol (2 × 3 min), 95 % ethanol (3 min), 70 % ethanol (3 min), and 50 % ethanol (3 min). Rinse under cold tap water.
-
2.
(Day 1) Microwave antigen retrieval : Place roughly 200 mL of the 1 % vector antigen unmasking solution in a glass Coplin jar (250 mL) and heat the solution in a microwave to 95 °C using a thermometer or temperature probe. At 95 °C, place the rack of slides into the antigen retrieval solution. Replace the Coplin jar into the microwave and ensure that the temperature is maintained at 95 °C to 100 °C by periodic heating of the microwave for 15 min. At the end of the 15 min, and using a glove to protect against the hot glass surface, remove the Coplin jar and place on the bench at RT for another 20 min. After 20 min, transfer the slide rack to a clean Coplin jar containing DPBS. Perform three DPBS washes of 5 min each of the slides. For anti-TF staining, antigen retrieval is carried out using antigen unmasking solution for 20 min at 98 °C.
-
3.
(Day 1) Permeabilization: Prepare a solution of 3 % Triton-X in DPBS. Place slides in Triton-X solution for 10 min at RT. At the completion of the permeabilization step, rinse slides in three washes of DPBS for 5 min each. For anti-TF staining, the slides are allowed to cool for 30 min after antigen retrieval and are subsequently washed with running tap water (10 min), TBS (2 × 5 min), and TBS containing 0.025 % Triton X-100 (2 × 5 min).
-
4.
(Day 1) Blocking unspecific signal: Prepare a solution of 1 % bovine serum albumin . Mix thoroughly and then filter using a 0.22 μm syringe filter. Remove each slide from DPBS, wipe away excess solution, and place 1 % BSA on the entire tissue. Place the slide with 1 % BSA in a humidified chamber and incubate for 30 min. For anti-TF staining the slides are blocked for 2 h with 10 % rabbit serum in TBS containing 1 % BSA.
-
5.
(Day 1) Primary antibody binding—first staining: While incubating in BSA, make the working solution of the primary antibody (see Note 5 ). For Endoglin staining a 1:100 dilution of goat anti-mouse Endoglin/CD105 (AF1320, mouse Endoglin Affinity Purified Goat IgG, R&D Systems, Minneapolis, MN, USA) in DPBS gives strong and specific signal. Maintain the antibody working solution on wet ice (4 °C) until ready for use. When the blocking step is complete, remove a slide from the humidified chamber, remove excess blocking buffer , and wipe the edge of the tissue with blotting paper. Without letting the tissue dry, add enough primary antibody to cover the tissue completely, and place it in the humidified chamber overnight at 4 °C. Complete the same procedure with the other slides. For anti-TF staining, the slides are incubated at 4 °C overnight in a humidified chamber with the TF primary antibody (sheep anti-human TF, SATF-IG, Affinity Biologicals) at 1:10,000 dilution in TBS containing 1 % BSA. While anti-TF staining can be combined with other multicolor protocols, it can also be reduced to a faster immunochistochemical cycle aiming at visualization of TF expression in tumor tissue. In such a case, the slides exposed to the primary antibody are processed through the following steps: the slides are washed (TBS containing 0.025 % Triton X-100 : 2 × 5 min) and then blocked for endogenous peroxidase activity for 1 h at 4 °C in a H2O2 solution; the Vectastain Elite kit for sheep IgG antibody detection is used according to the manufacturer’s instructions for incubation with the biotinylated secondary antibody and ABC reagent; and ImPACT DAB (3,3′-diaminobenzidine) peroxidase substrate was used to detect biotinylation prior to counterstaining with hematoxylin or methyl green .
-
6.
(Day 2) Secondary antibody—first staining: Continue with the double-staining protocol, remove the slides from the humidified chamber, and wash off the primary antibody by placing slides in a Coplin jar of DPBS for 5 min. Repeat the washes three times for 5 min each. Make up a solution of 1:200 dilution of the respective secondary antibodies. This includes the chicken anti-goat Alexa 488 antibody (for anti-CD105 staining). Remove the excess of DPBS by wiping the edge of the tissue and then apply enough of secondary antibody to completely cover the surface of the tissue section. Incubate in the humidified chamber for 45 min. Immediately thereafter, tip the slides to remove the secondary antibody and wash in DPBS in a Coplin jar for a total of five times in DPBS for 5 min each (also see Note 6 ). While the slides are still wet, verify the quality of staining under the fluorescent microscope (CD105-positive endothelial cells located around blood vessel lumens should exhibit bright and specific green fluorescence).
-
7.
(Day 2) Blocking tissues for the second staining: Second stain is intended to reveal the functional states of endothelial cells such as proliferation (Ki67 ) or association with pericytes /smooth muscle cells (αSMA), the latter a feature of mature blood vessels. For anti-Ki-67 and anti-αSMA antibodies, the blocking buffer consists of 5 % normal donkey serum (NDS). Make the solution of 5 % NDS (by diluting 200 μL of NDS in 4 mL of DPBS). Wipe excess solution from the edge with a tissue and place enough 5 % NDS to cover the tissue completely (see also Notes 2 – 4 ). Place the slide in a humidified chamber for 30 min.
-
8.
(Day 2) Primary antibody binding —second staining: Make up the working dilutions of the respective primary antibodies for the second staining. For the Ki-67 immunostaining, the primary antibody should be diluted at 1:200 in 0.3 % Triton X-100 in DPBS. For the αSMA staining make a 1:100 dilution using the blocking buffer as diluent. Before applying the antibody solutions, remove the blocking buffer by blotting the excess of liquid and add enough primary antibody to completely cover the tissue section (never wash the slides after the blocking step as the blocking buffer must remain on the tissue to prevent unspecific binding of the antibody). Place the slides in a humidified chamber and place in the refrigerator at 4 °C overnight.
-
9.
(Day 3) Secondary antibody —second staining: Prepare the solutions of the appropriate secondary antibodies. For staining with anti-Ki-67 and anti-αSMA primary rabbit antibodies, prepare the anti-rabbit secondary antibody (Alexa Fluor donkey anti-rabbit 594 IgG (H + L)) at a dilution of 1:200 in 0.3 % Triton X-100 in DPBS. Leave the solution on ice. Remove the slides from the refrigerator and place in 0.3 % Triton X-100/DPBS for five washes of 3 min each at room temperature (RT). Add the prepared secondary antibody solution to completely cover the tissue and place the slides in a humidified chamber at RT. Incubate with the secondary antibody for 45 min. When the incubation is complete, remove the slides and place them in 0.3 % Triton X-100 /DPBS for five washes of 3 min each. Using the fluorescent microscope (while the tissues are still wet), quickly examine whether proper staining has been achieved; for example Ki-67 will appear as bright red nuclear staining in dividing cells, and other patterns of staining such as diffuse or nonnuclear stain may signify lack of specificity.
-
10.
(Day 3) Mounting slides for storage for analysis: Mount slides by placing the cover slip over the tissue using Vectashield Hard Set Mounting Media with DAPI. This will highlight nuclei with blue fluorescence while protecting the tissue. Maintain slides in the dark and in a cold space (refrigerator, cold room) until used for analysis.
-
11.
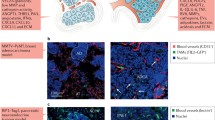
Slide viewing, interpretation, and image analysis: Stained tissues may be examined visually and micro photographed at different times after completion of the procedure. Image acquisition can be performed using appropriate fluorescent or confocal microscope while ensuring that any unnecessary and protracted exposure to microscope or laser light is avoided, as this may lead to photo-bleaching of the signal. Fluorescent double staining is particularly useful in seeking information as to vascular geometry (e.g., density) and functional state of cells within and around blood vessels . For example, the vascular caliber and density could be combined with the analysis of numbers, percentages, and distribution of proliferating endothelial cells (CD105+/Ki67+) versus their quiescent counterparts (CD105+/Ki67−). These features may exhibit regional differences within tumor tissue as shown in Fig. 1. In this image, proliferating endothelial cells can be found mostly in vascular sprouts, or in tumor regions undergoing vascular remodeling, such as segments within the wall of larger feeding vessels. Of note is the fact that dividing cancer cells surrounding blood vessels exhibit CD105−/Ki67+ staining pattern. In Fig. 2, double staining for CD105 and αSMA reveals blood vessels containing pericyte coverage (mature blood vessel). Layers of pericytes or mural cells (smooth muscle cells in larger vessels) are visualized as structures with the CD105−/aSMA+ staining pattern located in the outer aspect of the vessel wall. Immunostaining also offers the opportunity to perform some quantification of the features of interest. Using the protocol outlined above, dividing endothelial cells or mature blood vessels may be enumerated under high magnification (400×) and expressed as numbers per high-power field or per number of blood vessels. Such assessment is often performed to compare vascular features under different treatment conditions, in distinct tumor types, or between micro-regions within the same tumor (e.g., center and periphery, or in hypoxic regions). Another aspect of the vascular milieu is revealed by immunohistochemical staining for TF expression. In the protocol, described TF-positive cells are distinguished by brown coloration, often in regions of hypoxia , inflammation , or malignant growth. This signal can be quantified as percent area stained for TF multiplied by average staining intensity, as evaluated by independent blinded observers. The percent area is measured using the ImageJ software, and the staining intensity is assessed on an arbitrary scale of 1–4. However, the specific nature of tissue analysis depends on the underlying research question as described earlier [13, 15, 18, 28].
Fig 1 Immunofluorescent staining of tumors for proliferating endothelium. Since normal endothelial cells remain largely quiescent, proliferation of these cells is a hallmark of vascular growth (angiogenesis and arteriogenesis ). Human clear cell renal cell carcinoma (ccRCC) cells, 786-O , were injected subcutaneously into immune-deficient SCID mice and the resulting tumors were collected, fixed, sectioned, and co-stained for the endothelial cell marker (CD105, green) and proliferation marker (Ki-67, red). Nuclei of proliferating endothelial cells within a larger vessels (arteriogenesis) and capillary sprout (angiogenesis) are clearly positive for Ki-67, which also stains CD105-negative tumor cells. Image adapted with permission from [13]
Fig. 2 Immunofluorescent staining of tumors for endothelial and mural cells . Murine embryonic stem cells (R1) were injected into immune-deficient SCID mice to form aggressive teratoma. The tumor tissue was collected, fixed, and stained for markers of endothelial cells (CD105, green) and mural cells, such as pericytes and smooth muscle cells (αSMA, red). The image depicts the close association of mural and endothelial cells in a small arteriole. Image adapted from [29]
3.2 Tissue Factor Procoagulant Activity Assay (TF-PCA)
Measurement of TF procoagulant activity of cancer cells is useful and complementary to the aforementioned staining of tissues and cells for the TF antigen. The presence of TF staining does not always correspond to the cellular ability to activate the coagulation cascade as intracellular, encrypted TF, or alternatively spliced TF are inactive in this respect. The simple and effective quantitative TF-PCA can be performed on cultured cancer cells:
-
1.
Establishing adherent cultures of cancer cells. Plate each cell line or condition in quadruplicates in a 24-well plate, and grow cells to 70–80 % confluence. The cells should be seeded at densities that would not require prolonged culture to achieve these conditions (ideally cells should be grown for 1–3 days) or lead to uneven distribution of cells in wells, altered viability, or other unspecific differentials (see Note 7 ).
-
2.
Preparation of the TF standard curve and stock solutions. Once cell cultures are at optimal density, the 1-day TF-PCA begins with preparation of the TF standard curve. To accomplish this, serial dilutions of the Rabbit Brain Thromboplastin (RBT ; Sigma 44213) standard, a source of TF/thromboplastin activity, are prepared in TBS, pH 7.4, according to Table 5, and 200 μL aliquots of each dilution are added into empty wells of a 24-well plate. Prepare the stock solutions of all reagents in quantities sufficient for all wells containing cells or standard. From these ingredients, prepare the reagent mix containing 281 μL TBS , pH 7.4, 2 μL 5 nM FVIIa, and 2 μL 150 nM FXa per well.
Table 5 Dilution table -
3.
Setting up the TF-PCA reaction: Wash the cells three times with pre-warmed TBS, pH 7.4. Add 285 μL of reagent mix solution to each well. At 10-s intervals, add 15 μL of 100 mM CaCl2 to each well and incubate the cells with solutions for 30 min at 37 °C (this is important as TF activity reaction is time sensitive).
-
4.
Developing color reaction. While the cells are incubating, add 20 μL of chromogenic substrate to wells of 96-well plate. At 10-s intervals in the order as in the previous step, add 200 μL of solution from each well of the 24-well plate to the 20 μL substrate in the 96-well plate. This will mix the activated factor X (FXa) from wells containing cancer cells (or standard) with the chromogenic substrate, the cleavage of which results in a color reaction. This reaction is indicative of the FXa activity, which is quantitatively generated by the TF/VIIa complex on the surface of cancer cells. Incubate for 3–5 min at 37 °C. At 10-s intervals, add 20 μL of 50 % acetic acid to stop the reaction.
-
5.
Reading the TF-PCA reaction. Place the 96-well plate containing samples and RBT standards in a plate reader and record the absorbance at 405 nm.
-
6.
Normalization. Wash the 24-well plate twice with PBS , and lyse the cells for protein quantification. Interpolate the units of TF activity from the RBT standard curve, and normalize to total protein (μg). Additional modification, use of TF-neutralizing antibodies, and related methods to assess the interplay between the coagulation system and the tumor vasculature are reviewed elsewhere [1–3].
4 Notes
-
1.
It is essential to use positive and negative controls with every immunostaining (usually tissue samples known to exhibit the expression of the respective markers). It is also essential to pay attention to subcellular localization of the staining, e.g., membrane/cytoplasmic for CD105 or nuclear for Ki67 .
-
2.
Do not place the slides in direct contact with running water but rather allow water to flush over the slides.
-
3.
When applying the primary antibody completely cover the surface of tissue sections and never wash the slides after the blocking step, as the blocking buffer must remain on the tissue to prevent unspecific binding.
-
4.
When removing the blocking buffer , wipe the edge of the tissue with blotting paper and never let tissue dry.
-
5.
For double staining, carefully select the antibodies . For example, the secondary antibody of the second stain must be devoid of any cross-reactivity against all the primary and secondary antibodies used in the first stain. It must also be matched to the species and class of the primary immunoglobulin in the second stain, and carry a fluorophore that has non-overlapping emission spectra with the first staining cycle. It is also practical to avoid antibodies reactive with host tissue immunoglobulins (e.g., anti-mouse antibodies to stain mouse tumors), as blocking off these signals often complicates the protocol.
-
6.
After incubation with the secondary antibody and washing, and while slides are still wet, check one slide to verify, under fluorescence , if the staining was successful (vessels will appear bright green).
-
7.
For TF-PCA, having a proper cell culture and control cells and especially timing the reaction using 10-s intervals, as indicated, are essential as FXa generation and substrate cleavage are dynamic processes.
References
Folkman J (2007) Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov 6:273–286
Rak J (2009) Ras oncogenes and tumour vascular interface. In: Thomas-Tikhonenko A (ed) Cancer genome and tumor microenvironment. Springer, New York, pp 133–165
Carmeliet P, Jain RK (2011) Molecular mechanisms and clinical applications of angiogenesis. Nature 473:298–307
Nagy JA, Dvorak HF (2012) Heterogeneity of the tumor vasculature: the need for new tumor blood vessel type-specific targets. Clin Exp Metastasis 29:657–662
Irvin MW, Zijlstra A, Wikswo JP, Pozzi A (2014) Techniques and assays for the study of angiogenesis. Exp Biol Med (Maywood) 239:1476–1488
Jain RK, Schlenger K, Hockel M, Yuan F (1997) Quantitative angiogenesis assays: progress and problems. Nat Med 3:1203–1208
Goodwin AM (2007) In vitro assays of angiogenesis for assessment of angiogenic and anti-angiogenic agents. Microvasc Res 74:172–183
Welti J, Loges S, Dimmeler S, Carmeliet P (2013) Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer. J Clin Invest 123:3190–3200
Brat DJ, Van Meir EG (2004) Vaso-occlusive and prothrombotic mechanisms associated with tumor hypoxia, necrosis, and accelerated growth in glioblastoma. Lab Invest 84:397–405
Magnus N, Gerges N, Jabado N, Rak J (2013) Coagulation-related gene expression profile in glioblastoma is defined by molecular disease subtype. J Thromb Haemost 11:1197–1200
Tehrani M, Friedman TM, Olson JJ, Brat DJ (2008) Intravascular thrombosis in central nervous system malignancies: a potential role in astrocytoma progression to glioblastoma. Brain Pathol 18:164–171
Yu JL, Rak JW (2003) Host microenvironment in breast cancer development: inflammatory and immune cells in tumour angiogenesis and arteriogenesis. Breast Cancer Res 5:83–88
Meehan B, Dombrovsky A, Magnus N, Rak J (2015) Arteriogenic expansion of extratumoral macrovessels—impact of vascular ageing. Neoplasma 62:372–383
D’Asti E, Kool M, Pfister SM, Rak J (2014) Coagulation and angiogenic gene expression profiles are defined by molecular subgroups of medulloblastoma: evidence for growth factor-thrombin cross-talk. J Thromb Haemost 12:1838–1849
Klement H, St CB, Milsom C, May L, Guo Q, Yu JL, Klement P, Rak J (2007) Atherosclerosis and vascular aging as modifiers of tumor progression, angiogenesis, and responsiveness to therapy. Am J Pathol 171:1342–1351
Weidner N, Semple JP, Welch WR, Folkman J (1991) Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. N Engl J Med 324:1–8
Meehan B, Garnier D, Dombrovsky A, Lau K, D’Asti E, Magnus N, Rak J (2014) Ageing-related responses to antiangiogenic effects of sunitinib in atherosclerosis-prone mice. Mech Ageing Dev 140:13–22
Meehan B, Appu S, St CB, Rak-Poznanska K, Klotz L, Rak J (2011) Age-related properties of the tumour vasculature in renal cell carcinoma. BJU Int 107:416–424
Benjamin LE, Golijanin D, Itin A, Pode D, Keshet E (1999) Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest 103:159–165
Mancuso MR, Davis R, Norberg SM, O’Brien S, Sennino B, Nakahara T, Yao VJ, Inai T, Brooks P, Freimark B, Shalinsky DR, Hu-Lowe DD, McDonald DM (2006) Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest 116:2610–2621
Contrino J, Hair G, Kreutzer DL, Rickles FR (1996) In situ detection of tissue factor in vascular endothelial cells: correlation with the malignant phenotype of human breast disease. Nat Med 2:209–215
Yu JL, May L, Lhotak V, Shahrzad S, Shirasawa S, Weitz JI, Coomber BL, Mackman N, Rak JW (2005) Oncogenic events regulate tissue factor expression in colorectal cancer cells: implications for tumor progression and angiogenesis. Blood 105:1734–1741
van den Berg YW, Osanto S, Reitsma PH, Versteeg HH (2012) The relationship between tissue factor and cancer progression: insights from bench and bedside. Blood 119:924–932
Magnus N, Garnier D, Rak J (2010) Oncogenic epidermal growth factor receptor up-regulates multiple elements of the tissue factor signaling pathway in human glioma cells. Blood 116:815–818
Albrektsen T, Sorensen BB, Hjorto GM, Fleckner J, Rao LV, Petersen LC (2007) Transcriptional program induced by factor VIIa-tissue factor, PAR1 and PAR2 in MDA-MB-231 cells. J Thromb Haemost 5:1588–1597
Magnus N, Garnier D, Meehan B, McGraw S, Lee TH, Caron M, Bourque G, Milsom C, Jabado N, Trasler J, Pawlinski R, Mackman N, Rak J (2014) Tissue factor expression provokes escape from tumor dormancy and leads to genomic alterations. Proc Natl Acad Sci U S A 111:3544–3549
Erickson HS, Gillespie JW, Emmert-Buck MR (2008) Tissue microdissection. Methods Mol Biol 424:433–448
D’Asti E, Huang A, Kool M, Meehan B, Chan JA, Jabado N, Korshunov A, Pfister SM, Rak J (2016) Tissue factor regulation by miR-520g in primitive neuronal brain tumor cells: a possible link between oncomirs and the vascular tumor microenvironment. Am J Pathol 186:446–459
Yu J, May L, Milsom C, Anderson GM, Weitz JI, Luyendyk JP, Broze G, Mackman N, Rak J (2008) Contribution of host-derived tissue factor to tumor neovascularization. Arterioscler Thromb Vasc Biol 28:1975–1981
Staton CA, Reed MW, Brown NJ (2009) A critical analysis of current in vitro and in vivo angiogenesis assays. Int J Exp Pathol 90:195–221
Rak J, Mitsuhashi Y, Bayko L, Filmus J, Shirasawa S, Sasazuki T, Kerbel RS (1995) Mutant ras oncogenes upregulate VEGF/VPF expression: implications for induction and inhibition of tumor angiogenesis. Cancer Res 55:4575–4580
Liang CC, Park AY, Guan JL (2007) In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2:329–333
Ashby WJ, Wikswo JP, Zijlstra A (2012) Magnetically attachable stencils and the non-destructive analysis of the contribution made by the underlying matrix to cell migration. Biomaterials 33:8189–8203
Boyden S (1962) The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J Exp Med 115:453–466
Alessandri G, Raju K, Gullino PM (1983) Mobilization of capillary endothelium in vitro induced by effectors of angiogenesis in vivo. Cancer Res 43:1790–1797
Albini A, Benelli R, Noonan DM, Brigati C (2004) The “chemoinvasion assay”: a tool to study tumor and endothelial cell invasion of basement membranes. Int J Dev Biol 48:563–571
Arnaoutova I, Kleinman HK (2010) In vitro angiogenesis: endothelial cell tube formation on gelled basement membrane extract. Nat Protoc 5:628–635
Lawley TJ, Kubota Y (1989) Induction of morphologic differentiation of endothelial cells in culture. J Invest Dermatol 93:59S–61S
Kanzawa S, Endo H, Shioya N (1993) Improved in vitro angiogenesis model by collagen density reduction and the use of type III collagen. Ann Plast Surg 30:244–251
Weinandy S, Laffar S, Unger RE, Flanagan TC, Loesel R, Kirkpatrick CJ, Van ZM, Hermanns-Sachweh B, Klee D, Jockenhoevel S (2014) Biofunctionalized microfiber-assisted formation of intrinsic three-dimensional capillary-like structures. Tissue Eng Part A 20:1858–1869
Shin Y, Han S, Jeon JS, Yamamoto K, Zervantonakis IK, Sudo R, Kamm RD, Chung S (2012) Microfluidic assay for simultaneous culture of multiple cell types on surfaces or within hydrogels. Nat Protoc 7:1247–1259
Nakatsu MN, Hughes CC (2008) An optimized three-dimensional in vitro model for the analysis of angiogenesis. Methods Enzymol 443:65–82. doi:10.1016/S0076-6879(08)02004-1
Norrby KC (2011) Rat mesentery angiogenesis assay. J Vis Exp 52:pii: 3078
Ribatti D, Nico B, Vacca A, Presta M (2006) The gelatin sponge-chorioallantoic membrane assay. Nat Protoc 1:85–91
Ausprunk DH, Knighton DR, Folkman J (1975) Vascularization of normal and neoplastic tissues grafted to the chick chorioallantois. Role of host and preexisting graft blood vessels. Am J Pathol 79:597–618
Vogel HB, Berry RG (1975) Chorioallantoic membrane heterotransplantation of human brain tumors. Int J Cancer 15:401–408
Weinstein BM, Stemple DL, Driever W, Fishman MC (1995) Gridlock, a localized heritable vascular patterning defect in the zebrafish. Nat Med 1:1143–1147
Serbedzija GN, Flynn E, Willett CE (1999) Zebrafish angiogenesis: a new model for drug screening. Angiogenesis 3:353–359
Kenyon BM, Voest EE, Chen CC, Flynn E, Folkman J, D’Amato RJ (1996) A model of angiogenesis in the mouse cornea. Invest Ophthalmol Vis Sci 37:1625–1632
Gimbrone MA Jr, Leapman SB, Cotran RS, Folkman J (1973) Tumor angiogenesis: iris neovascularization at a distance from experimental intraocular tumors. J Natl Cancer Inst 50:219–228
Gimbrone MA Jr, Cotran RS, Leapman SB, Folkman J (1974) Tumor growth and neovascularization: an experimental model using the rabbit cornea. J Natl Cancer Inst 52:413–427
Akhtar N, Dickerson EB, Auerbach R (2002) The sponge/Matrigel angiogenesis assay. Angiogenesis 5:75–80
Passaniti A (2004) In vivo angiogenesis assays. In: Augustin H (ed) Methods in endothelial cell biology. Springer, Berlin, pp 207–222
Oikawa T, Sasaki M, Inose M, Shimamura M, Kuboki H, Hirano S, Kumagai H, Ishizuka M, Takeuchi T (1997) Effects of cytogenin, a novel microbial product, on embryonic and tumor cell-induced angiogenic responses in vivo. Anticancer Res 17:1881–1886
Sandison JC (1924) A new method for the microscopic study of living growing tissues by the introduction of transparent chamber in the rabbit's ear. Anat Rec 28:281–287
Clark ER, Kirby-Smith HT, Rex RO, Williams RG (1930) Recent modifications in the method of studying living cells and tissues in transparent chambers inserted in the rabbit’s ear. Anat Rec 47:187–211
Clark ER, Clark EL (1932) Observations on living preformed blood vessels as seen in transparent chamber inserted into the rabbit's ear. Am J Anat 49:441–477
Ide AG, Baker NH, Warren SL (1939) Vascularization of the Brown Pearce rabbit epithelioma transplant as seen in the transparent ear chamber. Am J Roentgenol 42:891–899
Algire GH (1943) An adaptation of the transparent-chamber technique to the mouse. J Natl Cancer Inst 4:1–11
Papenfuss HD, Gross JF, Intaglietta M, Treese FA (1979) A transparent access chamber for the rat dorsal skin fold. Microvasc Res 18:311–318
Endrich B, Asaishi K, Gotz A, Messmer K (1980) Technical report—a new chamber technique for microvascular studies in unanesthetized hamsters. Res Exp Med (Berl) 177:125–134
Lehr HA, Leunig M, Menger MD, Nolte D, Messmer K (1993) Dorsal skinfold chamber technique for intravital microscopy in nude mice. Am J Pathol 143:1055–1062
Baluk P, McDonald DM (2008) Markers for microscopic imaging of lymphangiogenesis and angiogenesis. Ann N Y Acad Sci 1131:1–12. doi:10.1196/annals.1413.001
Benjamin LE, Hemo I, Keshet E (1998) A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development 125:1591–1598
Laib AM, Bartol A, Alajati A, Korff T, Weber H, Augustin HG (2009) Spheroid-based human endothelial cell microvessel formation in vivo. Nat Protoc 4:1202–1215
Phillips RM, Pearce J, Loadman PM, Bibby MC, Cooper PA, Swaine DJ, Double JA (1998) Angiogenesis in the hollow fiber tumor model influences drug delivery to tumor cells: implications for anticancer drug screening programs. Cancer Res 58:5263–5266
Nicosia RF, Tchao R, Leighton J (1982) Histotypic angiogenesis in vitro: light microscopic, ultrastructural, and radioautographic studies. In Vitro 18:538–549
Aplin AC, Fogel E, Zorzi P, Nicosia RF (2008) The aortic ring model of angiogenesis. Methods Enzymol 443:119–36. doi:10.1016/S0076-68796879(08)02007-7
Burbridge MF, West DC (2001) Rat aortic ring: 3D model of angiogenesis in vitro. Methods Mol Med 46:185–204
Zhu WH, Nicosia RF (2002) The thin prep rat aortic ring assay: a modified method for the characterization of angiogenesis in whole mounts. Angiogenesis 5:81–86
Reed MJ, Karres N, Eyman D, Vernon RB (2007) Culture of murine aortic explants in 3-dimensional extracellular matrix: a novel, miniaturized assay of angiogenesis in vitro. Microvasc Res 73:248–252
Nicosia RF, Zhu WH, Fogel E, Howson KM, Aplin AC (2005) A new ex vivo model to study venous angiogenesis and arterio-venous anastomosis formation. J Vasc Res 42:111–119
Stiffey-Wilusz J, Boice JA, Ronan J, Fletcher AM, Anderson MS (2001) An ex vivo angiogenesis assay utilizing commercial porcine carotid artery: modification of the rat aortic ring assay. Angiogenesis 4:3–9
Lamfers ML, Aalders MC, Grimbergen JM, de Vries MR, Kockx MM, Van HV, Quax PH (2002) Adenoviral delivery of a constitutively active retinoblastoma mutant inhibits neointima formation in a human explant model for vein graft disease. Vascul Pharmacol 39:293–301
Bardy N, Karillon GJ, Merval R, Samuel JL, Tedgui A (1995) Differential effects of pressure and flow on DNA and protein synthesis and on fibronectin expression by arteries in a novel organ culture system. Circ Res 77:684–694
Deckers M, van der Pluijm G, Dooijewaard S, Kroon M, van HInsbergh V, Papapoulos S, Lowik C (2001) Effect of angiogenic and antiangiogenic compounds on the outgrowth of capillary structures from fetal mouse bone explants. Lab Invest 81:5–15
Cackowski FC, Anderson JL, Patrene KD, Choksi RJ, Shapiro SD, Windle JJ, Blair HC, Roodman GD (2010) Osteoclasts are important for bone angiogenesis. Blood 115:140–149
Brown KJ, Maynes SF, Bezos A, Maguire DJ, Ford MD, Parish CR (1996) A novel in vitro assay for human angiogenesis. Lab Invest 75:539–555
Jung SP, Siegrist B, Wade MR, Anthony CT, Woltering EA (2001) Inhibition of human angiogenesis with heparin and hydrocortisone. Angiogenesis 4:175–186
Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C (2003) VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol 161:1163–1177
Rezzola S, Belleri M, Gariano G, Ribatti D, Costagliola C, Semeraro F, Presta M (2014) In vitro and ex vivo retina angiogenesis assays. Angiogenesis 17:429–442
Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275:964–967
Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, Wu Y, Hicklin D, Zhu Z, Hackett NR, Crystal RG, Moore MA, Hajjar KA, Manova K, Benezra R, Rafii S (2001) Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med 7:1194–1201
Bertolini F, Shaked Y, Mancuso P, Kerbel RS (2006) The multifaceted circulating endothelial cell in cancer: towards marker and target identification. Nat Rev Cancer 6:835–845
Basile DP, Yoder MC (2014) Circulating and tissue resident endothelial progenitor cells. J Cell Physiol 229:10–16
Acknowledgements
Financial support: This work was supported by the operating grants from Canadian Institutes for Health Research (CIHR Foundation grant; CIHR; MOP 102736, MOP 111119), Cancer Research Society (CRS), and Canadian Cancer Society Innovation to Impact (CCSRI) to J.R., who is also a recipient of the Jack Cole Chair in Pediatric Hematology/Oncology. Studentship support for E.D. and infrastructure funds were provided by Fonds de Recherche du Québec en Santé (FRQS). E.D. was also supported by the Piccoli 401 Bike Challenge Fund.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this protocol
Cite this protocol
D’Asti, E., Meehan, B., Rak, J. (2016). Studies on the Tumor Vasculature and Coagulant Microenvironment. In: Ursini-Siegel, J., Beauchemin, N. (eds) The Tumor Microenvironment. Methods in Molecular Biology, vol 1458. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-3801-8_4
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3801-8_4
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-3799-8
Online ISBN: 978-1-4939-3801-8
eBook Packages: Springer Protocols