Abstract
Background
Inflammatory bowel disease is an intestinal disorder presented by recurrent inflammation in the gastrointestinal tract. It has been reported that modafinil, also known as an awakening drug, has anti-inflammatory characteristics. The objective of this experiment is to investigate the protective effects of modafinil on colitis induced by acetic acid in rat and the involvement of nitric oxide pathway.
Methods
Colitis was induced by intra-rectal instillation of 1 ml acetic acid (4%). After one h of colitis induction (first day), intraperitoneal injection of dexamethasone (1 mg/kg), modafinil (50, 100, and 150 mg/kg), nitric oxide synthase inhibitors (NOS)—N (G)-nitro-l-arginine methyl ester (L-NAME) 10 mg/kg, 7-nitroindazole 40 mg/kg, and aminoguanidine 50 mg/kg—was performed and continued for 2 consecutive days. Ultimately, macroscopic, microscopic, and biochemical assessments were performed.
Results
While induction of colitis caused severe macroscopic lesions, administration of dexamethasone and modafinil (100 and 150 mg/kg) significantly improved macroscopic ulcers. Interestingly, the combination of modafinil with NOS inhibitors reversed the beneficial effects of modafinil on macroscopic destructions. In addition, the elevated level of interleukin-1beta (IL-1β) and tumor necrosis factor-alpha (TNF-α) was decreased by modafinil. However, treatment with NOS inhibitors before modafinil neutralized the anti-inflammatory influence of modafinil. Additionally, histological disorders emerged by acetic acid in colon tissue remarkably were disappeared after treatment with modafinil.
Conclusions
In conclusion, modafinil has a protective effect on injuries induced by acetic acid in the colon of rat, which is presumably via the inhibition of inflammatory cascade and mediation of NO pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammatory bowel disease (IBD) is categorized into ulcerative colitis (UC) and Crohn’s disease (CD), both of which characterized by chronic, uncontrolled and recurrent intestinal inflammation [1, 2]. Genetic, environment and immune system have been considered as the essential etiological factors for IBD. In addition, it has been postulated that interplay between these agents leads to an excessive immune response against the normal flora of the intestine [3]. Consequently, provoked immune cells—neutrophil, macrophages and monocytes—secrete pro-inflammatory cytokines such as interleukin-1beta (IL-1β) and tumor necrosis factor-alpha (TNF-α), which play a crucial role in mucosal inflammation [4]. Generally, the management of inflammation is a key target in IBD treatment [5]. Ordinary pharmacotherapies for IBD are glucocorticoids and aminosalicylates. Additionally, immunosuppressive drugs and biological response modifiers are utilized as alternative therapies. Although these agents have been effective in control and treatment of IBD, they breed various adverse effects. Hence, supplementary researches to achieve new approaches and novel drugs should be conducted [6, 7].
Nitric oxide (NO) is synthesized from amino acid l-arginine by three isoforms of NO synthase (NOSs), two of which are constituently expressed in neuronal and endothelial tissues—(nNOS) and (eNOS), respectively. The third one, inducible NOS (iNOS), is induced within inflammation in various cells. An increase in the level of NO has been reported in inflammatory bowel disease [8, 9]. Some studies have demonstrated the protective effect and profitable role of NO in acute colitis [9, 10].
Modafinil 2-[(diphenylmethyl)sulfinyl] acetamide is a non-amphetamine stimulant drug prescribed to treat wakefulness diseases—narcolepsy, shift work sleep disorder (SWSD), and obstructive sleep apnea/hypopnea syndrome (OSAHS) [11]. Recently, it has been indicated that modafinil features anti-inflammatory properties and reduces the level of TNF-α and IL-1 [11,12,13]. One of the considered mechanisms by which modafinil acts is NO pathway [14]. In this regard, some animal studies reported that the effects of modafinil are neutralized by injection of N (G)-nitro-l-arginine methyl ester (L-NAME) and 7-nitroindazole—non-specific inhibitors and neuronal inhibitors of NOS, respectively [15]. Therefore, the aim of this experiment was to assess the impacts of modafinil on acetic acid-induced colitis and evaluate the presumable role of NO pathway in colonic inflammation in rat.
Materials and methods
Animals
The animal procedures were performed in accordance with National Institutes of Health guide for the care and use of Laboratory Animals (NIH Publications No. 8023, revised 1978) and were approved by Ethical Committee of Tehran University of Medical Sciences (No.: 982518). Ninety-six male Wistar rats weighing (200–250 g) were provided from Department of Pharmacology, Tehran University of Medical Sciences. They were kept under-controlled condition of 12 h light/dark cycles, temperature 20–23 °C, and humidity 50–60% with free access to standard rodent food and water.
Chemicals
Modafinil, L-NAME (nonspecific nitric oxide synthase inhibitor), 7-nitroindazole (a neuronal NOS inhibitor), aminoguanidine (an inducible NOS inhibitor), and dexamethasone were purchased from Sigma (St. Louis, MO, United State). Modafinil and 7-nitroindazole were dissolved in Tween 80 1% solution and 0.9% saline solution. L-NAME, aminoguanidine, and dexamethasone were dissolved in 0.9% saline solution—each mg/kg of drugs was calculated in 10 ml of solvent. Ketamine and Xylazine were provided from Alfasan Company (Woerden Holland), and formalin solution and acetic acid were bought from Merck Company.
Induction of colitis and surgical procedure
Before the induction of colitis, animals fasted for 24 h; however, they had free access to tap water. To induce colitis, we lightly anesthetized animals using ketamine (50 mg/kg, ip) and xylazine (10 mg/kg, ip). Afterwards, 1 ml of 4% acetic acid was intrarectally administered by means of a flexible catheter, with 2 mm of the diameter, which was inserted 8 cm into the rectum [16]. Then, animals were positioned upside down for 1 min in order for the prevention of acetic acid solution leakage. One h after the induction of colitis (first day), intraperitoneal administration of modafinil (50, 100, 150 mg/kg, ip) and dexamethasone (1 mg/kg, ip) were performed and continued for another two consecutive days. To elucidate the role of NO pathway in modafinil performance, intraperitoneal injection of non-effective doses of L-NAME (10 mg/kg, ip), 7-nitroindazole (40 mg/kg, ip), and aminoguanidine (50 mg/kg, ip) were also applied for 3 days—1 h after colitis induction and 15 min prior to the administration of the effective dose of modafinil. Finally, animals were sacrificed via cervical dislocation on the third day. Later on, the skin of abdomen was shaved and opened by surgical section. After exposing the colon, the last 5 cm of which was excised, opened longitudinally, and washed with normal saline. Ultimately, colon specimens were incised into two pieces: one of which was fixed by formalin 10% solution for histological analysis, and the other one was frozen in − 80 °C for biochemical assessments.
Experimental design
Animals were randomly allocated into 12 study groups, by 8 rats in each group. In the control group, colitis was induced and animals were administrated intraperitoneally with vehicle (normal saline 0.9% + tween 80 1%). Sham group was intrarectally administrated with 0.9% saline solution. Other groups, after induction of colitis, were injected with dexamethasone (1 mg/kg), modafinil (50, 100, and 150 mg/kg), NOS inhibitors + modafinil (150 mg/kg, ip), and NOS inhibitors alone—vehicle also was administrated in a separate injection along with NOS inhibitors in order for unifying the condition in terms of the number of injections in comparison with the NOS inhibitors + modafinil (150 mg/kg, ip) groups.
Assessment of macroscopic damage
For evaluating the macroscopic impacts of acetic acid on colon tissue and effects of various drugs on colitis induced by acetic acid, high-quality pictures were provided and scored by an observer blinded to the treatment according to macroscopic score [16] (Table 1).
Measurement of body weight
In all study groups, animals were weighed daily, and data were recorded to consider the changes in body weight within the study.
Biochemical analysis
To assess the anti-inflammatory effects of modafinil on acute colitis, we measured the colonic level of TNF-α and IL-1β by means of specific rat ELISA kits (Sigma, United State). To do so, tissue was homogenized in 50 mmol/l ice-cold potassium phosphate buffer; then, the homogenate was centrifuged—at 4000 rpm at 4 °C for 20 min. Finally, the supernatant was extracted and retained at − 80 °C until analysis.
Assessment of histological damage
To perform histologic studies, colon tissues, which had been fixed in neutral 10% formalin solution, was embedded in paraffin wax, cut into sections of four μm, and ultimately stained by hematoxylin and eosin (HE). Histopathologic studies were performed by a pathologist blinded to the experiment based on histopathologic scores, which evaluate inflammation severity, inflammation extent, tissue regeneration, crypt damage, and tissue involvement (Table 2).
Statistical analysis
All data were presented as mean ± SEM. To analyze the results, Statistical Package for the Social Sciences (SPSS), version 22, was used. Normal distribution of data was determined via Shapiro–Wilk test. Two-way ANOVA was applied to analyze the results of the sham and control groups. One-way ANOVA was used in order for comparing the results of control group and other treated groups. Tukey post hoc test was conducted to detect the differences between groups. p < 0.05 was considered significant.
Result
Effects of modafinil and dexamethasone administration on macroscopic appearance
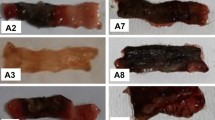
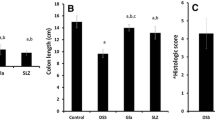
Macroscopic appearance of all study groups was illustrated in Fig. 1. No lesion was observed in sham group; by contrast, after colitis induction, tissue showed severe ulcers, and as depicted in Fig. 2a, analyses declared a significant increase in macroscopic score compared with the sham group (p < 0.001). However, after the injection of modafinil (100 and 150 mg/kg, ip) and dexamethasone (1 mg/kg, ip), ulcers were remarkably improved, and evaluating the results indicated a significant decrease in the macroscopic score (p < 0.001). Administration of modafinil (50 mg/kg, ip) did not have any protective effect towards the tissue destruction in comparison with the control group, and samples in this group revealed severe ulceration and necrosis (Fig. 2b).
Photographic images of colon tissue. a Sham, b control, c dexamethasone 1 mg/kg, d–f modafinil 50, 100, and 150, respectively, g L-NAME 10 mg/kg, h 7-nitoindazole 40 mg/kg, i aminoguanidine 50 mg/kg, j modafinil 150 mg/kg + L-NAME 10 mg/kg, k modafinil 150 mg/kg + 7-nitroindazole 40 mg/kg, l modafinil 150 mg/kg + aminoguanidine 50 mg/kg
Effect of acetic acid on colon tissue according to macroscopic scoring. Two-way ANOVA was applied to comparison between sham and control group. One-way ANOVA was used to compare between the control group and other treated groups. Bars represent mean ± SEM. &&&p < 0.001 significantly different from the sham group. +++p < 0.001 significantly different from the control group. ***p < 0.001 significantly different from the control group. ###p < 0.001, xxxp < 0.001, and αααp < 0.001 significantly different from modafinil 150 mg/kg group. The data are presented as mean ± SEM (8 rats per groups)
Effects of L-NAME, 7-nitroindazole, and aminoguanidine administration on macroscopic appearance
Macroscopic assessment of colon samples after administration of NOS inhibitors, alone and in combination with the effective dose of modafinil (150 mg/kg, ip), was conducted.
The intraperitoneal administration of the non-effective dose of L-NAME (10 mg/kg, ip) alone did not ameliorate macroscopic lesions compared with the control group. Furthermore, Treatment with L-NAME (10 mg/kg, ip) 15 min before the effective dose of modafinil (150 mg/kg, ip) reversed improving impact of modafinil, and analyses represented a significant increase in macroscopic score in modafinil (150 mg/kg, ip) + L-NAME (10 mg/kg, ip) group compared with modafinil (150 mg/kg, ip) group (p < 0.001) (Fig. 2c).
Figure 2d illustrates that the administration of the non-effective dose of 7-nitroindazole (40 mg/kg, ip) per se did not exert any protective influence on macroscopic ulcer in comparison with the control group. Moreover, injection of 7-nitroindazole (40 mg/kg, ip) before the administration of the effective dose of modafinil (150 mg/kg, ip) neutralized beneficial impacts of modafinil on macroscopic ulcer. Analyses represented a notable increase in macroscopic score in modafinil (150 mg/kg, ip) + 7-nitroindazole (40 mg/kg, ip) group compared with modafinil (150 mg/kg, ip) group (p < 0.001).
As depicted in Fig. 2e, injection of aminoguanidine (50 mg/kg, ip) alone did not exhibit any protective effect on macroscopic lesions compared with the control group, and samples in this group revealed severe ulceration, wall thickening, and tissue necrosis. Pretreatment with aminoguanidine (50 mg/kg, ip) before the effective dose of modafinil (150 mg/kg, ip) significantly raised macroscopic score compared with the modafinil (150 mg/kg, ip) group (p < 0.001).
Assessment the changes in body weight
At the end of the study, acetic acid-induced colitis caused noteworthy weight loss in the control group compared with the sham group (p < 0.001). By contrast, after the administration of modafinil (100 and 150 mg/kg, ip), animals showed significant gain weight (p < 0.01 and p < 0.001; respectively). Likewise, administration of dexamethasone (1 mg/kg, ip) resulted in a remarkable gain weight compared with the control group (p < 0.001). However, the amount of weight loss in acetic acid 4% + modafinil (50 mg/kg, ip) group and the groups, which received NOS inhibitors—L-NAME, 7-nitroindazole, and aminoguanidine—alone, was similar to the control group. In like manner, pretreatment with NOS inhibitors 15 min before modafinil (150 mg/kg, ip) administration caused considerable losing weight in comparison with modafinil (150 mg/kg, ip) group (p < 0.001; Fig. 3).
Percentage of body weight changes in all study groups. Two-way ANOVA was applied to comparison between sham and control group. One-way ANOVA was used to compare between the control group and other treated groups. Bars represent mean ± SEM (Mod: modafinil). &&&p < 0.001 significantly different from the sham group. +++p < 0.001 significantly different from the control group. **p < 0.001 and ***p < 0.001 significantly different from the control group. ###p < 0.001, xxxp < 0.001, and αααp < 0.001 significantly different from modafinil 150 mg/kg group
Effects of modafinil and dexamethasone administration on the level of TNF-α
Figure 4a represents the impression of the administration of modafinil (50, 100, 150 mg/kg, ip) and dexamethasone (1 mg/kg, ip) on the level of TNF-α. Analysis represented that after colitis induction, the level of TNF-α was considerably increased compared with the sham group (p < 0.001), whereas injection of modafinil (100 and 150 mg/kg, ip) significantly lowered the level of TNF-α (p < 0.01 and p < 0.001, respectively). Similarly, the administration of dexamethasone (1 mg/kg, ip) showed remarkable decrease in TNF-α level compared with the control group (p < 0.001). However, treatment with modafinil (50 mg/kg, ip) did not possess any influence on the level of TNF-α compared with the control group.
The level of TNF-alpha in colon tissue. Two-way ANOVA was applied to comparison between sham and control group. One-way ANOVA was used to compare between the control group and other treated groups. Bars represent mean ± SEM. &&&p < 0.001 significantly different from the sham group. +++p < 0.001 significantly different from the control group. **p < 0.001 and ***p < 0.001 significantly different from the control group. ###p < 0.001, xxxp < 0.001, and αααp < 0.001 significantly different from modafinil 150 mg/kg group. The data are presented as mean ± SEM (8 rats per groups)
Effects of L-NAME, 7-Nitroindazole, and Aminoguanidine administration on the level of TNF-α
The non-effective dose of L-NAME (10 mg/kg, ip) was applied as a non-specific nitric oxide synthase inhibitor. It was declared that intraperitoneal administration of L-NAME (10 mg/kg, ip) per se did not affect the level of TNF-α in comparison with the control group. Interestingly, the administration of L-NAME (10 mg/kg, ip) 15 min before modafinil (150 mg/kg, ip) neutralized the decreasing impact of modafinil on TNF-α level, and analyses reported a remarkable increase in the level of TNF-α in modafinil (150 mg/kg, ip) + L-NAME (10 mg/kg, ip) group compared with modafinil (150 mg/kg, ip) group (p < 0.001) (Fig. 4b).
Figure 4c indicates that the injection of 7-nitroindazole (40 mg/kg, ip) did not alter the level of TNF-α in comparison with the control group. Interestingly, pretreatment with 7-nitroindazole (40 mg/kg, ip) before modafinil (150 mg/kg, ip) remarkably raised the level of TNF-α compared with modafinil (150 mg/kg, ip) group (p < 0.001).
As demonstrated in Fig. 4d, administration of aminoguanidine (50 mg/kg, ip) alone did not attenuate the level of TNF-α compared with the control group. However, the administration of the non-effective dose of aminoguanidine (50 mg/kg, ip) before treatment with the effective dose of modafinil (150 mg/kg, ip) significantly increased the level of TNF-α in compared with the modafinil (150 mg/kg, ip) group (p < 0.001). In other words, the combination of modafinil (150 mg/kg, ip) with aminoguanidine (50 mg/kg, ip) reversed anti-inflammatory effects of modafinil.
Effects of modafinil and dexamethasone administration on the level of IL-1β
Figure 5a shows the impact of intraperitoneal injection of different doses of modafinil (50, 100, 150 mg/kg, ip) and dexamethasone (1 mg/kg, ip) on the level of IL-1β. After induction of colitis, the level of IL-1β remarkably increased in comparison with sham group (p < 0.001), while analysis indicated that the injection of modafinil (100 and 150 mg/kg, ip) significantly decreased the level of IL-1β (p < 0.01 and p < 0.001, respectively). Administration of dexamethasone (1 mg/kg, ip) also remarkably decreased the level of IL-1β compared with the control group (p < 0.001). Nevertheless, treatment with modafinil (50 mg/kg, ip) did not show any meaningful effect on reducing the level of IL-1β.
The level of IL-1 beta in colon tissue. Two-way ANOVA was applied to comparison between sham and control group. One-way ANOVA was used to compare between the control group and other treated groups. Bars represent mean ± SEM. &&&p < 0.001 significantly different from the sham group. +++p < 0.001 significantly different from the control group. **p < 0.001 and ***p < 0.001 significantly different from the control group. ###p < 0.001, xxxp < 0.001, and αααp < 0.001 significantly different from modafinil 150 mg/kg group. The data are presented as mean ± SEM (8 rats per groups)
Effects of L-NAME, 7-nitroindazole, and aminoguanidine administration on the level of IL-1β
The intraperitoneal injection of L-NAME (10 mg/kg, ip) did not change the level of IL-1β compared with the control group. However, the administration of L-NAME (10 mg/kg, ip) before modafinil (150 mg/kg, ip) reversed anti-inflammatory effects of modafinil, and analyses represented a significant increase in the level of IL-1β in modafinil (150 mg/kg, ip) + L-NAME (10 mg/kg, ip) group in comparison with modafinil (150 mg/kg, ip) group (p < 0.001) (Fig. 5b).
Figure 5c indicates that treatment with the non-effective dose of 7-nitroindazole (40 mg/kg, ip) alone did not exhibit any remarkable effect on the level of IL-1β compared with the control group. Amazingly, the injection of 7-nitroindazole (40 mg/kg, ip) before treatment with the effective dose of modafinil (150 mg/kg, ip) reversed anti-inflammatory effects of modafinil, and analyses declared a considerable increase in the level of IL-1β in modafinil (150 mg/kg, ip) + 7-nitroindazole (40 mg/kg, ip) group in comparison with modafinil (150 mg/kg, ip) group (p < 0.001).
As shown in Fig. 5d, the administration of aminoguanidine (50 mg/kg, ip) did not decrease the level of IL-1β in comparison with the control group; nevertheless, pretreatment with the non-effective dose of aminoguanidine (50 mg/kg, ip) before the effective dose of modafinil (150 mg/kg, ip) significantly raised the level of IL-1β in compared with the modafinil (150 mg/kg, ip) (p < 0.001).
Effects of modafinil and dexamethasone administration on histopathologic (microscopic) features
Figure 6 demonstrates the histologic appearance of samples in each study group. In the sham group, colon tissue was intact, and no evidence of inflammation and crypt damage was seen. By contrast, the induction of colitis by acetic acid in control group declared severe infiltration of inflammatory cells with transmural extension. Additionally, entire crypt and epithelium were destroyed. After treatment with modafinil (150 mg/kg, ip) and dexamethasone (1 mg/kg, ip), a significant improvement in inflammation and crypt damages was observed, and most parts of the samples were regenerated (p < 0.001). Administration of modafinil (100 mg/kg, ip) also remarkably caused reduction in inflammation, edema, wall thickening, and necrosis (p < 0.01). However, modafinil (50 mg/kg, ip) did not alter the inflammation and degeneration compared with the control group, and histologic slides in this group showed severe infiltration of inflammatory cells, which invaded mucosa and submucosa as well as in some areas had a transmural extension (Table 3).
Histologic images of rat’ colon tissue. a Sham, b control, c dexamethasone 1 mg/kg, d–f modafinil 50, 100, and 150 mg/kg, respectively, g L-NAME 10 mg/kg, h 7-nitoindazole 40 mg/kg, i aminoguanidine 50 mg/kg, j modafinil 150 mg/kg + L-NAME 10 mg/kg, k modafinil 150 mg/kg + 7-nitroindazole 40 mg/kg, l modafinil 150 mg/kg + aminoguanidine 50 mg/kg
Effects of L-NAME, 7-nitroindazole, and aminoguanidine administration on histopathologic (microscopic) features
The intraperitoneal administration of LAME (10 mg/kg, ip), 7-nitroindazole (40 mg/kg, ip), and aminoguanidine (40 mg/kg, ip) did not lead to any improvement in histologic lesions compared with the control group. Samples in these groups showed severe inflammation and crypt damage. Pretreatment with these NOS inhibitors before the administration of the effective dose of modafinil (150 mg/kg, ip) neutralized protective influence of modafinil (150 mg/kg, ip). Samples in these groups were significantly different from ones in modafinil (150 mg/kg, ip) group, and large amounts of inflammatory cells with transmural extension were observed in the histopathologic study. Additionally, crypt structures and goblet cells were severely destroyed.
Discussion
The present study demonstrated that intraperitoneal injection of modafinil reduced macroscopic, biochemical, and microscopic disorders in the rat model of acetic acid-induced colitis. Regarding macroscopic changes, induction of colitis resulted in severe ulcer and inflammation, while treatment with modafinil (100 and 150 mg/kg, ip) remarkably improved colonic lesions. Injection of NOS inhibitors—L-NAME, 7-nitroindazole, and aminoguanidine—per se did not have any ameliorating effect on ulcer and inflammation in colonic samples compared with the control group. Interestingly, administration of NOS inhibitors before modafinil completely reversed beneficial effects of modafinil on macroscopic ulcer and lesions. Given biochemical evaluations, IL-1β and TNF-α were increased after the induction of colitis; nevertheless, the administration of modafinil significantly decreased the level of aforementioned cytokines in colon tissue. Administration of NOS inhibitors did not show any noteworthy effect on reducing the inflammatory cytokines compared with the control group. Interestingly, the injection of NOS inhibitors 15 min prior to modafinil neutralized anti-inflammatory impacts of modafinil. In addition, the results of the microscopic assessments were in accordance with macroscopic changes. Histologic features such as infiltration and extension of inflammatory cells as well as the destruction of goblet cells and crypt structures showed that the protective effects of modafinil were neutralized by NOS inhibitors. These findings indicated that modafinil presumably via NO pathway exhibits a protective effect against the inflammation in colitis.
Crohn’s disease and ulcerative colitis are the constituents of inflammatory bowel disease, caused by an interaction between environment, genetic, and immune regulatory factors [2, 17]. Acetic acid has been applied in many animal studies to induce acute colitis, which is compatible with those observed in human IBD [18]. Some experimental studies have indicated macroscopic lesions after the induction of colitis by means of acetic acid. They reported severe ulcer and tissue necrosis in macroscopic appearance [19,20,21]. Our macroscopic assessments also showed similar damages after induction of colitis. The analysis of the mucosa from patients with ulcerative colitis and Crohn’s disease revealed an increase in the expression of certain pro-inflammatory cytokines such as TNF-α [4]. Kojouharoff et al. showed that TNF-α has an essential role in the pathogenesis of IBD. In addition, anti-TNF-α antibody had been effective for the prevention of inflammation in the colon of patients with Crohn’s disease [22]. Increased expression of IL-1β and TNF-α has been also detected in mucosal biopsies of patients with IBD [23]. In accordance with these findings, our study revealed that the level of IL-1β and TNF-α remarkably increased after the induction of colitis in colon tissue.
Moreover, it has been represented that colitis results in histological changes in colon tissue, including infiltration of acute inflammatory cells, hemorrhage of mucosa, destruction of crypts, and tissue necrosis [24,25,26]. In accordance with these findings, our experiment also showed similar microscopic changes.
Modafinil, otherwise known as Provigil, has been demonstrated as a drug for the treatment of excessive daytime sleepiness disorders such as obstructive sleep apnea, shift work sleep disorders, and narcolepsy [15]. Recently, some research has indicated that modafinil exerts anti-inflammatory influence. One study, which investigated atherosclerosis in an apoE deficient mouse model, indicated that modafinil stimulates anti-inflammatory cytokines; on the contrary, it suppresses the production of pro-inflammatory cytokines. Additionally, this study reported that modafinil results in the inhibition of macrophage proliferation [12]. In another animal study, Raineri et al. demonstrated that modafinil plays a protective role against methamphetamine-induced neuroinflammation [27]. In harmony with these studies, in our experiment after treatment with modafinil, the level of TNF-α and IL-1β were significantly decreased.
Currently, a great deal of attention has been attracted towards the role of NO in the pathogenesis of inflammatory bowel disease, and this is mostly in the light of an increase in the level of NO in IBD patients’ rectal dialysis [28]. NO which is synthesized by three isoforms of NOSs during IBD could have some protective effects towards inflammatory processes, and the loss of each isoform may accompany by severe damages. In this regard, one animal study represented that after inducing IBD by acetic acid, iNOS-deficient mice expressed severe inflammation in colonic tissue. [29]. Bruce et al. in 2004 also reported that eNOS knockout (KO) mice developed an exaggerated and prolonged inflammation after IBD induction [9]. Additionally, it was represented that nNOS plays a protective role in colitis model and the loss of nNOS expression resulting in severe intestinal damages [30]. As a matter of fact, NO can reduce the expression of adhesion molecules and interrupts interactions between leukocytes and endothelial cell; therefore, NO protects from tissue injury during inflammatory processes [31].
It could be assumed that Modafinil by mediating NO pathway exerts its anti-inflammatory influence. The review of literature also confirms this statement. For instance, Gupta et al. in 2014 investigated the role of NO in the mechanism of action of modafinil in mice. To do so, they induced hyperalgesia by injecting modafinil at different doses. To elucidate the role of NO, they applied the administration of NOS inhibitors—L-NAME and 7-nitroindazole. They observed that injection of NOS inhibitors before administration of modafinil, completely reversed hyperalgesia induced by modafinil; therefore, they suggested the involvement of NO pathway in the mechanism of action of modafinil [15]. Another animal study aimed to determine the impacts of modafinil on the threshold of seizure that was induced by pentylenetetrazole. They reported that modafinil at the doses of 50 and 80 mg/kg (ip) has anti-convulsant property. By measuring the level of NO, they observed an increased in the level of NO in the groups, which were treated by modafinil 50 and 80 mg/kg. Moreover, to delineate the role of NO pathway in the mechanism of action of modafinil, they injected NOS inhibitors—aminoguanidine, L-NAME, and 7-nitroindazole—before the administration of modafinil. It was observed that anti convulsant influence of modafinil was thoroughly blocked by NOS inhibitors. Hence, they concluded that modafinil via NO pathway exhibits its effects [14]. In harmony with previous studies, our experiment declared that pretreatment with NOS inhibitors—L-NAME, 7-nitroindazole, and aminoguanidine—before modafinil neutralize anti-inflammatory impacts of modafinil on colitis.
In conclusion, modafinil exerted anti-inflammatory influence on colitis induced by acetic acid, and administration of NOS inhibitors prior to modafinil nullified the anti-inflammatory impacts of modafinil. As a result, modafinil presumably through NO pathway could prevent inflammation and colon injury, as well as NO could be proposed as one of the mechanisms by which modafinil acts.
References
Levine A, Griffiths A, Markowitz J, Wilson DC, Turner D, Russell RK, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis. 2011;17:1314–21.
Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis. 2006;12:S3–9.
Monteleone G, Pallone F, MacDonald TT. Emerging immunological targets in inflammatory bowel disease. Curr Opin Pharmacol. 2011;11:640–5.
Papadakis KA, Targan SR. Role of cytokines in the pathogenesis of inflammatory bowel disease. Annu Rev Med. 2000;51:289–98.
Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369:1641–57.
Mousavizadeh K, Rahimian R, Fakhfouri G, Aslani F, Ghafourifar P. Anti-inflammatory effects of 5-HT3 receptor antagonist, tropisetron on experimental colitis in rats. Eur J Clin Invest. 2009;39:375–83.
Khalili H, Chan SS, Lochhead P, Ananthakrishnan AN, Hart AR, Chan AT. The role of diet in the aetiopathogenesis of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2018;15:525.
Hosoi T, Goto H, Arisawa T, Niwa Y, Okada N, Ohmiya N, et al. Role of nitric oxide synthase inhibitor in experimental colitis induced by 2,4,6-trinitrobenzene sulphonic acid in rats. Clin Exp Pharmacol Physiol. 2001;28:9–12.
Vallance BA, Dijkstra G, Qiu B, van der Waaij LA, van Goor H, Jansen PL, et al. Relative contributions of NOS isoforms during experimental colitis: endothelial-derived NOS maintains mucosal integrity. Am J Physiol Gastrointest Liver Physiol. 2004;287:G865–74.
Binion DG, Rafiee P, Ramanujam KS, Fu S, Fisher PJ, Rivera MT, et al. Deficient iNOS in inflammatory bowel disease intestinal microvascular endothelial cells results in increased leukocyte adhesion. Free Radic Biol Med. 2000;29:881–8.
Ballon JS, Feifel D. A systematic review of modafinil: potential clinical uses and mechanisms of action. J Clin Psychiatry. 2006;67:554–66.
Han J, Chen D, Liu D, Zhu Y. Modafinil attenuates inflammation via inhibiting Akt/NF-κB pathway in apoE-deficient mouse model of atherosclerosis. Inflammopharmacology. 2018;26:385–93.
Minzenberg MJ, Carter CS. Modafinil: a review of neurochemical actions and effects on cognition. Neuropsychopharmacology. 2008;33:1477.
Bahramnjead E, Roodsari SK, Rahimi N, Etemadi P, Aghaei I, Dehpour AR. Effects of modafinil on clonic seizure threshold induced by pentylenetetrazole in mice: involvement of glutamate, nitric oxide, GABA, and serotonin pathways. Neurochem Res. 2018;43:2025–37.
Gupta R, Gupta LK, Bhattacharya SK. Chronic administration of modafinil induces hyperalgesia in mice: reversal by L-NG-nitro-arginine methyl ester and 7-nitroindazole. Eur J Pharmacol. 2014;736:95–100.
Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803.
Hanić M, Trbojević-Akmačić I, Lauc G. Inflammatory bowel disease-glycomics perspective. Biochim Biophys Acta. 2019;1863:1595–601.
Goyal N, Rana A, Ahlawat A, Bijjem KRV, Kumar P. Animal models of inflammatory bowel disease: a review. Inflammopharmacology. 2014;22:219–33.
Daneshmand A, Mohammadi H, Rahimian R, Habibollahi P, Fakhfouri G, Talab SS, et al. Chronic lithium administration ameliorates 2,4,6-trinitrobenzene sulfonic acid-induced colitis in rats; potential role for adenosine triphosphate sensitive potassium channels. GastroHep. 2011;26:1174–81.
Minaiyan M, Hajhashemi V, Rabbani M, Fattahian E, Mahzouni P. Evaluation of anti-colitic effect of fluvoxamine against acetic acid-induced colitis in normal and reserpinized depressed rats. Eur J Pharmacol. 2015;746:293–300.
Fakhraei N, Javadian N, Rahimian R, Nili F, Rahimi N, Hashemizadeh S, et al. Involvement of central opioid receptors in protective effects of methadone on experimental colitis in rats. Inflammopharmacology. 2018;26:1399–413.
Kojouharoff G, Hans W, Obermeier F, Mannel D, Andus T, Scholmerich J, et al. Neutralization of tumour necrosis factor (TNF) but not of IL-1 reduces inflammation in chronic dextran sulphate sodium-induced colitis in mice. ClinExpImmunol. 1997;107:353–8.
Reinecker HC, Steffen M, Witthoeft T, Pflueger I, Schreiber S, MacDermott R, et al. Enhand secretion of tumour necrosis factor-alpha, IL-6, and IL-1β by isolated lamina ropria monouclear cells from patients with ulcretive cilitis and Crohn’s disease. ClinExpImmunol. 1993;94:174–81.
Fakhfouri G, Rahimian R, Daneshmand A, Bahremand A, Rasouli MR, Dehpour AR, et al. Granisetron ameliorates acetic acid-induced colitis in rats. Hum Exp Toxicol. 2010;29:321–8.
Daneshmand A, Rahimian R, Mohammadi H, Ejtemaee-Mehr S, Tavangar SM, Kelishomi RB, et al. Protective effects of lithium on acetic acid-induced colitis in rats. Dig Dis Sci. 2009;54:1901–7.
Fakhraei N, Abdolghaffari AH, Delfan B, Abbasi A, Rahimi N, Khansari A, et al. Protective effect of hydroalcoholic olive leaf extract on experimental model of colitis in rat: involvement of nitrergic and opioidergic systems. Phytother Res. 2014;28:1367–73.
Raineri M, Gonzalez B, Goitia B, Garcia-Rill E, Krasnova IN, Cadet JL, et al. Modafinil abrogates methamphetamine-induced neuroinflammation and apoptotic effects in the mouse striatum. PLoS One. 2012;7:e46599.
Roediger W, Lawson M, Nance S, Radcliffe B. Detectable colonic nitrite levels in inflammatory bowel disease-mucosal or bacterial malfunction? Digestion. 1986;35:199–204.
McCafferty DM, Mudgett JS, Swain MG, Kubes P. Inducible nitric oxide synthase plays a critical role in resolving intestinal inflammation. Gastroenterology. 1997;112:1022–7.
Beck P, Xavier R, Wong J, Ezedi I, Mashimo H, Mizoguchi A, et al. Paradoxical roles of different nitric oxide synthase isoforms in colonic injury. Am J Physiol Gastrointest Liver Physiol. 2004;286:G137–47.
Kubes P, Kurose I, Granger DN. NO donors prevent integrin-induced leukocyte adhesion but not P-selectin-dependent rolling in postischemic venules. Am J Physiol Heart Circ Physiol. 1994;267:H931–7.
Acknowledgements
This study was financially supported by National Institute for Medical Research Development (no. 982518).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dejban, P., Rahimi, N., Takzare, N. et al. Biochemical and histopathological evidence for the beneficial effects of modafinil on the rat model of inflammatory bowel disease: involvement of nitric oxide pathway. Pharmacol. Rep 72, 135–146 (2020). https://doi.org/10.1007/s43440-019-00054-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-019-00054-5