Abstract
Glioma is known as one of the most common primary intracranial tumors accounting for four-fifths of malignant brain tumors. There are several biological pathways that play a synergistic, pathophysiological role in glioma, including apoptosis, autophagy, oxidative stress, and cell cycle arrest. According to previous rese arches, the drugs used in the treatment of glioma have been associated with significant limitations. Therefore, improved and/or new therapeutic platforms are required. In this regard, multiple flavonoids and alkaloids have been extensively studied in the treatment of glioma. Berberine is a protoberberine alkaloid with wide range of pharmacological activities, applicable to various pathological conditions. Few studies have reported beneficial roles of berberine in glioma. Berberine exerts its pharmacological functions in glioma by controlling different molecular and cellular pathways. We reviewed the existing knowledge supporting the use of berberine in the treatment of glioma and its effects on molecular and cellular mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioma is known as one of the most common primary intracranial tumors, which represents almost four-fifths of malignant brain tumors (da Silva et al. 2019). Gliomas are neuroectodermal in origin, arising from glial cells and glial precursor (Ostrom et al. 2017). Based on 2016 WHO update, glioma is classified by WHO as grade I-IV, while grade IV glioblastoma multiform (GBM) is known as the most common type of glioma (Howlader 2011). It has been reported that microglia, the purinergic P2X7 receptor (P2X7R) and immunocompetent cells of CNS are involved in the tumor progression and pathology of glioma. P2X7R is overexpressed in tumor cells, infiltrating microglia in glioma cells both in vitro and in vivo (Kan et al. 2019). The exosomes and miRNAs also have important roles in the pathogenesis of GBM (Saadatpour et al. 2016). In this regard, it has been shown that miR-21, miR-124, and miR-155 are involved in the pathogenesis of glioma (Guo et al. 2019; Masoudi et al. 2018). The up-regulation of NQO1 decreases reactive oxygen species (ROS) and elevates the cell proliferation in glioma cells. In addition, overexpression of PINK1 represses the ROS and cell proliferation in glioma cells (Luo et al. 2018).
Oxidative stress induces interaction of apurinic/apyrimidinic endonuclease with ectonucleotide pyrophosphatase/phosphodiesterase 2 and pyruvate kinase M2 in glioma cells (Cholia et al. 2018). Hence, modulation of the oxidative stress can be considered as a treatment strategy for glioma (Marconi et al. 2019; Wu et al. 2019). Magnetic resonance imaging (MRI) is an ideal imaging modality for the diagnosis or monitoring of CNS malignancies (Senft et al. 2011).The standard therapy of newly diagnosed GBM is the combination of radiotherapy and temozolomide (TMZ) (Stupp et al. 2009). However, in accordance with high resistance of GBM to current treatments as well as the failure of prolonging overall survival, new therapeutic approaches are needed (Mooney et al. 2019). In a study, authors documented that 5-ALA fluorescence-guided-surgery (5-ALA-FGS) is able to improve glioma treatment (Picart et al. 2019). In another study, redox-sensitive micelles were confirmed to be effective in the treatment of glioma (Tian et al. 2019). Moreover, it has been reported that medical plants and their active compounds such as gynostemma pentaphyllum extract, resveratrol, and berberine have also beneficial effects on glioma cells (Schild et al. 2010; Filippi-Chiela et al. 2011; Tong et al. 2019).
Berberine, a protoberberine alkaloid, possesses several pharmacological activities, which could be used in the treatment of different diseases such as cancer. Current evidences supported that berberine has a pivotal role in the prevention and treatment of brain-related disorders. Kim et al. (Kim et al. 2014) indicated that berberine has beneficial effects on the improvement of memory and motor dysfunction in Parkinson’s disease through the modulation of apoptotic pathways. Berberine was stated to exert neuroprotective effects in an animal model of Alzheimer’s disease through the regulation of amyloid precursor protein processing (Durairajan et al. 2012). In addition, Bhutada et al. (2010) found that berberine has anticonvulsant activity. Berberine also has anticancer activities against different cancers. Berberine is able to inhibit enzyme cyclooxygenase-2 activity in colon cancer (Fukuda et al. 1999). In another study, it has been reported that berberine could decrease the expression of matrix metallopeptidase (MMP)-9 and subsequently reduce the cell invasion in breast cancer cells (Kim et al. 2008). Finally, it seems that berberine exerts anti-glioma effects via modulating different cellular and molecular mechanisms, including decreasing the extracellular-signal-regulated kinase (ERK) 1/2 activity and increasing apoptosis both in vitro and in vivo (Sun et al. 2018; Eom et al. 2010). In this regard, a better insight into anti-glioma effects of berberine and involved cellular and molecular pathways could provide new milestones in the treatment of glioma. Herein, we focus on reviewing the current scenario in an attempt to provide interested readers with an updated view in this field.
Berberine in the Combination with Radiotherapy
Radiotherapy is a treatment method, which could be employed in various malignancies (Delaney et al. 2005). Radiotherapy is known as a post-surgical treatment for patients with malignant glioma (Walker et al. 1980). A systematic review indicated that the total dose delivered to glioma cells should ranges from 50 to 60 Gy, in the fraction sizes of 1.8–2.0 Gy (Laperriere et al. 2002). Different studies have indicated the beneficial effects of berberine plus radiotherapy in the treatment of cancers. In a study, it was reported that berberine increased the cytotoxicity of radiation via increasing autophagy in lung cancer (Peng et al. 2008). In another study, berberine was stated to increase the radiosensitivity of esophageal cancer cells via down-regulation of RAD51 (Liu et al. 2011). Few studies have shown the effects of berberine in combination with radiotherapy on the treatment of glioma. Yount et al. (Yount et al. 2004) reported that berberine sensitized human glioma cells in response to ionizing radiation, which can lead to the death of GBM cells. Co-administration of berberine and the low energy laser markedly decreased the LD50 of berberine. Another study also indicated the treatment effect of berberine plus laser in glioma cell line (Chen et al. 1994). Further studies are needed to evaluate the effects of berberine in combination with chemotherapy and radiotherapy in the treatment of gliomas (Fig. 1).
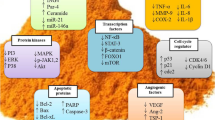
Schematic representation of the beneficial effects of berberine on different signaling pathways in glioma cells. AMPK 5′ AMP-activated protein kinase; Apaf-1 apoptotic protease activating factor-1; Bcl-2 B-cell lymphoma 2; Bax B-cell lymphoma 2-associated X; ERK extracellular-signal-regulated kinase; IL-1β interleukin-1β; IL-18 interleukin-18; JNK c-Jun N-terminal kinases; ULK1 Unc-51-like autophagy activating kinase 1
The Effects of Berberine on Various Molecular and Cellular Mechanisms in Glioma
Berberine and MAPKs Pathway in Glioma Cells
There are three main subfamilies of mitogen-activated protein kinases (MAPK), including the extracellular-signal-regulated kinases (ERK), MAPK14, and the c-jun N-terminal kinase or stress-activated protein kinases (JNK) (Dong et al. 2002). MAPK pathways are downstream pathways of different growth factor receptors such as epidermal growth factor (EGF) (Pece and Gutkind 2000). EGF activates both PKC-dependent and -independent pathways, thereby activating the Raf/MEK/ERK pathway (Chen and Davis 2003). It has been indicated that the Ras/Raf/MEK/ERK pathway is involved in the controlling of growth signals, cell proliferation, survival and invasion in different cancers (De Luca et al. 2012; Meier et al. 2005). Raf/MEK/ERK pathway is also implicated in the pathogenesis of glioma (See and Mukherjee 2018). In addition, ERK regulates cell proliferation in glioma cells (Jacques-Silva et al. 2004). ERK1/2 pathway regulates the BCL-2 proteins to promote cell survival in tumor cells (Balmanno and Cook 2009). In this pathway, protein kinase C (PKC) is able to activate the ERK for the migration of glioma cells (Besson et al. 2001). Lee et al. (2005) reported that the Ras/MEK/ERK pathway is involved in mediating H2O2-induced apoptosis in human glioma cells. Moreover, the activation of ERK plays an important role in the mediation of cisplatin-induced apoptosis in human glioma cells (Lee et al. 2005).
Few studies have addressed the presence of potential links between berberine and MAPKs. Indeed, available evidences still are not conclusive. Berberine decreases tumor growth and suppresses p-ERK1/2 and Ki-67 expression in glioma cells (Sun et al. 2018). In another study, berberine significantly inhibited the Caspase-1-Mediated IL-1β and IL-18 releasing via inhibition of ERK1/2 signaling in glioma cells (Tong et al. 2019). Berberine greatly reduces the epidermal growth factor receptor (EGFR). Downregulation of EGFR resulted in inhibiting the RAF-MEK-ERK signaling pathway, but AKT phosphorylation was not changed (Liu et al. 2015). More studies are also needed to evaluate the effects of berberine on different proteins of the MAPKs pathway in gliomas.
Berberine and MMPs Pathways in Glioma Cells
Known as zinc-dependent endopeptidases family, MMPs are involved in the progression of cancer via increasing the cancer cell growth, migration, invasion, metastasis, and angiogenesis (Egeblad and Werb 2002). MT1-MMP has an important role in the pathophysiology of human malignant gliomas (Forsyth et al. 1999). In a study, it was reported that MMP-2 is localized in vasculature cells and tumor cells of malignant astrocytomas as well (Sawaya et al. 1996). MMP-2 inhibition significantly decreases tumor cell invasion, migration, and tumor growth in glioma cells (Badiga et al. 2011). In addition, these MMPs could be utilized as prognosis factors in the treatment of glioma (Thorns et al. 2003). Inhibition of MMPs can be a strategy for controlling cancer cells. Nyormoi et al. (2003) indicated that the inhibitor of MMP-2/MMP-9 led to inducing of apoptosis in cancer cells. Moreover, inhibition of MMPs can sensitize glioma cells to TMZ (Ulasov et al. 2013). It has been reported that berberine has beneficial effects on MMPs in cancer. Berberine also prevents the cell migration through inhibition of MMP-1, -2, and -9 in gastric cancer (Lin et al. 2008a). In another study, it was documented that MMP-1 and MMP-9 could be inhibited by berberine in human breast cancer (Kim et al. 2012). Berberine enhances the anti-glioma effects of As2O3-; therefore, the treatment with As2O3 and berberine synergistically decreased the activation of PKC alpha and epsilon and triggered actin cytoskeleton rearrangements. Also, the levels of jun and myc, and MT1-MMP, and MMP-2 reduced (Lin et al. 2008b).
Berberine and Autophagy Pathway in Glioma Cells
Autophagy is a process of protein recycling, which initiates with sequestering of cytoplasmic organelles in the membrane of vacuole, a process named autophagosome (Mizushima 2007). PtdIns3K-Akt-mTORC1 is a very important signaling pathway which is related to autophagy (Wu et al. 2009). TOR acts as the main regulator of autophagy. In addition, AMPK inhibits mTORC1 activity (Kang et al. 2011), which leads to suppressing ULK1 (Kang et al. 2011). ULK1 induces the Beclin-1 phosphorylation, which can result in autophagy (Russell et al. 2013). The autophagy process act as a tumor suppressor in the early stage of tumor development (Kondo et al. 2005). It has been showed that the mTOR, AKT and Ki-67/MIB-1 expression increased in glioma cells with a rise in grade of malignancy (Annovazzi et al. 2009). Autophagy could be used as a therapeutic target in the treatment of several cancers (Kondo et al. 2005); therefore, the induction of autophagy can promote the radiosensitivity in glioma cells (Palumbo et al. 2012). TMZ is able to exert its anti-glioma effects via inducing autophagy (Fan and Weiss 2011). This chemotherapy agent induces autophagy through ATM-AMPK-ULK1 signaling pathways in glioma cells (Zou et al. 2014). It has been shown that berberine induces autophagy in various cancers (Wang et al. 2010; Yu et al. 2014). A study has reported that berberine dephosphorylated mTOR, leading to the induction of autophagy. Berberine activates AMPK, which negatively regulates the mTOR and enhances the autophagy. In addition, berberine treatment elevated the p-Beclin-1 Ser93 in GBM cells. Berberine increased phosphorylated ULK1, a downstream target of mTOR. These signaling pathways lead to induction of autophagy in GBM cells (Wang et al. 2016).
Berberine and Cell Cycle Arrest in Glioma Cells
In different phases of the cell cycle (G1, S/DNA synthesis, G2, M/mitosis) during development, DNA is duplicated and the chromosomes are distributed into two daughter cells (Pardee 1989). Insulin-like growth factor-binding protein (IGFBP)-3 inhibits the cell proliferation via stimulation of cell cycle arrest. The expression of IGFBP-3 leads to G1 arrest, whereby the levels of the cell cycle-regulated proteins including cyclin D1, cyclin D3, cyclin A, cyclin E, and cyclin-dependent kinase (CDK) 2 and CDK4 may be decreased (Kim et al. 2010). The acetylation of p53 is also involved in the cell cycle arrest (Li et al. 2012). It has been shown that the induction of G1 arrest could contribute to control of cancer (Pardee 1989). Berberine promotes the cell cycle arrest in different cancers by decreasing cyclin D1, cyclin D3, cyclin A, cyclin E, and cyclin-dependent kinase (CDK) 2 and CDK4 (Lin et al. 2006; Mantena et al. 2006). In addition, berberine induces the G1 arrest through an increase in the expression of P27 and a decrease in the cyclin-dependent kinase (CDK) 2, CDK4, cyclin E proteins, and cyclin D (Eom et al. 2010).
Berberine and Apoptosis Pathways in Glioma Cells
Apoptosis contributes to tumor pathogenesis by different pathways (Wong 2011). There is an inverse relationship between cell apoptosis and tumor progression (Symonds et al. 1994). The changes in the ratio of pro- and anti-apoptotic proteins are involved in cell death. There are two general classes of apoptosis pathways, including intrinsic and extrinsic ones (Fulda and Debatin 2006). Caspases are important parts of apoptosis pathways (Leist et al. 1997). Thus, the change in caspases activities affects apoptosis and carcinogenesis in different cancers (Ghavami et al. 2009). Death receptors and their ligands have important roles in the external apoptosis pathway, including TNF, TNFR1, TNFR1-associated death domain protein (TRADD), and TNFR-associated factor (TRAFs). In addition, FAS (CD95) and FASL ligand (CD95L) are members of the extrinsic pathway (Ashkenazi 2008). Additionally, intrinsic pathways are related to non-receptor proteins, which stimulate apoptosis through intracellular signals that influence different targets within the cell or mitochondria. These proteins mainly includes the B-cell lymphoma protein 2 (Bcl-2) family proteins and cytochrome c (Wu and Bratton 2013). Various malignant glioma cells also express apoptotic proteins in intrinsic and extrinsic pathways (Song et al. 2003). It has been reported that apoptotic proteins are appropriate targets in controlling cancers (Wu and Bratton 2013).
Berberine increases the ROS and intracellular Ca (2 +) and also induces ER stress. In addition, this natural compound markedly increases apoptosis by induction of a higher ratio of Bax/Bcl-2 proteins, the activation of caspase-3 and -9, and the cleavage of the poly(ADP-ribose) polymerase (PARP) (Eom et al. 2010). Berberine treatment markedly increases the apoptosis via induction of a higher ratio of the Bax/Bcl-2 proteins, mitochondrial membrane potential disruption, and activation of procaspase-9, caspase-3, caspase-9, and poly (ADP-ribose) polymerase (PARP). Berberine inhibits the glioma cell proliferation through promoting G1 arrest and apoptosis (Eom et al. 2008). Berberine increases the production of ROS and Ca2 + in glioma cells. Berberine inhibits Bcl-2 but increases Bax. The caspase-8, -9, and -3 were activated by berberine in glioma cells (Chen et al. 2009).
Berberine and Arylamine N-acetyltransferases in Glioma Cells
There are two related genes on chromosome 8, which have an important role in encoding the human arylamine N-acetyltransferases (NAT1 and NAT2). NATs are known as polymorphic drug-metabolizing enzymes (Sim et al. 2014). NAT1 and NAT2 from this family are expressed in different tissues in the body (Windmill et al. 2000). These enzymes add an acetyl group from the O to the N group of arylacetohydroxates, which leads to the activation of arylamine carcinogens (Kabir and Rehman 2018). NAT1 is involved in cancer cell growth, since it is over€expressed in several cancers, leading to an increase in the resistance to chemotherapy (Butcher and Minchin 2012). Therefore, human arylamine N-acetyltransferases can be a drug target (Butcher and Minchin 2012). It has been reported that berberine inhibits the expression of NAT in cancer (LIN et al. 2005; Chung et al. 1999). In addition, berberine suppresses NAT1 in GBM cells (Wang et al. 2002). Further studies are needed to estimate the effects of berberine on NATs in gliomas.
Conclusions
Increasing evidence shows that berberine has pharmacological effects on tumor growth, cell proliferation, glycolytic capacity, migration, invasion, G1 arrest, NAT1, apoptosis, and autophagy signaling in glioma cells. In addition, berberine in combination with radiotherapy has beneficial effects on gliomas. Therefore, berberine could be used as an adjuvant therapy in the treatment of glioma. Further clinical and pre-clinical studies are needed to assess the therapeutic effects of berberine in the treatment of glioma.
References
Annovazzi L, Mellai M, Caldera V, Valente G, Tessitore L, Schiffer D (2009) mTOR, S6 and AKT expression in relation to proliferation and apoptosis/autophagy in glioma. Anticancer Res 29(8):3087–3094
Ashkenazi A (2008) Targeting the extrinsic apoptosis pathway in cancer. Cytokine Growth Factor Rev 19(3–4):325–331
Badiga AV, Chetty C, Kesanakurti D, Are D, Gujrati M, Klopfenstein JD, Dinh DH, Rao JS (2011) MMP-2 siRNA inhibits radiation-enhanced invasiveness in glioma cells. PLoS ONE 6(6):e20614
Balmanno K, Cook S (2009) Tumour cell survival signalling by the ERK1/2 pathway. Cell Death Differ 16(3):368
Besson A, Davy A, Robbins SM, Yong VW (2001) Differential activation of ERKs to focal adhesions by PKC ε is required for PMA-induced adhesion and migration of human glioma cells. Oncogene 20(50):7398
Bhutada P, Mundhada Y, Bansod K, Dixit P, Umathe S, Mundhada D (2010) Anticonvulsant activity of berberine, an isoquinoline alkaloid in mice. Epilepsy Behav 18(3):207–210
Butcher NJ, Minchin RF (2012) Arylamine N-acetyltransferase 1: a novel drug target in cancer development. Pharmacol Rev 64(1):147–165
Chen D-b, Davis JS (2003) Epidermal growth factor induces c-fos and c-jun mRNA via Raf-1/MEK1/ERK-dependent and-independent pathways in bovine luteal cells. Mol Cell Endocrinol 200(1–2):141–154
Chen K, Hao D, Liu Z, Chen Y, You Z (1994) Effect of berberine alone or in combination with argon ion laser treatment on 9L rat glioma cell line. Chin Med J 107(11):808–812
Chen T-C, Lai K-C, Yang J-S, Liao C-L, Hsia T-C, Chen G-W, Lin J-J, Lin H-J, Chiu T-H, Tang Y-J (2009) Involvement of reactive oxygen species and caspase-dependent pathway in berberine-induced cell cycle arrest and apoptosis in C6 rat glioma cells. Int J Oncol 34(6):1681–1690
Cholia RP, Dhiman M, Kumar R, Mantha AK (2018) Oxidative stress stimulates invasive potential in rat C6 and human U-87 MG glioblastoma cells via activation and cross-talk between PKM2, ENPP2 and APE1 enzymes. Metab Brain Dis 33(4):1307–1326
Chung J, Wu L, Chu C, Jan J, Ho C, Tsou M, Lu H, Chen G, Lin J, Wang T (1999) Effects of berberine on arylamine N-acetyltransferase activity in human bladder tumour cells. Food Chem Toxicol 37(4):319–326
da Silva AB, Coelho PLC, das Neves Oliveira M, Oliveira JL, Amparo JAO, da Silva KC, Soares JRP, Pitanga BPS, dos Santos Souza C, de Faria Lopes GP (2019) The flavonoid rutin and its aglycone quercetin modulate the microglia inflammatory profile improving antiglioma activity. Brain Behav Immun 85:170–185
De Luca A, Maiello MR, D'Alessio A, Pergameno M, Normanno N (2012) The RAS/RAF/MEK/ERK and the PI3K/AKT signalling pathways: role in cancer pathogenesis and implications for therapeutic approaches. Expert Opin Ther Targets 16(sup2):S17–S27
Delaney G, Jacob S, Featherstone C, Barton M (2005) The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer 104(6):1129–1137
Dong C, Davis RJ, Flavell RA (2002) MAP kinases in the immune response. Annu Rev Immunol 20(1):55–72
Durairajan SSK, Liu L-F, Lu J-H, Chen L-L, Yuan Q, Chung SK, Huang L, Li X-S, Huang J-D, Li M (2012) Berberine ameliorates β-amyloid pathology, gliosis, and cognitive impairment in an Alzheimer's disease transgenic mouse model. Neurobiol Aging 33(12):2903–2919
Egeblad M, Werb Z (2002) New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2(3):161
Eom K-S, Hong J-M, Youn M-J, So H-S, Park R, Kim J-M, Kim T-Y (2008) Berberine induces G1 arrest and apoptosis in human glioblastoma T98G cells through mitochondrial/caspases pathway. Biol Pharm Bull 31(4):558–562
Eom KS, Kim H-J, So H-S, Park R, Kim TY (2010) Berberine-induced apoptosis in human glioblastoma T98G cells is mediated by endoplasmic reticulum stress accompanying reactive oxygen species and mitochondrial dysfunction. Biol Pharm Bull 33(10):1644–1649
Fan Q-W, Weiss WA (2011) Autophagy and Akt promote survival in glioma. Autophagy 7(5):536–538
Filippi-Chiela EC, Villodre ES, Zamin LL, Lenz G (2011) Autophagy interplay with apoptosis and cell cycle regulation in the growth inhibiting effect of resveratrol in glioma cells. PLoS ONE 6(6):e20849
Forsyth P, Wong H, Laing TD, Rewcastle N, Morris D, Muzik H, Leco K, Johnston R, Brasher P, Sutherland G (1999) Gelatinase-A (MMP-2) gelatinase-B (MMP-9) and membrane type matrix metalloproteinase-1 (MT1-MMP) are involved in different aspects of the pathophysiology of malignant gliomas. Br J Cancer 79(11):1828
Fukuda K, Hibiya Y, Mutoh M, Koshiji M, Akao S, Fujiwara H (1999) Inhibition by berberine of cyclooxygenase-2 transcriptional activity in human colon cancer cells. J Ethnopharmacol 66(2):227–233
Fulda S, Debatin K-M (2006) Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 25(34):4798
Ghavami S, Hashemi M, Ande SR, Yeganeh B, Xiao W, Eshraghi M, Bus CJ, Kadkhoda K, Wiechec E, Halayko AJ (2009) Apoptosis and cancer: mutations within caspase genes. J Med Genet 46(8):497–510
Guo Y, Hong W, Wang X, Zhang P, Körner H, Tu J, Wei W (2019) MicroRNAs in microglia: How do MicroRNAs affect activation, inflammation, polarization of microglia and mediate the interaction between microglia and glioma? Front Mol Neurosci 12:125
Howlader N (2011) SEER cancer statistics review, 1975–2008. http://seercancergov/csr/1975_2008/
Jacques-Silva MC, Bernardi A, Rodnight R, Lenz G (2004) ERK, PKC and PI3K/Akt pathways mediate extracellular ATP and adenosine-induced proliferation of U138-MG human glioma cell line. Oncology 67(5–6):450–459
Kabir S, Rehman A (2018) Carcinogenic potential of arylamine N-acetyltransferase in Asian populations: A review. J Cancer Res Pract 5:131–135
Kan LK, Williams D, Drummond K, O'Brien T, Monif M (2019) The role of microglia and P2X7 receptors in gliomas. J Neuroimmunol 332:138–146
Kang R, Zeh H, Lotze M, Tang D (2011) The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ 18(4):571
Kim H-S, Lee W, Lee S, Chae H-W, Kim D, Oh Y (2010) Insulin-like growth factor binding protein-3 induces G1 cell cycle arrest with inhibition of cyclin-dependent kinase 2 and 4 in MCF-7 human breast cancer cells. Horm Metab Res 42(03):165–172
Kim M, Cho K-H, Shin M-S, Lee J-M, Cho H-S, Kim C-J, Shin D-H, Yang HJ (2014) Berberine prevents nigrostriatal dopaminergic neuronal loss and suppresses hippocampal apoptosis in mice with Parkinson's disease. Int J Mol Med 33(4):870–878
Kim S, Choi JH, Kim JB, Nam SJ, Yang J-H, Kim J-H, Lee JE (2008) Berberine suppresses TNF-α-induced MMP-9 and cell invasion through inhibition of AP-1 activity in MDA-MB-231 human breast cancer cells. Molecules 13(12):2975–2985
Kim S, Han J, Lee SK, Choi M-Y, Kim J, Lee J, Jung SP, Kim JS, Kim J-H, Choe J-H (2012) Berberine suppresses the TPA-induced MMP-1 and MMP-9 expressions through the inhibition of PKC-α in breast cancer cells. J Surg Res 176(1):e21–e29
Kondo Y, Kanzawa T, Sawaya R, Kondo S (2005) The role of autophagy in cancer development and response to therapy. Nat Rev Cancer 5(9):726
Laperriere N, Zuraw L, Cairncross G, Group CCOPGIN-ODS (2002) Radiotherapy for newly diagnosed malignant glioma in adults: a systematic review. Radiother Oncol 64(3):259–273
Lee WC, Choi CH, Cha SH, Oh HL, Kim YK (2005) Role of ERK in hydrogen peroxide-induced cell death of human glioma cells. Neurochem Res 30(2):263–270
Leist M, Volbracht C, Kühnle S, Fava E, Ferrando-May E, Nicotera P (1997) Caspase-mediated apoptosis in neuronal excitotoxicity triggered by nitric oxide. Mol Med 3(11):750
Li T, Kon N, Jiang L, Tan M, Ludwig T, Zhao Y, Baer R, Gu W (2012) Tumor suppression in the absence of p53-mediated cell-cycle arrest, apoptosis, and senescence. Cell 149(6):1269–1283
Lin C-C, Kao S-T, Chen G-W, Chung J-G (2005) Berberine decreased N-acetylation of 2-aminofluorene through inhibition of N-acetyltransferase gene expression in human leukemia HL-60 cells. Anticancer Res 25(6B):4149–4155
Lin J-P, Yang J-S, Lee J-H, Hsieh W-T, Chung J-G (2006) Berberine induces cell cycle arrest and apoptosis in human gastric carcinoma SNU-5 cell line. World J Gastroenterol: WJG 12(1):21
Lin J-P, Yang J-S, Wu C-C, Lin S-S, Hsieh W-T, Lin M-L, Yu F-S, Yu C-S, Chen G-W, Chang Y-H (2008a) Berberine induced down-regulation of matrix metalloproteinase-1,-2 and-9 in human gastric cancer cells (SNU-5) in vitro. In Vivo 22(2):223–230
Lin T-H, Kuo H-C, Chou F-P, Lu F-J (2008b) Berberine enhances inhibition of glioma tumor cell migration and invasiveness mediated by arsenic trioxide. BMC Cancer 8(1):58
Liu Q, Jiang H, Liu Z, Wang Y, Zhao M, Hao C, Feng S, Guo H, Xu B, Yang Q (2011) Berberine radiosensitizes human esophageal cancer cells by downregulating homologous recombination repair protein RAD51. PLoS ONE 6(8):e23427
Liu Q, Xu X, Zhao M, Wei Z, Li X, Zhang X, Liu Z, Gong Y, Shao C (2015) Berberine induces senescence of human glioblastoma cells by downregulating the EGFR–MEK–ERK signaling pathway. Mol Cancer Ther 14(2):355–363
Luo S, Lei K, Xiang D, Ye K (2018) NQO1 Is Regulated by PTEN in Glioblastoma, Mediating Cell Proliferation and Oxidative Stress. Oxidat Med Cell Longev 201:9146528–9146528
Mantena SK, Sharma SD, Katiyar SK (2006) Berberine, a natural product, induces G1-phase cell cycle arrest and caspase-3-dependent apoptosis in human prostate carcinoma cells. Mol Cancer Ther 5(2):296–308
Marconi GD, Gallorini M, Carradori S, Guglielmi P, Cataldi A, Zara S (2019) The Up-Regulation of Oxidative Stress as a Potential Mechanism of Novel MAO-B Inhibitors for Glioblastoma Treatment. Molecules 24(10):2005
Masoudi MS, Mehrabian E, Mirzaei H (2018) MiR-21: A key player in glioblastoma pathogenesis. J Cell Biochem 119(2):1285–1290
Meier F, Schittek B, Busch S, Garbe C, Smalley K, Satyamoorthy K, Li G, Herlyn M (2005) The RAS/RAF/MEK/ERK and PI3K/AKT signaling pathways present molecular targets for the effective treatment of advanced melanoma. Front Biosci 10(2986–3001):2986–3001
Mizushima N (2007) Autophagy: process and function. Genes Dev 21(22):2861–2873
Mooney J, Bernstock JD, Ilyas A, Ibrahim A, Yamashita D, Markert JM, Nakano I (2019) Current approaches and challenges in the molecular therapeutic targeting of glioblastoma. World Neurosurg
Nyormoi O, Mills L, Bar-Eli M (2003) An MMP-2/MMP-9 inhibitor, 5a, enhances apoptosis induced by ligands of the TNF receptor superfamily in cancer cells. Cell Death Differ 10(5):558
Ostrom QT, Gittleman H, Liao P, Vecchione-Koval T, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS (2017) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro-oncology 19(suppl_5):1–88
Palumbo S, Pirtoli L, Tini P, Cevenini G, Calderaro F, Toscano M, Miracco C, Comincini S (2012) Different involvement of autophagy in human malignant glioma cell lines undergoing irradiation and temozolomide combined treatments. J Cell Biochem 113(7):2308–2318
Pardee AB (1989) G1 events and regulation of cell proliferation. Science 246(4930):603–608
Pece S, Gutkind JS (2000) Signaling from E-cadherins to the MAPK pathway by the recruitment and activation of epidermal growth factor receptors upon cell-cell contact formation. J Biol Chem 275(52):41227–41233
Peng P-l, Kuo W-H, Tseng H-C, Chou F-P (2008) Synergistic tumor-killing effect of radiation and berberine combined treatment in lung cancer: the contribution of autophagic cell death. Int J Radiat Oncol Biol Phys 70(2):529–542
Picart T, Berhouma M, Dumot C, Pallud J, Metellus P, Armoiry X, Guyotat J (2019) Optimization of high-grade glioma resection using 5-Ala fluorescence-guided surgery: literature review and practical recommendations from the Neuro-Oncology Club Of The French Society Of Neurosurgery. Neurochirurgie 65:164–177
Russell RC, Tian Y, Yuan H, Park HW, Chang Y-Y, Kim J, Kim H, Neufeld TP, Dillin A, Guan K-L (2013) ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat Cell Biol 15(7):741
Saadatpour L, Fadaee E, Fadaei S, Mansour RN, Mohammadi M, Mousavi S, Goodarzi M, Verdi J, Mirzaei H (2016) Glioblastoma: exosome and microRNA as novel diagnosis biomarkers. Cancer Gene Ther 23(12):415
Sawaya RE, Yamamoto M, Gokaslan ZL, Wang SW, Mohanam S, Fuller GN, McCutcheon IE, Stetler-Stevenson WG, Nicolson GL, Rao JS (1996) Expression and localization of 72 kDa type IV collagenase (MMP-2) in human malignant gliomas in vivo. Clin Exp Metas 14(1):35–42
Schild L, Chen B, Makarov P, Kattengell K, Heinitz K, Keilhoff G (2010) Selective induction of apoptosis in glioma tumour cells by a Gynostemma pentaphyllum extract. Phytomedicine 17(8–9):589–597
See WL, Mukherjee J (2018) Targeting the RAS-RAF-MEK-ERK signaling pathway in gliomas. In: Newton HB (ed) Handbook of brain tumor chemotherapy, molecular therapeutics, and immunotherapy. Elsevier, New York, pp 323–332
Senft C, Bink A, Franz K, Vatter H, Gasser T, Seifert V (2011) Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncol 12(11):997–1003
Sim E, Abuhammad A, Ryan A (2014) Arylamine N-acetyltransferases: from drug metabolism and pharmacogenetics to drug discovery. Br J Pharmacol 171(11):2705–2725
Song JH, Song DK, Pyrzynska B, Petruk KC, Van Meir EG, Hao C (2003) TRAIL triggers apoptosis in human malignant glioma cells through extrinsic and intrinsic pathways. Brain Pathol 13(4):539–553
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10(5):459–466
Sun Y, Yu J, Liu X, Zhang C, Cao J, Li G, Liu X, Chen Y, Huang H (2018) Oncosis-like cell death is induced by berberine through ERK1/2-mediated impairment of mitochondrial aerobic respiration in gliomas. Biomed Pharmacother 102:699–710
Symonds H, Krall L, Remington L, Saenz-Robles M, Lowe S, Jacks T, Van Dyke T (1994) p53-dependent apoptosis suppresses tumor growth and progression in vivo. Cell 78(4):703–711
Thorns V, Walter G, Thorns C (2003) Expression of MMP-2, MMP-7, MMP-9, MMP-10 and MMP-11 in human astrocytic and oligodendroglial gliomas. Anticancer Res 23(5A):3937–3944
Tian C, Asghar S, Hu Z, Qiu Y, Zhang J, Shao F, Xiao Y (2019) Understanding the cellular uptake and biodistribution of a dual-targeting carrier based on redox-sensitive hyaluronic acid-ss-curcumin micelles for treating brain glioma. Int J Biol Macromol 136:143–153
Tong L, Xie C, Wei Y, Qu Y, Liang H, Zhang Y, Xu T, Qian X, Qiu H, Deng H (2019) Antitumor effects of berberine on gliomas via inactivation of caspase-1-mediated IL-1β and IL-18 release. Front Oncol 9:364
Ulasov I, Thaci B, Sarvaiya P, Yi R, Guo D, Auffinger B, Pytel P, Zhang L, Kim CK, Borovjagin A (2013) Inhibition of MMP 14 potentiates the therapeutic effect of temozolomide and radiation in gliomas. Cancer Med 2(4):457–467
Walker MD, Green SB, Byar DP, Alexander E Jr, Batzdorf U, Brooks WH, Hunt WE, MacCarty CS, Mahaley MS Jr, Mealey J Jr (1980) Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med 303(23):1323–1329
Wang D, Yeh C, Lee J, Hung C, Chung J (2002) Berberine inhibited arylamine N-acetyltransferase activity and gene expression and DNA adduct formation in human malignant astrocytoma (G9T/VGH) and brain glioblastoma multiforms (GBM 8401) cells. Neurochem Res 27(9):883–889
Wang J, Qi Q, Feng Z, Zhang X, Huang B, Chen A, Prestegarden L, Li X, Wang J (2016) Berberine induces autophagy in glioblastoma by targeting the AMPK/mTOR/ULK1-pathway. Oncotarget 7(41):66944
Wang N, Feng Y, Zhu M, Tsang CM, Man K, Tong Y, Tsao SW (2010) Berberine induces autophagic cell death and mitochondrial apoptosis in liver cancer cells: the cellular mechanism. J Cell Biochem 111(6):1426–1436
Windmill KF, Gaedigk A, de la M, Hall P, Samaratunga H, Grant DM, McManus ME (2000) Localization of N-acetyltransferases NAT1 and NAT2 in human tissues. Toxicol Sci 54(1):19–29
Wong RS (2011) Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res 30(1):87
Wu C-C, Bratton SB (2013) Regulation of the intrinsic apoptosis pathway by reactive oxygen species. Antioxid Redox Signal 19(6):546–558
Wu H, Fu X, Cao W, Xiang W, Hou Y, Ma J, Wang Y, Fan C (2019) Induction of apoptosis in human glioma cells by fucoxanthin via triggering of ROS-mediated oxidative damage and regulation of MAPKs and PI3K-AKT pathways. J Agric Food Chem 67(8):2212
Wu Y-T, Tan H-L, Huang Q, Ong C-N, Shen H-M (2009) Activation of the PI3K-Akt-mTOR signaling pathway promotes necrotic cell death via suppression of autophagy. Autophagy 5(6):824–834
Yount G, Qian Y, Moore D, Basila D, West J, Aldape K, Arvold N, Shalev N, Haas-Kogan D (2004) Berberine sensitizes human glioma cells, but not normal glial cells, to ionizing radiation in vitro. J Exp Ther Oncol 4(2):137–143
Yu R, Zhang Z-q, Wang B, Jiang H-x, Cheng L, Shen L-m (2014) Berberine-induced apoptotic and autophagic death of HepG2 cells requires AMPK activation. Cancer Cell Int 14(1):49
Zou Y, Wang Q, Li B, Xie B, Wang W (2014) Temozolomide induces autophagy via ATM-AMPK-ULK1 pathways in glioma. Mol Med Rep 10(1):411–416
Funding
No specific source of funding is associated with this work.
Author information
Authors and Affiliations
Contributions
ZA, MB, M-AP, HM, and Z-SR and O-RT contributed in the conception or design of the work and drafting of the manuscript. All authors confirmed the final version for submission.
Corresponding author
Ethics declarations
Competing interest
All authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Asemi, Z., Behnam, M., Pourattar, M.A. et al. Therapeutic Potential of Berberine in the Treatment of Glioma: Insights into Its Regulatory Mechanisms. Cell Mol Neurobiol 41, 1195–1201 (2021). https://doi.org/10.1007/s10571-020-00903-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-020-00903-5