Abstract
Background
Glioblastoma multiforme (GBM), as the broadest cerebrum tumor, is resistant to current medical interventions, particularly chemo/radiation. Hence, it necessitates further therapeutic options that could enhance the efficacy of existing modalities.
Methods
A comprehensive and systematic review of literature on the NF-κB signaling pathway-contributed in the pathogenesis of GBM with a focus on natural products was carried out.
Results
Several examinations have shown that nuclear factor (NF)-κB is participated in apoptosis, cellular proliferation, angiogenesis, metastasis, invasion, and many other processes implicated in GBM pathobiology. Recent studies have provided that NF-κB regulation is the primary pharmacological target for GBM therapy. Specific natural products are involved in several signaling pathways implicated in tumor growth and apoptosis of GBM cells.
Conclusion
In the current review, we elaborate on the role of NF-κB as a promising target in GBM and discuss some natural products affecting the NF-κB signaling pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastoma multiforme (GBM), known as the terminator of the brain with an average survival rate of 1 year, is an incurable inflammatory brain tumor, regardless of maximal standard chemo/radiation therapy [1, 2]. Molecular pathogenesis of GBM is believed to contain various genetic modifications resulting in aberrant pathway activity involving cell motility, angiogenesis, regulation of the cell cycle, micrometastasis, and apoptosis [3, 4]. The current therapeutic interventions, particularly temozolomide (TMZ, an alkylating agent) and bevacizumab (an antiangiogenic agent), remain insufficient to ablate the invasive and metastatic behavior of GBM [5, 6]. Therefore, considering this extremely invasive nature, new therapeutic modalities for GBM patients are essential.
The recent trials indicate that the activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), as a characteristic feature of inflammation, causes an unfavorable prognosis in GBM patients [7]. Interestingly, it has lately been noted that inflammatory symptoms of GBM (brain edema and necrosis of surrounding tissues affected by highly invasive nature of GBM cells) are identified through macrophages/microglia infiltrations, inflammatory cytokines production, and NF-κB activity, boosting GBM development and chemoresistance [8]. While aberrant activation of the NF-κB cascade is commonly discovered in GBM, its vital functions against tumor growth stay obscure.
Many oncogenic mechanisms are present in GBM, and several prior studies addressed how gliomagenesis can be suppressed by promoting procedures varying from cellular proliferation to invasion and metastasis [9]. Herein, this study will concentrate specifically on the participation of natural products affected NF-κB mechanism and the consequences of aberrant NF-κB activity in GBM.
Structural and functional properties of NF-κB
NF-κB is a cluster of proteins responsible for regulating cytokine production, survival, and DNA synthesis [10]. All proteins of the NF-κB family contain a Rel homology domain in their N-terminus. Based on its structure, NF-κB, as a multi-subunit transcriptional factor, is comprised by heterodimers and homodimers of the five members of the Rel family, including p65 (RelA), RelB, c-Rel, p52 (NF-κB2, p100), and p50 (NF-κB1, p105) [11, 12]. RelA, RelB, and c-Rel have a transactivation domain in their C-termini. On the other hand, the NF-κB1 and NF-κB2 proteins are synthesized as significant precursors, p105, and p100, which are processed to generate the mature NF-κB subunits, p50 and p52, respectively. The p50 and p52 proteins have no intrinsic ability to promote transcription and have, therefore, been indicated to behave as transcriptional repressors when interacting as homodimers κB components [13].
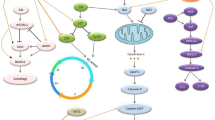
The inhibitors of kappa B (IκBs) are a class of associated enzymes with an N-terminal structural region, accompanied by six or more ankyrin chains and a PEST domain close to their C terminus. Although the IκB family consists of IκBε, IκBβ, IκBα, and Bcl-3, the best-studied and major IκB protein is IκBα [14]. Due to its contact with the inhibitor IκBα, NF-κB is kept inactive in unstimulated cells, and the structure is generally situated in the cytoplasm. In reaction to stimulators, such as cytokines (TRAIL, tumor necrosis factor [TNF]-α), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), and DNA damage, IκB kinases (IKKα or IKKβ), are triggered and phosphorylate IκBα, causing to its degradation by a K48 ubiquitin-mediated proteasomal process [13]. As shown in Fig. 1, we demonstrated the mechanism of NF-κB action in human cancer.
Mechanism of action of NF-κB in cells. The NF-κB subunits, including Rel and p50 proteins, are utilized as an example. In an inactivated situation, NF-κB is complexed to IκBα (an inhibitory protein). A variety of signals might induce the enzyme IκB kinase (IKK), leading to phosphorylation of the IκBα protein, resulting in ubiquitination, dissociation of IκBα from NF-κB, and eventual proteasome degradation of IκBα. Then, the activated NF-κB translocated into the nucleus and bound to specific sequences of DNA. The structure of DNA/NF-κB then produces other enzymes such as coactivators and RNA polymerase that transcribe downstream DNA into mRNA. The result will be a shift in the feature of the cell, mRNA being transformed into the protein. TNF-α tumor necrosis factor-alpha, IL-1β interleukin 1 beta, ROS reactive oxygen species, IκBα inhibitor of kappa B, RelA RELA proto-oncogene, NF kappa B subunit, IKK inhibitor of NF-kappa B kinase
A range of stimuli might trigger the mammalian NF-κB signaling mechanisms, including stress, cytokines (IL-1β and TNF–α), ultraviolet and ionizing irradiation, pathogen-associated molecular patterns, DNA damage, oncogenic stress, reactive oxygen species (ROS), and growth factors [12, 15]. The NF-κB family is involved in numerous mechanisms, such as apoptosis, reprogramming of metabolism, immunity, cell cycle progression, tumor progression, invasion, metastasis, angiogenesis, cell survival, chemoresistance, and inflammation (Fig. 2) [16, 17]. Upregulation of inflammatory cytokines, including IL-8, IL-6, IL-11, IL-1β, IL-15, and C–C motif chemokine ligand (CCL)-2 and genes with diverse pathobiological activities, including proteolysis (TFPI2, PLAU), cell adhesion (CD44), cell cycle modulators (Cyclin D1), and cyclooxygenase (COX)-2 are the well-known targets of the NF-κB pathway [18]. In addition to nuclear translocation, the regulation of NF-κB signaling involves some other regulatory processes, including post-translatory alterations of particular NF-κB subunits, protein–protein interactions found in specific gene regulatory locations, and nuclear export mechanisms. Thereby, a complex combination of specific processes that might differ in their upstream stimuli and/or downstream targets typically results in different cellular responses to NF-κB [15].
The NF-κB-based mechanisms involved in GBM
P50 and p65 are the main dimers for NF-κB found in resting GBM cells. P50 is formed from parental protein p105 in a co-translational manner. Since p50 does not have a C-terminal transactivation domain, it works at an inhibitory capacity except when it is either dimerized with a C-terminal transactivation domain subunit such as p65 or linked with a transactivating coregulator [19]. Even though p65 is frequently retained in the cytoplasm at rest, a high amount of cytokine and oncogene activation occurs in malignant cells, which results in an enhanced IKK activation and translocation of the nuclear p65. Given the crucial role that p65 played to promote the transcriptional activity of NF-κB, this subunit has been the focus of the majority of the studies examined for NF-κB in GBM [20].
As mentioned, NF-κB is the center of intracellular signal transductions implicated in influencing numerous physiological and pathological procedures, such as cell proliferation, angiogenesis, and apoptosis [21]. A study indicated that several gene promoters or enhancers, such as Cyclin D1, intercellular adhesion molecule-1 (ICAM-1), C-Myc, Bcl-xL, and Bcl-2 were correlated with cell apoptosis and proliferation. Consequently, NF-κB can lead to the expression of these genes, which causes anti-apoptosis and other biological impacts [22]. In addition, in a multitude of malignant cancers, such as pancreatic cancer, prostate cancer, melanoma, and GBM, NF-κB is progressively expressed [23]. Over the past few years, a vast amount of research has shown that by adjusting associated genetic variables, the NF-κB signaling pathway can mediate the development and progression of GBM cells [16, 24, 25]. The genetic alterations detected most frequently in GBM include p53 downregulation, epidermal growth factor receptor (EGFR, as a receptor tyrosine kinase) amplification and mutation, INK4A loss, phosphoinositide 3 kinase (PI3K)/protein kinase B (Akt) upregulation, phosphatase and tensin homolog (PTEN) loss, and neurofibromin 1 (NF1, as a consequence of PI3K/Akt activation) downregulation, and MDM2 amplification [11, 26]. Many trials have indicated that oncogenic EGFR pathways significantly contribute to tumor development and invasion of GBM, implicating a pivotal role for NF-κB in at least some of the tumor-inducing functions of this receptor [27].
It has been shown that an oncogenic upstream activator of NF-κB, receptor-interacting protein 1 (RIP1), upregulates MDM2, a specific inhibitor of p53, indicating a mechanical relation between NF-κB and p53. It is noteworthy to say that both MDM2 and RIP1 are usually overexpressed in GBM [28, 29]. It has been established that EGFR variant III (EGFRvIII) induces GBM growth and angiogenesis through the IL-8, as a common proangiogenic gene, and NF-κB signaling pathway [21, 30]. In addition to this, NF-κB induces the VEGF expression, a significant factor of angiogenesis. Consistently, GBM growth and angiogenesis significantly decreased in nude mice by inhibiting the signal of NF-κB [31].
Numerous studies have indicated that EGFR promotes NF-κB by way of AKT (especially mTORC2 complex), and this signaling cascade induces chemoresistance in GBM cells [32]. AKT utilizes at least three separate processes to activate the transactivation capability of NF-κB. First, the IKK α-component, which enhances IKK action, can be phosphorylated by AKT. Second, AKT stimulates IKK by stimulating the phosphorylation of the mitogen-activated protein kinase (MAPK). Finally, AKT can also target the transactivation domain of RelA, improving the activation of NF-κB. This stimulation tends to be integrated into a positive feedback loop, where Akt activation of NF-κB further stimulates Akt via down-regulation of the PTEN as a PI3K negative modulator [33, 34]. The Grb2-associated binder 1 (Gab1) protein and the tyrosine phosphatase SHP-2 are two main molecules that are crucial for the association of EGFR to NF-κB transcriptional activity via the PI3K/Akt signaling cascade in GBM cells. It is found that the Akt/NF-κB signaling pathway regulates cell survival, apoptosis, proliferation, and malignant transformation, including GBM [35]. Therefore, in agreement with Kapoor et al.’s study, deletion (but not mutated) of IκB impacts in GBM pathogenesis and poor survival are comparable similar to EGFR amplification [36]. It has also been demonstrated in the latest research that NF-κB p65 in GBM has often been phosphorylated; p65 was phosphorylated in 20 out of the 23 GBM tumors tested [37].
Numerous chemical agents have cytotoxic effects on GBM through NF-κB blocking. Sulfasalazine, as a potent inhibitor of NF-κB activation, induced apoptosis, and blocked the cell cycle in some GBM cells and primary cultures [38]. In addition, the mechanism of action of anthelmintic niclosamide revealed inhibitory effects through NF-κB signaling pathways in GBM cells [39]. In another research that focused on GBM cells (U138MG, C6, U87, and U373), the mitochondrial-dependent apoptosis induced by the MG132, as a proteasome blocker, was specifically inhibited by both PI3K and NF-κB pathways [40]. Other chemical agents, including BAY117082 and arsenic trioxide, have also been revealed to induce apoptosis of GBM cells through NF-κB inhibition [40, 41]. These combined observations fortify the role of the NF-κB signaling pathway and provide a mechanistic explanation in the pathogenesis of GBM. In the next section, we discussed some natural products affecting the NF-κB signaling pathway in GBM.
Therapeutic natural targeting of the NF-κB pathway in GBM
There are currently at least 120 distinct natural substances isolated from plants that are regarded to be significant medicines, which is utilized as anticancer agents [4, 42,43,44,45,46]. Some of the examples include vincristine, vinblastine, and paclitaxel [47, 48]. To date, several medicinal plants and natural products are used against inflammatory diseases, including cancer. For instance, oral administration of Scutellaria solution, as a genus of flowering plants in the mint family, postponed the development of F98 GBM in F344 rats through inhibition of glycogen synthase kinase (GSK)-3α/β, Akt, and NF-κB phosphorylation [49]. In addition, Avarol and its derivates, as sesquiterpenoid hydroquinone, has potent cytotoxicity on different cancer cells [50,51,52,53,54], including U251 GBM cells [44].
It is recognized that several nutritional chemopreventive compounds, including curcumin, resveratrol, and some potential flavonoids, prevent the initiation of NF-κB [55,56,57,58]. These surveys highly favor the hypothesis that NF-κB is a functionally appropriate target for chemopreventive medicines and nutritional compounds, displaying their feature in the earliest oncogenesis phases [59]. The importance of natural products as modulators of NF-κB in the treatment of GBM may be a beneficial strategy; therefore, we reviewed some natural products that have been used to block NF-κB signaling.
Resveratrol
Resveratrol is a polyphenolic compound that exhibits antitumor, anti-inflammation, immunomodulation, and anti-invasion activities in multiple cancer cells [60]. Many studies have demonstrated that resveratrol effectively suppressed NF-κB signaling by inhibiting the actions of NF-κB and IκB kinase, providing a novel strategy for treating cancer [55, 61]. Jiao et al. proved that resveratrol inhibited PI3K/Akt/NF-κB signaling pathway and the subsequent suppression of matrix metalloproteinase (MMP)-2 expression, leading to inhibition of invasion in GBM-initiating cells [62]. In line with this, it has been discovered that the connection between NF-κB action and GBM invasiveness is owing to the processing of fibronectin by MMPs, which enables the integration of this matrix element directly into the surrounding tumor cells [63]. Besides, resveratrol reversed TMZ resistance by downregulation of O-6-methylguanine-DNA methyltransferase (MGMT) in GBM cells (T98G) by the NF-κB-dependent pathway [64]. Numerous studies have indicated that NF-κB activation in various cancer cells, mainly related to drug resistance, is mediated by many chemotherapy drugs and radiation due to its role in MGMT transcription [65]. NF-κB-p65, a subunit of NF-κB, has led to enhanced expression of MGMT in a study reported, while NF-κB inhibitor abolishes the elevated MGMT expression [66, 67]. Hence, targeting NF-κB, IκB kinase, and MGMT might be useful in the anti-GBM potential of resveratrol.
Quercetin
Quercetin, as a plant-derived phenolic compound, induces cell death of breast, liver, and brain cancer cells, and reports to have antihypertensive, anticarcinogenic, anti-inflammatory, and antioxidant properties [68]. Notably, quercetin regulates various protein kinases, especially the PI3K signaling pathway [69]. Kiekow et al. showed quercetin induces apoptosis in GBM cells by modulating caspase-3 activation and NF-κB nuclear translocation, suggesting that quercetin is a potential flavonoid compound to develop a new anti-GBM treatment [70]. It has been proved that overexpression of phospholipase D (PLD) induces expression of MMP-2, and consequently, GBM cell invasion via protein kinase C and protein kinase A/NF-κB-mediated signaling pathways [71, 72]. In line with this, a study indicated that quercetin suppressed NF-κB-induced PLD-1 expression via the mitigation of NF-κB transactivation [73].
Apigenin
Apigenin, as a dietary flavonoid, presents in tea leaves and fruits, exerting various biological effects, including immunoregulatory, antioxidant, cytotoxic, anti-viral, anti-inflammation, and anticancer (lung, breast, liver, and prostate) effects [74, 75]. It has been proved that the immunoregulatory of apigenin is mediated by the inhibition of PI3K/Akt/NF-κB (regulation of IκBα and IKK) axis in multiple human cancers [75,76,77], leading to a reduction in invasion and metastasis. Chen and co-workers in their study have shown that apigenin mitigated the proliferation of GBM cells through suppression of NF-κB and inhibited metastasis via the downregulation of MMP-9 [78].
Isothiocyanates
Isothiocyanates, derived from cruciferous vegetables, possess anti-invasion, anti-inflammatory, and anticancer effects, suppressing pancreatic cancer, myeloma, and breast cancer [79, 80]. Recently, the neuroprotective and anti-inflammatory impacts of isothiocyanates were evaluated in various cancer cells. Studies have shown that the anti-inflammatory activities of isothiocyanate might be through inhibition of c-Jun N-terminal kinase (JNK)/NF-κB/TNF-α signaling cascade [81]. A study has shown that subsequent treatment of phenethyl isothiocyanate increases the sensitivity of TMZ resistant T98, U87, and U373 cells by inhibiting expression of MGMT via the NF-κB pathway [82]. Moreover, Lee et al. have shown that isothiocyanates exerted an inhibitory effect on MMP-9 transcription levels through the mitigation of NF-κB and activator protein-1 (AP-1), preventing the invasion and migration of C6 GBM cells [83].
Sulforaphane
Sulforaphane, a phytochemical in broccoli sprouts, is considered to exert cancer prevention impacts by detoxifying and improving anti-oxidation ability [84, 85]. Recently, it is found that suppression of NF-κB and NF-κB-regulated gene expression by sulforaphane is through IκBα, IKK pathway in human cancer cells [86]. The studies have shown sulforaphane changed Bax/Bcl-2 ratio, caspase-3 activity, morphological features, intracellular Ca2+, DNA fragmentation, calpain activity, the release of cytochrome C, caspase-9/-12 cleavage, NF-κB, and IκBa protein levels in GBM U87MG and T98G cells [87]. Furthermore, sulforaphane caused a down-regulation of NF-κB expression through inhibition of inhibitor-of-apoptosis proteins (IAPs), and the up-regulation of IκBα in GBM cells [88].
Alantolactone
Alantolactone, as a sesquiterpene lactone isolated from Inula helenium, has a broad variety of pharmacological impacts, such as anti-inflammatory, antifungal, antibacterial, and anticancer activities [89]. The antitumor effects of alantolactone have been shown in liver cancer, lung cancer, colorectal cancer, brain tumors, and chronic myelogenous leukemia [90,91,92]. It was found that alantolactone causes cell death in GBM cells via ROS generation, mitochondrial dysfunction, and glutathione depletion [92]. The other mechanisms triggered by alantolactone include inhibition of COX-2 and iNOS expression and downregulation of AP-1 and NF-κB via the MyD88 signaling pathways [89]. In line with this, Wang et al. have reported that the antitumor effect of alantolactone against GBM is mediated by blocking IKKβ kinase activity and interrupting NF-κB/COX-2-mediated signaling pathways [93]. Hence, alantolactone might be a potential natural agent against GBM due to its dual inhibitory effects on IKKβ and NF-κB expression.
Baicalein
Baicalein, as a bioactive flavonoid initially isolated from the Scutellaria baicalensis root and has historically been used in anticancer therapies. Numerous studies have demonstrated that this compound inhibits NF-κB nuclear translocation, representing a useful anti-inflammatory agent in various cancer cell lines [94, 95]. It was shown that the NF-κB-p65 expression and activity was significantly inhibited after baicalein treatment in U251 GBM cells, suggesting that baicalein is a potential therapeutic natural product against GBM [96].
Parthenolide
Parthenolide, a significant sesquiterpene lactone derived from Tanacetum parthenium, suppresses NF-κB by inhibiting the IκB kinase and modifying the p65 subunit [97]. Parthenolide has been utilized to cure migraine and rheumatoid arthritis due to its low toxicity characteristics and anti-inflammatory effects [98]. The impact of parthenolide therapy on animal malignancies has also been explored in many studies [15, 99]. Parthenolide suppresses invasion, angiogenesis, and proliferation of GBM cells (U373 and U87MG). Yu et al. have shown that suppression of NF-κB causes anti-GBM activity and inhibits TMZ-initiated chemoresistance by down-regulation of MGMT gene expression [100]. Molecular trials have demonstrated that parthenolide inhibits angiogenesis as well as reduces Akt phosphorylation and activated mitochondrial signaling, implying that the antitumor activity of parthenolide may be mediated by the inhibition of NF-κB, inhibition of Akt signaling, and induction of apoptosis [101]. Although, some evidence has shown that treatment of GBM cells with parthenolide causes rapid apoptosis through caspase-3/-7 without affecting NF-κB regulation [102].
Nepalolide A
A plant of Chinese traditional medicine Carpesium nepalense is a source of sesquiterpene lactone nepalolide A. In C6 rat glioma cells, nepalolide A is found to suppress signaling induced by lipopolysaccharide and cytokine and inhibit IκB protein phosphorylation. Therefore, inhibition of NF-κB activation by nepalolide A was mediated by blockade of the degradation of IκB, leading to inhibition of the iNOS expression [103].
Curcumin
Curcumin, as a polyphenol isolated from the root of the rhizome, Curcuma longa, possesses antioxidant and anti-inflammatory activities through a reduction in AP-1 and NF-κB activity [104]. Curcumin has been lately initiated in phase I clinical trials to treat several high-risk cancers [105], The newest literature indicates that curcumin may have many beneficial impacts in GBM cells, including inhibition of angiogenesis, invasion, and cell development [106,107,108]. The outcomes of curcumin on GBM development were examined in human (U87MG, T67, and T98G) and rat (C6) GBM cell lines. Curcumin reduced cell survival in a caspase- and p53-independent manner, an effect correlated with the inhibition of AP-1 and NF-κB signaling pathways via the prevention of constitutive JNK and Akt activation [109].
Furthermore, curcumin augments the antitumor activity of nimustine (an alkylating agent) against GBM by suppressing the PI3K/Akt and NF-κB/COX-2 signaling cascades [110]. Besides, in a study was done by Fratantonio et al., curcumin potentiates the anti-GBM activity of paclitaxel in rat C6 cells through inhibition of NF-κB activation [111]. Recently, demethoxycurcumin (DMC, a curcuminoid) has shown anti-proliferative impacts by inhibition of the Akt/NF-κB pathway in U87 cells [112]. As shown in Table 1, we summarized the potential natural product affecting the NF-κB signaling pathway in GBM.
As mentioned, despite numerous therapeutic approaches, only minimal survival improvements have been made for GBM patients [2, 4, 44]. Chemotherapy is commonly approved for the treatment of many tumors, but the Blood–Brain Barrier (BBB), as a specific framework, prevents the majority of chemotherapeutic agents from reaching the tumor [114]. Hence, the function of nano-technology is motivated by the need to mask the physicochemical characteristics of therapeutic drugs to extend half-life through the BBB [115]. This might be achieved by encapsulating some natural products discussed here (such as resveratrol and curcumin) in many different types of nanosystems, such as liposomes, lipid, and polymeric nanoparticles.
Concluding comments
Increasing proof supports the critical functions of the NF-κB signaling pathway in the pathobiology of GBM. Since NF-κB primarily induces an aggressive phenotype, considerable effort has been made to incorporate NF-κB inhibition into GBM therapy; however, currently, no specific success has been achieved. Although some small molecule inhibitors of the NF-κB pathway, mainly inhibitors of IKK proteins, are already accessible, more particular inhibitors of IKK and other upstream kinases need to reach clinical studies to prove their effectiveness in GBM patients (Fig. 3).
In the current study, we have reviewed a series of studies on the NF-κB signaling pathway in GBM, and a new strategy to anti-GBM therapy based on natural products. It is believed that curcumin (as a first clinically tested agent in GBM patients), parthenolide, resveratrol, and quercetin have promising NF-κB modulatory effects against GBM. Since the NF-κB modulators have shown low toxicity against normal astrocytes, which imply their cancer cell selectivity, we strongly suggest that the natural products reviewed here are potential agents to develop specific clinical trials, consequently find a better solution for treating GBM.
References
Xiao H, Bai J, Yan M, Ji K, Tian W, Liu D, et al. Metastatic glioblastoma multiforme: a rare case of long-term survival. Biomed Res. 2018;29:1120–2.
Afshari AR, Karimi Roshan M, Soukhtanloo M, Ghorbani A, Rahmani F, Jalili-Nik M, et al. Cytotoxic effects of auraptene against a human malignant glioblastoma cell line. Avicenna J Phytomed. 2019;9:334–46.
Afshari AR, Jalili-Nik M, Soukhtanloo M, Ghorbani A, Sadeghnia HR, Mollazadeh H, et al. Auraptene-induced cytotoxicity mechanisms in human malignant glioblastoma (U87) cells: role of reactive oxygen species (ROS). EXCLI J. 2019;18:576–90.
Jalili-Nik M, Sabri H, Zamiri E, Soukhtanloo M, Roshan MK, Hosseini A, et al. Cytotoxic effects of ferula latisecta on human glioma U87 cells. Drug Res (Stuttg). 2019;69:665–70.
Guan X, Hasan MN, Maniar S, Jia W, Sun D. Reactive astrocytes in glioblastoma multiforme. Mol Neurobiol. 2018;55:6927–38.
Hintenlang LL, Miller DH, Kaleem T, Patel N, May BC, Tzou KS, et al. Treatment of a glioblastoma multiforme dural metastasis with stereotactic radiosurgery: a case report and select review of the literature. J Clin Neurosci. 2018;48:118–21.
Gray GK, McFarland BC, Nozell SE, Benveniste EN. NF-kappaB and STAT3 in glioblastoma: therapeutic targets coming of age. Expert Rev Neurother. 2014;14:1293–306.
Murray PG, Flavell JR, Baumforth KR, Toomey SM, Lowe D, Crocker J, et al. Expression of the tumour necrosis factor receptor-associated factors 1 and 2 in Hodgkin’s disease. J Pathol. 2001;194:158–64.
Messaoudi K, Clavreul A, Lagarce F. Toward an effective strategy in glioblastoma treatment. Part I: resistance mechanisms and strategies to overcome resistance of glioblastoma to temozolomide. Drug Discov Today. 2015;20:899–905.
Taniguchi K, Karin M. NF-kappaB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol. 2018;18:309–24.
Puliyappadamba VT, Hatanpaa KJ, Chakraborty S, Habib AA. The role of NF-kappaB in the pathogenesis of glioma. Mol Cell Oncol. 2014;1:e963478.
Sun SC. Non-canonical NF-kappaB signaling pathway. Cell Res. 2011;21:71–85.
Park MH, Hong JT. Roles of NF-kappaB in cancer and inflammatory diseases and their therapeutic approaches. Cells. 2016;5:15.
Zhou Y, Eppenberger-Castori S, Eppenberger U, Benz CC. The NFkappaB pathway and endocrine-resistant breast cancer. Endocr Relat Cancer. 2005;12(Suppl 1):S37–46.
Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006;25:6680–4.
Dolcet X, Llobet D, Pallares J, Matias-Guiu X. NF-kB in development and progression of human cancer. Virchows Arch. 2005;446:475–82.
Grivennikov SI, Karin M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010;21:11–9.
Richmond A, Yang J. The role of NF-kB in modulating antitumor immunity. Oncoimmunology. 2016;5:e1005522.
Yamini B, Yu X, Dolan ME, Wu MH, Darga TE, Kufe DW, et al. Inhibition of nuclear factor-kappaB activity by temozolomide involves O6-methylguanine induced inhibition of p65 DNA binding. Cancer Res. 2007;67:6889–98.
Nagai S, Washiyama K, Kurimoto M, Takaku A, Endo S, Kumanishi T. Aberrant nuclear factor-kappaB activity and its participation in the growth of human malignant astrocytoma. J Neurosurg. 2002;96:909–17.
Galvani E, Sun J, Leon LG, Sciarrillo R, Narayan RS, Sjin RT, et al. NF-kappaB drives acquired resistance to a novel mutant-selective EGFR inhibitor. Oncotarget. 2015;6:42717–32.
Takada Y, Kobayashi Y, Aggarwal BB. Evodiamine abolishes constitutive and inducible NF-kappaB activation by inhibiting IkappaBalpha kinase activation, thereby suppressing NF-kappaB-regulated antiapoptotic and metastatic gene expression, up-regulating apoptosis, and inhibiting invasion. J Biol Chem. 2005;280:17203–12.
Xia Y, Shen S, Verma IM. NF-kappaB, an active player in human cancers. Cancer Immunol Res. 2014;2:823–30.
Atkinson GP, Nozell SE, Benveniste ET. NF-kappaB and STAT3 signaling in glioma: targets for future therapies. Expert Rev Neurother. 2010;10:575–86.
Song L, Liu L, Wu Z, Li Y, Ying Z, Lin C, et al. TGF-beta induces miR-182 to sustain NF-kappaB activation in glioma subsets. J Clin Investig. 2012;122:3563–78.
Karimi Roshan M, Soltani A, Soleimani A, Rezaie Kahkhaie K, Afshari AR, Soukhtanloo M. Role of AKT and mTOR signaling pathways in the induction of epithelial-mesenchymal transition (EMT) process. Biochimie. 2019;165:229–34.
Salminen A, Lehtonen M, Suuronen T, Kaarniranta K, Huuskonen J. Terpenoids: natural inhibitors of NF-kappaB signaling with anti-inflammatory and anticancer potential. Cell Mol Life Sci. 2008;65:2979–99.
Meylan E, Burns K, Hofmann K, Blancheteau V, Martinon F, Kelliher M, et al. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-kappa B activation. Nat Immunol. 2004;5:503–7.
Festjens N, Vanden Berghe T, Cornelis S, Vandenabeele P. RIP1, a kinase on the crossroads of a cell’s decision to live or die. Cell Death Differ. 2007;14:400–10.
Bonavia R, Inda MM, Vandenberg S, Cheng SY, Nagane M, Hadwiger P, et al. EGFRvIII promotes glioma angiogenesis and growth through the NF-kappaB, interleukin-8 pathway. Oncogene. 2012;31:4054–66.
Xie T-X, Xia Z, Zhang N, Gong W, Huang S. Constitutive NF-κB activity regulates the expression of VEGF and IL-8 and tumor angiogenesis of human glioblastoma. Oncol Rep. 2010;23:725–32.
Shostak K, Chariot A. EGFR and NF-kappaB: partners in cancer. Trends Mol Med. 2015;21:385–93.
Dan HC, Cooper MJ, Cogswell PC, Duncan JA, Ting JP, Baldwin AS. Akt-dependent regulation of NF-{kappa}B is controlled by mTOR and Raptor in association with IKK. Genes Dev. 2008;22:1490–500.
Bai D, Ueno L, Vogt PK. Akt-mediated regulation of NFkappaB and the essentialness of NFkappaB for the oncogenicity of PI3K and Akt. Int J Cancer. 2009;125:2863–70.
Kapoor GS, Zhan Y, Johnson GR, O’Rourke DM. Distinct domains in the SHP-2 phosphatase differentially regulate epidermal growth factor receptor/NF-kappaB activation through Gab1 in glioblastoma cells. Mol Cell Biol. 2004;24:823–36.
Pearson JRD, Regad T. Targeting cellular pathways in glioblastoma multiforme. Signal Transduct Target Ther. 2017;2:17040.
Puliyappadamba VT, Chakraborty S, Chauncey SS, Li L, Hatanpaa KJ, Mickey B, et al. Opposing effect of EGFRWT on EGFRvIII-mediated NF-kappaB activation with RIP1 as a cell death switch. Cell Rep. 2013;4:764–75.
Robe PA, Bentires-Alj M, Bonif M, Rogister B, Deprez M, Haddada H, et al. In vitro and in vivo activity of the nuclear factor-kappaB inhibitor sulfasalazine in human glioblastomas. Clin Cancer Res. 2004;10:5595–603.
Wieland A, Trageser D, Gogolok S, Reinartz R, Hofer H, Keller M, et al. Anticancer effects of niclosamide in human glioblastoma. Clin Cancer Res. 2013;19:4124–36.
Zanotto-Filho A, Braganhol E, Battastini AM, Moreira JC. Proteasome inhibitor MG132 induces selective apoptosis in glioblastoma cells through inhibition of PI3K/Akt and NFkappaB pathways, mitochondrial dysfunction, and activation of p38-JNK1/2 signaling. Investig New Drugs. 2012;30:2252–62.
Zanotto-Filho A, Braganhol E, Schroder R, de Souza LH, Dalmolin RJ, Pasquali MA, et al. NFkappaB inhibitors induce cell death in glioblastomas. Biochem Pharmacol. 2011;81:412–24.
Dutta S, Mahalanobish S, Saha S, Ghosh S, Sil PC. Natural products: An upcoming therapeutic approach to cancer. Food Chem Toxicol. 2019;128:240–55.
Pejin B, Simonovic M, Talevska A, Glumac M, Jakimov D, Kojic V. A neglected natural source for targeting glioblastoma. Nat Prod Res. 2019. https://doi.org/10.1080/14786419.2019.1638386.
Pejin B, Tommonaro G, Glumac M, Jakimov D, Kojic V. The redox couple avarol/avarone in the fight with malignant gliomas: the case study of U-251 MG cells. Nat Prod Res. 2018;32:616–20.
Pejin B, Glumac M. A brief review of potent anti-CNS tumourics from marine sponges: covering the period from 1994 to 2014. Nat Prod Res. 2018;32:375–84.
Pejin B, Jovanovic KK, Mojovic M, Savic AG. New and highly potent antitumor natural products from marine-derived fungi: covering the period from 2003 to 2012. Curr Top Med Chem. 2013;13:2745–66.
Weaver BA. How Taxol/paclitaxel kills cancer cells. Mol Biol Cell. 2014;25:2677–81.
Gascoigne KE, Taylor SS. How do anti-mitotic drugs kill cancer cells? J Cell Sci. 2009;122:2579–85.
Parajuli P, Joshee N, Chinni SR, Rimando AM, Mittal S, Sethi S, et al. Delayed growth of glioma by Scutellaria flavonoids involve inhibition of Akt, GSK-3 and NF-kappaB signaling. J Neurooncol. 2011;101:15–24.
Tommonaro G, Pejin B, Iodice C, Tafuto A, De Rosa S. Further in vitro biological activity evaluation of amino-, thio- and ester-derivatives of avarol. J Enzyme Inhib Med Chem. 2015;30:333–5.
Pejin B, Iodice C, Tommonaro G, De Rosa S. Synthesis and biological activities of thio-avarol derivatives. J Nat Prod. 2008;71:1850–3.
Tommonaro G, Garcia-Font N, Vitale RM, Pejin B, Iodice C, Canadas S, et al. Avarol derivatives as competitive AChE inhibitors, non hepatotoxic and neuroprotective agents for Alzheimer’s disease. Eur J Med Chem. 2016;122:326–38.
Pejin B, Iodice C, Tommonaro G, Stanimirovic B, Ciric A, Glamoclija J, et al. Further in vitro evaluation of antimicrobial activity of the marine sesquiterpene hydroquinone avarol. Curr Pharm Biotechnol. 2014;15:583–8.
Pejin B, Iodice C, Kojic V, Jakimov D, Lazovic M, Tommonaro G. In vitro evaluation of cytotoxic and mutagenic activity of avarol. Nat Prod Res. 2016;30:1293–6.
Ren Z, Wang L, Cui J, Huoc Z, Xue J, Cui H, et al. Resveratrol inhibits NF-κB signaling through suppression of p65 and IB kinase activities. Pharmazie. 2013;68:689–94.
Cheemanapalli S, Chinthakunta N, Shaikh NM, Shivaranjani V, Pamuru RR, Chitta SK. Comparative binding studies of curcumin and tangeretin on up-stream elements of NF-kB cascade: a combined molecular docking approach. Netw Model Anal Health Inform Bioinform. 2019;8:15.
Khan H, Ullah H, Castilho P, Gomila AS, D’Onofrio G, Filosa R, et al. Targeting NF-kappaB signaling pathway in cancer by dietary polyphenols. Crit Rev Food Sci Nutr. 2019. https://doi.org/10.1080/10408398.2019.1661827.
Kumar D, Dwivedi DK, Lahkar M, Jangra A. Hepatoprotective potential of 7,8-dihydroxyflavone against alcohol and high-fat diet induced liver toxicity via attenuation of oxido-nitrosative stress and NF-kappaB activation. Pharmacol Rep. 2019;71:1235–43.
Luqman S, Pezzuto JM. NFkappaB: a promising target for natural products in cancer chemoprevention. Phytother Res. 2010;24:949–63.
El-Readi MZ, Eid S, Abdelghany AA, Al-Amoudi HS, Efferth T, Wink M. Resveratrol mediated cancer cell apoptosis, and modulation of multidrug resistance proteins and metabolic enzymes. Phytomedicine. 2019;55:269–81.
Vervandier-Fasseur D, Latruffe N. The potential use of resveratrol for cancer prevention. Molecules. 2019;24:4506.
Jiao Y, Li H, Liu Y, Guo A, Xu X, Qu X, et al. Resveratrol inhibits the invasion of glioblastoma-initiating cells via down-regulation of the PI3K/Akt/NF-kappaB signaling pathway. Nutrients. 2015;7:4383–402.
Westhoff MA, Zhou S, Nonnenmacher L, Karpel-Massler G, Jennewein C, Schneider M, et al. Inhibition of NF-kappaB signaling ablates the invasive phenotype of glioblastoma. Mol Cancer Res. 2013;11:1611–23.
Huang H, Lin H, Zhang X, Li J. Resveratrol reverses temozolomide resistance by downregulation of MGMT in T98G glioblastoma cells by the NF-kappaB-dependent pathway. Oncol Rep. 2012;27:2050–6.
Li F, Sethi G. Targeting transcription factor NF-kappaB to overcome chemoresistance and radioresistance in cancer therapy. Biochim Biophys Acta. 2010;1805:167–80.
Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003.
Arepalli SK, Choi M, Jung JK, Lee H. Novel NF-kappaB inhibitors: a patent review (2011–2014). Expert Opin Ther Pat. 2015;25:319–34.
Reyes-Farias M, Carrasco-Pozo C. The anti-cancer effect of quercetin: molecular implications in cancer metabolism. Int J Mol Sci. 2019;20:3177.
Liu Y, Tang ZG, Lin Y, Qu XG, Lv W, Wang GB, et al. Effects of quercetin on proliferation and migration of human glioblastoma U251 cells. Biomed Pharmacother. 2017;92:33–8.
Kiekow CJ, Figueiro F, Dietrich F, Vechia LD, Pires EN, Jandrey EH, et al. Quercetin derivative induces cell death in glioma cells by modulating NF-kappaB nuclear translocation and caspase-3 activation. Eur J Pharm Sci. 2016;84:116–22.
Park MH, Ahn BH, Hong YK, Min do S. Overexpression of phospholipase D enhances matrix metalloproteinase-2 expression and glioma cell invasion via protein kinase C and protein kinase A/NF-kappaB/Sp1-mediated signaling pathways. Carcinogenesis. 2009;30:356–65.
Tang W, Liang R, Duan Y, Shi Q, Liu X, Liao Y. PLD1 overexpression promotes invasion and migration and function as a risk factor for Chinese glioma patients. Oncotarget. 2017;8:57039–46.
Park MH, Min do S. Quercetin-induced downregulation of phospholipase D1 inhibits proliferation and invasion in U87 glioma cells. Biochem Biophys Res Commun. 2011;412:710–5.
Yan X, Qi M, Li P, Zhan Y, Shao H. Apigenin in cancer therapy: anti-cancer effects and mechanisms of action. Cell Biosci. 2017;7:50.
Erdogan S, Doganlar O, Doganlar ZB, Serttas R, Turkekul K, Dibirdik I, et al. The flavonoid apigenin reduces prostate cancer CD44(+) stem cell survival and migration through PI3K/Akt/NF-kappaB signaling. Life Sci. 2016;162:77–86.
Qin Y, Zhao D, Zhou HG, Wang XH, Zhong WL, Chen S, et al. Apigenin inhibits NF-kappaB and snail signaling, EMT and metastasis in human hepatocellular carcinoma. Oncotarget. 2016;7:41421–31.
Chang X, He H, Zhu L, Gao J, Wei T, Ma Z, et al. Protective effect of apigenin on Freund’s complete adjuvant-induced arthritis in rats via inhibiting P2X7/NF-kappaB pathway. Chem Biol Interact. 2015;236:41–6.
Chen XJ, Wu MY, Li DH, You J. Apigenin inhibits glioma cell growth through promoting microRNA-16 and suppression of BCL-2 and nuclear factor-kappaB/MMP9. Mol Med Rep. 2016;14:2352–8.
Brunelli D, Tavecchio M, Falcioni C, Frapolli R, Erba E, Iori R, et al. The isothiocyanate produced from glucomoringin inhibits NF-kB and reduces myeloma growth in nude mice in vivo. Biochem Pharmacol. 2010;79:1141–8.
Prawan A, Saw CL, Khor TO, Keum YS, Yu S, Hu L, et al. Anti-NF-kappaB and anti-inflammatory activities of synthetic isothiocyanates: effect of chemical structures and cellular signaling. Chem Biol Interact. 2009;179:202–11.
Subedi L, Venkatesan R, Kim SY. Neuroprotective and anti-inflammatory activities of allyl isothiocyanate through attenuation of JNK/NF-kappaB/TNF-alpha signaling. Int J Mol Sci. 2017;18:1423.
Guo Z, Wang H, Wei J, Han L, Li Z. Sequential treatment of phenethyl isothiocyanate increases sensitivity of Temozolomide resistant glioblastoma cells by decreasing expression of MGMT via NF-κB pathway. Am J Transl Res. 2019;11:696.
Lee CS, Cho HJ, Jeong YJ, Shin JM, Park KK, Park YY, et al. Isothiocyanates inhibit the invasion and migration of C6 glioma cells by blocking FAK/JNK-mediated MMP-9 expression. Oncol Rep. 2015;34:2901–8.
Cheung KL, Kong A-N. Molecular targets of dietary phenethyl isothiocyanate and sulforaphane for cancer chemoprevention. AAPS J. 2010;12:87–97.
Clarke JD, Dashwood RH, Ho E. Multi-targeted prevention of cancer by sulforaphane. Cancer Lett. 2008;269:291–304.
Xu C, Shen G, Chen C, Gelinas C, Kong AN. Suppression of NF-kappaB and NF-kappaB-regulated gene expression by sulforaphane and PEITC through IkappaBalpha, IKK pathway in human prostate cancer PC-3 cells. Oncogene. 2005;24:4486–95.
Lenzi M, Fimognari C, Hrelia P. Sulforaphane as a promising molecule for fighting cancer. Cancer Treat Res. 2014;159:207–23.
Huang TY, Chang WC, Wang MY, Yang YR, Hsu YC. Effect of sulforaphane on growth inhibition in human brain malignant glioma GBM 8401 cells by means of mitochondrial- and MEK/ERK-mediated apoptosis pathway. Cell Biochem Biophys. 2012;63:247–59.
Chun J, Choi RJ, Khan S, Lee DS, Kim YC, Nam YJ, et al. Alantolactone suppresses inducible nitric oxide synthase and cyclooxygenase-2 expression by down-regulating NF-kappaB, MAPK and AP-1 via the MyD88 signaling pathway in LPS-activated RAW 264.7 cells. Int Immunopharmacol. 2012;14:375–83.
Wei W, Huang H, Zhao S, Liu W, Liu CX, Chen L, et al. Alantolactone induces apoptosis in chronic myelogenous leukemia sensitive or resistant to imatinib through NF-kappaB inhibition and Bcr/Abl protein deletion. Apoptosis. 2013;18:1060–70.
Rasul A, Khan M, Ali M, Li J, Li X. Targeting apoptosis pathways in cancer with alantolactone and isoalantolactone. Sci World J. 2013;2013:248532.
Khan M, Yi F, Rasul A, Li T, Wang N, Gao H, et al. Alantolactone induces apoptosis in glioblastoma cells via GSH depletion, ROS generation, and mitochondrial dysfunction. IUBMB Life. 2012;64:783–94.
Wang X, Yu Z, Wang C, Cheng W, Tian X, Huo X, et al. Alantolactone, a natural sesquiterpene lactone, has potent antitumor activity against glioblastoma by targeting IKKbeta kinase activity and interrupting NF-kappaB/COX-2-mediated signaling cascades. J Exp Clin Cancer Res. 2017;36:93.
Seo MB, Lee SK, Jeon YJ, Im JS. Inhibition of p65 nuclear translocation by baicalein. Toxicol Res. 2011;27:71–6.
Yu X, Liu Y, Wang Y, Mao X, Zhang Y, Xia J. Baicalein induces cervical cancer apoptosis through the NF-kappaB signaling pathway. Mol Med Rep. 2018;17:5088–94.
Jiang G, Zhang L, Wang J, Zhou H. Baicalein induces the apoptosis of U251 glioblastoma cell lines via the NF-kB-p65-mediated mechanism. Anim Cells Syst. 2016;20:296–302.
Kwok BHB, Koh B, Ndubuisi MI, Elofsson M, Crews CM. The anti-inflammatory natural product parthenolide from the medicinal herb Feverfew directly binds to and inhibits IκB kinase. Chem Biol. 2001;8:759–66.
Heptinstall S. Feverfew—an ancient remedy for modern times? J R Soc Med. 1988;81:373.
Haffner MC, Berlato C, Doppler W. Exploiting our knowledge of NF-kappaB signaling for the treatment of mammary cancer. J Mammary Gland Biol Neoplasia. 2006;11:63–73.
Yu Z, Chen Y, Wang S, Li P, Zhou G, Yuan Y. Inhibition of NF-kappaB results in anti-glioma activity and reduces temozolomide-induced chemoresistance by down-regulating MGMT gene expression. Cancer Lett. 2018;428:77–89.
Nakabayashi H, Shimizu K. Involvement of Akt/NF-kappaB pathway in antitumor effects of parthenolide on glioblastoma cells in vitro and in vivo. BMC Cancer. 2012;12:453.
Anderson KN, Bejcek BE. Parthenolide induces apoptosis in glioblastomas without affecting NF-kappaB. J Pharmacol Sci. 2008;106:318–20.
Wang CN, Shiao YJ, Lin YL, Chen CF. Nepalolide A inhibits the expression of inducible nitric oxide synthase by modulating the degradation of IkappaB-alpha and IkappaB-beta in C6 glioma cells and rat primary astrocytes. Br J Pharmacol. 1999;128:345–56.
Saberi-Karimian M, Katsiki N, Caraglia M, Boccellino M, Majeed M, Sahebkar A. Vascular endothelial growth factor: an important molecular target of curcumin. Crit Rev Food Sci Nutr. 2019;59:299–312.
Cheng A-L, Hsu C-H, Lin J-K, Hsu M-M, Ho Y-F, Shen T-S, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–900.
Ambegaokar SS, Wu L, Alamshahi K, Lau J, Jazayeri L, Chan S, et al. Curcumin inhibits dose-dependently and time-dependently neuroglial cell proliferation and growth. Neuro Endocrinol Lett. 2003;24:469.
Gao X, Deeb D, Jiang H, Liu YB, Dulchavsky SA, Gautam SC. Curcumin differentially sensitizes malignant glioma cells to TRAIL/Apo2L-mediated apoptosis through activation of procaspases and release of cytochrome c from mitochondria. J Exp Ther Oncol. 2005;5:39–48.
Kim SY, Jung SH, Kim HS. Curcumin is a potent broad spectrum inhibitor of matrix metalloproteinase gene expression in human astroglioma cells. Biochem Biophys Res Commun. 2005;337:510–6.
Dhandapani KM, Mahesh VB, Brann DW. Curcumin suppresses growth and chemoresistance of human glioblastoma cells via AP-1 and NFkappaB transcription factors. J Neurochem. 2007;102:522–38.
Zhao J, Zhu J, Lv X, Xing J, Liu S, Chen C, et al. Curcumin potentiates the potent antitumor activity of ACNU against glioblastoma by suppressing the PI3K/AKT and NF-kappaB/COX-2 signaling pathways. Onco Targets Ther. 2017;10:5471–82.
Fratantonio D, Molonia MS, Bashllari R, Muscara C, Ferlazzo G, Costa G, et al. Curcumin potentiates the antitumor activity of Paclitaxel in rat glioma C6 cells. Phytomedicine. 2019;55:23–30.
Kumar R, Lal N, Nemaysh V, Luthra PM. Demethoxycurcumin mediated targeting of MnSOD leading to activation of apoptotic pathway and inhibition of Akt/NF-kappaB survival signalling in human glioma U87 MG cells. Toxicol Appl Pharmacol. 2018;345:75–93.
Karmakar S, Weinberg MS, Banik NL, Patel SJ, Ray SK. Activation of multiple molecular mechanisms for apoptosis in human malignant glioblastoma T98G and U87MG cells treated with sulforaphane. Neuroscience. 2006;141:1265–80.
Mollazadeh H, Afshari AR, Hosseinzadeh H. Review on the potential therapeutic roles of nigella sativa in the treatment of patients with cancer: involvement of apoptosis:-black cumin and cancer. J Pharmacopunct. 2017;20:158–72.
Gerstner ER, Fine RL. Increased permeability of the blood-brain barrier to chemotherapy in metastatic brain tumors: establishing a treatment paradigm. J Clin Oncol. 2007;25:2306–12.
Acknowledgements
The authors would like to honor Dr. Sima Khosravi, a kind physician and a great mother to the corresponding author (A. R. A), who lost her battle with GBM disease in the fall of 2016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Soukhtanloo, M., Mohtashami, E., Maghrouni, A. et al. Natural products as promising targets in glioblastoma multiforme: a focus on NF-κB signaling pathway. Pharmacol. Rep 72, 285–295 (2020). https://doi.org/10.1007/s43440-020-00081-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-020-00081-7