Abstract
Tyro3, Axl, and Mertk (TAM) receptors are a subfamily of receptor tyrosine kinases. TAM receptors have been implicated in mediating efferocytosis, regulation of immune cells, secretion of inflammatory factors, and epithelial-to-mesenchymal transition in the tumor microenvironment, thereby serving as a critical player in tumor development and progression. The pro-carcinogenic role of TAM receptors has been widely confirmed, overexpression of TAM receptors is tied to tumor cells growth, metastasis, invasion and treatment resistance. Nonetheless, it is surprising to detect that inhibiting TAM signaling is not all beneficial in the tumor immune microenvironment. The absence of TAM receptors also affects anti-tumor immunity under certain conditions by modulating different immune cells, as the functional diversification of TAM signaling is closely related to tumor immunotherapy. Glioblastoma is the most prevalent and lethal primary brain tumor in adults. Although research regarding the crosstalk between TAM receptors and glioblastoma remains scarce, it appears likely that TAM receptors possess potential anti-tumor effects rather than portraying a total cancer-driving role in the context of glioblastoma. Accordingly, we doubt whether TAM receptors play a double-sided role in glioblastoma, and propose the Janus-faced TAM Hypothesis as a conceptual framework for comprehending the precise underlying mechanisms of TAMs. In this study, we aim to cast a spotlight on the potential multidirectional effects of TAM receptors in glioblastoma and provide a better understanding for TAM receptor-related targeted intervention.
Video Abstract
Similar content being viewed by others
Introduction
Glioblastoma (GBM) is the most common and fatal primary brain tumor in adults and has a preference for occuring in men and the elderly. GBM accounts for 45.2% of primary malignant brain tumors, with the annual incidence of approximately 3 people per 100,000 person worldwide [1, 2]. GBM develops as a result of a malignant transformation of astrocytoma and represents the most high-grade malignancy of glioma (World Health Organization (WHO) grade IV) [3]. Additionally, GBM is characterized by strong invasiveness, high rates of recurrence and poor sensitivity to therapeutics [4, 5]. In recent decades, continuous advances have been made in the treatment of GBM, including maximal surgical resection, concurrent chemoradiation therapy, adjuvant temozolomide (TMZ) or carmustine wafers, bevacizumab targeted therapy and immunotherapy [6,7,8,9]. However, despite the current aggressive treatment protocol, no remarkable improvements have been obtained with regard to the survival rate of GBM patients. Overall, the 2 and 5-year survival rates are still only 27% and 9.8%, respectively, with a mean overall survival of approximately 15 months [9,10,11].
With newer discoveries and a more in-depth study of cancer immune evasion mechanisms, immunotherapy is appeared to be an effective therapeutic option, in addition to traditional surgery, radiotherapy and chemotherapy [12, 13]. Correspondingly, the successive emergence of immune checkpoint inhibitors (ICIs), such as programmed cell death 1 (PD-1), programmed cell death 1 ligand 1 (PD-L1) and cytotoxic lymphocyte antigen 4 (CTLA4) has achieved a remarkable breakthrough in immuno-oncology [14,15,16]. Therefore, immunotherapy holds great promise for the treatment of aggressive and malignant GBM, particularly considering that the traditional treatments of GBM are restricted [8, 17, 18]. To date, a myriad of clinical trials concerning GBM immunotherapy have been conducted on a large global scale [19, 20]. Unfortunately, no obvious clinical benefit has been observed thus far [19, 21].
Tyro3, Axl, and Mertk (TAM) receptors are significant players in both the immune and nervous system [22]. Vast literature data indicates the autonomous tumorigenic effect of TAM receptors in tumor immunity microenvironment (TIME), thus TAM inhibition has been explored as a potential anti-tumor strategy a decade ago [23,24,25]. Similarly, in recent years, the crosstalk between TAM receptors and GBM has increasingly attracted widespread attention. The upregulation of TAM signaling is usually associated with GBM development, progression and poor prognosis [26,27,28]. Plentiful studies have reached a consensus that TAM receptors have an immunosuppressive and carcinogenic role in the progression of GBM [27, 29,30,31]. Accordingly, a myriad of clinical trials regarding the specific small molecule inhibitors of Axl in the treatment of recurrent GBM have been registered on clinicaltrials.gov and are currently underway, and many combined treatments of anti-TAM therapy and other immunotherapeutic have been carried out [32].
However, most contemporary research focuses on the impact of TAM receptors on tumors, the exploration of changes in tumor immunity remains limited. In this complicated tumor microenvironment (TME), it is almost impossible that one tyrosine receptor kinases (RTKs) family has only one direction of influence on cancer development. Correspondingly, not all evidence supports that blockage of TAM signaling will favor an anti-tumor TME [33]. Especially in some inflammation-driving tumors, TAM blockers may even cause tumor-promoting effects [34]. In addition, it is a surprising finding that TAM signaling serves as adjusters of cancer-related endothelial recruitment, restraining tumor growth through the inhibition of angiogenesis [35]. Importantly, in the context of GBM, the highly malignant and refractory brain tumor that warranting pioneering ideas and span-new treatment strategies, TAM inhibitors have been researched widely. However, several controversial areas remain, including the continued reports that TAM receptor inhibitors have a limited therapeutic effect on GBM and some patients with better prognosis overexpress Mertk receptors [36, 37]. Moreover, recent studies have found that inhibiting inflammation has the potential to substantially prevent the progression of GBM [38]. The emergence of these contradictory observations makes us wonder whether TAM receptors play a dual role in GBM? Therefore, more detailed cellular and molecular mechanisms are urgently needed to further clarify the role of TAM receptors in GBM, so that more precise interventions can be made.
High heterogeneity and special immune microenvironment of glioblastoma
GBM is the most aggressive and common primary malignant brain tumor in adults [2]. Recent high-throughput data have revealed a wide range of genetic and epigenetic alterations in GBM [39]. According to gene expression profiles, researchers have divided GBMs into multiple different subgroups. Genetic alterations are widespread in GBM, including commonly the loss of heterozygosity (LOH) at 10q, isocitrate dehydrogenase (IDH) mutations, O6-methyl guanine-DNA methyltransferase (MGMT) promoter methylation, epidermal growth factor receptor (EGFR) amplification, tumor protein 53 (TP53) mutations [40, 41]. These alterations represent the histological and morphological hallmarks of GBM, encompassing numerous abnormal cell types, increased cell density, local necrosis, and formidable angiogenesis [42]. Gene expression changes and deregulated genetic pathways are also closely related to the biological behavior of the tumor (e.g., rapid proliferation, abnormal differentiation and angiogenesis) and resistance to treatments [43, 44]. These diverse heterogeneity contribute to the difficulty of GBM treatment.
Different from other solid tumors, GBM, which belongs to central nervous system (CNS) tumors, has a unique neuro-immunology known as “immunological privilege” [45, 46]. Besides, the GBM microenvironment lacks lymphocyte infiltration and activated T cells, forming an immunosuppressive TME [46, 47]. Furthermore, the existence of the blood–brain barrier (BBB) prevents chemotherapeutic and immunotherapeutic agents from reaching the tumor site or reaching effective therapeutic concentrations, which is also a potential reason for the failure of some current clinical trials [48, 49]. However, in recent years, with the discovery of the CNS lymphatic vessels [50] and the development of new delivery strategies, e.g., nanoparticle-based drug delivery system [51, 52], for CNS tumor, pharmacotherapy targeting GBM across the BBB seems to be promising [42, 47].
Overview of TAM receptors
General features of TAM receptors and their ligands
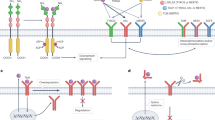
TAM receptors, a subgroup of RTKs family, consist of Tyro3, Axl, and Mertk receptors [53,54,55,56]. TAM receptors are distinguished from other RTKs due to the presence of a unique conserved sequence in their kinase domain named KW(I/L)A(I/L)ES and a distinctive extracellular domain, which combines two N-terminal immunoglobulin-like domains followed by two fibronectin type-III (FN-III) domains [53,54,55]. Therefore, due to the presence of its unique domain, Axl, a 140 kDa protein, was first identified in 1991 [53]. Subsequently, Tyro3 and Mertk were also identified [57, 58]. The two most well-known ligands for TAM receptors include growth arrest-specific 6 (Gas6) and protein S (ProS), which act as bridging factors for TAM receptors indirectly combine with phospholipids including externalized phosphatidylserine (PtdSer, a phospholipid localized in the plasma membrane) on apoptotic cells [59, 60]. The N-terminal gamma-carboxyglutamic acid domain structure of ligands interact with PtdSer,while their laminin G (LG) domains bind to the extracellular immunoglobulin-like domains of TAM receptors, opsonizing downstream TAM signaling functions (Fig. 1) [61, 62]. ProS is only able to bind to Tyro3 and Mertk, Gas6 can bind to all three TAM receptors (Axl > Tyro3 >>> Mertk), whereas, in a specific tumor microenvironment such as the presence of PtdSer, Mertk and Tyro3 are hyperactivated but their affinities for Gas6 are lower than Axl [60, 62, 63].
The structural features of TAM receptors and their ligands. TAM receptors have a unique intracellular protein tyrosine kinase domain (named KW(I/L)A(I/L)ES) that distinguishes them from other RTKs. TAM receptors use their extracellular N-terminal immunoglobulin-like domains to bind laminin G (LG) domains of TAM ligands. Gas6 and ProS are the most well-known soluble circulating proteins ligands for TAM receptors, which own a similar N-terminal gamma-carboxyglutamic acid domain structure. TAM receptors, Tyro3, Axl and Mertk receptors; RTKs, receptor tyrosine kinases; Gas6, growth arrest-specific 6; ProS, protein S; FNIII, fibronectin type-III domains. LG domains, laminin G domains; EGF-like domains, epidermal growth factor-like domains
TAM receptors are widely expressed in human cells, especially in hematopoietic cells, and carry out similar functions [64,65,66,67]. They have also been reported to be expressed in the CNS [22, 68, 69], reproductive system [70, 71] and immune system [64, 72]. Interestingly, TAM receptors have been reported to be overexpressed in myeloma cells and acute myeloid leukemia patients as early as two decades ago [73, 74], and have even been shown to participate in disease progression [75].
The role TAM receptors play in cancer development and immune regulation
Previous studies reveal that TAM receptors regulate the occurrence and development of various diseases [26, 76,77,78]. There is large quantity evidence showing a relation between autoimmune diseases and abnormal expression of TAM receptors [79, 80]. The absence of the TAM signaling pathway prevents the optimal phagocytosis of apoptotic cells, bringing about disarray in homeostasis that results in autoimmune diseases [79, 81]. The role of TAM signaling has been detected in autoimmune diseases such as rheumatoid arthritis (RA) [82], systemic lupus erythematosus (SLE) [83, 84] and multiple sclerosis (MS) [85]. Furthermore, TAM receptors are also closely associated with several types of human cancer [72]. In particular, high expression of Axl receptor has been observed in advanced colorectal cancer [86], prostate cancer [87] and osteosarcoma [77, 88], which correlates with advanced tumor cell invasion and migration. Moreover, Tyro3 and Mertk receptors have also been found to be upregulated in various tumors such as leukemia and melanoma [74, 89,90,91]. However, surprisingly, TAM receptors seem to have a two-tier regulatory effect: on the one hand, they promote tumorigenesis and progression; on the other hand, they are implicated in the anti-tumor response of different immune cells [72]. Besides, a report by Wium M et al. showed that increased TAM signaling pathway activity was associated with drug resistance, an unfavorable prognosis, and metastasis in cancer patients, while the loss of TAM receptor functions led to the development of autoimmune diseases [26]. Accordingly, TAM receptors play a significant and paradoxical role in oncogenesis and immune regulation.
The role of TAM receptors in immuno-oncology
TAM receptors and efferocytosis
TAM receptors are primarily expressed on myeloid hematopoietic cells, including antigen-presenting cells (APCs, such as macrophages and dendritic cells (DCs)), natural killer (NK) cells and platelets [92,93,94]. In addition to having a significant function in autoimmune disease [80], overexpression of TAMs in various cancers exerts essential roles in macrophage polarization and efferocytosis [25, 95]. Efferocytosis is defined as the process of using phagocytes to accurately recognize and engulf apoptotic cells [96,97,98]. Apoptosis refers to programmed cell death under physiological or pathological conditions, and phagocytes are capable of recognizing and engulfing apoptotic cells in order to maintain the integrity of the cell membrane and avoid secondary necrosis [96, 99].
TAM receptor-mediated efferocytosis was first detected in mice macrophages [71]. Since the overexpression of one or more TAM receptors was identified in various tumor tissues [25], TAM receptor-mediated efferocytosis in the TME has been widely studied, especially Mertk-mediated efferocytosis [100]. Efferocytosis has tumor-promoting functions, including immunosuppression, metastasis and treatment resistance [101]. Efferocytosis is initiated by recognizing apoptotic cells that emit a "find me" signal, thus promoting the aggregation of surrounding phagocytes, including macrophages, monocytes and DCs [102, 103]. Following the “find me” signal, the cell starts to display an "eat me" signal on its surface, urging phagocytes to precisely recognize apoptotic cells [102, 104]. The most extensively studied "eat me" signal is PtdSer. During apoptosis, PtdSer can be transferred from the inner leaflet of the plasma membrane to the outer leaflet [105]. Once there, PtdSer interacts with the bridge ligand Gas6/ProS1, thus indirectly binding to the TAM receptors on the surface of phagocytes (Fig. 2) [62, 106]. As phagocytic receptors, TAM receptors perform different functions, but the activation of Axl and Mertk receptors kinase are indispensably dedicated to the PtdSer-dependent phagocytosis of apoptotic cells [107]. Researchers have found that PS-targeting antibody partially inhibited TAM receptors–mediated efferocytosis [60]. Studies have demonstrated that Axl and Mertk-mediated efferocytosis restrain the innate immune response in macrophages and DCs [103, 108], which creates a TME conducive to tumor development and metastasis [105, 109]. Following the identification and binding of phagocytes to apoptotic cells, TAM receptors become phosphorylated, which leads to the activation of downstream signaling pathways and regulation of cytoskeletal rearrangements, resulting in the engulfment of apoptotic cells [99].
TAM receptor-mediated efferocytosis. Efferocytosis is the process of using phagocytes to accurately recognize and engulf apoptotic cells. Apoptotic cells send out "find me" signals (e.g., lipids, proteins, and peptides) and "eat me" signals (e.g., PtdSer) to promote the recruitment of phagocytes. PtdSer of the apoptotic cells migrates to the outer leaflet of the plasma membrane during apoptosis, interacting with the bridge ligand Gas6/ProS1 to indirectly bind to TAM receptors on the surface of phagocytes. Subsequently, phosphorylation of TAM receptors activates PI3K/Akt and other downstream signaling pathways, regulates cytoskeletal rearrangements to engulf the apoptotic cells, and leads to M2 macrophages polarization, production of anti-inflammatory cytokines. PtdSer, phosphatidylserine; PI3K/Akt, phosphatidylinositol 3 kinase/protein serine threonine kinase; M2 macrophages, M2-like phenotype of macrophages
Post-engulfment, under the action of various cytokines, tumor-associated macrophages lean towards M2 macrophage polarization, a wound-healing phenotype, and halting of their anti-tumor immunity [96, 100]. Phosphatidylinositol 3 kinase (PI3K)/protein serine threonine kinase (Akt) pathway is the most common downstream signaling pathway following TAM receptor phosphorylation and contributes to the macrophage polarization [25]. Mechanically, TAM receptors can directly bind to a subunit of PI3K, which causes PI3K to phosphorylates Akt. This leads to macrophage polarization towards an M2 phenotype while dampening polarization of M1 macrophages [110, 111]. On one hand, M2 macrophages encourage the secretion of immunosuppressive cytokines such as transforming growth factor β (TGF-β), interleukin (IL)-10, and IL-13, which recruit regulatory T cells and suppress the response of CD4+ and CD8+ effector T cells in the TME. On the other hand, they downregulate the expression of pro-inflammatory cytokines such as tumor necrosis factor α (TNF-α), IL-6, IL-12, IL-15, and IL-18 [100, 112]. This change in the expression of cytokines sets up an immunosuppressed TME and promotes tumor progression and invasion, which correlates with poor survival [113].
In addition, the process of efferocytosis can, in turn, upregulates the expression of TAM receptors in tumor APCs, inducing their polarization to immunosuppressive phenotype [100]. The transform in APC phenotypes leads to weakened antigen presentation to T cells, lessened activation of T cells, undermined the effect of antigen-dependent anti-tumor immunity, yielding a more aggressive and tolerogenic TME [100, 114]. Extensive studies have established a consensus that the expression and function of TAM receptors are related to tumor progression, poor survival and drug resistance [22, 23, 25]. Furthermore, Keating AK et al. have identified that knockdown of Mertk and Axl receptors enhances the apoptotic response and drug-sensitivity of astrocytoma cells [115]. Overall, the immunosuppression and pro-tumor environment induced by TAM receptor-mediated efferocytosis play an essential role in immuno-oncology.
TAM receptors regulate PD-L1/PD-L2 expression and are associated with resistance to anti-PD-1 therapy
PD-1 receptor, which is expressed on tumor-infiltrating activated T cells, binds to the ligands PD-L1 and PD-L2 present on APCs. This binding leads to negative regulation of tumor-reactive T cell activation and a weakening of the anti-tumor T cell responses [116,117,118]. In various kinds of human cancers, it is well-known that PD-1 or PD-L1 and PD-L2 are negative prognostic factors [119,120,121]. Over the years, in cancer-immunotherapy, studies have found that TAM receptors play key roles in modulating PD-1 axis-related immune checkpoint signals [122].
In 2014, researchers identified that Mertk induces upregulation of PD-L1 transcription in apoptotic cells, which subsequently regulates Mertk-mediated efferocytosis and immune balance for tumor progression [95]. Next, surprisingly, researchers discovered that PtdSer potentiates the effects of PD-L1 signaling to T cells, thus proving the existence of a PtdSer-TAM-PD-L1-PI3k/Akt signaling axis in breast cancer, which contributes to tumor immune escape and chemoresistance [60]. In addition, Mertk significantly upregulated the expression of the coinhibitory ligands PD-L1 and PD-L2 on monocytes/macrophages in the leukemia microenvironment [123]. Inversely, Mertk blockers downregulated the PD-1 receptor on T cells and subsequently induced the activation of tumor-infiltrating T cells, yielding the anti-leukemia immunity [123]. Similarly, Axl was detected to promote epithelial-mesenchymal transition (EMT), which is associated with resistance to anti-PD-1 therapy in metastatic melanoma [124]. A recent analysis has demonstrated that through Axl and PI3K signaling, PD-L1 expression has increased in HPV-negative head and neck squamous cell carcinoma (HNSCC) cells, which correlates with radiotherapy resistance, leading to local treatment failure and higher mortality in HNSCC [125].
Therefore, anti-TAM strategy combined with anti-PD-1/PD-L1 therapeutics represents a novel direction for immune checkpoint inhibitor therapeutics in immune-oncology.
TAM receptors and associated anti-tumor responses
As mentioned earlier, TAM receptors exert a significant tumorigenic role across a variety of tumors [126]. However, continued studies have revealed that TAM receptor-mediated signaling wields new inhibitory roles in tumor development and angiogenesis [34, 35, 127].
In 2017, Lee EH et al. made a surprising discovery that Axl receptors generate effective anti-tumor immunity by upregulating the expression of LIGHT in the T lymphoma TME [127]. LIGHT, a member of TNF superfamily ligand, is a 29 kDa transmembrane protein produced by activated T cells and can compete with herpesvirus envelope glycoprotein D (gD) to bind T-cell herpesvirus entry mediator (HVEM) receptors [128, 129]. LIGHT exerts its immunomodulatory effect by promoting T lymphocyte infiltration, enhancing T-cell proliferation and cytokine secretion and thereby inhibiting tumor growth and progression [130, 131]. In mouse EL4-Axl T lymphoma cells, Axl receptors mediate the expression of LIGHT through the PI3K/Akt/Sp1 axis and promote the secretion of immunocyte regulatory factors such as chemokine C–C motif ligand 5 (CCL5) and its receptor CCR5, thereby enhancing cytotoxic T lymphocytes (CTLs) and NK cells activity in the TME, leading to the suppression of tumor [127].
It is well-known that Axl and Mertk promote the formation of an immunosuppressive microenvironment and tumor evasion immunity by reducing the release of pro-inflammatory factors and inhibiting anti-inflammatory response in the TME [96, 113]. However, evidence also suggests that particular inflammatory conditions can affect tumor promotion [132]. Hence, the definite impact of immuno-inflammatory responses on tumorigenesis remains elusive [133]. Due to its paradoxical nature, researchers have also demonstrated that Axl and Mertk reduce the release of pro-inflammatory factors and limit the phagocytosis of apoptotic neutrophils, thereby inhibiting long-term chronic tumor-promoting inflammation and lowering the incidence of colorectal cancer [34]. Moreover, the inhibitory mechanism of Gas6/TAM in intestinal tumors has also been gradually discovered. Interestingly, one study revealed that Gas6–/– mice possessed stronger azoxymethane/dextran sulfate sodium (DSS)-induced tumorigenesis and poor survival, compared to Gas6+/+ mice. The inhibitory effect of Gas6 on intestinal tumors may be related to the suppression of colonic stromal cellular immune response [33]. This study demonstrated that an increase in local Gas6 can activate TLR/Gas6/TAM signaling, limiting the secretion of pro-inflammatory factors such as TNF-α, CXCL1 and CCL2 and the activation of NF-κB. Expression of the pro-tumor factors c-Myc and Cox2 (Ptgs2), which are downstream of NF-κB, were also downregulated. In addition, this pathway also induces the activation of SOCS1/3 and inhibits the immune response of cells that derive from stromal monocyte lineage (such as macrophages) in order to limit intestinal inflammation [33]. In conclusion, Gas6/TAM signaling has been demonstrated to reduce local immune inflammatory responses through the mechanisms outlined above, exert potential intestinal tumor suppression, and prolong the survival of colorectal cancer patients.
In addition, the expression of TAM receptors has also been detected in vascular endothelial cells and vascular smooth muscle cells [82]. Gas6/Axl signaling is involved in vascular homeostasis and function via downstream PI3K/Akt signaling [134]. Pros/Mertk signaling is engaged in the aggregation, proliferation, migration, invasion of endothelial cell, moreover, TAM signaling inhibits endothelial cell recruitment and angiogenesis, represses vascular endothelial growth factor (VEGF) receptor 2–mediated vascularization [135]. Therefore, TAM signaling exhibits a potential inhibitory role in tumor development through the hindrance of angiogenesis [35, 135].
Taken together, TAM receptors may trigger anti-tumor immune responses by activating downstream pathways that enhance the anti-tumor activity of immune cells, lessen local inflammation against inflammation-driving cancers, and impede tumor angiogenesis [35, 135]. Therefore, the overall impact of TAM signaling on carcinogenesis may rest with the combination of all the distinctive cell responses in TME (Fig. 3). In this context, TAM inhibitors may be counterproductive and even promote tumor progression [33].
The Role of TAM Receptors in Immuno-oncology. TAM receptors play a bidirectional role in immuno-oncology, not only promoting tumorigenesis but also activating anti-tumor activity. TAM receptors, Tyro3, Axl, and Mertk receptors; TGF-β, transforming growth factor β; IL, interleukin; M2 macrophages, M2-like phenotype of macrophages; PD-L1, programmed cell death 1 ligand 1; PD-L2, programmed cell death 1 ligand 2; CTLs, cytotoxic T lymphocytes; NK cells, nature kill cells
Are TAM receptors foes or friends in glioblastoma?
In the past several years, multiple reports have demonstrated the significant roles of TAM receptors in GBM development and prognosis. Thus, they are attractive as innovative therapeutic targets (Table 1) [23, 136, 137]. As limited research concerning TAM receptors in GBM has been conducted, their specific mechanisms of action have not been thoroughly understood yet. Considering the aforementioned contradictory experimental observations concerning TAM receptors in carcinogenesis, we wonder whether TAM receptors are foes or friends in GBM? Is it possible that TAM signaling plays a dual role in GBM?
TAM receptors as foes for glioblastoma patients
Over a decade ago, researchers discovered that TAM receptors and related ligands were highly expressed and activated in GBM tissue, and this was associated with poor prognosis [115, 138]. Nowadays, with the development of clinical utilization of TAM inhibitors, specific blockers of TAM receptors for GBM are gradually entering the field of vision.
In particular, the role of Mertk receptor has been extensively described and has emerged as an attractive therapeutic approach in GBM [139]. Blocking Mertk signals, which creates a pro-inflammatory anti-tumor environment by reducing M2 macrophage polarization, hinders GBM survival and destroys tumor cells [140]. Furthermore, reports have also described that the activation of Mertk plays an important role in GBM growth and invasion, which is the reason why a variety of Mertk inhibitors have been developed to effectively promote cell autophagy and apoptosis and significantly increase the chemosensitivity of GBM to temozolomide [115, 136]. Additional research has demonstrated that overexpression of Mertk receptors in GBM can enhance the infiltration and anti-apoptotic activity of tumor cells [141]. Interestingly, the literature reveals that Mertk signaling mediates the migration of GBM cells and alters cellular morphology, leading to therapeutic resistance in GBM [142].
Similar to Mertk, the role of Axl has also been studied in the context of GBM. Axl and Gas6 are upregulated in gliomas and involved in neovascularization of GBMs, leading to poor prognosis in patients with GBM and reduced recurrence/progression time from 9 to 4 months [143]. Sadahiro H et al. first detected that ProS1-mediated Axl signaling, which not only mediates progress and survival of glioma stem cells but also regulates the immune microenvironment, results in aggressive GBM progression [122]. In a GBM model, Wang J et al. demonstrated that knockdown of Axl receptor increases TMZ sensitivity and decreases tumorigenesis. Moreover, exogenous Axl upregulation induces TMZ resistance [144]. Therefore, some Axl-targeted inhibitors have been found to effectively block the invasion and migration of GBM [145, 146], and even improve apoptotic response and chemosensitivity [115].
In conclusion, dysfunction of the expression, activation and regulation of TAM family members has been confirmed in GBM. Although the research regarding the specific mechanism of TAM signaling in GBM remains limit, its close relationship with the development, metastasis, prognosis and treatment resistance of GBM has been extensively testified. Thus, To a certain extent, TAM receptors act as foes in the TME of GBM.
TAM receptors as friends for glioblastoma patients
Interestingly, TAM receptors are not totally harmful to GBM patients. In fact, some studies have gradually manifested the potential inhibitory roles of TAM receptors on GBM progression.
Current research believes that pan-RTK inhibitors (such as Sunitinib), which simultaneously target multiple RTKs, have a better clinical treatment effect in GBM [147, 148]. Surprisingly, a study by Martinho O et al. recently identified that activation of Axl by its ligand can modulate the response of sunitinib, causing Axl-positive GBM cell lines to become more sensitive to sunitinib [36]. Therefore, Axl has emerged as having a novel role as a sunitinib response modulator.
Skoda et al. analyzed the HGG-02 GBM cell line derived from a patient who experienced a favorable survival outcome and whose event-free survival was nearly 34 months. They observed a significant upregulation of Mertk receptor and down-regulation of Axl phosphorylation in the HGG‑02 cells [37], although a large number of studies have shown that Mertk is correlated with poor prognosis of GBM patients [139]. In brief, this paradoxical results implied that the comprehensive effect of TAM receptors on oncogenesis might rely on the combination of the complicated immune response.
As mentioned earlier, TAM receptors play a tumor-suppressive role in inflammation-mediated tumors [34]. Interestingly, a similar situation may exist in GBM. The notion of immune privilege of the CNS has been reached a consensus for decades. Whereas, in recent years, CNS lymphatics, such as meningeal lymphatic vessels, have been discovered as drainage channels between the CNS and peripheral immune system [50, 149]. Therefore, it provides a possibility for peripheric immune cells, such as T cells and NK cells, to enter into the CNS, which has been demonstrated in the pathological process of meningiomas [150]. Notably, inflammation is an important stimulating factor of GBM [151], and researchers have demonstrated that inhibiting the inflammatory microenvironment in GBM can effectively repress tumor cells proliferation, migration and angiogenesis by activating microRNA-93 [38]. Therefore, TAM receptors, which have been reported to play anti-inflammatory and immunosuppressive roles in the TIME of GBM [122], perhaps have the potential to obstruct the progression of GBM through the regulation of TIME.
On the whole, TAM receptors may be friends in GBM under certain conditions. The positive role of TAM receptors in GBM seems to be confined, on account of numbered studies on the specific mechanism of TAM signaling. However, the contradictory results provide us with innovative ideas of the role of TAM signaling in GBM. As a consequence, the ambivalent role of TAM receptors in GBM needs to be further researched. It is signally important to further clarify the cellular and molecular mechanisms of TAM signaling in GBM.
Janus-faced TAM hypothesis
Overall, TAM receptors exert multifarious roles in immunity modulation, homeostasis maintenance and tumor progression, in addition to serving as oncogene signals and predictors of poor prognosis in cancer [126, 152]. As is well-known, in various types of tumors, including GBM, the upregulation of TAM signaling is closely linked to tumor invasiveness, metastasis, therapeutic tolerance and poor prognosis [26,27,28]. Therefore, for treatment of GBM, analogous to other tumors, various TAM inhibitors have been developed and implemented to regulate the immune microenvironment, limit tumor progression, and restore the sensitivity to treatment [115, 142, 153].
In inflammation-driving tumors, e.g., colorectal cancer, it has been shown that the Axl and Mertk receptors have potential cancer suppression effects [64]. They create an inflammation-suppressive immune TME through the TAM signaling pathway, regulate the secretion of immune regulatory factors and activate immune cells, thereby resulting in tumor suppression [34]. In GBM, the mechanism of tumor-promoting inflammation has not yet been detailedly revealed. However, recent studies have shown that inflammation is a driver of GBM, and inhibiting inflammation can effectively curb tumor invasion [38, 151]. Therefore, in the context of GBM, the immunosuppressive and anti-inflammatory TAM receptors may also portray an anti-tumor role under certain circumstances. Besides, TAM signaling has potential anti-tumor effects by suppressing angiogenesis [35], a necessary condition for tumor nutrition, metabolism and metastasis, although this anti-angiogenic efficacy has not been verified in GBM.
As is mentioned above, it is interesting that researchers detected that Axl is a modulator in GBM, as it regulates the therapeutic sensitivity of GBM cells to sunitinib [36]. Researchers have also detected that the expression of Mertk is upregulated in a GBM patient with a good prognosis [37]. Importantly, Tyro3 is relatively highly expressed in CNS compared with Axl and Mertk [27], however, there are less pointed studies. Hence, some potential regulative mechanisms may not have been discovered yet in TIME.
Overall, in immuno-oncology, TAM receptors have received extensive attention. However, their specific mechanisms and predictive biomarkers of efficacy remain to be fully elucidated. In this study, we discuss the potential dual effect of TAM receptors and put forward a Janus-faced TAM Hypothesis to understand the potential two-tier role of TAM receptors in GBM, thus providing a fresh perspective for the treatment of this aggressive tumor. On one hand, they motivate GBM immune escape and resistance to therapy. On the other hand, they have the potential to activate GBM-related immune cells and inhibit tumor angiogenesis, thus yielding anti-tumor effects and prolonging survival.
Conclusion and perspectives
TAM receptors are widely expressed in human cells and are upregulated in various tumors. They can indirectly bind to PtdSer through bridging ligand to mediate efferocytosis, which induces an immunosuppressive environment for tumor survival and growth. Moreover, TAM receptors upregulate the expression of PD-L1 and PD-L2 and increase resistance to anti-PD-1 therapy. However, they can also portray anti-tumor roles by modulating the activity of immune cells and inhibiting angiogenesis. Similarly, in the context of GBM, TAM receptors seem to be a key player in tumor cell growth, metastasis, invasion, and treatment-resistance. Nevertheless, conflicting observations and complicated TME imply that TAM receptors may not only play a one-way cancer-promoting effect in GBM. They positively modulate the therapeutic sensitivity of pan-RTK inhibitors. More importantly, they impede tumor angiogenesis and even also induce anti-tumor immune response under certain conditions. Accordingly, we first propose the Janus-faced TAM Hypothesis to uncover the potential bidirectional role of TAM receptors in GBM and provide a new research direction for this highly malignant and refractory glioma.
TAM-dependent immunomodulatory functions are attractive strategies for cancer immunotherapy. Nevertheless, the different regulatory roles of TAM receptors are dependent on the intricate cellular context. Tumor status, level of inflammation, and the type of immune cells in TME may possess a paradoxical dual role during the treatment of different tumors. Actually, most clinical trials regarding TAM receptors target Axl. In view of the context-dependent characteristics of TAM and the unique molecular signaling mechanism of the three receptors, targeting one or a combination of multiple TAM receptors may have different therapeutic effects, which indeed warrants necessary further research in the future. Hence, due to the duality of TAM receptors, future studies will have to focus on how to determine the sensitivity of selected patients, the efficacy-associated predictive biomarkers, and how to implement precise treatment. Besides, although it has been reported that TAM receptors can be revitalized by a virus infection, and TAM agonists play potential roles in preventing viral encephalitis [134], how to specifically activate TAM receptors is still a challenge.
Furthermore, TAM receptors are involved in the PD-1 axis-related therapeutic resistance and regulate diverse immune cells to exert anti-tumor immunity in selected tumors. Yet, these mechanisms have not been discovered in GBM. Consequently, further research is needed. Additionally, the highly intratumoral heterogeneity lead GBM insensitive to single-targeted therapy or single-agent therapy, so appropriate drugs combination with TAM-targeted therapy is worth exploring. Therefore, combined treatment with another immunotherapy such as anti-PD-L1 treatment appears promising for TAM-based cancer immunotherapy.
Availability of data and materials
Not applicable.
Abbreviations
- TAM:
-
Tyro3, Axl, and Mertk receptors
- GBM:
-
Glioblastoma
- WHO:
-
World Health Organization
- TMZ:
-
Temozolomide
- ICIs:
-
Immune checkpoint inhibitors
- PD-1:
-
Programmed cell death 1
- PD-L1:
-
Programmed cell death 1 ligand 1
- CTLA4:
-
Cytotoxic lymphocyte antigen 4
- TIME:
-
Tumor immunity microenvironment
- TME:
-
Tumor microenvironment
- RTKs:
-
Receptor tyrosine kinases
- LOH:
-
Loss of heterozygosity
- IDH:
-
Isocitrate dehydrogenase
- MGMT:
-
O6-methyl guanine-DNA methyltransferase
- EGFR:
-
Epidermal growth factor receptor
- TP53:
-
Tumor protein 53
- CNS:
-
Central nervous system
- BBB:
-
Blood–brain barrier
- FN III domains:
-
Fibronectin type-III domains
- Gas6:
-
Growth arrest-specific 6
- ProS:
-
Protein S
- PtdSer:
-
Phosphatidylserine
- LG domains:
-
Laminin G (LG) domains
- RA:
-
Rheumatoid arthritis
- SLE:
-
Systemic lupus erythematosus
- MS:
-
Multiple sclerosis
- APCs:
-
Antigen presenting cells
- DCs:
-
Dendritic cells
- NK cells:
-
Natural killer cells
- M2 macrophages:
-
M2-like phenotype of macrophages
- PI3K/Akt pathway:
-
Phosphatidylinositol 3 kinase/protein serine threonine kinase pathway
- M1 macrophages:
-
M1-like phenotype of macrophages
- TGF-β:
-
Transforming growth factor β
- IL:
-
Interleukin
- TNF-α:
-
Tumor necrosis factor α
- EMT:
-
Epithelial-mesenchymal transition
- HPV:
-
Human papillomavirus
- HNSCC:
-
Head and neck squamous cell carcinoma
- gD:
-
Herpesvirus envelope glycoprotein D
- HVEM:
-
Herpesvirus entry mediator
- CCL5:
-
Chemokine C–C motif ligand 5
- CTLs:
-
Cytotoxic T lymphocytes
- DSS:
-
Dextran sulfate sodium
- VEGF:
-
Vascular endothelial growth factor
References
Ostrom QT, Gittleman H, Farah P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 2013;15:ii1-56.
Batash R, Asna N, Schaffer P, Francis N, Schaffer M. Glioblastoma multiforme, diagnosis and treatment; recent literature review. Curr Med Chem. 2017;24(27):3002–9.
Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109.
Wirsching HG, Galanis E, Weller M. Glioblastoma. Handbook Clin Neurol. 2016;134:381–97.
Kim EL, Sorokin M, Kantelhardt SR, et al. Intratumoral heterogeneity and longitudinal changes in gene expression predict differential drug sensitivity in newly diagnosed and recurrent glioblastoma. Cancers (Basel). 2020;12(2):520.
Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507.
Brown TJ, Brennan MC, Li M, et al. Association of the extent of resection with survival in glioblastoma: a systematic review and meta-analysis. JAMA Oncol. 2016;2(11):1460–9.
Ampie L, Woolf EC, Dardis C. Immunotherapeutic advancements for glioblastoma. Front Oncol. 2015;5:12.
Gilbert MR, Wang M, Aldape KD, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013;31(32):4085–91.
Weller M, van den Bent M, Tonn JC, et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017;18(6):e315–29.
Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–66.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74.
Old AJ. Immunotherapy for cancer. Sci Am. 1996;275(3):136–43.
Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Investig. 2015;125(9):3335–7.
Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350–5.
Li Z, Song W, Rubinstein M, Liu D. Recent updates in cancer immunotherapy: a comprehensive review and perspective of the 2018 China Cancer Immunotherapy Workshop in Beijing. J Oncol Hematol. 2018;11(1):142.
Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–66.
Linz U. Commentary on Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial (Lancet Oncol. 2009;10:459–466). Cancer 2010;116(8):1844–1846.
Lim M, Xia Y, Bettegowda C, Weller M. Current state of immunotherapy for glioblastoma. Nat Rev Clin Oncol. 2018;15(7):422–42.
Sahebjam S, Sharabi A, Lim M, Kesarwani P, Chinnaiyan P. Immunotherapy and radiation in glioblastoma. J Neurooncol. 2017;134(3):531–9.
Nduom EK, Weller M, Heimberger AB. Immunosuppressive mechanisms in glioblastoma. Neuro-oncology. 2015;17:vii9–14.
Shafit-Zagardo B, Gruber RC, DuBois JC. The role of TAM family receptors and ligands in the nervous system: from development to pathobiology. Pharmacol Therapeut. 2018;188:97–117.
Linger RM, Keating AK, Earp HS, Graham DK. Taking aim at Mer and Axl receptor tyrosine kinases as novel therapeutic targets in solid tumors. Expert Opin Ther Targets. 2010;14(10):1073–90.
Verma A, Warner SL, Vankayalapati H, Bearss DJ, Sharma S. Targeting Axl and Mer kinases in cancer. Mol Cancer Therapeut. 2011;10(10):1763–73.
Linger RM, Keating AK, Earp HS, Graham DK. TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv Cancer Res. 2008;100:35–83.
Wium M, Paccez JD, Zerbini LF. The dual role of tam receptors in autoimmune diseases and cancer: an overview. Cells. 2018;7(10):166.
Pierce AM, Keating AK. TAM receptor tyrosine kinases: expression, disease and oncogenesis in the central nervous system. Brain Res. 2014;1542:206–20.
Onken J, Vajkoczy P, Torka R, et al. Phospho-AXL is widely expressed in glioblastoma and associated with significant shorter overall survival. Oncotarget. 2017;8(31):50403–14.
Cheng P, Phillips E, Kim SH, et al. Kinome-wide shRNA screen identifies the receptor tyrosine kinase AXL as a key regulator for mesenchymal glioblastoma stem-like cells. Stem Cell Rep. 2015;4(5):899–913.
Che Mat MF, Abdul Murad NA, Ibrahim K, et al. Silencing of PROS1 induces apoptosis and inhibits migration and invasion of glioblastoma multiforme cells. Int J Oncol. 2016;49(6):2359–66.
Rothlin CV, Carrera-Silva EA, Bosurgi L, Ghosh S. TAM receptor signaling in immune homeostasis. Annu Rev Immunol. 2015;33(1):355–91.
Desai K, Hubben A, Ahluwalia M. The role of checkpoint inhibitors in glioblastoma. Target Oncol. 2019;14(4):375–94.
Akitake-Kawano R, Seno H, Nakatsuji M, et al. Inhibitory role of Gas6 in intestinal tumorigenesis. Carcinogenesis. 2013;34(7):1567–74.
Bosurgi L, Bernink JH, Delgado Cuevas V, et al. Paradoxical role of the proto-oncogene Axl and Mer receptor tyrosine kinases in colon cancer. Proc Natl Acad Sci USA. 2013;110(32):13091–6.
Png KJ, Halberg N, Yoshida M, Tavazoie SF. A microRNA regulon that mediates endothelial recruitment and metastasis by cancer cells. Nature. 2011;481(7380):190–4.
Martinho O, Zucca LE, Reis RM. AXL as a modulator of sunitinib response in glioblastoma cell lines. Exp Cell Res. 2015;332(1):1–10.
Skoda J, Neradil J, Zitterbart K, Sterba J, Veselska R. EGFR signaling in the HGG-02 glioblastoma cell line with an unusual loss of EGFR gene copy. Oncol Rep. 2014;31(1):480–7.
Hübner M, Moellhoff N, Effinger D, et al. MicroRNA-93 acts as an “anti-inflammatory tumor suppressor” in glioblastoma. Neurooncol Adv. 2020;2(1):vdaa047.
Lee E, Yong RL, Paddison P, Zhu J. Comparison of glioblastoma (GBM) molecular classification methods. Semin Cancer Biol. 2018;53:201–11.
Aldape K, Zadeh G, Mansouri S, Reifenberger G, von Deimling A. Glioblastoma: pathology, molecular mechanisms and markers. Acta Neuropathol. 2015;129(6):829–48.
Haque A, Banik NL, Ray SK. Molecular alterations in glioblastoma: potential targets for immunotherapy. Prog Mol Biol Sci Transl. 2011;98:187–234.
Shergalis A, Bankhead A, Luesakul U, Muangsin N, Neamati N. Current challenges and opportunities in treating glioblastoma. Pharmacol Rev. 2018;70(3):412–45.
Stoyanov GS, Dzhenkov DL. On the Concepts and history of glioblastoma multiforme—morphology, genetics and epigenetics. Folia Med. 2018;60(1):48–66.
Stoyanov GS, Dzhenkov D, Ghenev P, Iliev B, Enchev Y, Tonchev AB. Cell biology of glioblastoma multiforme: from basic science to diagnosis and treatment. Med Oncol. 2018;35(3):27.
Medawar PB. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol. 1948;29(1):58–69.
Heimberger AB, Sampson JH. Immunotherapy coming of age: what will it take to make it standard of care for glioblastoma? Neuro-oncology. 2011;13(1):3–13.
Geraldo LHM, Garcia C, da Fonseca ACC, et al. Glioblastoma therapy in the age of molecular medicine. Trends Cancer. 2019;5(1):46–65.
Zhang F, Xu CL, Liu CM. Drug delivery strategies to enhance the permeability of the blood-brain barrier for treatment of glioma. Drug Des Dev Ther. 2015;9:2089–100.
Balça-Silva J, Matias D, Carmo AD, Sarmento-Ribeiro AB, Lopes MC, Moura-Neto V. Cellular and molecular mechanisms of glioblastoma malignancy: implications in resistance and therapeutic strategies. Semin Cancer Biol. 2019;58:130–41.
Louveau A, Smirnov I, Keyes TJ, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523(7560):337–41.
Auffinger B, Morshed R, Tobias A, Cheng Y, Ahmed AU, Lesniak MS. Drug-loaded nanoparticle systems and adult stem cells: a potential marriage for the treatment of malignant glioma? Oncotarget. 2013;4(3):378–96.
Yao Y, Zhou Y, Liu L, et al. Nanoparticle-based drug delivery in cancer therapy and its role in overcoming drug resistance. Front Mol Biosci. 2020;7:193.
O’Bryan JP, Frye RA, Cogswell PC, et al. Axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell Biol. 1991;11(10):5016–31.
Lai C, Gore M, Lemke G. Structure, expression, and activity of Tyro 3, a neural adhesion-related receptor tyrosine kinase. Oncogene. 1994;9(9):2567–78.
Graham DK, Dawson TL, Mullaney DL, Snodgrass HR, Earp HS. Cloning and mRNA expression analysis of a novel human protooncogene, c-mer. Cell Res Differ Growth Mol Biol J Am Assoc Cancer. 1994;5(6):647–57.
Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141(7):1117–34.
Jia R, Hanafusa H. The proto-oncogene of v-eyk (v-ryk) is a novel receptor-type protein tyrosine kinase with extracellular Ig/GN-III domains. J Biol Chem. 1994;269(3):1839–44.
Lai C, Lemke G. An extended family of protein-tyrosine kinase genes differentially expressed in the vertebrate nervous system. Neuron. 1991;6(5):691–704.
Tsou WI, Nguyen KQ, Calarese DA, et al. Receptor tyrosine kinases, TYRO3, AXL, and MER, demonstrate distinct patterns and complex regulation of ligand-induced activation. J Biol Chem. 2014;289(37):25750–63.
Kasikara C, Kumar S, Kimani S, et al. Phosphatidylserine sensing by TAM receptors regulates AKT-dependent chemoresistance and PD-L1 expression. Mol Cancer Res. 2017;15(6):753–64.
Stitt TN, Conn G, Gore M, et al. The anticoagulation factor protein S and its relative, Gas6, are ligands for the Tyro 3/Axl family of receptor tyrosine kinases. Cell. 1995;80(4):661–70.
Hafizi S, Dahlbäck B. Gas6 and protein S. Vitamin K-dependent ligands for the Axl receptor tyrosine kinase subfamily. FEBS J. 2006;273(23):5231–44.
Lew ED, Oh J, Burrola PG, et al. Differential TAM receptor-ligand-phospholipid interactions delimit differential TAM bioactivities. Elife. 2014;3:e03385.
Rothlin CV, Carrera-Silva EA, Bosurgi L, Ghosh S. TAM receptor signaling in immune homeostasis. Annu Rev Immunol. 2015;33:355–91.
Shao WH, Zhen Y, Eisenberg RA, Cohen PL. The Mer receptor tyrosine kinase is expressed on discrete macrophage subpopulations and mainly uses Gas6 as its ligand for uptake of apoptotic cells. Clin Immunol (Orlando Fla). 2009;133(1):138–44.
Hilliard BA, Zizzo G, Ulas M, Linan MK, Schreiter J, Cohen PL. Increased expression of Mer tyrosine kinase in circulating dendritic cells and monocytes of lupus patients: correlations with plasma interferon activity and steroid therapy. Arthritis Res Ther. 2014;16(2):R76.
Gould WR, Baxi SM, Schroeder R, et al. Gas6 receptors Axl, Sky and Mer enhance platelet activation and regulate thrombotic responses. J Thrombosis Haemostasis JTH. 2005;3(4):733–41.
Schulz NT, Paulhiac CI, Lee L, Zhou R. Isolation and expression analysis of tyro3, a murine growth factor receptor tyrosine kinase preferentially expressed in adult brain. Mol Brain Res. 1995;28(2):273–80.
Prieto AL, O’Dell S, Varnum B, Lai C. Localization and signaling of the receptor protein tyrosine kinase Tyro3 in cortical and hippocampal neurons. Neuroscience. 2007;150(2):319–34.
Wang H, Chen Y, Ge Y, et al. Immunoexpression of Tyro 3 family receptors–Tyro 3, Axl, and Mer–and their ligand Gas6 in postnatal developing mouse testis. J Histochem Cytochem. 2005;53(11):1355–64.
Lu Q, Gore M, Zhang Q, et al. Tyro-3 family receptors are essential regulators of mammalian spermatogenesis. Nature. 1999;398(6729):723–8.
Paolino M, Penninger JM. The role of TAM family receptors in immune cell function: implications for cancer therapy. Cancers. 2016;8(10):97.
De Vos J, Couderc G, Tarte K, et al. Identifying intercellular signaling genes expressed in malignant plasma cells by using complementary DNA arrays. Blood. 2001;98(3):771–80.
Crosier PS, Hall LR, Vitas MR, Lewis PM, Crosier KE. Identification of a novel receptor tyrosine kinase expressed in acute myeloid leukemic blasts. Leuk lymphoma. 1995;18:443–9.
Ben-Batalla I, Schultze A, Wroblewski M, et al. Axl, a prognostic and therapeutic target in acute myeloid leukemia mediates paracrine crosstalk of leukemia cells with bone marrow stroma. Blood. 2013;122(14):2443–52.
Zhu H, Sun X, Zhu L, et al. The expression and clinical significance of different forms of Mer receptor tyrosine kinase in systemic lupus erythematosus. J Immunol Res. 2014;2014:431896.
Wu G, Ma Z, Hu W, et al. Molecular insights of Gas6/TAM in cancer development and therapy. Cell Death Disease. 2017;8(3):e2700.
Burstyn-Cohen T, Maimon A. TAM receptors, Phosphatidylserine, inflammation, and cancer. Cell CCS Signal Commun. 2019;17(1):156.
Rothlin CV, Lemke G. TAM receptor signaling and autoimmune disease. Curr Opin Immunol. 2010;22(6):740–6.
Lu Q, Lemke G. Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science. 2001;293(5528):306–11.
Seitz HM, Camenisch TD, Lemke G, Earp HS, Matsushima GK. Macrophages and dendritic cells use different Axl/Mertk/Tyro3 receptors in clearance of apoptotic cells. J Immunol. 2007;178(9):5635–42.
O’Donnell K, Harkes IC, Dougherty L, Wicks IP. Expression of receptor tyrosine kinase Axl and its ligand Gas6 in rheumatoid arthritis: evidence for a novel endothelial cell survival pathway. Am J Pathol. 1999;154(4):1171–80.
Recarte-Pelz P, Tàssies D, Espinosa G, et al. Vitamin K-dependent proteins GAS6 and Protein S and TAM receptors in patients of systemic lupus erythematosus: correlation with common genetic variants and disease activity. Arthritis Res Ther. 2013;15(2):R41.
Zhu H, Sun X, Zhu L, et al. The expression and clinical significance of different forms of Mer receptor tyrosine kinase in systemic lupus erythematosus. J Immounal Res. 2014;2014:431896.
Weinger JG, Omari KM, Marsden K, Raine CS, Shafit-Zagardo B. Up-regulation of soluble Axl and Mer receptor tyrosine kinases negatively correlates with Gas6 in established multiple sclerosis lesions. Am J Pathol. 2009;175(1):283–93.
Uribe DJ, Mandell EK, Watson A, et al. The receptor tyrosine kinase AXL promotes migration and invasion in colorectal cancer. PLoS ONE. 2017;12(7):e0179979.
Shiozawa Y, Pedersen EA, Patel LR, et al. GAS6/AXL axis regulates prostate cancer invasion, proliferation, and survival in the bone marrow niche. Neoplasia. 2010;12(2):116–27.
Han J, Tian R, Yong B, et al. Gas6/Axl mediates tumor cell apoptosis, migration and invasion and predicts the clinical outcome of osteosarcoma patients. Biochem Biophys Res Commun. 2013;435(3):493–500.
Tworkoski KA, Platt JT, Bacchiocchi A, et al. MERTK controls melanoma cell migration and survival and differentially regulates cell behavior relative to AXL. Pigment Cell Melanoma Res. 2013;26(4):527–41.
Demarest SJ, Gardner J, Vendel MC, et al. Evaluation of Tyro3 expression, Gas6-mediated Akt phosphorylation, and the impact of anti-Tyro3 antibodies in melanoma cell lines. Blood. 2013;52(18):3102–18.
Lee-Sherick AB, Eisenman KM, Sather S, et al. Aberrant Mer receptor tyrosine kinase expression contributes to leukemogenesis in acute myeloid leukemia. Oncogene. 2016;35(48):6270.
Shao WH, Zhen Y, Eisenberg RA, Cohen PL. The Mer receptor tyrosine kinase is expressed on discrete macrophage subpopulations and mainly uses Gas6 as its ligand for uptake of apoptotic cells. Clin Immunol. 2009;133(1):138–44.
Gould WR, Baxi SM, Schroeder R, et al. Gas6 receptors Axl, Sky and Mer enhance platelet activation and regulate thrombotic responses. J Thromb Haemost. 2005;3(4):733–41.
Paolino M, Choidas A, Wallner S, et al. The E3 ligase Cbl-b and TAM receptors regulate cancer metastasis via natural killer cells. Nature. 2014;507(7493):508–12.
Nguyen KQ, Tsou WI, Calarese DA, et al. Overexpression of MERTK receptor tyrosine kinase in epithelial cancer cells drives efferocytosis in a gain-of-function capacity. J Biol Chem. 2014;289(37):25737–49.
Yunxiang Z, Yihan Y, Yongchuan D, Anwen S. Regulation of efferocytosis as a novel cancer therapy. Cell Commun Signal. 2020;18(1):71.
deCathelineau AM, Henson PM. The final step in programmed cell death: phagocytes carry apoptotic cells to the grave. Essays Biochem. 2003;39:105–17.
Duvall E, Wyllie AH, Morris RG. Macrophage recognition of cells undergoing programmed cell death (apoptosis). Immunology. 1985;56(2):351–8.
Kumar S, Calianese D, Birge RB. Efferocytosis of dying cells differentially modulate immunological outcomes in tumor microenvironment. Immunol Rev. 2017;280(1):149–64.
Werfel TA, Cook RS. Efferocytosis in the tumor microenvironment. Semin Immunopathol. 2018;40(6):545–54.
Roy S, Bag AK, Dutta S, et al. Macrophage-derived neuropilin-2 exhibits novel tumor-promoting functions. Cancer Res. 2018;78(19):5600–17.
Ravichandran KS. Beginnings of a good apoptotic meal: the find-me and eat-me signaling pathways. Immunity. 2011;35(4):445–55.
Bosurgi L, Cao YG, Cabeza-Cabrerizo M, et al. Macrophage function in tissue repair and remodeling requires IL-4 or IL-13 with apoptotic cells. Science. 2017;356(6342):1072–6.
Nagata S. Apoptosis and clearance of apoptotic cells. Annu Rev Immunol. 2018;36:489–517.
Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148(7):2207–16.
van der Meer JH, van der Poll T, van’t Veer C. TAM receptors, Gas6, and protein S: roles in inflammation and hemostasis. Blood. 2014;123(16):2460–9.
Zagórska A, Través PG, Lew ED, Dransfield I, Lemke G. Diversification of TAM receptor tyrosine kinase function. Immunol Nat. 2014;15(10):920–8.
Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131(6):1124–36.
Cook RS, Jacobsen KM, Wofford AM, et al. MerTK inhibition in tumor leukocytes decreases tumor growth and metastasis. J Clin Invest. 2013;123(8):3231–42.
Lan Z, Wu H, Li W, et al. Transforming activity of receptor tyrosine kinase tyro3 is mediated, at least in part, by the PI3 kinase-signaling pathway. Blood. 2000;95(2):633–8.
Vergadi E, Ieronymaki E, Lyroni K, Vaporidi K, Tsatsanis C. Akt signaling pathway in macrophage activation and M1/M2 polarization. J Immunol. 2017;198(3):1006–14.
Fadok VA, Bratton DL, Guthrie L, Henson PM. Differential effects of apoptotic versus lysed cells on macrophage production of cytokines: role of proteases. J Immunol. 2001;166(11):6847–54.
Myers KV, Amend SR, Pienta KJ. Targeting Tyro3, Axl and MerTK (TAM receptors): implications for macrophages in the tumor microenvironment. Mol Cancer. 2019;18(1):94.
Graham DK, DeRyckere D, Davies KD, Earp HS. The TAM family: phosphatidylserine sensing receptor tyrosine kinases gone awry in cancer. Nat Rev Cancer. 2014;14(12):769–85.
Keating AK, Kim GK, Jones AE, et al. Inhibition of Mer and Axl receptor tyrosine kinases in astrocytoma cells leads to increased apoptosis and improved chemosensitivity. Mol Cancer Ther. 2010;9(5):1298–307.
Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–34.
Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7–H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800.
Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99(19):12293–7.
Wang H, Yao H, Li C, et al. PD-L2 expression in colorectal cancer: Independent prognostic effect and targetability by deglycosylation. Oncoimmunology. 2017;6(7):e1327494.
Yamamoto R, Nishikori M, Kitawaki T, et al. PD-1-PD-1 ligand interaction contributes to immunosuppressive microenvironment of Hodgkin lymphoma. Blood. 2008;111(6):3220–4.
Thompson RH, Dong H, Kwon ED. Implications of B7–H1 expression in clear cell carcinoma of the kidney for prognostication and therapy. Clin Can Res. 2007;13:709s–15s.
Sadahiro H, Kang KD, Gibson JT, et al. Activation of the Receptor Tyrosine Kinase AXL regulates the immune microenvironment in glioblastoma. Cancer Res. 2018;78(11):3002–13.
Lee-Sherick AB, Jacobsen KM, Henry CJ, et al. MERTK inhibition alters the PD-1 axis and promotes anti-leukemia immunity. JCI Insight. 2018;3(21):e97941.
Hugo W, Zaretsky JM, Sun L, et al. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell. 2016;165(1):35–44.
Skinner HD, Giri U, Yang LP, et al. Integrative analysis identifies a novel AXL-PI3 kinase-PD-L1 signaling axis associated with radiation resistance in head and neck cancer. Clin Cancer Res. 2017;23(11):2713–22.
Akalu YT, Rothlin CV, Ghosh S. TAM receptor tyrosine kinases as emerging targets of innate immune checkpoint blockade for cancer therapy. Immunol Rev. 2017;276(1):165–77.
Lee EH, Kim EM, Ji KY, et al. Axl acts as a tumor suppressor by regulating LIGHT expression in T lymphoma. Oncotarget. 2017;8(13):20645–55.
Mauri DN, Ebner R, Montgomery RI, et al. LIGHT, a new member of the TNF superfamily, and lymphotoxin alpha are ligands for herpesvirus entry mediator. Immunity. 1998;8(1):21–30.
Zhai Y, Guo R, Hsu TL, et al. LIGHT, a novel ligand for lymphotoxin beta receptor and TR2/HVEM induces apoptosis and suppresses in vivo tumor formation via gene transfer. J Clin Invest. 1998;102(6):1142–51.
Tamada K, Shimozaki K, Chapoval AI, et al. Modulation of T-cell-mediated immunity in tumor and graft-versus-host disease models through the LIGHT co-stimulatory pathway. Nat Med. 2000;6(3):283–9.
Yu P, Lee Y, Liu W, et al. Priming of naive T cells inside tumors leads to eradication of established tumors. Nat Immunol. 2004;5(2):141–9.
Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–44.
Mantovani A, Garlanda C, Allavena P. Molecular pathways and targets in cancer-related inflammation. Ann Med. 2010;42(3):161–70.
Wang ZY, Wang PG, An J. The multifaceted roles of TAM receptors during viral infection. Virol Sin. 2020;31:103–9.
Fraineau S, Monvoisin A, Clarhaut J, et al. The vitamin K-dependent anticoagulant factor, protein S, inhibits multiple VEGF-A-induced angiogenesis events in a Mer- and SHP2-dependent manner. Blood. 2012;120(25):5073–83.
Sufit A, Lee-Sherick AB, DeRyckere D, et al. MERTK inhibition induces polyploidy and promotes cell death and cellular senescence in glioblastoma multiforme. PLoS ONE. 2016;11(10):e0165107.
Yen SY, Chen SR, Hsieh J, et al. Biodegradable interstitial release polymer loading a novel small molecule targeting Axl receptor tyrosine kinase and reducing brain tumour migration and invasion. Oncogene. 2016;35(17):2156–65.
Vajkoczy P, Knyazev P, Kunkel A, et al. Dominant-negative inhibition of the Axl receptor tyrosine kinase suppresses brain tumor cell growth and invasion and prolongs survival. Proc Natl Acad Sci USA. 2006;103(15):5799–804.
Knubel KH, Pernu BM, Sufit A, Nelson S, Pierce AM, Keating AK. MerTK inhibition is a novel therapeutic approach for glioblastoma multiforme. Oncotarget. 2014;5(5):1338–51.
Wu J, Frady LN, Bash RE, et al. MerTK as a therapeutic target in glioblastoma. Neuro Oncol. 2018;20(1):92–102.
Wang Y, Moncayo G, Morin P, et al. Mer receptor tyrosine kinase promotes invasion and survival in glioblastoma multiforme. Oncogene. 2013;32(7):872–82.
Rogers AE, Le JP, Sather S, et al. Mer receptor tyrosine kinase inhibition impedes glioblastoma multiforme migration and alters cellular morphology. Oncogene. 2012;31(38):4171–81.
Hutterer M, Knyazev P, Abate A, et al. Axl and growth arrest-specific gene 6 are frequently overexpressed in human gliomas and predict poor prognosis in patients with glioblastoma multiforme. Clin Cancer Res. 2008;14(1):130–8.
Wang J, Zuo J, Wang MD, et al. Receptor tyrosine kinase AXL is correlated with poor prognosis and induces temozolomide resistance in glioblastoma. CNS Neurosci Ther. 2019;26:777.
Vouri M, An Q, Birt M, Pilkington GJ, Hafizi S. Small molecule inhibition of Axl receptor tyrosine kinase potently suppresses multiple malignant properties of glioma cells. Oncotarget. 2015;6(18):16183–97.
Onken J, Torka R, Korsing S, et al. Inhibiting receptor tyrosine kinase AXL with small molecule inhibitor BMS-777607 reduces glioblastoma growth, migration, and invasion in vitro and in vivo. Oncotarget. 2016;7(9):9876–89.
De Witt Hamer PC. Small molecule kinase inhibitors in glioblastoma: a systematic review of clinical studies. Neuro-oncology. 2010;12(3):304–16.
Martinho O, Silva-Oliveira R, Miranda-Gonçalves V, et al. In vitro and in vivo analysis of rtk inhibitor efficacy and identification of its novel targets in glioblastomas. Transl Oncol. 2013;6(2):187–96.
Aspelund A, Antila S, Proulx ST, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212(7):991–9.
Fang L, Lowther DE, Meizlish ML, et al. The immune cell infiltrate populating meningiomas is composed of mature, antigen-experienced T and B cells. Neuro Oncol. 2013;15(11):1479–90.
Shalapour S, Karin M. Immunity, inflammation, and cancer: an eternal fight between good and evil. J Clin Investig. 2015;125(9):3347–55.
Peeters MJW, Rahbech A, Thor SP. TAM-ing T cells in the tumor microenvironment: implications for TAM receptor targeting. Cancer Immunol Immunother CII. 2020;69(2):237–44.
Liu J, Zhang W, Stashko MA, et al. UNC1062, a new and potent Mer inhibitor. Eur J Med. 2013;65:83–93.
Acknowledgements
This work was funded by National Natural Science Foundation of China (81701144, 81371433), National Key Research and Development Program of China (2017YFC1308500), and Key Program of Science and Technology Development of Zhejiang (2017C03021).
Author information
Authors and Affiliations
Contributions
YXZ and AWS conceptualized the research project. YLW, HLC, YYX and YL drafted the manuscript; YXZ, YLW and YCD reviewed and modified the manuscript. AWS and YCD supervised the research, led the discussion. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval and consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhou, Y., Wang, Y., Chen, H. et al. Immuno-oncology: are TAM receptors in glioblastoma friends or foes?. Cell Commun Signal 19, 11 (2021). https://doi.org/10.1186/s12964-020-00694-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12964-020-00694-8