Abstract
RNA interference, the process in which small interfering RNAs (SiRNAs) silence a specific gene and thus inhibit the associated protein, has opened new doors for the treatment of a wide range of diseases. However, efficient delivery of SiRNAs remains a challenge, especially due to their instability in biological environments and their inability to cross cell membranes. To protect and deliver SiRNAs to mammalian cells, a variety of polymeric nanocarriers have been developed. Among them, the polysaccharide chitosan has generated great interests. This derivative of natural chitin is biodegradable and biocompatible, and can complex SiRNAs into nanoparticles on account of its positive charges. However, chitosan presents some limitations that need to be taken into account when designing chitosan/SiRNA nanoparticles. Here, we describe a method to prepare SiRNA/chitosan nanoparticles with high gene silencing efficiency and low cytotoxicity by using the ionic gelation technique.
Access provided by CONRICYT – Journals CONACYT. Download protocol PDF
Similar content being viewed by others
Key words
- Chitosan
- SiRNA
- Nanoparticles
- Ionic gelation
- RNA interference
- Gene silencing
- Nucleic acid delivery
- PEGylated chitosan
1 Introduction
Chitosan is one of the most studied polymers in non-viral SiRNA delivery, on account of several unique attributes: (1) its polycationic nature under slightly acidic conditions allows for complexation of SiRNA into nanoparticles (NPs) via a fast, easy, and gentle process [1]; (2) chitosan is biodegradable and biocompatible, which is critical to in vivo administration [2, 3]; and (3) its versatile chemical structure enables functionalization to impart the polymer with new properties and/or to enhance its performance [4]. The molecular characteristics of chitosan and formulation parameters are both decisive for the gene silencing effect of SiRNA NPs and need to be optimized. In particular, the molecular weight (MW) and the degree of deacetylation (DD) (defined as the percentage of deacetylated primary amine groups) are two important features that influence siRNA NP formation and efficacy. On account of its stiff molecular topology and short length, SiRNA needs a high number of positive charges to remain efficiently bound. Therefore, chitosans with DD equal or higher than 80 % are typically used to form SiRNA NPs. Regarding the MW, it was described that chitosan molecules that were 5–10 times the length of SiRNA, i.e., chitosan of MW from 65 to 170 kDa, were most suitable for NP formation [5]. Indeed, the MW should be high enough to efficiently complex SiRNA into stable NPs, but low enough to allow SiRNA unpacking and release into the cells. Two main methods for chitosan/siRNA NP formulation are described in the literature: simple complexation where SiRNA is mixed with chitosan alone and ionic gelation where a cross-linker agent, e.g., sodium tripolyphosphate, is added. Generally, ionic gelation is preferred since the NPs show higher stability compared to the simple complexation method [6]. Finally, an important parameter that needs to be determined experimentally is the positive/negative charge ratio. This ratio is decisive for the formation of NPs of suitable size.
While chitosan has demonstrated significant promise for in vitro SiRNA delivery, some limitations remain for in vivo administration. First, chitosan suffers from low water solubility at pH values higher than 6.5, due to partial protonation of the primary amino groups, which can weaken SiRNA binding and affect the stability of the NPs [7]. In addition, the transfection efficiency mediated by chitosan is restricted by its poor buffering capacity and inability to trigger endosomal escape [8, 9]. To address these limitations, a number of chitosan derivatives as well as formulation improvements have been described. Among them, the use of poly(ethylene glycol) (PEG) grafted chitosan (CPEG) efficiently improves polymer solubility as well as the NP colloidal stability and the addition of endosomal disrupting agents, such as primary amine group-rich compounds: poly(ethylene imine) has shown to increase transfection efficiency [10].
In this chapter, we describe a method to formulate efficient chitosan/siRNA NPs by ionic gelation. The formulation was optimized in terms of chitosan characteristics, formulation process and addition of excipients in order to obtain NPs of 100–200 nm diameter that display high in vitro gene silencing efficiency in multiple cell culture models.
2 Materials
2.1 Nanoparticle Preparation
-
1.
Chitosan derived from crustaceans: MW 190–310 kDa, DD 75–85 %.
-
2.
Isocyanate terminated methoxy-poly(ethylene)glycol (mPEG-ISC): Mn = 1000 g/mol.
-
3.
Dimethylformamide (DMF).
-
4.
Hydrazine, anhydrous.
-
5.
Phthalic anhydride.
-
6.
siRNAs, purified by HPLC: Luc SiRNA (MW 13,300 g/mol): sense 5′-CUUACGCUGAGUACUUCGAtt-3′; control (ctrl) SiRNA (MW 13,300 g/mol): sense 5′-AUAGUGCAACGAUGAGCUCtt-3′ and EGFP SiRNA (MW 13,490 g/mol): sense 5′-pACCCUGAAGUUCAUCUGCAcc-3′. Lower case letters = deoxyribonucleotides and p = phosphate residues.
-
7.
Sodium tripolyphosphate (TPP).
-
8.
Poly(ethylene imine) (PEI ), branched, 25 kDa.
-
9.
Sodium acetate and acetic acid.
-
10.
Transfection reagent: INTERFERin®, Polyplus transfection™.
-
11.
Zetasizer ZS Malvern Instruments Ltd.
-
12.
Zeta potential cuvette, Malvern Instruments Ltd.
-
13.
1 % Sodium dodecyl sulfate (SDS) solution: Weigh 1 g of SDS and add 99 ml of water.
-
14.
0.2 M Sodium acetate, pH 4.5: Add 11.5 ml of 3 M sodium acetate and 3.7 ml of 17.5 M acetic acid 17.5 M into 484.8 ml of RNase-free water.
-
15.
1 mg/ml Chitosan solution, pH 5.8: Weigh 40 mg of chitosan in a conical tube. Add 40 ml of 0.2 M sodium acetate buffer to obtain a final chitosan concentration of 1 mg/ml. Vortex until chitosan is completely dissolved. Adjust the pH to 5.8 using NaOH 10 M solution. Filter the solution using 0.45 μm syringe filter (see Note 1 ).
-
16.
1 mg/ml CPEG solution: Weigh 40 mg of CPEG in a conical tube. Add 40 ml of RNAse-free water. Vortex until CPEG is completely dissolved. Filter the solution using 0.45 μm syringe filter.
-
17.
1 mg/ml PEI solution: Weigh PEI in a conical tube using a plastic pipette, add RNase-free water to obtain 1 mg/ml final solution. Sonicate the solution. Adjust the pH to 7.4 using acetic acid 1.75 M. Filter the solution using 0.22 μm syringe filter.
-
18.
1 mg/ml TPP solution: weigh 40 mg of TPP in a conical tube, add 40 ml of RNase-free water, vortex. Filter the solution using a syringe filter 0.22 μm (see Note 2 ).
-
19.
50 μM SiRNA solution in RNase-free water: Briefly centrifuge the tubes to ensure that the dried SiRNA is at the bottom of the tube. Add RNase-free water to obtain a final SiRNA concentration of 50 μM, gently vortex and make aliquots of 200 μl. Store the SiRNA aliquots at −20 °C.
2.2 Materials for Cell Culture
-
1.
B16F10 luc cells: Murine melanoma cell line B16F10 stably expressing firefly luciferase.
-
2.
H1299 EGFP obtained from [11]: Human non-small-cell lung carcinoma cell line H1299 expressing the destabilized enhanced green fluorescence protein.
-
3.
Complete cell culture medium for B16F10 luc cells: Minimum essential Medium (MEM) with Glutamax supplemented with 10 % fetal bovine serum (FBS), and 1 % 10,000 U/ml penicillin-streptomycin.
-
4.
Complete cell culture medium for H1299 EGFP cells: RPMI 1640 supplemented with 10 % FBS and 1 % 10,000 U/ml penicillin-streptomycin.
-
5.
Cell culture plates: Black 96-well plates and transparent 12-well.
-
6.
Phosphate-buffered saline (PBS).
-
7.
Luminometer, GloMax Promega.
-
8.
Fluorescence-activated cell sorting (FACS), FACSCalibur BD.
3 Methods
3.1 Preparation of PEGylated Chitosan (CPEG)
PEG chains were grafted onto the hydroxyl groups of chitosan as described in detail in [10].
-
1.
To protect the amine groups of chitosan, transfer 1 g of chitosan into a glass tube containing 5 ml of anhydrous DMF, add 2.4 g of phthalic anhydride and stir at 130°C for 7 h.
-
2.
Rapidly pour the chitosan–phthalimide solution in a water/ice mixture under vigorous stirring.
-
3.
Filter, lyophilize, and store the chitosan–phthalimide under N2 at 6 °C until further use.
-
4.
To functionalize with PEG, dissolve 500 mg of chitosan–phthalimide in 25 ml DMF in a round bottom flask, then add 0.48 g mPEG-ISC, and let the reaction go overnight at room temperature.
-
5.
To deprotect the amino groups, add 0.8 ml of hydrazine, increase the temperature to 110 °C, and let the deprotection reaction go for 3 h.
-
6.
Dry the CPEG under vacuum, solubilize the collected residues into sodium acetate buffer and remove the phthalimide derivatives by filtration.
-
7.
Adjust the pH of the polymer solution to 10, dialyze against water until neutral pH and lyophilize.
3.2 Nanoparticle Formulation
-
1.
In an Eppendorf tube, mix 125 μl (125 μg) of TPP and 14.8 μl (10 μg) of EGFP SiRNA or 15 μl (10 μg) of luc or control SiRNA. This is Eppendorf A.
-
2.
In another Eppendorf tube, mix 250 μl (250 μg) of chitosan, 41.6 μl (41.6 μg) of CPEG and 50 μl (50 μg) of PEI. This is Eppendorf B.
-
3.
Add all solution in Eppendorf A to Eppendorf B. Mix vigorously by pipetting and vortex for 30 s.
-
4.
Leave the nanoparticle mix for 1 h at room temperature.
-
5.
Add RNase-free water to the NPs up to a volume of 1 ml.
-
6.
Use as is or centrifuge the mix for 30 min at 17,860 × g, 25 °C, and suspend the NPs into 1 ml RNase-free water.
-
7.
For each assay, prepare a formulation containing a luc or EGFP SiRNA and another one containing ctrl SiRNA (see Notes 3 – 5 ).
3.3 Nanoparticle Characterization
-
1.
Size measurements using dynamic light scattering (e.g., Nanosizer ZS): Transfer the diluted NP suspension to a cuvette adapted to the equipment. Let the suspension equilibrate for 2 min at 25 °C before performing the measurements (see Table 1).
Table 1 Typical physicochemical characteristics of the chitosan-based NPs loaded with siRNA (n ≥ 3) -
2.
Zeta potential measurements using electrophoretic mobility: Transfer the diluted NP suspension to a zeta potential cuvette. Let the suspension equilibrate for 2 min at 25 °C before performing the measurements (see Table 1).
-
3.
Encapsulation efficiency (EE): After centrifugation of the NP suspension (17,860 × g, 30 min), transfer 100 μl of the supernatant to a 96-well plate and mix with 100 μl of a diluted solution of Oligreen® (Life technologies, 1:200 in buffer TE 1×). Repeat this in triplicate. Incubate for 5 min in dark and measure the fluorescence (wavelengths: excitation 480 nm and emission 520 nm). The amount of SiRNA in the supernatant (siRNAsupernatant) is determined from a standard curve (recommended concentration range: 0.5 μg/ml to 0.1 ng/ml) and the encapsulation efficiency is calculated using the following equation where SiRNA is the initial quantity of SiRNANPs is the initial quantity of SiRNA in the nanoparticles:
3.4 Determination of In Vitro Gene Silencing Using Luciferase System
-
1.
The day before the experiment, plate B16F10 luc cells on black or white 96-well plates at a cell density of 8000 cells per well in complete cell culture medium.
-
2.
Prepare 1 ml NPs/medium mix: add 135 μl NPs to 865 μl of cell medium in an Eppendorf tube. Mix. Add 100 μl of the mix to the wells to obtain a final concentration of 100 nM SiRNA (n = 8).
-
3.
As a positive control, mix 1 μl INTERFERin® with 0.2 μl SiRNA (final concentration of 100 nM) following the manufacturer’s protocol.
-
4.
Incubate for 4 h at 37 °C.
-
5.
After 4 h, aspirate the medium and add 100 μl of fresh complete medium/well. Incubate at 37 °C for 48 h.
-
6.
At 48 h post-transfection, add 100 μl of ONE-Glo™ (Promega) reagent/well.
-
7.
Measure the luminescence within 30 min.
-
8.
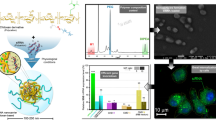
To determine the percentage of luciferase inhibition, use the following equation: \( \%\mathrm{inhibition}=100-\left(\left(100\times {\mathrm{RLU}}_{\mathrm{luc}}\right)/{\mathrm{RLU}}_{\mathrm{ctl}}\right) \) where RLUluc is the mean of relative light unit (RLU) for luc SiRNA and RLUctl is the mean of RLU for control SiRNA (Fig. 1).
3.5 Determination of In Vitro Gene Silencing Using EGFP System
-
1.
The day before the experiment, plate H1299 EGFP cells on a 12-well plate at a cell density of 105 cells/well in complete cell culture medium.
-
2.
Add 135 μl of NPs and 865 μl of complete cell culture medium (final SiRNA concentration of 100 nM) and incubate for 4 h. As a negative control, prepare the same NPs loaded with the ctrl SiRNA. As a positive control, use INTERFERin® transfection reagent.
-
3.
After 4 h, remove the medium and add 1 ml of fresh medium/well.
-
4.
After 48 h, wash the cells with 1 ml PBS, and add 200 μl trypsin for 5 min.
-
5.
Neutralize trypsin with 800 μl of medium, transfer the cell suspension to an Eppendorf tube, and centrifuge at 500 × g for 5 min.
-
6.
Add 300 μl of PBS and transfer to a tube for FACS analysis.
-
7.
For each measurement, perform the FACS analysis on 10,000 cells.
-
8.
Using FlowJo software, determine the FL1 median (median of the EGFP peak) for each fluorescence histogram.
-
9.
Calculate the percentage of EGFP silencing using the following equation:
$$ \%\mathrm{EGFPsilencing}=100-\left(\mathrm{F}\mathrm{L}1{\mathrm{median}}_{\mathrm{NPEGFP}}\times 100/\mathrm{F}\mathrm{L}1{\mathrm{median}}_{\mathrm{NPCTRL}}\right). $$
4 Notes
-
1.
Throughout all of the experiments, strict RNase-free conditions must be applied: use RNase-free tubes, gloves, tips with filter, and a dedicated pipette set. Before the experiment, spray the bench and the gloves with a solution of 1 % SDS.
-
2.
The chitosan, PEI, and TPP solutions can be stored for up to 1 month at 4 °C. After this time, variations of the NP characteristics (larger size, high PdI) might be observed.
-
3.
It is recommended to let the chitosan, PEI, and TPP solutions stabilize at room temperature for 1 h before making the NPs.
-
4.
To optimize the NP characteristics, it is recommended to set the SiRNA and the chitosan amounts constant and to vary the amount of TPP.
-
5.
It is recommended to prepare the NPs just before performing the in vitro tests.
References
Mao S, Sun W, Kissel T (2010) Chitosan-based formulations for delivery of DNA and siRNA. Adv Drug Deliv Rev 62:12–27
Baldrick P (2010) The safety of chitosan as a pharmaceutical excipient. Regul Toxicol Pharmacol 56:290–299
Kean T, Thanou M (2010) Biodegradation, biodistribution and toxicity of chitosan. Adv Drug Deliv Rev 62:3–11
Raemdonck K, Martens TF, Braeckmans K, Demeester J, De Smedt SC (2013) Polysaccharide-based nucleic acid nanoformulations. Adv Drug Deliv Rev 65:1123–1147
Liu X, Howard KA, Dong M, Andersen MO, Rahbek UL, Johnsen MG, Hansen OC, Besenbacher F, Kjems J (2007) The influence of polymeric properties on chitosan/siRNA nanoparticle formulation and gene silencing. Biomaterials 28:1280–1288
Katas H, Alpar HO (2006) Development and characterisation of chitosan nanoparticles for siRNA delivery. J Control Release 115:216–225
Ragelle H, Vandermeulen G, Preat V (2013) Chitosan-based siRNA delivery systems. J Control Release 172:207–218
Kim TH, Kim SI, Akaike T, Cho CS (2005) Synergistic effect of poly(ethylenimine) on the transfection efficiency of galactosylated chitosan/DNA complexes. J Control Release 105:354–366
Lai WF, Lin MC (2009) Nucleic acid delivery with chitosan and its derivatives. J Control Release 134:158–168
Ragelle H, Riva R, Vandermeulen G, Naeye B, Pourcelle V, Le Duff CS, D’Haese C, Nysten B, Braeckmans K, De Smedt SC, Jerome C, Preat V (2014) Chitosan nanoparticles for siRNA delivery: optimizing formulation to increase stability and efficiency. J Control Release 176:54–63
Jensen DM, Cun D, Maltesen MJ, Frokjaer S, Nielsen HM, Foged C (2010) Spray drying of siRNA-containing PLGA nanoparticles intended for inhalation. J Control Release 142:138–145
Acknowledgement
This work was supported by the Walloon Region (BioWin project TARGETUM).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this protocol
Cite this protocol
Ragelle, H., Vanvarenberg, K., Vandermeulen, G., Préat, V. (2016). Chitosan Nanoparticles for SiRNA Delivery In Vitro. In: Shum, K., Rossi, J. (eds) SiRNA Delivery Methods. Methods in Molecular Biology, vol 1364. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-3112-5_12
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3112-5_12
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-3111-8
Online ISBN: 978-1-4939-3112-5
eBook Packages: Springer Protocols