Abstract
The preparation of safe and efficient siRNA carriers remains a challenge that has limited the therapeutic applications of siRNA. In this study, the design of a new small interfering RNA (siRNA) carrier based on diisopropylaminoethyl-chitosan was devised for application in non-viral gene therapy. Polycations having varied proportions (11–32%) of diisopropylethylamine groups (DIPEA) and grafted with polyethylene glycol (1–3%) were synthesized and characterized. The physicochemical and biological properties of the polymers and their nanoparticles were evaluated at pH 6.3 and pH 7.4. The degrees of ionization at pH 7.4 were precisely controlled by the composition and increased from 13% for chitosan to 47% for the more substituted derivative. Nanoparticles with very low toxicities and sizes in the range of 100–200 nm, remained stable up to 24 h after their preparation in both the evaluated pHs under plasma osmolality. As probed by scanning electron and confocal microscopies, an efficient cell uptake of spherical nanoparticles mediated a TNFα knockdown of almost 60% in RAW 264.7 macrophages, and mRNA silence levels higher than the Lipofectamine (up to 90%) in HeLa cells. Overall, the results showed that these derivatives are promising vectors for in vivo studies under physiological conditions.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
RNA interference is the basic mechanism in which siRNA molecules interfere in the expression of specific genes by degrading targeted messenger RNA (mRNA) after transcription [1]. The application of therapies based on small interfering RNA (siRNA) in clinical trials has been greatly limited by its susceptibility to rapid degradation by nucleases, limited blood stability, off-target effects and an innate inability to diffuse through the cell membrane [1, 2]. Therefore, despite the progress made in improving the pharmacological activities and stability of these molecules, the administration of siRNAs is largely dependent on suitable nanocarriers for their efficient intracellular delivery. However, non-viral gene therapy, including three therapeutic treatments using siRNA, has already been approved by the US Food and Drug Administration [3].
In this scenario, vectors based on chitosan have been recognized as promising for the delivery of siRNA molecules due to their good biological properties, such as the low toxicity and its biodegradability [4,5,6,7,8]. However, the application of chitosan as a transfection agent has been partially limited due to its low solubility and low degree of ionization at neutral pH [9, 10]. The grafting with additional amino groups increases the degree of ionization at physiological pH and improves the transfection efficiency of its nanoparticles [11,12,13]. Also, owing to the different pKa values of the amine groups grafted onto the polymer chain, the polycation can bind siRNA molecules and acts as a proton scavenger along the endocytic pathway [14].
In this context, our research group has hypothesized that the precise grafting of chitosan with diisopropylaminoethyl (DIPEA) groups, could be an alternative to build efficient carriers for siRNA delivery. This strategy is supported by the interesting properties obtained with synthetic polycations having 2-(diisopropylamino)ethyl methacrylate (DPA) units in their structures, which provide a high sensitivity to pH [15, 16] that, once combined with primary amines and polyethylene glycol (PEG), has resulted in promising siRNA delivery systems [17,18,19]. Moreover, the diisopropylamine group is present in several commercially available medicines, like disopyramide, propantheline, and acotiamide, so it was also expected that its combination with chitosan would be non-toxic.
To improve the vector circulation lifetime and avoid unwanted interactions, polyethylene glycol (PEG) coatings have been widely used [20,21,22]. For example, Wang’s group showed that the grafting with PEG (2 kDa) up to five molecules per polyethylenimine chain (PEI; 25 kDa) increased its transfection efficiency compared to non-PEGylated PEI, however, the grafting with PEG must be precisely controlled [23, 24]. More recently, it has been shown that grafting with a small fraction (3.8%) of PEG (2 kDa) to chitosan allows the formation of siRNA nanoparticles that mediate the silencing efficiencies of the targeted gene by up to 80% [25]. In addition, it is worth noting that PEG is a non-biodegradable polymer, so its fast excretion from the body is recommended to avoid effects from long-term exposure [26]. This can be achieved through the use of 1.5–4.0 kDa PEG, because PEGs in this MW range were quickly cleared through glomerular filtration membranes [28]. Hence, the grafting with PEG chains via labile bonds may allow the release of PEG coatings via intracellular stimuli [29]. The use of labile disulfide linkages, for instance, is a promising strategy that takes advantage of the high concentration of reduced glutathione (GSH), present in the intracellular environment, as a stimulus for the nanoparticle disassembly favoring the endosomal escape of siRNA and the renal clearance of PEG [30,31,32]. Therefore, in addition to the DIPEA grafting, our group has hypothesized that the low-density grafting of 2 kDa PEG via disulfide bonds could increase the biological stability of nanoparticles with minimal exposure effects and without impairing the endosomal escape.
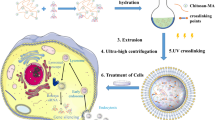
In this study, the effect of increasing proportions of DIPEA (11–32%) on the chitosan was evaluated by monitoring the physicochemical and biological properties of polymers and their nanoparticles (Fig. 1). Additionally, the effect of PEGylation via a disulfide linkage at a low density (1–3%) on DIPEA derivatives was also evaluated. The siRNA loaded nanoparticles were obtained by the coacervation process and evaluated in two different saline buffers at pH 6.3 and pH 7.4 with ionic strength adjusted to 150 mmol L−1. To evaluate the knockdown efficiency of nanocarriers formulated at pH 7.4, the silencing of the tumor necrosis factor alpha (TNFα) cytokine was evaluated in LPS stimulated RAW 264.7 macrophages treated with NPs loaded with anti-TNFα siRNA. In immune-mediated diseases, the production of TNFα by macrophages plays a central role in aggravating the inflammatory framework [33, 34] and siRNAs aimed at inhibiting the production of this cytokine emerge as a promising strategy for future clinical treatments [35]. The transfection studies for nanocarriers formulated at pH 6.3 were performed in HeLa cells and had, as a biological target, the mRNA of Sjögren syndrome antigen B (SSB), an abundant, essential and ubiquitously expressed ribonucleoprotein overexpressed in tumor cells [36]. Figure 1 summarizes the key characteristics of the siRNA nanocarriers, including their ability to efficiently condense the siRNA, neutralize its negative charge, avoid destructive interactions with serum proteins and promote efficient gene knockdown.
Materials and methods
Materials
Chitosans of high (400 kDa) and medium (208 kDa) molecular weight were purchased from Honeywell (Morris Plains, USA) and Polymar (Fortaleza, Brazil), respectively. O-(2-mercaptoethyl)-O-methyl-polyethylene glycol (PEG-SH), 2-chloro-N,N-diisopropylethylamine hydrochloride (DIPEA-Cl), bovine serum albumin (BSA), cellulose dialysis tubing of 14 kDa molecular weight cut-off (WMCO), universal negative siRNA control (MISSION®) labeled with cyanine 5 (siRNA-Cy5) and fluorescein amidite (siRNA-FAM), deuterium chloride (DCl), deuterium oxide (D2O), high-glucose DMEM medium, fetal bovine serum (FBS), and N-succinimidyl 3-(2-pyridyldithio) propionate (SPDP) were purchased from Sigma-Aldrich (St. Louis, USA). Monobasic and dibasic phosphates salt, potassium chloride, sodium chloride, sodium hydroxide, and tris(hydroxymethyl) aminomethane were purchased from Dinâmica Química Contemporânea Ltda. (Indaiatuba, Brazil). Dimethylsulfoxide (DMSO), glacial acetic acid, and hydrochloric acid were purchased from Synth (Diadema, Brazil). Antibiotic–antimycotic solution, DMEM medium, Lipofectamine 2000®, penicilin-streptomicin (PS) mixture, TRIzol® reagent, and siRNA anti-Tumor Necrosis Factor alpha (TNFα; NCBI reference sequence: NM_013693.3) were purchased from Thermo Fisher Scientific (Waltham, USA). siRNA anti-Sjögren syndrome type B antigen (SSB; NCBI reference sequence: NM_009278.4) was provided by Merck and Co. Inc. (Kenilworth, USA). CellTiter96® Aqueous One Solution kit composed of 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) and an phenazine ethosulfate (PES) was purchased from Promega Corporation (Wisconsin, USA). A murine TNF-α standard TMB ELISA development kit was obtained from Peprotech (Rocky Hill, USA). QuantiTect® Reverse transcription and SYBR Green PCR kits were purchased from Qiagen (Hilden, Germany). HeLa (folate receptor-positive), RAW 264.7, and 3T3/NIH cell lines were obtained from BCRJ (Rio de Janeiro, Brazil) and ATCC® (Rockville, USA).
Synthesis and characterization of polymers
Synthesis of diisopropylethylamine-chitosan derivatives grafted by PEG-thiol
Chitosans were grafted using varied proportions of 2-chloro-N,N-diisopropylethylamine hydrochloride (DIPEA-Cl) in the feed to obtain DIPEA-derivatives with increasing degrees of substitution (Table 1). The highly deacetylated starting chitosans (CM and CH) were obtained by the deacetylation of chitosans (medium MW; 208 kDa and high MW; 400 kDa, nominal value), following a previously reported method [37]. Briefly, chitosans of medium (CM) and high (CH) molecular weight were solubilized in a stoichiometrically equivalent volume of HCl 0.1 mol L−1. Next, the molar ratio of 2-chloro-N,N-diisopropylethylamine hydrochloride (DIPEA-Cl) to chitosan was set to obtain varied degrees of grafting (Table 1) and the pH was adjusted to 8.0 or 12.0 using 5.0 mol L−1 NaOH. The reaction mixture was heated to 70 °C and kept under magnetic stirring for 90 min under a nitrogen atmosphere with pH adjusted throughout the course of the reaction. Next, DIPEA derivatives were dialyzed (14 kDa MWCO), the first day against NaOH 0.05 mol L−1 and them against deionized water, until neutral pH. Thereafter, polymers were recovered by freeze-drying and stored at room temperature.
Selected derivatives were grafted with PEG (2 kDa) via disulfide bonds. Initially, 200 mg of polymer was solubilized in 10 mL of 0.3 mol L−1 acetic acid and diluted with 10 mL of PBS buffer pH 7.4. Then, 5 mol L−1 NaOH was added to adjust the pH to 7.4 and the proper amount (Table 1) of N-succinimidyl 3-(2-pyridyldithio) propionate (SPDP), solubilized in 1 mL of DMSO, was dripped into the polymer solution under stirring. After 3 h under magnetic stirring at room temperature, O-(2-mercaptoethyl)-O-methyl-polyethylene glycol (PEG-SH) set at molar ratio 1:1 with SPDP [38], was dissolved in 1 mL of PBS and added to the reaction. The reaction mixture was kept at 40 °C under intense stirring for 16 h. Finally, the DIPEA/PEG derivatives were purified by dialysis using a semi-permeable membrane tubing (14 kDa MWCO), for 3 days against PBS buffer pH 7.4 and another 3 days against a pH 8–9 NaOH solution. The derivatives were dried by lyophilization and stored at room temperature.
Polymer composition: 1H NMR and 13C NMR analysis
1H-NMR analyses were performed on a 400/54 Premium Shielded NMR spectrometer (Agilent Technologies, Santa Clara, USA), with a 4.0–5.0 s acquisition time and relaxation delay of 1.0 s. The samples were solubilized in DCl/D2O (2.8%, v/v) to obtain a polymer concentration of 10 g L−1. About 60–160 scans were accumulated at 70 °C. Water 1H NMR signal (4.7 ppm) was used as a reference [39]. The degrees of substitution by DIPEA (DSDIPEA) were determined from the areas of methyl hydrogens of DIPEA (H-DIPEA) at δ 1.6–1.9 ppm and anomeric hydrogens of all polymer unities (H1), at δ 4.9–5.5 ppm (Fig. 2a), using Eq. 1 [11]. The term ‘12’ (Eq. 1) refers to the number of hydrogens from the methyl groups of DIPEA.
The degree of substitution by PEG (DSPEG) was determined using Eq. 2, where HPEG refers to methylene protons (–OCH2CH2–) of PEG repeating units at δ 3.9–4.0 ppm [25]. The value 172 (Eq. 2) refer to amount of HPEG in 2 kDa PEG, determined based on its MW.
The NMR 13C measurements were taken on a 400 MHz Avance II NMR spectrometer (Bruker, Billerica, USA) at a 13C-frequency of 150.9 MHz and with 12° pulse width. The acquisition time was 0.4 s and relaxation delay was 2.0 s. About 20 000 scans were accumulated at 20 °C. The samples were solubilized in DCl/D2O (5%, v/v) to obtain a polymer concentration of 70 g L−1.
Molecular weight measurements by gel permeation chromatography (GPC)
Gel permeation chromatography (GPC) was used to determine molecular weight \({\overline{M} }_{\mathrm{w}}\), \({\overline{M} }_{n}\) and polydispersity (Mw/Mn) of the polymers, using acetate buffer (0.3 mol L−1 acetic acid/ 0.2 mol L−1 sodium acetate) pH 4.5 on an LC-20A chromatograph (Shimadzu, Kyoto, Japan), with refractive index detection model RID-10A (Fig. S15). Two columns in series: SB-803 HQ and SB-805-HQ (Shodex), with sizes of 0.8 cm × 30 cm, were used for separation of the polymer chains. The analysis was performed at 40 °C, using as eluent acetate buffer with a flow rate of 0.8 mL min−1. Pullulan monodisperse standards with molecular weight varying from 6.2 to 805 kDa were applied to build the analytical curve [40].
Buffer capacity and degree of ionization
The polymer solutions with 2.36 × 10–4 mol of total amines were prepared by the dissolution of dried polycation samples in 40 mL of 0.01 mol L−1 HCl with the ionic strength adjusted to 150 mmol L−1 by NaCl. Next, the buffer capacity (BC) and the degree of ionization (α) were, respectively, determined by monitoring the pH (Fig. S16) and conductivity (Fig. S17) after each addition of 0.1 mol L−1 standard NaOH solution.
The polymer BC was estimated by the buffer index (\(\beta\)), which represents, in this case, the amount (mol) of base (\(\mathrm{d}{N}_{\mathrm{B}}\)) necessary to promote a partial change in pH (\(\mathrm{dpH}\)) of the polymeric solution, as shown in Eq. 3. The \(\mathrm{dpH}\) refers to a pH variation of at least 0.25 units [41].
The α as a function of pH was determined using Eq. 4. Where \({N}_{\mathrm{pH}}\) is the number of mols of NaOH added into the polymer solution to have a specific pH value and \({N}_{\mathrm{S}}\) and \({N}_{\mathrm{E}}\) are the amounts (mol) of NaOH needed to start (α = 100%) and conclude (α = 0%) the polymer deprotonation, respectively. In this procedure, \({N}_{\mathrm{S}}\) and \({N}_{\mathrm{E}}\) were determined by conductometric titration [11].
Preparation and characterization of chitosan nanoparticles
Polymer/siRNA nanoparticle formulation
The nanoparticles were formulated by the coacervation process (Fig. 1), which is driven by the electrostatic interaction between the polycations and the negatively charged siRNA [42]. Polycation stock solutions (0.5–2.0 g L−1) were prepared by dissolution of polymers in 0.1 mol L−1 HCl followed by dilution with phosphate buffer pH 6.3 or 7.4, both having ionic strength (I) of 150 mmol L−1 (by NaCl) and a phosphate salt concentration of 50 mmol L−1. Then, 0.5 μg (for electrophoresis) or 5.0 μg (all other experiments) of siRNA was diluted in phosphate buffer and injected with small volumes of the stock polymer solutions to obtain nanoparticles at a specific N/P ratio (N: amine groups from polymer; P: phosphate groups from siRNA). The number of moles of phosphate groups was determined based on siRNA concentration (determined by a NanoDrop instrument) and the number of phosphate groups (42) per siRNA molecule; the number of moles of amino groups was determined based on the weighted mass of the derivatives and their compositions determined by NMR spectroscopy, considering two amino groups for glucosamine units substituted by DIPEA. Finally, the nanoparticles were kept for 30 min at room temperature before analysis [11]. In all analyses, the siRNAs anti-TNFα and anti-SSB were used for the formulations at pH 7.4 and 6.3, respectively.
Gel retardation assay: monitoring of siRNA unloading from the nanocarriers
In this study, the siRNA content was fixed at 0.5 µg and the nanoparticle/siRNA solution volume was kept at 10 µL. The free siRNA and nanoparticles prepared at increasing N/P ratio (1–10) were applied at 0.8% (m/v) agarose gel containing ethidium bromide (0.4 µg mL−1) for siRNA staining. Immediately prior to their application in the agarose gel, the samples were mixed with 1.6 µL of a loading dye solution (0.25% of bromophenol blue and 25% Ficoll® 400, m/v). The electrophoresis was performed for 75 min at 80 V, using a TAE solution as a running buffer. Finally, the electrophoresis gel was exposed to UV light and photographed by a gel documentation system.
Dynamic light scattering (DLS) measurements: zeta potential (ζ), hydrodynamic diameter (D h) and stability of nanoparticles
Nanoparticles (NPs) were formulated to load 5 µg of siRNA under varied N/P ratios for DLS measurements, which were evaluated at 25 °C on a Nano ZS Zetasizer with a red laser (λ = 633 nm) light source and detection optics at 173° (Malvern Panalytical; Malvern, UK). The final volume of the nanoparticle solution was adjusted to 1 mL and the Dh measurements were based on Z-average size. The results were expressed as (mean ± SD) from triplicate (Dh and ζ) or duplicate (stability) formulations, which were always measured three times. In stability studies without proteins, the Dh of nanoparticles formulated at 10 N/P ratio was measured over time, up to 24 h.
The biological stability of nanocarriers was estimated by monitoring their size distribution after being in the presence of proteins [15]. For this purpose, after NPs formulation at 10 N/P ratio (0.9 mL; 5 µg of siRNA), 200 µL of 220 g L−1 bovine serum albumin (BSA) solution (filtered twice in 0.22 µm filter) was added to obtain a final BSA concentration of 40 g L−1. The size distribution was measured immediately (Fig. S23) and then 7 h after the BSA addition (Fig. 6h). During the analysis interval, the solution was kept under slowly orbital stirring at 37 °C. A solution containing BSA only was also evaluated.
SEM-FEG microscopy
The nanocarrier solution was prepared at 10 N/P ratio, as described in 2.3.3. Then, less than one microliter of this solution was dripped onto the surface of a silicon plate, which was kept at room temperature in a desiccator until the solvent evaporated. Next, the nanoparticles were coated with a carbon layer and their morphology and size were examined by Scanning Electron Microscopy with a Field Emission Gun (SEM-FEG) using a 7500F electron microscope (JEOL, Akishima, Japan), under an acceleration voltage of 2.0 kV [43].
Biological studies: cell viability, cellular uptake and in vitro transfection efficiency
Cell cultures
HeLa (ATCC®) cervical carcinoma human cells were cultivated in DMEM medium supplemented with a mixture of 10% (v/v) FBS and 1% (v/v) PS. RAW 264.7 macrophages (BCRJ) were grown in a high-glucose DMEM medium incorporated with 10% (v/v) FBS and 1% (v/v) antibiotic–antimycotic solution. 3T3/NIH fibroblast (ATCC®) murine cells were cultivated in high-glucose DMEM containing 10% (v/v) FBS and 1% (v/v) PS. All cells were evaluated at a subcultivation ratio of 1:2–1:6 in a humidified chamber (37 °C) with a 5% CO2 enriched atmosphere.
Cell viability assay
Cell viability of different cell lines in the presence of polymers and nanoparticles were evaluated by CellTiter96® Aqueous One Solution kit (Promega Corporation, Madison, USA) following the manufacturer instructions, as previously described [38]. Initially, HeLa and 3T3/NIH cells were seeded in a 96-well plate at a density of 1 × 104 and 2 × 104 cell/well, respectively. After overnight incubation, the medium over cells was replaced by 200 µL of polymers solution (0.01–0.5 g L−1) prepared in complete medium, aiming at evaluating the polymers cytotoxicity. For evaluation of nanocarriers cytotoxicity, the medium over cells was replaced by 50 µL of nanocarrier solution at increasing N/P ratios (1–20) plus 150 µL of complete medium. After 24 h under incubation, the cell medium was changed by 120 µL of an MTS/DEMEM solution (1:5, v/v) and the well absorbances at 490 nm were measured using an ELx 808 microplate reader (Biotek Instruments, Winoosky, USA). As controls: wells containing 3% (m/v) of sodium dodecyl sulphate (SDS), free siRNA, phosphate buffer (used to prepare the nanoparticles) and acid/DMEM solution were used to solubilize the polymers. All experiments were performed in triplicate and the results were expressed as (mean ± SD) by comparison with nontreated cells (100% cell viability).
Cellular uptake of fluorescent-labeled siRNA loaded nanocarriers
RAW 264.7 macrophages (3.8 × 105 cells/well) were previously seeded over glass coverslips arranged in a 6-well plate and then the grown medium was replaced by 1 mL of nanocarrier solution plus 3 mL of incomplete medium. After 4 h in incubation, the medium was removed and the cells were washed with PBS buffer, fixed with 1 mL of 4% paraformaldehyde for 15 min, and had their nuclei stained by treatment with 1 mL of 1 mg L−1 4′,6-diamidine-2′-phenylindole (DAPI) for 10 min. At the end of the procedure, the glass coverslips were washed with PBS and placed on glass slides for image capturing using an LSM 780 microscope (Zeiss, Oberkochen, Germany) using ZEN 2.3 software. The nanoparticles were prepared at 10 N/P ratio and formulated to load 200 pmol (final concentration of 50 nmol L−1) of siRNA-FAM or siRNA-Cy5 [38].
siRNA anti-TNFα interference experiments on RAW 264.7 macrophages
The transfection efficiency on RAW 264.7 macrophages treated with siRNA anti-TNFα nanoparticles was determined based on TNFα protein quantity expressed by cells, which was determined using a Murine TNFα Standard TMB ELISA Development kit (Prepotech, Cranbury, USA), following the manufacturer instructions. The cells were seeded in a 24-well plate one day before experiments at a density of 1.8 × 105 cells/well. Then, the medium was removed and the macrophages received 450 µL of nanocarrier solution plus 1.35 mL of incomplete medium. The plates were incubated for 5 h, then, the supernatant was replaced by 1 mL of complete medium. 24 h after the start of transfection, the medium was replaced with 300 µL of 100 ng mL−1 lipopolysaccharide (LPS) in supplemented DMEM and the cells were incubated for 4 more hours to TNFα over production. After that, the cells were harvested, centrifuged at 12,000 RCF and stored at − 20 °C for measuring TNFα using the ELISA kit the next day, after the proper dilution (1:40) of the supernatant with 1× PBS buffer. In this study, the nanocarriers were formulated to load 5 µg of siRNA (200 nmol L−1 in the cells) anti-TNFα under physiological conditions of pH (7.4) and ionic strength. Experiments were performed in triplicate and the results were expressed as (mean ± SD) by comparison with macrophages treated with LPS only, which represent 100% of TNFα expression. Additionally, the amount of TNFα protein (pg mL−1) in each sample was also expressed using the ELISA TNFα standards and the results can be found in the supplementary information (Fig. S23).
SSB silencing on HeLa cells treated by anti-SSB loaded nanoparticles
SSB gene silencing efficacy on HeLa cells was evaluated by quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR). Briefly, FR-positive HeLa cells were grown in six-well plates at a density of 1 × 105 cells/well and then, 24 h after the seeding, the wells were washed three times with DMEM and received 500 µL of nanocarrier solution plus 500 µL of medium. After 4 h in incubation, the cells received 2 mL of enriched DMEM medium and were incubated again. The next day, the cell supernatant was replaced by 3 mL of complete medium and plates were kept under incubation for another 48 h. After that, the wells were washed with PBS buffer and the cells treated with TRIzol® reagent for RNA extraction, according to manufacturer specifications. Finally, the cDNA was obtained from the RNA and quantified by means of QuantiTect® Reverse transcription and SYBR Green PCR kits, as previously described [13, 14]. The nanocarriers, prepared at increasing N/P ratio (1–10), were formulated to load 5 µg de siRNA (final concentration of 125 nmol L−1 in the cells) anti-SSB at pH 6.3 (pH into the range of tumor environmental) under I = 150 mmol L−1. SSB RNAm expression level was expressed by comparison with nontreated cells.
Data analysis
All experiments were performed in at least three independent assays and data are summarized as mean ± SD. Statistical analysis of results was performed using the GraphPad Prism 8 software. Statistical significance (α 0.05) was assessed by multiple t test with adjusted correction for multiple comparisons using the Holm–Sidak method, *p < 0.05, **p < 0.01, ***p < 0.0001, and NS: not significant.
Results and discussion
Characterization of chitosan derivatives
Polymer structure and molecular weight analysis
The commercial chitosans went through a deacetylation procedure to provide starting polymers (CM and CH) with degrees of deacetylation (DD) higher than 95% (Table 1), which were determined by 1H NMR analysis (Fig. 2b) using the areas of 3 methyl hydrogens of acetylated unit (H-Ac) at δ 2.3 ppm and anomeric hydrogens (H1) at δ 4.8–5.4 ppm as previously described [12].
The degrees of grafting by DIPEA (DSDIPEA) and PEG (DSPEG) were evaluated by 1H NMR spectroscopy according to Eqs. (1) and (2), respectively. Some representative NMR spectra are shown in Fig. 2 to highlight the main signals used for characterization. The detailed signals and their corresponding integrations for all NMR spectra are found in the supplementary material (Fig. S1–S14). As compared to unmodified chitosan, the DIPEA-derivatives exhibited new signals at δ 1.6–1.9 ppm attributed to the resonance of 12 methyl hydrogens from diisopropyl groups (H-DIPEA), and new signals at δ 3.5–4.5 ppm corresponding to the resonances of methylene (CH2) and methine (CH) hydrogens, that are in the same region as the H2–H6 hydrogens of chitosan rings. The attachment of DIPEA groups to the chitosan framework brings further changes in the 1H NMR spectrum of chitosan and the anomeric proton H1 undergoes a downfield shift, from δ 5.2 ppm to δ 5.4 ppm (Fig. 2c). Thus, the total area in the range of δ 4.9–5.4 ppm, comprising all H1 signals, was used as a reference to calculate the DD, DSDIPEA, and DSPEG. Additionally, it is worth mentioning that DIPEA-derivative synthesis does not lead to quaternized units, because the voluminous isopropyl groups hinder the nucleophilic attack on DIPEA itself. Hence, the quaternization reaction is avoided and the compositions can be easily tuned.
As shown in Table 1, polymers with DSDIPEA in the range of 11–36 mol% were obtained. Derivatives with low proportions of DIPEA graft were the focus, aiming at avoiding the cytotoxic effects reported for chitosans highly grafted by tertiary amine groups [12]. Moreover, for grafting levels of about 15 and 25% with DEAE groups, stable formulations and favorable transfection properties were obtained at pH 6.3 [13] and pH 7.4, respectively [38]. Hence, selected derivatives were grafted with 2 kDa PEG by SPDP activation, and PEGylation was confirmed by 1H NMR spectroscopy. The PEG signal appears at δ 3.95 ppm (Fig. 2d), which corresponds to the resonance of the methylene hydrogens (HPEG) of the PEG chain [25]. The degrees of substitution by PEG (DSPEG) for all polymers were kept around 1–3% (mol/mol) of polycation units (Table 1).
Additionally, chitosan (CH) and selected DIPEA-derivatives were also evaluated by 13C NMR spectroscopy. The signals of the CH spectrum were assigned according to Fujita and Sakairi [45]: δ 97.5 ppm (C1), δ 77 ppm (C4), δ 75 ppm (C5), δ 70 ppm (C3), δ 60 ppm (C6) and δ 56 ppm (C2) (Fig. 2f). The derivatives also exhibited new 13C NMR signals from the DIPEA group: at δ 16–18 ppm, corresponding to its methylic carbons (C12–C15), at δ 42–44 ppm due to its methylene carbons (C8 and C9) and at δ 55–57 ppm corresponding to its methine carbons (C10 and C11), merging with the C2 signal from the chitosan framework (Fig. 2g, h). Moreover, as seen in the 1H NMR spectra, the substitution by DIPEA also led to changes in the C1 signal and, for the 13C NMR spectrum of CHD32, a second low-intensity upfield signal is observed at δ 96.5 ppm (Fig. 2h). Lastly, it has been reported that substitution at the hydroxyl groups of the carbon C6 gives origin to new signals near 65 ppm [46, 47]. As no new signal was observed in this region (Fig. S12–S14), the non-occurrence of DIPEA grafting at the C6 hydroxyl can be inferred, as expected due to the high reactivity of the chitosan primary amine group [48]. This result confirms the good control of the derivative composition, which is important considering that chitosan derivatives with the same substituent grafted at different functional groups may have different properties and applications [47, 49].
Chitosans and its DIPEA derivatives were also evaluated by GPC (Fig. S15) aiming at estimating their MW and the results are shown in Table 1. The derivatives synthesized from the higher molecular weight chitosan (CH) exhibited MWs of about 200 kDa, whilst the CM-polymers displayed molecular weights close to 120 kDa. Here, it is important to note that the deacetylation of commercial chitosans led to their chain degradation, that is, the MW decreased from 400 to 200 kDa for CH, and from 200 to 120 kDa for CM. Also, the grafting by DIPEA decreased the molecular weight (MW) compared to deacetylated chitosans due to reaction conditions (70 °C), which contributed to an increased polydispersity index (\({\overline{M} }_{\mathrm{w}}/{\overline{M} }_{\mathrm{n}}\)).
Buffer capacity (BC) and degree of ionization (α)
In general, the cell uptake via endocytosis drives the nanocarriers via an acid-dependent pathway that may degrade the therapeutic genes by the action of hydrolases [50]. However, the damage to nucleic acid during its internalization can be avoided using nanovectors composed of amino-protonable groups, which consume the H+ pumped inward to endosomal/lysosomal compartments during endocytosis leading to (i) an increase in osmotic pressure inside these biological transporter units and (ii) an increment of the nanocarrier positive charge surface [26]. These events combined give origin to the mechanism known as the “proton sponge” effect, which has been thoroughly reported as one of the main hypotheses to explain the endosomal evasion of drugs carried by polycations [14, 51].
First, the ability of the chitosan derivatives to consume protons during the endocytic pathway, in which vesicles are gradually acidified through early and late endosomes (pH 5.5), was evaluated. This ability is directly proportional to the polycation buffering performance, which was measured by the buffer index (β) of polymeric solutions after each addition of the standard NaOH solution [38, 52]. The greater the buffering performance of the polymer, more protons (and counterions) will be pumped into the transport compartment, which leads to a disturbance of the endosomal membrane due to the increase in the osmotic pressure within it [26]. As shown in Fig. 3a, b, all derivatives presented the maximum β (βmax) value in the pH range from 7.4 to 5.5, which make them promising candidates in providing an early endosomal escape for siRNA. This is in agreement with the high buffer capacity (BC) reported for chitosan, which can induce a proton-sponge effect similar to PEI, in the same pH range [41]. However, the growth of DSDIPEA caused decreases in βmax values compared to unmodified polymers and it is associated with both the conversion of primary amines of chitosan into secondary amines and the DIPEA group itself whose pKa is greater than 8.5 [53].
Buffering capacity, degree of ionization and electrophoretic mobility of siRNA: a, b Buffering index (β) B-spline plotted and c, d degree of ionization of polycations as a function of pH. e Gel electrophoresis images of siRNA released from nanoparticles at increasing N/P ratio in pH 6.3 and 7.4 (I = 150 mmol L−1), red rectangles indicate the released siRNA
Next, the degree of ionization (α) of the polymer as a function of pH was evaluated. The degree of ionization (α) is another equally important property in defining the nanovector features such as the ability to condense siRNA, the charge surface of nanoparticles and, accordingly, the cell uptake and endosomal escape [20, 54]. The results showed that the positive charge on polymers is directly proportional to DIPEA content, especially in neutral pH (Fig. 3c, d). In particular, at pH 7.4, the α values increased from approx. 13% for non-modified chitosans (CH and CM), to 22%, 31%, 32%, and 47% for CHD11, CMD14, CHD16, and CHD32, respectively. Accordingly, among all the polymers, CHD32 is expected to interact more strongly with siRNA to form positively charged nanoparticles at pH 7.4. In addition, at pH values lower than that found in early endosomes (pH 6.5), the α values for derivatives are higher than 65%, which may facilitate electrostatic interaction with internal endosome membrane during endocytosis, contributing to gene evasion [20, 50]. Both starting chitosans (CM and CH) exhibited 50% of ionization at pH 6.6, in agreement with the reported pKa value [55]. Also, the grafting with DIPEA promoted a visible increase in the solubility at neutral pH that is associated with the increase in α (Fig. S18), overcoming the very low solubility of chitosan at pH 7.4.
It must be considered that the increase in the degree of substitution (DS) with tertiary amino groups such as diethylaminoethyl [12], PEI [56] and also quaternized derivatives can increase nanocarrier toxicity [57]. Therefore, a precise control of DS is needed to maintain the balance between polymer characteristics like BC and α to achieve an optimized carrier for gene therapy.
Nanoparticle physicochemical properties at physiological and tumor environmental conditions
Gel retardation assay
It has been reported that good gene carriers have the ability to neutralize the negative charges of siRNA molecules by preventing their electrostatic repulsion against the plasma membrane, favoring their cell internalization [4]. To evaluate the siRNA/polycation interactions, the electrophoretic mobility study of nanocarriers formulated up to an N/P ratio of 10 was carried out (Fig. 3e). When siRNA is not released from nanoparticles, it can be inferred that siRNA molecules have been efficiently complexed by polycations chains and that the siRNA-negative charges have been neutralized by the polycation [58]. At pH 7.4 and I = 150 mmol L−1, the unmodified chitosan (CH) released the siRNA for all N/P ratios studied, which indicates a partial neutralization and poor complexation of siRNA molecules. On the other hand, chitosans grafted with DIPEA displayed an improved ability to retain siRNA into the wells, i.e. from N/P ratios 2 and 3 no siRNA release was observed for CHD32 and CHD16 formulations, respectively. Here, it is important to note the correlation between DSDIPEA and α values with the electrophoretic mobility of siRNA: (i) at pH 7.4 derivatives CHD32 and CHD16 were able to condense the nucleic acid at low N/P ratios compared to chitosan; (ii) at pH 6.3 α values are about 80% for all polymers (Fig. 3c and d) and no significant differences were observed in the siRNA released from nanoparticles. These results confirm the important role of the degree of ionization (charge density) in driving the condensation of the siRNA molecules and for the formation of nanoparticles. A modest effect of MW is also noted: CHD11 and CMD14 have similar proportions of DIPEA but the derivative with the higher MW (CHD11) retained the siRNA (Fig. 3e) more efficiently, which is in agreement with the general trend of stronger chitosan-nucleic acid interactions with increasing molecular weights [8, 55, 59].
To design nanocarriers with an enhanced lifetime in a biological medium by preventing unintended interactions with proteins, some derivatives were also grafted with 2 kDa PEG via a disulfide linkage. It has been established that PEGylation can decrease the ability of polycations to interact with genetic material [24] and after PEGylation, chitosan displayed an even weaker interaction with siRNA at pH 7.4 (Fig. S19). On the contrary, for DIPEA-chitosan, the PEG grafts caused no or minimal change to siRNA release and for the most substituted derivative, CHD32 with DSPEG of 1.3 and 2.6%, the N/P ratio of 2 was enough to retain siRNA in the well (Fig. 3e), whereas PEGylation of CHD16 decreased its capacity to condense the siRNA, i.e., the maximum N/P ratios at which siRNA was retained into the wells increased from 2.0 for CHD16 to 7.0 for CHD16-P3.3. These results make the positive effect of DIPEA groups in the nanocarrier formulations clear, especially under neutral conditions (pH 7.4 and I = 150 mmol L−1).
Nanoparticle morphology, zeta potential (ζ) and hydrodynamic diameter (D h)
The biological fate of nanoparticles is strongly influenced by their size and surface charge. While chitosan-based NPs with sizes up to 200–300 nm and positively charged surfaces are efficiently uptaken by cells and have an enhanced lysosomal escape in different cell lines [8, 25, 60], nanostructures with negatively charged surfaces are poorly internalized [61]. As chitosan nanocarrier properties depend greatly on the N/P ratio, pH and the ionic strength in which they are prepared [8, 62], the formulations in this study were prepared at pH 6.3 or 7.4 and ionic strength (I) of 150 mmol L−1 aiming at selecting suitable compositions for efficient in vitro knockdown in both pH environments.
Under the conditions of this study, the unmodified chitosans (CM and CH) were not able to complex with siRNA to form nanoparticles at pH 6.3 up to N/P ratio 10, i.e. their nanoparticles consisted of large aggregates with slightly negative charged surfaces (Fig. 4a, b). This behavior can be explained taking into account the ionic strength of 150 mmol L−1, which, besides shielding the attractive siRNA-chitosan electrostatic interaction, may condense on the particle surface, decreasing the zeta potential of the nanoparticles [62]. The DIPEA-derivatives (Fig. 4c–f), on the other hand, generated positively charged NPs (ζ 8–12 mV) with Dh smaller than 200 nm from N/P ratio 5 for formulations prepared in both pH 6.3 and 7.4. Also, positive zeta potentials were obtained for N/P ratios in which siRNA was not released in agarose gel electrophoresis (N/P ≥ 5.0). Additionally, the polydispersity indices for DIPEA-chitosan/siRNA nanoparticles were lower than 0.4 (Fig. S20).
DLS measurements. a–i Hydrodynamic diameter (Dh—black circles) and Zeta potential (ζ—red squares) for nanoparticles of a, b unmodified chitosans, c–f DIPEA and g–i DIPEA/PEG derivatives, built by coacervation with siRNA-TNFα under a–d pH 6.3 and e–i pH 7.4, ionic strength of 150 mmol L−1; \(n=9\). j Scheme depicting the positive effects of DIPEA groups on the nanoparticle properties
The PEGylation resulted in a partial neutralization of the polycation charges [26] and induced changes on Dh and ζ of the nanoparticles, especially for CHD16. Hence, the N/P ratio for obtaining positively charged nanocarriers was increased from N/P 5.0 for CHD16 to N/P 10 for CHD16-P3.3 (Fig. 3e). On other hand, the PEGylation did not affect the ability of CHD32 to complex the siRNA and nanoparticles with good physicochemical properties, i.e., appropriate size and ζ were obtained from N/P ratio 3 (Fig. 4f). This suggests that the weakening of electrostatic interaction caused by PEGylation can be compensated by the increase in the DIPEA content.
The morphology of nanoparticles prepared with CHD11, CHD32 and siRNA-TNFα (N/P ratio 10) were evaluated by SEM-FEG microscopy and revealed spheroid particles with sizes in agreement with light scattering analysis (Fig. 5). Dh values observed by SEM-FEG remained in the range of 50–200 nm, where the spherical shape may provide a more efficient internalization than rod-shaped NPs, for instance [63]. Nanoparticles of chitosan for siRNA delivery are often prepared and analyzed under varied conditions, usually in water or under pH lower than 7. In this study, nanoparticles with 200 nm and positive ζ were obtained at an N/P ratio of 5, at pH 6.3 and 7.4 and 150 mmol L−1 of ionic strength, which underscores the importance of the DIPEA groups in obtaining chitosan/siRNA nanoparticles with potential for in vivo applications.
Colloidal and biological stability of nanoparticles
The colloidal stability of the siRNA nanocarriers formulated at N/P ratio 10 at pH 7.4 or 6.3 (I = 150 mmol L−1) were evaluated by monitoring their hydrodynamic diameter (Dh) over time. All nanoparticles evaluated at pH 6.3 kept sizes less than 200 nm even after 24 h, including those prepared with the less-substituted derivatives (CMD14, 115.8 kDa and CHD11, 230 kDa), regardless of their MW (Fig. 6c). On the contrary, as seen in Fig. 4a, b, unmodified chitosans were not capable of building nanoparticles even in the first minutes after the mixing, which highlights the positive effect of DIPEA groups in providing stable nanoparticles at neutral pH. Moreover, the most substituted derivatives, CHD16 and CHD32, formed siRNA nanoparticles whose stability was not limited to the lower pH 6.3, and kept their initial size (100–200 nm) even after 7 h of their formulation at pH 7.4 and physiological ionic strength (Fig. 6a, b). Finally, as shown in Fig. 6d–f, the polydispersity index (PdI) of nanoparticles remained near or less than 0.4 without the addition of stabilizers. However, after 24 h, both Dh and PDI values increased as a result of a nanoparticle aggregation process.
Colloidal and biological stability of nanoparticles. a–c Hydrodynamic diameter (Dh) and d–f nanoparticles polydispersity index (PdI) as function of time; \(n=6\). Representative size-distribution of the nanoparticles g without and h in the presence of proteins (BSA; 40 g L−1) after 7 h of BSA/nanoparticles interaction; \(n=2\). The nanoparticles were formulated at N/P ratio 10, under 150 mmol L−1 of ionic strength and pH adjusted at a, b, d, e, g, h 7.4 or c, f 6.3
Another hurdle that limits the in vivo knockdown are the unwanted interactions between nanocarriers and proteins, which in human plasma consists of proteins like serum albumin, immunoglobulins, fibrinogen, apolipoproteins [20, 64]. Hence, to check the ability of PEGylated nanoparticles to avoid the interaction with proteins, the changes in the hydrodynamic diameter of NP upon binding to proteins were probed by DLS via the monitoring of the size distribution after their mixing with BSA (40 g L−1) under pH 7.4 and I = 150 mmol L−1 [15]. In the absence of protein, the scattered light of PEGylated nanoparticles, CHD32-P2.6 and CHD16-P3.2, was centered at about 200 nm (Fig. 6g). 7 h after the addition of BSA, the size distributions for BSA (approx. 10 nm) and nanoparticles were not changed and the signals for both remained without any significant differences, indicating that a good resistance to BSA adsorption was provided by PEGylation of the derivatives (Fig. 6h).
Biological studies: cell viability, cellular uptake and in vitro transfection efficiency
The structure of chitosan has been modified with many chemical groups aiming to combine its non-toxic property for different applications. It has been shown that solubility at neutral pH, biocompatibility and low toxicity are essential to act as a nucleic acid carrier [65]. To verify the effects of grafting DIPEA and PEG on cytotoxicity of chitosan derivatives, viabilities of NIH/3T3 fibroblasts and HeLa cells in the presence of the polymers and nanoparticles were evaluated by MTS assay. HeLa was the cell line used for transfection under moderately acid environmental conditions (pH 6.3), and the fibroblasts are abundant cells in the body, so they are more prone to interact with nanoparticles during their in vivo distribution [66]. As shown in Fig. 7, cell viabilities in the presence of all polymers and their nanoparticles remained higher than 80%, irrespective of DIPEA/PEG contents and the cell line studied (NIH/3T3 or HeLa). The cytotoxicity polymers and nanoparticles were also evaluated in RAW 264.7 macrophages and viabilities higher than 80% were also obtained for all polymers (0.02–0.5 g L−1) and their nanoparticles prepared at an N/P ratio of 10 (Fig. S22). It is worth noting that the polymer cytotoxicity against the mentioned cells lines was evaluated in concentrations higher than those used for in vitro transfection studies. For example, CHD32-P2.6/siRNA nanoparticles prepared at N/P ratio 20 require a polymer amount equivalent to 0.18 g L−1 and viabilities of both cell lines were near 100% in the presence of CHD32-P2.6 up to 0.5 g L−1(Fig. 7a, c). Overall, these findings indicate that DIPEA-chitosan derivatives have retained the low cytotoxicity profile displayed by plain chitosan.
The macrophage uptake of nanoparticles formed by DIPEA-derivatives and fluorescent-labeled siRNA-FAM or siRNA-Cy5 was investigated by confocal microscopy. Figure 8a shows an efficient uptake rate of CHD32-P1.3/siRNA-FAM nanoparticles by RAW 264.7 cells as observed fluorescent green dots (siRNA-FAM) in the cytoplasm area, after four hours of transfection. To avoid the overlap of siRNA-FAM with DAPI staining and better visualize the internalization of the vectors, a 3D confocal image (Fig. 8b) of the CHD32/siRNA-Cy5 nanoparticles was also done and its Z-stacked image obtained in the range of 2.6–10.96 μm. As can be seen in Fig. 8b, siRNA-Cy5 loaded nanoparticles appeared as red dots in all Z-stacked confocal images, which represent different horizontal slices from the cell, confirming the efficient internalization of the nanocarriers by macrophages.
Cell uptake and transfections studies. a Confocal microscopy images of RAW 264.7 macrophages treated with CHD32-P1.3/siRNA-FAM nanoparticles—the yellow dots are estimating the cell area based on white-light image. b Z-stacked confocal microscopy images (merged DAPI, siRNA-Cy5 and white-light channels) of a RAW 264.7 macrophage treated with CHD32/siRNA-Cy5 nanoparticles. c Transfection study based on knockdown of TNFα in RAW 264.7 macrophages measured via ELISA assay and expressed by comparison with cells treated LPS-only (100% TNFα expression). d In vitro transfection on HeLa cells measured via RT-PCR and expressed by comparison with nontreated cells (100% of SSB RNAm expression). Statistical analysis was performed using the unpaired Student test (α 0.05): in c the control group used for comparison was the cells treated only with LPS; in d the control group was the lipofectamine (in free FBS medium). *p < 0.05, **p < 0.01, ***p < 0.0001 and NS not significant
It has been reported that only a small fraction of internalized siRNA can evade from transporter compartments to cell cytoplasm [67]. Thus, to evaluate the efficiency of nanoparticles to deliver siRNA, the knockdown of TNFα of stimulated RAW 264.7 macrophages and the silencing of SSB RNAm on HeLa cells were evaluated by ELISA and qRT-PCR measurements, respectively (Fig. 8c, d). The transfection efficiencies were evaluated at pH 6.3 for the less-substituted derivatives (CHD11 and CMD14) with HeLa cells, because they displayed higher colloidal stability at this pH and most cancer tissues usually have a lower extracellular pH than normal cells with differences varying from 0.3 to 0.7 pH units [68]. On the other hand, the transfection ability at pH 7.4 was tested with NPs formulated with CHD16, CHD32 and their PEGylated versions for RAW macrophages (Fig. 8 c).
Overall, the results are in accordance with the physicochemical properties of the formulations, i.e., the nanocarriers that showed efficient siRNA condensation, smaller sizes and positive surface charges presented the best gene knockdown efficiencies. These physical properties were strongly dependent on the N/P ratio and DSDIPEA especially at pH 7.4. As can be seen in Fig. 8c, TNFα expression decreased gradually with increasing N/P. For CHD16 and CHD16-P3.3 at N/P 5.0 particle sizes were ~ 1.0 μm (Fig. 4c), while nanoparticles prepared at N/P 10 displayed Dh values of around 150 nm and silencing effects of 18% and 42%, respectively.
The large difference between the transfection efficiencies mediated by cell lines (macrophages vs HeLa) must be highlighted. The best knockdown rate with CHD16 nanoparticles on macrophages was lower than 20% while, for HeLa cells, the knockdown values for CHD11 nanoparticles were as large as 80% and increased to 92% for the lower MW derivative, CMD14 (Fig. 8d). Moreover, in the presence of FBS, the levels of mRNA for SSB expression in Hela cells treated by nanoparticles were close to that of lipofectamine 2000® (Fig. 8d). This difference can be partially explained by the higher zeta potentials of nanoparticles prepared at pH 6.3 (ξ = + 12 mV) compared to pH 7.4 (ξ = + 8 mV) used for transfection of macrophages. Besides, another important point is the impact of DIPEA content on TNFα-gene silencing efficiency, which becomes clear by comparing CHD16 versus CHD32 nanoparticles at N/P 10 (Fig. 4c) where, as DSDIPEA was doubled, the TNFα expression decreased to less than half. Accordingly, this result emphasizes the importance of a precise control of DIPEA grafting and of the degree of polycation ionization to efficiently condense the siRNA and build nanoparticles with appropriate surface charge. The transfection efficiency of the most efficient vector applied to RAW macrophages (CHD32) was not significantly affected by PEGylation and the low levels of grafting used in this study (1.3 and 2.6 mol%, Fig. 8c) increased the resistance to protein adsorption, resulting in TNFα expression levels very close to that of lipofectamine (Fig. S23). This result is supported by the literature, which indicates that, while low degrees of grafting by PEG favor the transfection efficiency, higher degrees can reduce cell uptake and final siRNA knockdown efficiency [69]. However, in general, PEGylation has been reported to improve the colloidal stability of nanocarriers based on chitosan [25] and its derivatives [70,71,72] with varied cell lines.
Overall, this approach has confirmed that a robust carrier can be built by combining DIPEA and chitosan to obtain nanoparticles with promising physicochemical properties, such as positive surface charge, smaller sizes and improved colloidal/biological stability under physiological conditions. Besides, they could be PEGylated without any loss in their knockdown efficiency, which confers potential to these vectors for in vivo studies.
Conclusions
A new carrier based on chitosan was developed to formulate electrostatically complexed nanoparticles which are easily uptaken by cells at neutral pH and capable of delivering therapeutic siRNA molecules. The insertion of DIPEA groups in this polysaccharide resulted in siRNA nanoparticles with positive charge surfaces and enhanced colloidal stability at blood (pH 7.4) and slightly acid (pH 6.3) environmental conditions. This study showed that the association of DIPEA and PEG to highly deacetylated chitosan provides formulations with transfection-favorable characteristics at very low N/P ratios. The cell viability studies showed that, even after DIPEA and PEG substitutions, the polymers and their nanocarriers kept the non-cytotoxic characteristic of deacetylated chitosans, with cell viabilities higher than 80%. Confocal microscopy evaluations showed an efficient internalization of nanoparticles by RAW 264.7 macrophages—data that were supported by: the reduction of more than 60% on TNFα expression by macrophages; and, also, by the high silencing levels (up to 90%) of SSB mRNA on HeLa cells. Additionally, the composition of the nanocarriers proposed in the present study can be tuned to maximize the transfection efficiency and colloidal stability. Overall, it may be concluded that the chitosan DIPEA/PEG derivatives presented in this research are promising candidates for in vivo studies.
References
Mirzaei, S., Mahabady, M.K., Zabolian, A., et al.: Small interfering RNA (siRNA) to target genes and molecular pathways in glioblastoma therapy: current status with an emphasis on delivery systems. Life Sci. 275, 119368 (2021)
Mirzaei, S., Gholami, M.H., Hashemi, F., et al.: Employing siRNA tool and its delivery platforms in suppressing cisplatin resistance: approaching to a new era of cancer chemotherapy. Life Sci. 277, 119430 (2021)
Rossi, J.J., Rossi, D.J.: siRNA drugs: here to stay. Mol. Ther. 29, 431 (2021)
Tavakoli, N., Divsalar, A., Haertlé, T., et al.: Milk protein-based nanodelivery systems for the cancer treatment. J. Nanostruct. Chem. 11, 483–500 (2021)
Ashrafizadeh, M., Delfi, M., Hashemi, F., et al.: Biomedical application of chitosan-based nanoscale delivery systems: potential usefulness in siRNA delivery for cancer therapy. Carbohydr. Polym. 260, 117809 (2021)
Lavertu, M., Méthot, S., Tran-Khanh, N., Buschmann, M.D.: High efficiency gene transfer using chitosan/DNA nanoparticles with specific combinations of molecular weight and degree of deacetylation. Biomaterials 27, 4815 (2006)
Jennings, J.A., Bumgarden, J.D.: Chitosan Based Biomaterials Volume 2: Tissue Engineering and Therapeutics. Woodhead Publishing, Amsterdam (2017)
Alameh, M., Lavertu, M., Tran-Khanh, N.: siRNA delivery with chitosan: influence of chitosan molecular weight, degree of deacetylation, and amine to phosphate ratio on in vitro silencing efficiency, hemocompatibility, biodistribution, and in vivo efficacy. Biomacromol 19, 112–131 (2018)
Dehousse, V., Garbacki, N., Jaspart, S., et al.: Comparison of chitosan/siRNA and trimethylchitosan/siRNA complexes behaviour in vitro. Int. J. Biol. Macromol. 46, 342–349 (2010)
Soliman, O.Y., Alameh, M.G., De Cresenzo, G., et al.: Efficiency of chitosan/hyaluronan-based mRNA delivery systems in vitro: influence of composition and structure. J. Pharm. Sci. 109, 1581–1593 (2020)
Oliveira, F.D.P.P., Picola, I.P.D., Shi, Q., et al.: Synthesis and evaluation of diethylethylamine-chitosan for gene delivery: composition effects on the in vitro transfection efficiency. Nanotechnology 24, 055101 (2013)
Picola, I.P.D., Shi, Q., Fernandes, J.C., et al.: Chitosan derivatives for gene transfer: effect of phosphorylcholine and diethylaminoethyl grafts on the in vitro transfection efficiency. J. Biomater. Sci. Polym. Ed. 27, 1611–1630 (2016)
De Souza, R.H.F.V., Picola, I.P.D., Shi, Q., et al.: Diethylaminoethyl-chitosan as an efficient carrier for siRNA delivery: improving the condensation process and the nanoparticles properties. Int. J. Biol. Macromol. 119, 186–197 (2018)
Jones, C.H., Chen, C.K., Ravikrishnan, A., et al.: Overcoming nonviral gene delivery barriers: perspective and future. Mol. Pharm. 10, 4082–4098 (2013)
Giacomelli, F.C., Stepánek, P., Giacomelli, C., et al.: pH-triggered block copolymer micelles based on a pH-responsive PDPA (poly[2-(diisopropylamino)ethyl methacrylate]) inner core and a PEO (poly(ethylene oxide)) outer shell as a potential tool for the cancer therapy. Soft Matter 7, 9316–9325 (2011)
Zhou, K., Liu, H., Zhang, S., et al.: Multicolored pH-tunable and activatable fluorescence nanoplatform responsive to physiologic pH stimuli. J. Am. Chem. Soc. 134, 7803–7811 (2012)
Zhou, G., Xu, Y., Chen, M., et al.: Tumor-penetrating peptide modified and pH-sensitive polyplexes for tumor targeted siRNA delivery. Polym. Chem. 7, 3857–3863 (2016)
Cao, Y., Tan, Y.F., Wong, Y.S., et al.: Recent advances in chitosan-based carriers for gene delivery. Mar. Drugs 17, 381 (2019)
Tiera, M.J., Shi, Q., Winnik, F.M., Fernandes, J.C.: Polycation-based gene therapy: current knowledge and new perspectives. Curr. Gene Ther. 11, 288–306 (2011)
Blanco, E., Shen, H., Ferrari, M.: Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 33, 941–951 (2015)
Abbaszadeh, F., Moradi, O., Norouzi, M., Sabzevari, O.: Improvement single-wall carbon nanotubes (SWCNTs) based on functionalizing with monomers 2-hydroxyethylmethacryate (HEMA) and N-vinylpyrrolidone (NVP) for pharmaceutical applications as cancer therapy. J. Ind. Eng. Chem. 20, 2895–2900 (2014)
Partikel, K., Korte, R., Stein, N.C., et al.: Effect of nanoparticle size and PEGylation on the protein corona of PLGA nanoparticles. Eur. J. Pharm. Biopharm. 141, 70–80 (2019)
Tang, G.P., Zeng, J.M., Gao, S.J., et al.: Polyethylene glycol modified polyethylenimine for improved CNS gene transfer: effects of PEGylation extent. Biomaterials 24, 2351–2362 (2003)
Rheiner, S., Bae, Y.: Increased poly(ethylene glycol) density decreases transfection efficacy of siRNA/poly(ethylene imine) complexes. AIMS Bioeng. 3, 454–467 (2016)
Yang, C., Gao, S., Dagnæs-Hansen, F., et al.: Impact of PEG chain length on the physical properties and bioactivity of PEGylated chitosan/siRNA nanoparticles in vitro and in vivo. ACS Appl. Mater. Interfaces 9, 12203–12216 (2017)
Cabral, H., Miyata, K., Osada, K., Kataoka, K.: Block copolymer micelles in nanomedicine applications. Chem. Rev. 118, 6844–6892 (2018)
Agirre, M., Zarate, J., Ojeda, E., et al.: Low molecular weight chitosan (LMWC)-based polyplexes for pDNA delivery: from bench to bedside. Polymers 6, 1727–1755 (2014)
Du, B., Jiang, X., Huang, Y., et al.: Tailoring kidney transport of organic dyes with low-molecular-weight PEGylation. Bioconj. Chem. 31, 241–247 (2019)
Zhang, Y., Satterlee, A., Huang, L.: In vivo gene delivery by nonviral vectors: overcoming hurdles? Mol. Ther. 20, 1298–1304 (2012)
Tiera, M.J., Shi, Q., Barbosa, H.F.G., Fernandes, J.C.: Polymeric systems as nanodevices for siRNA delivery. Curr. Gene Ther. 13, 358–369 (2013)
Tamura, A., Yui, N.: Lysosomal-specific cholesterol reduction by biocleavable polyrotaxanes for ameliorating niemann-pick type C disease. Sci. Rep. 4, 1–8 (2014)
Wang, X., Cai, X., Hu, J., et al.: Glutathione-triggered “off-on” release of anticancer drugs from dendrimer-encapsulated gold nanoparticles. J. Am. Chem. Soc. 135, 9805–9810 (2013)
Adriaansen, J., Vervoordeldonk, M.J.B.M., Tak, P.P.: Gene therapy as a therapeutic approach for the treatment of rheumatoid arthritis: innovative vectors and therapeutic genes. Rheumatology 45, 656–668 (2006)
Ta, W., Chawla, A., Pollard, J.W.: Origins and hallmarks of macrophages: development, homeostasis, and disease. Nature 496, 445–455 (2013)
Pandi, P., Jain, A., Raju, S., Khan, W.: Therapeutic approaches for the delivery of TNF-α siRNA. Ther. Deliv. 8, 343–355 (2017)
Staudacher, A.H., Al-Ejeh, F., Fraser, C.K., et al.: The La antigen is over-expressed in lung cancer and is a selective dead cancer cell target for radioimmunotherapy using the La-specific antibody APOMAB®. EJNMMI Res. 4, 1–13 (2014)
Tiera, M.J., Qiu, X.P., Bechaouch, S., et al.: Synthesis and characterization of phosphorylcholine-substituted chitosans soluble in physiological pH conditions. Biomacromol 7, 3151–3156 (2006)
Martins, G.O., Petrônio, M.S., Lima, A.M.F., et al.: Amphipathic chitosans improve the physicochemical properties of siRNA-chitosan nanoparticles at physiological conditions. Carbohydr. Polym. 216, 332–342 (2019)
Gunn, J., Paranji, R.K., Zhang, M.: A simple and highly sensitive method for magnetic nanoparticle quantitation using 1H-NMR spectroscopy. Biophys. J. 97, 2640–2647 (2009)
Gabriel, J.D.S., Tiera, M.J., Tiera, V.A.D.O.: Synthesis, characterization, and antifungal activities of amphiphilic derivatives of diethylaminoethyl chitosan against Aspergillus flavus. J. Agric. Food Chem. 63, 5725–5731 (2015)
Richard, I., Thibault, M., De Crescenzo, G., et al.: Ionization behavior of chitosan and chitosan-DNA polyplexes indicate that chitosan has a similar capability to induce a proton-sponge effect as PEI. Biomacromol 14, 1732–1740 (2013)
Rai, R., Alwani, S., Badea, I.: Polymeric nanoparticles in gene therapy: new avenues of design and optimization for delivery applications. Polymers 11, 745 (2019)
Barreto, J.C.G., Tita, D.L., Orlandia, M.O.: Development of an automated method to perform a quantitative study of particle size distribution and the effect of a conductive layer in scanning electron microscopy. Quim. Nova 42, 447–452 (2019)
Fernandes, J.C., Qiu, X., Winnik, F.M., et al.: Low molecular weight chitosan conjugated with folate for siRNA delivery in vitro: optimization studies. Int. J. Nanomed. 7, 5833 (2012)
Fujita, S., Sakairi, N.: Water soluble EDTA-linked Chitosan as a zwitterionic flocculant for pH sensitive removal of Cu(II) ion. RSC Adv. 6, 10385–10392 (2016)
Fiamingo, A., Campana-Filho, S.P.: Structure, morphology and properties of genipin-crosslinked carboxymethylchitosan porous membranes. Carbohydr. Polym. 143, 155–163 (2016)
Zhang, K., Helm, J., Peschel, D.: NMR and FT Raman characterisation of regioselectively sulfated chitosan regarding the distribution of sulfate groups and the degree of substitution. Polymer 51, 4698–4705 (2010)
Zhang, J., Xia, W., Liu, P., et al.: Chitosan modification and pharmaceutical/biomedical applications. Mar. Drugs 8, 1962–1987 (2010)
Jain, A., Gulbake, A., Shilpi, S., et al.: A new horizon in modifications of chitosan: syntheses and applications. Crit. Rev. Ther. Drug Carr. Syst. 30, 91 (2013)
Thomas, D.P., William, C.E., Jennifer, L.S., Graham, T.J.: Cell Biology, 3rd edn. Elsevier, Philadelphia (2016)
Vermeulen, L.M., De Smedt, S.C., Remaut, K., Braeckmans, K.: The proton sponge hypothesis: fable or fact? Eur. J. Pharm. Biopharm. 129, 184–190 (2018)
Casey, J.R., Grinstein, S., Orlowski, J.: Sensors and regulators of intracellular pH. Nat. Rev. Mol. Cell Biol. 11, 50–61 (2010)
Doriti, A., Brosnan, S.M., Weidner, S.M., Schlaad, H.: Synthesis of polysarcosine from air and moisture stable N-phenoxycarbonyl-N-methylglycine assisted by tertiary amine base. Polym. Chem. 7, 3067–3070 (2016)
Serrano-Sevilla, I., Artiga, Á., Mitchell, S.G., et al.: Natural polysaccharides for siRNA delivery: nanocarriers based on chitosan, hyaluronic acid, and their deerivatives. Molecules 24, 2570 (2019)
Layek, B., Singh, J.: Chitosan for DNA and gene therapy. In: Jennings, J.A., Bumgarden, J.D. (eds.) Chitosan Based Biomaterials Volume 2: Tissue Engineering and Therapeutics, pp. 209–244. Woodhead Publishing, Amsterdam (2017)
Chen, H., Cui, S., Zhao, Y., et al.: Grafting chitosan with polyethylenimine in an ionic liquid for efficient gene delivery. PLoS ONE 10, e0121817 (2015)
Kean, T., Roth, S., Thanou, M.: Trimethylated chitosans as non-viral gene delivery vectors: cytotoxicity and transfection efficiency. J. Control. Release 103, 643–653 (2005)
Layek, B., Singh, J.: Caproic acid grafted chitosan cationic nanocomplexes for enhanced gene delivery: effect of degree of substitution. Int. J. Pharm. 447, 182–191 (2013)
Liu, X., Howard, K.A., Dong, M., et al.: The influence of polymeric properties on chitosan/siRNA nanoparticle formulation and gene silencing. Biomaterials 28, 1280–1288 (2007)
Yue, Z.G., Wei, W., Lv, P.P., et al.: Surface charge affects cellular uptake and intracellular trafficking of chitosan-based nanoparticles. Biomacromol 12, 2440–2446 (2011)
Makvandi, P., Chen, M., Sartorius, R., et al.: Endocytosis of abiotic nanomaterials and nanobiovectors: Inhibition of membrane trafficking. Nano Today 40, 101279 (2021)
Picola, I.P.D., Busson, K.A.N., Casé, A.H., et al.: Effect of ionic strength solution on the stability of chitosan-DNA nanoparticles. J. Exp. Nanosci. 8, 703–7016 (2013)
Behzadi, S., Serpooshan, V., Tao, W., et al.: Cellular uptake of nanoparticles: journey inside the cell. Chem. Soc. Rev. 46, 4218–4244 (2017)
Saptarshi, S.R., Duschl, A., Lopata, A.L.: Interaction of nanoparticles with proteins: relation to bio-reactivity of the nanoparticle. J. Nanobiotechnology. 11, 1–12 (2013)
Carlos, M.I.S., Zheng, K., Garrett, N., et al.: Limiting the level of tertiary amines on polyamines leads to biocompatible nucleic acid vectors. Int. J. Pharm. 526, 106–124 (2017)
Gormley, A.J., Ghandehari, H.: Evaluation of toxicity of nanostructures in biological systems. In: Sahu, S.C., Casciano, D.A. (eds.) Nanotoxicity: From In Vivo and In Vitro Models to Health Risks, pp. 115–159. Wiley, Hoboken (2009)
Kim, B., Park, J.H., Sailor, M.J.: Rekindling RNAi therapy: materials design requirements for in vivo siRNA delivery. Adv. Mater. 31, 1903637 (2019)
Hao, G., Xu, Z.P., Li, L.: Manipulating extracellular tumour pH: an effective target for cancer therapy. RSC Adv. 8, 22182–22192 (2018)
Guţoaia, A., Schuster, L., Margutti, S., et al.: Fine-tuned PEGylation of chitosan to maintain optimal siRNA-nanoplex bioactivity. Carbohydr. Polym. 143, 25–34 (2016)
Ping, Y., Liu, C., Zhang, Z., et al.: Chitosan-graft-(PEI-β-cyclodextrin) copolymers and their supramolecular PEGylation for DNA and siRNA delivery. Biomaterials 32, 8328–8341 (2011)
Germershaus, O., Mao, S., Sitterberg, J., et al.: Gene delivery using chitosan, trimethyl chitosan or polyethylenglycol-graft-trimethyl chitosan block copolymers: establishment of structure-activity relationships in vitro. J. Control. Release 125, 145–154 (2008)
Ragelle, H., Riva, R., Vandermeulen, G., et al.: Chitosan nanoparticles for siRNA delivery: optimizing formulation to increase stability and efficiency. J. Control. Release 176, 54–63 (2014)
Acknowledgements
This research was supported by a grant from the São Paulo Research Foundation, FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) grant n. 2017/10331-5. A. M. Martinez-Junior acknowledge the support of the National Council for the Improvement of Higher Education, CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) grant n. 2017/10331-5. A. M. Martinez-Junior and M. S. Petrônio acknowledge the support of FAPESP (grants 2019/27801-0 and 2015/05148-1). J. Fernandes and M. Benderour would like to thank the Ministère de l’Économie, de la Science et de l’Innovation du Québec, (PSR-SIIRI-960) and the Chaire de Recherche en Orthopédie de l’Université de Montréal à l’Hôpital du Sacré-Cœur de Montréal). The authors would also like to thank: the CMIB-UNESP (EMU-FAPESP project nº 2009/53989-4) for instrumentation access and Dr. F. R. de Moraes for his help with 13C NMR analysis; Dr. S. C. M. Agustinho and A. L. Tognon (MSc) for help with 1H NMR analysis (IQSC-USP); the LMA-IQ (UNESP) laboratory for providing SEM facilities and Dr. D. Tita for his assistance with Scanning Electron Microscopy; Dr. M. F. Lima (LQBOA Group-UNESP/IBILCE), Dr. M. P. S Cabrera (Peptides Research Group-UNESP/IBILCE) and Dr. C. R. B. Domingos (LHGDH Group-UNESP/IBILCE) for access to facilities and instrumentation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
MJT, JCF, AMMJ, RHFVS and MB have filed for a patent on the nanoparticles described in this study. The authors report no other conflicts of interest in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
40097_2022_487_MOESM1_ESM.docx
Supplementary file1 1H and 13C NMR spectra, dynamic light scattering measurements, cytotoxicity in RAW 264.7 cells, 3D confocal images, GPC chromatograms, and titrations data can be found in the supplementary information of the manuscript. (DOCX 5337 KB)
Rights and permissions
About this article
Cite this article
Martinez Junior, A.M., de Souza, R.H.F.V., Petrônio, M.S. et al. Double-grafted chitosans as siRNA nanocarriers: effects of diisopropylethylamine substitution and labile-PEG coating. J Nanostruct Chem 13, 605–624 (2023). https://doi.org/10.1007/s40097-022-00487-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40097-022-00487-0