Abstract
We have developed reproducible micropropagation, callus culture, phytochemical, and antioxidant analysis protocols for the wild passion fruit species P. tenuifila, and P. setacea, native to the Brazilian endangered biomes Atlantic Forest, Cerrado, and Caatinga, by using seeds and explants from seedlings and adult plants. Genotype and explant origin-linked differences are visible amongst the Passiflora species concerning callus production, total phenolics, and antioxidant activity. The protocols developed for screening phytochemicals and antioxidants in P. tenuifila and P. setacea callus extracts have shown their potential for phenolic production and antioxidant activity. The high level of phenolic compounds seems to account for the antioxidant activity of methanolic extracts of P. tenuifila derived from 45-day-old immature seed callus. The methanolic extracts of callus derived from P. setacea seedling leaf node and cotyledonary node explants have shown the highest antioxidant activity despite their lower content of phenolics, as compared to cotyledon callus extracts. The optimized micropropagation and callus culture protocols have great potential to use cell culture techniques for further vegetative propagation, in vitro germplasm conservation, and secondary metabolite production using biotic and abiotic elicitors.
Access provided by CONRICYT – Journals CONACYT. Download protocol PDF
Similar content being viewed by others
Key words
1 Introduction
Passiflora setacea and Passiflora tenuifila are endemic to the endangered Brazilian biome Cerrado, Caatinga, and the Atlantic Forest. Their fruits have great potential for commercial use by nutritional, pharmaceutical, and cosmetic industries due to the high content of phenolic compounds which are antioxidants, especially P. tenuifila [1]. P. tenuifila is a potential source of resistant genes for genetic improvement as it is resistant to the pathogens Xanthomonas axonopodis pv. passiflorae and Cladosporium herbarum. The main constraints for plant production of several Passiflora species as P. setacea (occurring in Caatinga, Cerrado, Atlantic Forest) and P. tenuifila (occurring in Cerrado and Atlantic Forest) are the low seed germination rate, slow seedling emergence, and lack of efficient seed conservation protocols [2–4]. In vitro propagation techniques have been established for several Passiflora species such as P. alata, P. caerulea, P. cincinnata, P. edulis, P. setacea, P. foetida, and P. suberosa. These techniques have been used for the rapid multiplication of healthy and disease-free elite plants, source of fruits, and medicinal products as the antioxidants and other biologically active compounds [5–7]. However, plant tissue culture systems for secondary metabolite and antioxidant assessment have been established for P. quadrangularis [5] and P. alata Curtis [8, 9]. The protocols described herein are based on efficient and reproducible methods for P. tenuifila and P. setacea shoot culture initiation, callus culture establishment, and evaluation of callus phenolic content and antioxidant activity. We have optimized in vitro callus culture systems of P. tenuifila and P. setacea according to the antioxidant activity [10]. In vitro culture and antioxidant responses are genotype, explant type , and callus age dependent. The phytochemical and antioxidant screening carried out on P. tenuifila and P. setacea callus extracts demonstrates potent antioxidant activity.
2 Materials
2.1 Plant Material
-
1.
Collect seeds from stock mother plants of P. tenuifila and P. setacea from the germplasm collection maintained at the experimental station, Embrapa Cerrados, Empresa Brasileira de Pesquisa Agropecuária (Embrapa), Unidade Planaltina (Planaltina, DF, Brazil).
2.2 Culture Media
-
1.
Murashige and Skoog [11] powder basal medium (MS), plant cell culture tested (Table 1).
Table 1 Murashige and Skoog basal medium composition -
2.
59 mM Sucrose grade I, plant cell culture tested.
-
3.
88.5 mM D-(+)-glucose, plant cell culture tested.
-
4.
88.5 mM D-(−)-fructose, plant cell culture tested.
-
5.
2 g/L Phytagel, plant cell culture tested.
-
6.
Plant growth regulators for plant cell culture : gibberellic acid (GA3 ), indole-3-butyric acid (IBA ), α-naphthaleneacetic acid (NAA ), 2,4-dichlorophenoxyacetic acid (2,4-D ).
2.3 Extraction and Estimation of Total Phenolics
-
1.
Folin-Ciocalteu reagent.
- 2.
-
3.
20 % (w/v) sodium carbonate solution: Prepare a stock solution and store at 4 °C.
-
4.
80 % (v/v) methanol, high-performance liquid chromatography (HPLC ) grade.
-
5.
Ultraviolet-visible (UV-Vis) microplate reader.
2.4 Free Radical Scavenging Effect of 2,2-Diphenyl-1-picrylhydrazyl
-
1.
2,2-Diphenyl-1-picrylhydrazyl (DPPH ) reagent (see Note 3 ).
-
2.
80 % (v/v/) methanol, high-performance liquid chromatography (HPLC ) grade.
-
3.
3,5-Di-tert-4-butylhydroxytoluene analytical standard (BHT).
-
4.
Ultraviolet-visible (UV-Vis) microplate reader.
3 Methods
3.1 Preparation of Culture Medium
-
1.
Prepare standard aqueous solutions of plant growth regulators (see Subheading 2.2, item 6) by adding two drops of 1 M NaOH.
-
2.
Prepare appropriate culture medium by adding the recommended concentration of the carbon source (see Tables 2, 3, 4, 5, 6, 7, and 8) together with plant growth regulators (see Notes 4 , 5 , and 9 ).
Table 2 Composition of culture media used for shoot culture initiation and callus induction from different explant type s of Passiflora tenuifila Table 3 Composition of culture media used for in vitro shoot culture growth from seedling and adult plant shoot tip s of Passiflora tenuifila Table 4 Composition of culture media used for in vitro biomass production of P. tenuifila callus derived from different explant type s cultured in light Table 5 Composition of culture media used for in vitro biomass production, total phenolics , and percentage of DPPH * inhibition of P. tenuifila callus extracts derived from different explant type s cultured in light Table 6 Composition of culture media used for shoot culture initiation and callus induction of Passiflora setacea Table 7 Composition of culture media used for in vitro shoot growth from shoot tip culture of P. setacea Table 8 Composition of culture media used for in vitro biomass production, total phenolics , and percentage of DPPH * inhibition of P. setacea callus extracts derived from different seedling explant type s cultured in light -
3.
Adjust medium pH to 5.8 with 1 M NaOH before adding 2 g/L Phytagel. Dispense into 25 × 150 mm glass culture tubes (8 mL/tube) and autoclave at 121 °C for 18 min.
3.2 Culture Condition
-
1.
Seal vessels with transparent polypropylene film (76 × 76 mm) and maintain the cultures at 25 °C, 70 % relative humidity (RH) under a 16-h photoperiod, photosynthetic photon flux of 20–25 μmol/m2/s supplied by fluorescent light tubes. In all protocols use these culture conditions.
3.3 Establishment of Shoot Cultures of P. tenuifila
3.3.1 Ex Vitro Seed Germination
-
1.
Collect the seeds from fresh mature fruits of P. tenuifila immediately after harvesting.
-
2.
Remove the seed arils.
-
3.
Immerse P. tenuifila seeds completely in 2 mL 2.5 % (w/v) gibberellic acid aqueous solution at 25 °C for 5 days (see Note 5 ).
-
4.
Remove P. tenuifila seeds from the GA3 aqueous solution and sow in 50 mL plastic cups containing 28 g soil. Add 8 mL distilled water.
-
5.
Place plastic cups in 23.5 cm × 16.9 cm × 10 cm polystyrene trays.
-
6.
Maintain the trays either at 25 °C or in the greenhouse with a natural photoperiod at 16 °C during the night and 40 °C during the day (see Note 5 ).
3.3.2 Surface Sterilization of Seedling and Adult Plant Shoot Tips
-
1.
Excise shoot tip s, 3–4 cm in length comprising one apical bud and two axillary buds, from 45- to 60-day-old seedlings (see Fig. 1a) or greenhouse-grown 15-month-old adult P. tenuifila plants.
Fig. 1 Ex vitro-germinated seedlings of Passiflora tenuifila (a). In vitro shoot culture s originated from seedling shoot tip explants of Passiflora tenuifila cultured on MS basal medium with 2 mg/L Phytagel, 59 mM sucrose (b); 59 mM sucrose and 1.25 μM GA3 (c) after 90 days; 88.5 mM glucose and 1.25 μM IBA (d) after 45 days. Bars = 25 mm
-
2.
Rinse shoot tip s in 100 mL tap water, containing 2–3 drops of commercial detergent.
-
3.
Wash four times with distilled water.
-
4.
Surface sterilize shoot tip s of P. tenuifila seedlings for 1 min 30 s in 70 % (v/v) alcohol, rinse for 3 min in sterile distilled water, and immerse in commercial bleach (2.5 % active chlorine) with 2–3 drops of Tween 20 for 2 min 30 s.
-
5.
Surface sterilize shoot tip s of P. tenuifila adult plants for 3 min in 70 % (v/v) alcohol, rinse for 3 min in sterile distilled water, and immerse in commercial bleach (2.5 % active chlorine) with 2–3 drops of Tween 20 for 4 min.
-
6.
Rinse the shoot tip s three times each for 10 min in sterile distilled water.
3.3.3 Shoot Initiation and Multiplication
-
1.
Place surface-sterilized shoot tip s of P. tenuifila vertically on the MS basal medium containing 59 mM sucrose and 2 g/L Phytagel (see Note 5 , Fig. 1b).
-
2.
Promote in vitro shoot elongation by transferring the 30-day-old in vitro shoots to MS basal medium with 59 mM sucrose , 2 g/L Phytagel, and either 1.25 μM IBA (P. tenuifila seedling shoot tip s) or 1.25 μM GA3 (P. tenuifila adult plant shoot tips) (see Note 7 ) (Fig. 1c).
-
3.
Evaluate the percentage of shoot formation, number of shoots, shoot length, and number of nodes initiated per explant after 12 weeks. The results for shoot induction and growth of P. tenuifila are shown in Tables 2 and 3.
-
4.
For shoot multiplication and shoot culture stock maintenance remove 2 cm long shoot tip s and single-leaf node explants (ca. 1.0 cm long) from the 8-week-old P. tenuifila plantlets originated from the shoot tips cultured according to the procedures 1–2 (see Note 8 ).
-
5.
Place explants vertically on the MS basal medium supplemented with 88.5 mM glucose, 2 g/L Phytagel, and 1.25 μM IBA . The results for in vitro shoot growth of P. tenuifila are shown in Fig. 1d (see Note 8 ).
-
6.
Subculture after every 60-day culture cycle.
3.4 Establishment of Callus Cultures
3.4.1 Establishment of Callus Cultures from Immature Seeds
-
1.
Harvest immature fruits (4-week-old after anthesis) from P. tenuifila greenhouse-grown plants.
-
2.
Rinse fruits in 100 mL tap water containing 2–3 drops of commercial detergent.
-
3.
Surface sterilize fruits for 5 min in commercial alcohol in the flow cabinet.
-
4.
Remove the seeds and place them on MS basal medium amended with 59 mM sucrose , 2 g/L Phytagel, and 2.5 μM 2,4-D (see Notes 9 and 10 ).
-
5.
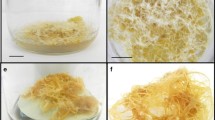
After 45 days evaluate the callus induction. Example results of callus induction are shown in Table 2, Fig. 2a.
Fig. 2 Callus originated from immature seeds (a, b), from shoot tip s of seedlings (c), adult plants (d, e), and stem segment explants of in vitro -formed shoots (f) of Passiflora tenuifila cultured on MS basal medium with 59 mM sucrose , 2 mg/L Phytagel, and 2.5 μM 2,4-D (a, b); 2.5 μM NAA (c–e); or 88.5 mM sucrose and 2.5 μM 2,4-D (f), after 50-day culture. Bars = 5 mm (a, b, e, f). Bars = 25 mm (c, d)
-
6.
Subculture callus (ca. 50 mg fresh weight) every 30-day cycle to MS basal medium containing 88.5 mM sucrose , 2 g/L Phytagel, and 2.5 μM 2,4-D (see Note 11 ).
-
7.
After 45 days evaluate callus fresh and dry mass. The results for callus growth are shown in Table 4, Fig. 2b (see Notes 10 and 11 ).
3.4.2 Establishment of Callus Cultures from Seedling and Adult Plant Shoot Tips
-
1.
Surface sterilize shoot tip s of P. tenuifila following the procedures described in Subheading 3.3.2.
-
2.
Place explants vertically on the MS basal medium supplemented with 59 mM sucrose , 2 g/L Phytagel, and 1.25 μM NAA (see Note 9 ).
-
3.
Evaluate the percentage of callus induction and fresh and dry mass after 45 days. The results for callus induction are shown in Table 2, Fig. 2c–e (see Notes 10 and 11 ).
3.4.3 Establishment of Callus Cultures from Stem Segments of In Vitro-Formed Shoots
-
1.
Remove stem segments (2–3 mm in length) from 60-day-old shoot culture s of P. tenuifila established according to the procedures described in Subheading 3.3.2 (Fig. 1).
-
2.
Place the explants on MS basal medium containing 88.5 mM of either sucrose , glucose, or fructose, 2 g/L Phytagel, and either 1.25–2.5 μM NAA or 2,4-D (see Note 9 ).
-
3.
After 45 days, evaluate the percentage of callus induction, fresh mass, and dry mass. The results for callus induction and growth are shown in Tables 2 and 4, Fig. 2f (see Notes 10 and 11 ).
3.5 Establishment of Shoot Cultures of P. setacea
3.5.1 Ex Vitro Seed Germination
-
1.
Collect the seeds from fresh mature fruits of P. setacea immediately after harvesting.
-
2.
Remove the seed arils.
-
3.
Sow P. setacea seeds in 50 mL plastic cups containing 28 g soil. Add 8 mL distilled water.
-
4.
Place plastic cups in 23.5 cm × 16.9 cm × 10 cm polystyrene trays.
-
5.
Keep the trays in the greenhouse with natural photoperiod and temperatures of 16 °C during the night and 40 °C during the day (see Note 6 ).
3.5.2 Shoot Initiation and Multiplication
-
1.
Surface sterilize ex vitro-grown seedling shoot tip s of P. setacea (see Subheading 3.3.2, steps 1–4; Fig. 3a).
Fig. 3 Ex vitro-germinated seedlings of Passiflora setacea (a). In vitro shoots of Passiflora setacea originated from seedling shoot tip explants cultured on MS basal medium with 2 mg/L Phytagel and either 59 mM sucrose (b, c) or 88.5 mM glucose μM IBA (d) after 45 days. In vitro-germinated seedling of Passiflora setacea. Bars = 25 mm
-
2.
Place surface-sterilized shoot tip s of P. setacea vertically on the MS basal medium supplemented with 59 mM sucrose and 2 g/L Phytagel (see Note 5 ).
-
3.
Evaluate the percentage of shoot formation, number of shoots, shoot length, and number of nodes initiated per explant after 12 weeks. The results of shoot induction and growth are shown in Tables 6 and 7, Fig. 3b (see Note 12 ).
-
4.
For shoot elongation, multiplication and maintenance of shoot culture stocks of P. setacea follow the procedures described in Subheading 3.3.3, steps 4–6 (see Note 8 ). The results for in vitro shoot growth of P. setacea are shown in Fig. 3c (see Note 8 ).
3.6 Establishment of Callus Cultures
3.6.1 Seed Surface Sterilization and In Vitro Seed Germination of P. setacea
-
1.
Rinse seeds of selected P. setacea plants in 100 mL tap water with 2–3 drops of commercial detergent, and wash four times with distilled water.
-
2.
Surface sterilize seeds for 10 min in commercial bleach (2.5 % active chlorine). Add 2–3 drops of Tween 20.
-
3.
Rinse three times for 10 min in sterile distilled water and culture the seeds on MS basal medium supplemented with 59 mM sucrose and 2 g/L Phytagel (see Note 6 ).
3.6.2 Establishment of Callus Cultures from Axenic Seedling Explants
-
1.
Remove segments (1 cm long) of either root, hypocotyl, epicotyl, cotyledonary node, or leaf node obtained from 60-day-old aseptically grown seedlings of P. setacea (Fig. 3d).
-
2.
Place the explants horizontally on MS basal medium with 88.5 mM sucrose , 2 g/L Phytagel, and 2.5 μM 2,4-D (see Note 13 ).
-
3.
After 45 days, take callus fresh and dry weight. The results of callus induction are shown in Table 6 (see Notes 11 and 12 ).
-
4.
Subculture callus at 30-day interval by following the procedures described in Subheading 3.4.1, step 6. The results for callus growth are shown in Table 8, Fig. 4 (see Notes 10 and 11 ).
3.7 Phytochemical Analysis and Antioxidant DPPH Test
3.7.1 Preparation of Callus Extract for Estimation of Total Phenolic Compounds
-
1.
Grind 1 g fresh callus material in 10 mL 80 % (v/v) methanol in water and leave the extract for 1 h in the darkness.
-
2.
Centrifuge contents at 11.76 × g for 5 min. The supernatant solution is filtered under vacuum into a volumetric flask and the filtrate is saved.
3.7.2 Estimation of Total Phenolics Contents in the Callus Extract
-
1.
Mix 40 μL methanolic extract with 3.16 mL deionized water and add 200 μL 10 % Folin-Ciocalteu reagent.
-
2.
After 6 min the reaction is neutralized with 600 μL 20 % sodium carbonate solution. The color will develop after incubation for 2 h at the room temperature in the darkness.
-
3.
300 μL of each sample and control are transferred to 96-well microplate and the absorbance is detected at 760 nm on a UV-visible microplate reader. The measurements are compared to the standard curve for gallic acid (0–1000 μg/mL) (see Notes 1 and 2 ).
-
4.
The results for total phenolics are expressed as microgram of gallic acid equivalent per gram of dry callus. The results of total phenolic contents in callus of P. tenuifila (Table 5) and P. setacea (Table 8) are shown (see Note 14 ).
3.7.3 Free Radical Scavenging Effect on 2,2-Diphenyl-1-picrylhydrazyl
-
1.
Mix samples of 10 μL methanolic extract with 290 μL 0.05 mM methanolic solution of DPPH in a 96-well microplate (see Note 3 ).
-
2.
A DPPH blank sample, without extract, containing 10 μL 80 % methanol and 290 μL DPPH solutions is prepared and assayed as control. The positive control is the DPPH solution plus tert-butylhydroxytoluene (BHT).
-
3.
After incubation in the darkness at room temperature for 2 h, the absorbance of the reaction mixture is measured at 515 nm using a UV-visible microplate reader.
-
4.
The percentage decrease in the absorbance at 515 nm is recorded for each sample and the percentage of quenching of the DPPH * radical is calculated on the basis of the observed decrease of the radical according to the formula DPPH* inhibition percentage = [(A DPPH − A Extr)/A DPPH] × 100 where A DPPH is the absorbance value of the DPPH blank sample (control) and A Extr is the absorbance value in the presence of the extract. The results for the percentage of DPPH scavenged of callus extracts of P. tenuifila (Table 5) and P. setacea (Table 8) are shown (see Note 15 ).
4 Notes
-
1.
Prepare a stock solution of gallic acid by dissolving 100 mg gallic acid in 100 mL deionized distilled water. Firstly dissolve the reagent in 1 mL 80 % methanol and increase the volume to 100 mL by adding deionized distilled water. Store this stock solution in an amber glass flask in a refrigerator to use as fresh working standards. Stock solution should be maintained at room temperature before use. Analyze total phenolics in the callus extract spectrophotometrically by using Folin-Ciocalteu reagent [12].
-
2.
Prepare working standards of 50–1000 μg/mL standard gallic acid solution. Total phenolics is expressed as microgram of gallic acid equivalent per gram of dry extract (μg GAE/g) using a standard curve (0–1000 μg/mL) of gallic acid.
-
3.
Prepare fresh 0.05 mM DPPH * stock solution in 80 % methanol (w/v) and store in the darkness at 4 °C in a flask, covered with aluminum foil. DPPH radical has been widely used to evaluate the free radical scavenging capacity (antioxidant activity) of plant and microbial extracts [13, 14].
-
4.
The media and plant growth regulator solutions are prepared in double-distilled water. Adjustment of pH is done before the addition of Phytagel. Store all the stock solutions at 4 °C until use.
-
5.
To optimize the Passiflora tenuifila and P. setacea shoot and callus culture protocols, determine the most appropriate concentration of sucrose , glucose, IBA , GA3 , NAA , and 2,4-D . The optimum culture conditions for shoot culture initiation and growth are shown in tables (see Tables 2, 3, 6, and 7).
-
6.
Passiflora tenuifila seeds fail to germinate in vitro . Seeds can be germinated in soil either at 25 °C or in the greenhouse (natural day light, minimum temperature 16 °C, maximum temperature 40 °C), after immersion for 5 days in 2.5 % GA3 aqueous solution. The germination rates of the GA3-treated seeds are 80–90 % within 20 days and mechanical scarification was unnecessary. We have successfully modified the originally proposed P. alata seed germination method [7].
-
7.
P. tenuifila in vitro shoots are produced in 90–100 % shoot tip s originated either from seedling or adult plant. IBA (for seedling shoot tip) and GA3 (for adult plant shoot tip) are effective promoters of in vitro shoot elongation and growth (see Table 3). The multiplication rates varied from 10 to 20 propagules per culture cycle depending on the shoot tip origin. Rooting is achieved in 40–50 % in vitro shoots cultured in IBA-containing medium . After 8 weeks of culture, in vitro shoots formed highest rooting rate (see Table 3).
-
8.
P. tenuifila and P. setacea shoot culture stocks are successfully maintained by subculturing shoot tip s and leaf node segments on MS basal medium containing 88.5 mM glucose, 1.25 μM IBA , and 2 g/L Phytagel for 24 months without decreasing the shoot proliferation capacity (see Figs. 1 and 3) (8-week subculture time).
-
9.
The best culture conditions for P. tenuifila callus induction (Table 2) and growth (Table 4) are shown. NAA is effective for callus induction from shoot tip s while 2,4-D is effective for callus induction from immature seeds.
-
10.
Consistent biomass (fresh and dry mass) is produced by the callus culture systems developed for P. tenuifila and maximum callus dry mass is obtained from immature seed-derived callus after ca. 45-day subculture cycle (see Table 5). P. setacea callus growth is affected by explant type and consistent callus biomass (fresh and dry mass) is produced by all callus culture systems developed for P. setacea. Callus derived from root and cotyledon segments explants shows the highest biomass accumulation (see Table 8). Maximum callus biomass is obtained after a 30–45-day subculture cycle.
-
11.
The subculture of P. tenuifila callus cultures originated from in vitro -formed shoot stem segments and from shoot tip s, either from seedling or adult plants, is not as effective as the subculture of immature seed-derived callus. The maintenance of P. tenuifila immature seed callus and P. setacea seedling explant callus culture stocks is carried out successfully for at least 18 months through periodic subculture (30-day time) on MS basal medium supplemented with 88.5 mM sucrose , 2.5 μM 2,4-D , and 2 mg/L Phytagel (see Tables 4 and 8).
-
12.
P. setacea in vitro shoots are produced in 100 % explants, originated from seedling shoot tip s. Addition of 1.25 μM IBA efficiently promotes in vitro shoot growth (number of shoots, shoot length, and number of nodes/micro shot) and rooting (see Table 7). The multiplication rate is ca. 15–18 propagules per culture cycle. Rooting is achieved in 60 % of the in vitro shoots. Approximately 8-week time is required to achieve the highest rooting rate from P. setacea in vitro shoots (see Table 7).
-
13.
The best culture conditions for P. setacea callus induction (Table 6) and growth (Table 8) are shown. Maximum callus induction rate (100 %) was achieved from all explant types.
-
14.
The levels of total phenolic compounds in P. tenuifila callus extracts are shown in Table 5. Callus age and explant -type linked differences are observed. The analysis shows that phenolic compound contents are higher in 45-day-old callus originated from immature seeds of P. tenuifila. The levels of total phenolic compounds in P. setacea callus extracts are shown in Table 8. The phytochemical analysis of callus extracts reveals explant-type linked differences and suggests that phenolic compound contents are superior in callus originated from cotyledon segments.
-
15.
The antioxidant activity of P. tenuifila callus extract is explant type and callus age dependent. The highest antioxidant activity is observed in callus originated from immature seeds which show the highest levels of total phenolics (see Table 5). The DPPH * test of P. setacea callus extracts also reveals explant-type linked differences and the highest antioxidant activity is detected in leaf node callus. Therefore, the maximum level of antioxidant activity is not dependent on the highest content of phenolic compounds (see Table 8).
References
Oliveira CM, Dianese AC, Frizzas MR et al (2014) First report on an insect pest on Passiflora tenuifila Killip (Passifloraceae). Phytoparasitica 42(5):677–680
Delanoy M, van Damme P, Scheldeman X et al (2006) Germination of Passiflora mollissima (Kunth) L. H. Bailey, Passiflora tricuspis Mast and Passiflora nov sp. Seeds. Sci Hortic 110:198–203
Mediondo GM, Garcia MTA (2006) Emergence of Passiflora caerulea seeds simulating possible natural densities. Fruits 61:251–258
Pires MV, de Almeida AAF, de Figueiredo AL et al (2012) Germination and seedling growth of ornamental species of Passiflora under artificial shade. Acta Sci Agron 2:67–75
Antognoni F, Zheng S, Pagnucco C et al (2007) Induction of flavonoid production by UV-B radiation in Passiflora quadrangularis callus cultures. Fitoterapia 78:345–352
Ozarowski M, Thiem B (2013) Progress in micropropagation of Passifloraspp to produce medicinal plants: a mini-review. Rev Bras Farmacogn 23(6):937–947
Zucolotto SM, Carize F, Reginatto FH et al (2012) Analysis of C-glycosyl flavonoids from South American Passiflora species by HPLC-DAD and HPLC-MS. Phytochem Anal 23:231–239
Pacheco G, Garcia R, Lugato D et al (2012) Plant regeneration, callus induction and establishment of cell suspension cultures of Passiflora alata Curtis. Sci Hortic 144:42–47
Lugato D, Simão MJ, Garcia R et al (2014) Determination of antioxidant activity and phenolic content of extracts from in vivo plants and in vitro materials of Passiflora alata Curtis. Plant Cell Tiss Org Cult 118:339–346
Sozo JS (2014) Secondary metabolite profile and antioxidant activity of fruits, seeds and in vitro cultured calluses of Passiflora setacea and Passiflora tenuifila (Passifloraceae). MSc Thesis, Federal University of Santa Catarina, Florianopolis, SC, Brazil (in Brazilian Portuguese with English summary)
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Rhandir R, Preethi S, Kalidas S (2002) L-DOPA and total phenolic stimulation in dark germinated fava beans in response to peptide and phytochemical elicitors. Process Biochem 37:1247–1256
Kim DO, Jeong SW, Lee CY (2003) Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem 81:231–326
Kim YK, Guo Q, Packer L (2002) Free radical scavenging activity of red ginseng aqueous extracts. Toxicology 172:149–156
Acknowledgement
The authors acknowledge the support of the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brasil), the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (PET/CAPES/Ministry of Education/Brazil), Embrapa Cerrados, Empresa Brasileira de Pesquisa Agropecuária (Embrapa) (Planaltina, DF, Brazil), and Dr. Erica E Benson for language revision.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this protocol
Cite this protocol
Sozo, J.S. et al. (2016). In Vitro Culture and Phytochemical Analysis of Passiflora tenuifila Killip and Passiflora setacea DC (Passifloraceae). In: Jain, S. (eds) Protocols for In Vitro Cultures and Secondary Metabolite Analysis of Aromatic and Medicinal Plants, Second Edition. Methods in Molecular Biology, vol 1391. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-3332-7_2
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3332-7_2
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-3330-3
Online ISBN: 978-1-4939-3332-7
eBook Packages: Springer Protocols