Abstract
Epilepsy is the third most common chronic brain disorder. Modafinil is an awakening agent approved for narcolepsy. In addition to its clinical uses some reports revealed that modafinil was associated with some alterations in seizure threshold. The purpose of this study was to clarify the effect of acute administration of modafinil in clonic seizure threshold (CST) induced by pentylenetetrazole in mice and the involvement of glutamate, nitric oxide, gamma amino butyric acid (GABA), and serotonin systems in this feature. Modafinil at 80 and 150 mg/kg showed anti- and pro-convulsant effects respectively and expressed maximum anti- and pro-convulsant activities at 30 min after injection. Both modulatory effects were blunted by pretreatment of l-NAME [nonspecific nitric oxide synthase (NOS) inhibitor; 10 mg/kg, i.p.], 7-nitroindazole (a neuronal NOS inhibitor; 40 mg/kg, i.p.), and aminoguanidine (an inducible NOS inhibitor; 50 mg/kg, i.p.). Injection of the NOS precursor l-arginine (60 mg/kg, i.p.) before modafinil did not change the anti-convulsant effect, while thoroughly reversed the pro-convulsant one. Our experiments displayed that administration of diazepam (a GABAA receptor agonist; 0.02 mg/kg, i.p.) and MK-801 (a NMDA receptor antagonist; 0.05 mg/kg, i.p.) before different doses of modafinil significantly increased CST. Finally, pretreatment of citalopram (a selective serotonin reuptake inhibitor; 0.1 mg/kg, i.p.) did not modify the convulsant activities of modafinil. Therefore, nitric oxide system may mediate anti-convulsant activity, while glutamate, nitric oxide, and GABA pathways may involve in pro-convulsant property. Serotonin receptors have no role on convulsant effects of modafinil.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epilepsy is a disorder characterized by frequent seizures. Roughly 10% of the populations encounter a seizure at some time in their life [1]. Epileptic seizures often impair consciousness, hurt the individual, and create problems in receiving education and finding jobs [2]. In spite of the fact that there are a lot of antiepileptic drugs, which are efficacious in patients with epilepsy, novel drugs with the specificity on anti-convulsant characteristic and low adverse effect profile are still required [3, 4]. Numerous molecular mechanisms have been documented to be involved in seizures including N-methyl-d-aspartate (NMDA) receptors, nitric oxide (NO) pathway, gamma amino butyric acid (GABA) release, and serotonin receptors [5,6,7,8].
Modafinil is a non-amphetamine stimulant agent approved for the treatment of narcolepsy, shift work sleep problems, and obstructive sleep apnea [9, 10]. It is categorized as psychostimulants [11,12,13,14], hence its use may be resulted in seizures, even at therapeutic doses [15]. There has been an increase in off-label use of modafinil for a variety of indications such as attention deficit hyperactivity disorder [16]. Recently, some reports in both clinical trials and animal studies showed that modafinil can exert both anti- and pro-convulsant activities in human or rodent paradigm and can modulate the effects of some antiepileptic drugs in an animal model [17,18,19]. Although data about the effects of modafinil on seizure are inconclusive (and sometimes conflicting), some animal data suggest that it influences several and different neurotransmitter pathways in the brain, including dopamine transporters, NO pathway, glutamate, GABA, serotonin, and histamine. Thus, the exact mechanisms related to its therapeutic effects are complicated and not well clarified [9, 10, 12, 20].
Glutamate is an excitatory amino acid, which is found throughout the mammalian central nervous system where they are considered to promote excitatory synaptic transmission. Glutamate has two kinds of receptors: ligand-gated and G protein coupled receptors. NMDA receptor is a heteromeric ligand-gated glutamatergic receptor which has a big role in seizure [21]. NR1, NR2, and NR3 are 3 subunits of NMDA receptors and most of its heterogeneity is based on NR2. The affinity of NMDA receptors with different subunits for agonists and antagonists vary slightly and greatly respectively [22]. Over-activation of NMDA receptors may play an important part in etiology and expression of epilepsy. Antagonizing the NMDA receptors are also believed as a mechanism of action of some anti-convulsant agents [5]. In addition, it seems that modafinil performs its anti-narcoleptic effect through activation of NMDA receptors [17].
Nitric oxide is a signaling molecule, which is produced from the amino acid l-arginine by three kinds of nitric oxide synthases (NOS). It operates as a neurotransmitter in the nervous system. Besides, several prior literatures have proposed that nitric oxide is a modifier of seizure threshold with very different anti- and pro-convulsant effects in distinct seizure models [23, 24]. One research revealed that the hyperalgesic effect of modafinil in mice was prevented by nitric oxide synthase inhibitors, so the involvement of nitric oxide system in this feature is plausible [20].
Gamma amino butyric acid (GABA) is an inhibitory amino acid which may play a crucial role in lowering the seizure threshold. Anti-seizure effect of benzodiazepines like diazepam, drug of choice for status epilepticus, happens through GABAA receptors which leads to increase in chloride current and hyperpolarization [25, 26]. One of the probable mechanisms in simulative effect of modafinil is a reduction in GABA release [17].
Serotonin is a neurotransmitter which is involved in huge processes in mammalian nervous system. There is a big group of receptor subtypes (5-HT) responsible for serotonin actions. It is believed that modafinil increases the extracellular 5-HT significantly in the frontal cortex, central nucleus of the amygdala, and dorsal raphe nucleus. This neurotransmitter is involved in major depressive disorder and selective serotonin reuptake inhibitors (SSRIs) drugs such as citalopram are approved as antidepressant drugs. A dose dependent anti- and pro-convulsant properties of citalopram has been observed in rodent models and it was demonstrated that 5-HT3 receptors exert this bimodal effect on seizure susceptibility [7, 10, 27].
Because of complicated and not well clarified convulsant activities of modafinil and its mechanisms, we designed this experiment to determine the anti- and pro-convulsant properties of modafinil in clonic seizure threshold induced by intravenous administration of pentylenetetrazole in mice and evaluate the possible involvement of glutamate, nitric oxide, GABA, and serotonin pathways in these activities.
Materials and Methods
Chemicals
The drugs and agents, which we used are: modafinil, pentylenetetrazole (PTZ), citalopram, MK-801, diazepam, N(G)-nitro-l-arginine methyl ester(l-NAME), 7-nitroindazole, aminoguanidine, l-arginine (All were purchased from Sigma, St. Louis, MO, USA). 7-nitroindazole and modafinil were suspended in Tween 80 1% solution, but all other drugs were freshly dissolved in sterile isotonic saline before the experiments. The dosage range, route of drug administration, and injection time of various agents were based on preceding published researches, and pilot experiments. Except PTZ, which was administered via intravenous (i.v.) route, all other injections were done through intraperitoneal (i.p.) route and with a volume of 10 ml/kg body weight.
Animals
Eight-week-old male NMRI strain mice with weights between 25 and 30 g (Tehran University of medical sciences, Pharmacology Department, Tehran, Iran) were used in our study. The animals were housed in groups of 10 and were allowed freely access to food and water except for the short time that animals were removed from their cages for testing. All experiments were conducted during the period between 10:00 a.m. and 13:00 p.m. with normal room light (12-h regular light/dark cycle) and temperature (22 ± 1 °C). All procedures were carried out in accordance with the institutional guidelines for animal care and in compliance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978). Each mouse was used only once, and each treatment group consisted of at least 12 animals.
Determination of Clonic Seizure Threshold (CST)
In order to evaluate the seizure susceptibility of mice we induced seizure with Intravenous infusion of PTZ which represents a standard experimental model of clinical myoclonic petit mal seizures and construct validity for myoclonic seizures. The infusion of PTZ was carried out by interpolating a 30-gauge butterfly needle into the tail vein of mice which was fixed by an adhesive tape and the PTZ (0.5%) flow infused at a constant rate of 1 ml/min to animals by an infusion pump (NE 1000, New Era Pump System, Inc.). A 40-cm flexible tube as a connector used between syringe and butterfly needle which provided an unrestrained freely moving condition. Infusion was stopped when forelimb clonus followed by full clonus of the body was spotted and the dose of PTZ (mg/kg of mice weight) was calculated according to the following formula:
The animals were anesthetized by increasing carbon dioxide concentrations and killed by cervical displacement [5,6,7, 28, 29].
Experiments
In experiment 1, mice in distinguished groups received a single injection of various doses of modafinil (0 [saline], 20, 50, 80, and 150 mg/kg, i.p.) at 30 min (based on prior study [17]) before determination of the PTZ-induced seizure threshold. In this step, the potent anti- and pro-convulsant doses of modafinil were measured for further experiments.
In experiment 2, in order to determine the best time of modafinil activity on PTZ-induced seizure threshold, mice received an acute i.p. injection of saline or modafinil (80 and 150 mg/kg) at 15, 30, and 45 min prior to establishment of PTZ-induced clonic seizure threshold. This dose was chosen based on our prior experiment. Control group was calculated by the average of separate groups of saline (5 ml/kg) injections in different time (15, 30, and 45 min).
In experiment 3, non-effective dose of NMDA antagonist (MK-801 0.05 mg/kg, i.p) [6] was administered 15 min before 0 (saline), 20, 50, 80, and 150 mg/kg i.p. of modafinil injections in distinct groups of mice, and seizure tests were performed 30 min after injections of saline or modafinil doses.
In experiment 4, 5, and 6 animals in different groups received an injection non-effective dose of nonspecific NOS inhibitor, l-NAME (10 mg/kg, i.p.), as well as aminoguanidine as an inducible NOS inhibitor (50 mg/kg, i.p.), and 7-nitroindazole as a neuronal NOS inhibitor (40 mg/kg, i.p.), 15 min before 0 (saline), 20, 50, 80, and 150 mg/kg, i.p. of modafinil respectively. The doses of L-NAME [24], 7-nitroindazole, and aminoguanidine were chosen based on prior literatures [26, 30]. The PTZ-induced seizure threshold was assessed 30 min after the injections of saline or modafinil doses.
In experiment 7, animals received an injection of ineffective dose of the precursor of nitric oxide (l-arginine 60 mg/kg, i.p.), according to prior publications [5, 24, 30], 15 min before 0 (saline), 20, 50, 80, and 150 mg/kg i.p. of modafinil, then after 30 min seizure tests were conducted.
In experiment 8, mice in separated groups were injected sub-effective dose of GABA agonist (diazepam 0.02 mg/kg, i.p.) which was shown in previous study [26] 15 min before 0 (saline), 20, 50, 80, and 150 mg/kg of modafinil (45 min prior to PTZ-induced clonic seizure test).
In experiment 9, animals in different group received non-effective dose of citalopram (0.1 mg/kg, i.p.) ,which was selected based on previous study [7], 15 min before 0 (saline), 20, 50, 80, and 150 mg/kg of modafinil, then after 30 min of saline or modafinil injections the PTZ-induced clonic seizure tests were carried out (Scheme 1).
Hippocampal Nitrite Assay
To specify the nitric oxide levels in the hippocampus, we measured nitrite levels as the result of the nitric oxide end product. After the seizure tests in 4, 5, 6, and 7 experiments animals were decapitated, and then the hippocampi were dissected on ice-cold surface and instantly immersed in liquid nitrogen. Tissue homogenates were prepared, and nitrite levels were measured by using a colorimetric assay based on the Griess reaction. Initially, each well was loaded with 100-µl samples, which were then mixed with 100-µl Griess reagent. Following 10-min incubation at room temperature, absorbance was measured at 540 nm in an automated plate reader. Concentration of nitrite was determined by referring to a standard curve of sodium nitrite and normalized to the weight of each sample [31, 32].
Statistical Analysis
Data are expressed as mean ± S.E.M. of clonic seizure threshold in each experimental group and were analyzed using SPSS 25 software. The one-way ANOVA followed by Post hoc Tukey’s tests were used to analyze the data. Tests of homogeneity of variance were used to ensure normal distribution of the data. The P value of less than 0.05 was considered statistically significant.
Results
Effect of Different Doses of Modafinil on CST
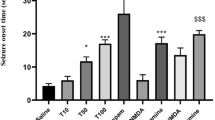
Figure 1 illustrates the dose-dependent effect of acute intraperitoneal administration of different doses of modafinil (20, 50, 80, and 150 mg/kg, i.p) on PTZ-induced clonic seizure threshold. The seizure sensitivity determination was done 30 min after modafinil injection. One-way ANOVA revealed a significant anti-convulsant activity for modafinil at doses of 50 (*P < 0.05) and 80 mg/kg (***P < 0.001) with maximal effect at 80 mg/kg and pro-convulsant effect at dose of 150 mg/kg (***P < 0.001) compared with saline-treated animals. Based on this experiment 20 mg/kg did not produce any significant anti-convulsant effect compared with saline group (P > 0.05), and was selected as sub-effective dose for subsequent experiments.
Effect of different doses of modafinil in PTZ-induced seizures. Effect of acute administration of modafinil (20, 50, 80, and 150 mg/kg, i.p., 30 min before PTZ infusion) on clonic seizure threshold (CST) in PTZ-induced seizures in mice. Values are expressed as mean ± SEM analyzed by one-way ANOVA followed by Tukey’s multiple comparison test. *P < 0.05, ***P < 0.001 vs. control (saline-injected) group
Impact of Different Times of Modafinil Injection on CST
Figure 2 displays the time course of the effective anti- and pro-convulsant doses of modafinil (80 and 150 mg/kg respectively). Administration of modafinil (80 and 150 mg/kg) 15 min before PTZ infusion, did not alter clonic seizure threshold (P > 0.05). Injection of the same doses of modafinil 30 min before seizure induction significantly changed the CST (***P < 0.001), but 45 min before the test, did not influence the CST. Data show that modafinil exerts anti- and pro-convulsant effects with maximal activity at 30 min after administration compared with saline-treated animals.
Time course of modafinil in PTZ-induced clonic seizure. Effect of acute administration of modafinil (80 and 150 mg/kg) on clonic seizure threshold (CST) in PTZ-injected mice. Modafinil (80 and 150 mg/kg) was injected 15, 30, and 45 min prior to PTZ test. Values are expressed as the mean ± S.E.M. analyzed by one-way ANOVA followed by Tukey’s multiple comparison test. ***P < 0.001 vs. control group. Control group was calculated as an average of different data of control groups at 15, 30, and 45 min
Effect of the NMDA Receptor Antagonist on the Different Doses of Modafinil
For investigation of the role NMDA receptors in the anti- and pro-convulsant activity of modafinil, MK-801, a NMDA antagonist, (0.05 mg/kg) was administered 15 min prior to modafinil (20, 50, 80, and 150 mg/kg) or saline. Data in Fig. 3 show that MK-801 at this specific dose did not alter the CST in control animals. However, MK-801 in combination with modafinil (20, 50, and 80 mg/kg) significantly enhanced the seizure threshold (**P < 0.01 ***P < 0.001 compared with saline-treated group, %%%P < 0.001, $$$P < 0.001, ##P < 0.01 compared with modafinil-treated (20, 50, and 80 mg/kg) groups respectively). Also, injection of this dose of MK-801 15 min before modafinil (150 mg/kg, i.p.) completely blocked the pro-convulsant effect of modafinil (&&&P < 0.001 in comparison with modafinil-treated group).
Effect of MK-801 injection on clonic seizure threshold of different doses of modafinil. MK-801 (0.05 mg/kg, i.p,) was administered 15 min before modafinil (20, 50, 80, 150 mg/kg, i.p,). Data are expressed as mean ± SEM analyzed by one-way ANOVA followed by Tukey’s multiple comparison test. *P < 0.05, **P < 0.01, ***P < 0.001 compared with saline-treated group. %%%P < 0.001 compared with modafinil-treated (20 mg/kg) group. $$$P < 0.001 compared with modafinil-treated (50 mg/kg) group. ##P < 0.01 compared with modafinil-treated (80 mg/kg) group. &&&P < 0.001 compared with modafinil-treated (150 mg/kg) group
Impact of l-NAME on the Anti- and Pro-Convulsant Activity of Modafinil
To explore the role of nitric oxide on the anti- and pro-convulsant effects of modafinil, we examined the impact of nonspecific nitric oxide synthase inhibitor, l-NAME, on the seizure threshold alteration of modafinil. Figure 4 shows the effect of selected doses of l-NAME (10 mg/kg, i.p.) and modafinil (80 and 150 mg/kg, i.p.) alone or in combination on the clonic seizure threshold induced by PTZ. The l-NAME (10 mg/kg) was administered 15 min prior to modafinil (80 and 150 mg/kg) or saline injection. Our data exhibit that l-NAME (10 mg/kg) alone did not alter the CST while co-administration of modafinil (80 mg/kg) with l-NAME (10 mg/kg) completely reversed the anti-convulsant effect of modafinil, compared with modafinil-treated (80 mg/kg) group (###P < 0.001). Moreover, combination of l-NAME (10 mg/kg) with modafinil (150 mg/kg) significantly decreased the pro-convulsant activity of modafinil (*P < 0.05, &&P < 0.01 compared with saline-treated and modafinil-treated (150 mg/kg) groups respectively). There are no statistical differences between saline and 20, 50 mg/kg groups which is why their data have not been shown.
Impact of l-NAME on both anti- and pro-convulsant effects of modafinil. l-NAME (10 mg/kg, i.p,) administered 15 min before modafinil (80 and 150 mg/kg, i.p,). Data are expressed as mean ± SEM analyzed by one-way ANOVA followed by Tukey’s multiple comparison test. *P < 0.05, ***P < 0.001 compared with saline-treated group. ###P < 0.001 compared with modafinil-treated (80 mg/kg) group. &&P < 0.01 compared with modafinil-treated (150 mg/kg) group
Impact of Aminoguanidine on the Effective Doses of Modafinil
As shown in Fig. 5, the specific inducible nitric oxide synthase inhibitor aminoguanidine (50 mg/kg, i.p.) did not modify the seizure threshold (P > 0.05). However, pretreatment with the same dose of aminoguanidine 15 min before modafinil (80 mg/kg, i.p.) totally blocked the anti-convulsant effect of modafinil in comparison with modafinil-treated (80 mg/kg) animals (###P < 0.001). Administration of aminoguanidine (30 mg/kg, i.p.) 15 min before modafinil (150 mg/kg, i.p.) significantly lower the pro-convulsant effect of modafinil (**P < 0.01, &&P < 0.01 compared with saline and modafinil-treated (150 mg/kg) groups respectively). There are no significant differences between saline and 20, 50 mg/kg groups, so their data have not been shown.
Effect of aminoguanidine on both anti- and pro-convulsant features of modafinil. Aminoguanidine (50 mg/kg, i.p,) was administered 15 min before modafinil (80 and 150 mg/kg, i.p,). Data are expressed as mean ± SEM analyzed by one-way ANOVA followed by Tukey’s multiple comparison test. **P < 0.01, ***P < 0.001 compared with saline-treated group. ###P < 0.001 compared with modafinil-treated (80 mg/kg) group. &&P < 0.01 compared with modafinil-treated (150 mg/kg) group
Effect of 7-Nitroindazole on the Effective Doses of Modafinil
As depicted in Fig. 6, the acute administration of 7-nitroindazole (40 mg/kg, i.p.) 15 min before modafinil (80 and 150 mg/kg) completely reversed the anti- and pro-convulsant exertions of modafinil compared with modafinil-treated (80 and 150 mg/kg) groups (###P < 0.001 and &&&P < 0.001 respectively). There are no meaningful differences between saline and 20, 50 mg/kg groups, thus their data have not been displayed.
Effect of 7-nitroindazole on both anti- and pro-convulsant effects of modafinil. 7-nitroindazole (40 mg/kg, i.p,) was administered 15 min before modafinil (80 and 150 mg/kg, i.p,). Data are expressed as mean ± SEM analyzed by one-way ANOVA followed by Tukey’s multiple comparison test. ***P < 0.001 compared with saline-treated group. ###P < 0.001 and &&&P < 0.001 compared with modafinil-treated (80 and 150 mg/kg respectively) groups
Impact of l-Arginine on the Anti- and Pro-Convulsant Effects of Modafinil
To evaluate the effect of the nitric oxide precursor l-arginine (60 mg/kg, i.p.) alone and in combination with effective doses of modafinil (80 and 150 mg/kg) on the clonic seizure threshold induced by PTZ, l-Arginine (60 mg/kg) was administered 15 min prior to modafinil (80 and 150 mg/kg) or saline. Analysis exposes that l-arginine alone did not revamp the CST in comparison with control animals (P > 0.05). Combination of l-arginine with anti-convulsant dose of modafinil (80 mg/kg) did not alter the CST compared with modafinil-treated (80 mg/kg) mice (P > 0.05). However, injection of l-arginine at the same dose completely reversed pro-convulsant effect of modafinil (***P < 0.001, &&&P < 0.001 in comparison with saline and modafinil-treated (150 mg/kg) groups respectively) (Fig. 7). The differences between saline and 20, 50 mg/kg groups are insignificant and because of that their data have not been exhibited.
Impact of l-arginine on both anti- and pro-modulatory effects of modafinil in seizure threshold induced by PTZ. l-arginine (60 mg/kg, i.p,) was administered 15 min before modafinil (80 and 150 mg/kg, i.p,). Data are expressed as mean ± SEM analyzed by one-way ANOVA followed by Tukey’s multiple comparison test. ***P < 0.001 compared with saline-treated group. &&&P < 0.001 compared with modafinil-treated (150 mg/kg) group
Effect of GABAA Receptor Agonist on Different Doses of Modafinil
Figure 8 expresses the influence of GABAA receptor agonist on seizure regulating property of modafinil. Diazepam at the dose of 0.02 mg/kg injected 15 min before modafinil (20, 50, 80, and 150 mg/kg) and saline. In comparison with control animal diazepam alone did not change the CST. Co-administration of diazepam (0.02 mg/kg) with modafinil (20 mg/kg) significantly escalated the CST (*P < 0.05, %%P < 0.01 compared with saline and modafinil-treated (20 mg/kg) animals respectively). Moreover, injection of the same dose of diazepam with modafinil (50 and 80 mg/kg) again thoroughly enhanced the seizure threshold (***P < 0.001, $$$P < 0.001, ##P < 0.01 compared with saline and modafinil-treated (50 and 80 mg/kg) groups respectively). In addition, the same dose of diazepam with modafinil (150 mg/kg) totally deescalated the pro-convulsant effect of modafinil in contrast with modafinil-treated (150 mg/kg) group (&&&P < 0.001).
Effect of co-administration of diazepam before different doses of modafinil on clonic seizure threshold. Diazepam (0.02 mg/kg, i.p,) was administered 15 min before modafinil (20, 50, 80, 150 mg/kg, i.p,). Data are expressed as mean ± SEM analyzed by one-way ANOVA followed by Tukey’s multiple comparison test. *P < 0.05 ***P < 0.001 compared with saline-treated group. %%P < 0.01 compared with modafinil-treated (20 mg/kg) group. $$$P < 0.001 compared with modafinil-treated (50 mg/kg) group. ##P < 0.01 compared with modafinil-treated (80 mg/kg) group. &&&P < 0.001 compared with modafinil-treated (150 mg/kg) group
Effect of Co-administration of Citalopram and Modafinil on PTZ-Induced CST
Citalopram (0.1 mg/kg) was injected 45 min before PTZ-induced clonic seizure threshold determination. At this dose citalopram did not remake seizure threshold compared with saline group (P > 0.05). Pretreatment with citalopram (0.1 mg/kg, i.p.) 15 min before administration of ineffective and effective anti-convulsant doses of modafinil (20, 50, and 80 mg/kg respectively, i.p.) failed to influence this property of modafinil (Fig. 9). In addition, combination of citalopram (0.1 mg/kg) with pro-convulsant dose of modafinil (150 mg/kg) did not change the seizure threshold in comparison with modafinil-treated (150 mg/kg) animals (P > 0.05).
Effect of citalopram administration on clonic seizure threshold of various doses of modafinil. Citalopram (0.1 mg/kg, i.p,) was administered 15 min before modafinil (20, 50, 80, and 150 mg/kg, i.p,). Data are expressed as mean ± SEM analyzed by one-way ANOVA followed by Tukey’s multiple comparison test. ***P < 0.001 compared with saline-treated group
Hippocampal Nitrite Concentrations in Different Groups
To analyze the role of nitric oxide in modulatory feature of modafinil on CST induced by PTZ we measured nitrite concentrations in hippocampi. Table 1 flaunts that nitrite levels significantly were escalated in modafinil-treated (80 and 150 mg/kg) groups compared with saline-treated group (60.39 ± 2.15 P < 0.001 and 56.49 ± 0.63 P < 0.05 respectively). To substantiate this speculation we examined the effect of pretreatment of l-NAME (10 mg/kg), aminoguanidine (50 mg/kg), and 7-nitroindazole (40 mg/kg) 15 min before the modafinil (80 and 150 mg/kg) injection on hippocampi nitrite levels. Our analysis exposed that all NOS inhibitors reversed the escalation that had been occurred in modafinil-treated (80 and 150 mg/kg) groups. Moreover, nitrite concentrations in animals administered l-arginine (60 mg/kg) 15 min prior to modafinil (150 mg/kg) were elevated in comparison with control mice (59.68 ± 1.794 P < 0.001). But the same dose of l-arginine injection before anti-convulsant dose (80 mg/kg) of modafinil did not alter the nitrite level. Thus, it can be concluded that modafinil augmented the amount of nitric oxide in hippocampus in both anti- and pro-convulsant effects.
Discussion
We studied the effects of different doses of modafinil and the involvement of glutamate, nitric oxide, GABA, and serotonin systems in clonic seizure threshold induced by pentylenetetrazole in mice. Our data divulged that modafinil has both anti- and pro-convulsant effects on PTZ-induced CST. The results of the present study demonstrated that acute pretreatment of l-NAME, a nonspecific NOS inhibitor, 7-nitroindazole, a neuronal NOS inhibitor, and aminoguanidine, an inducible NOS inhibitor, blocked the anti- and pro-convulsant effects of modafinil. Administration of NOS precursor l-arginine did not alter the anti-convulsant effect, but completely reversed the pro-convulsant effect of modafinil. Results of nitric oxide metabolite quantity revealed that nitric oxide level increased in both anti- and pro-convulsant activities of modafinil, which reversed after treatment with all NOS inhibitors. Moreover, co-administration of MK-801, a NMDA antagonist, and diazepam, a GABAA agonist, prior to different doses of modafinil augmented the effect of modafinil on CST enhancement, but completely blocked the pro-convulsant activity of this drug. Finally, injection of citalopram, a selective serotonin reuptake inhibitor, before anti- and pro-convulsant doses of modafinil did not have any effect on the seizure threshold.
The substantial increase in off-label use of modafinil has happened in recent decades [16], and it has been used for treatment of a veriety of psychiatery disorders including depression and hyperactivity [10, 33]. The paradox records and safety concerns was arisen in patients with seizures [19]. Zolkowska and his collegues (2015) reported that a single administration of modafinil with dose 75 mg/kg (also its metabolites) increased the sizure threshold in the maximal electroshock seizure threshold model in mice. also, they exhibited that injection of modafinil at dose 50 mg/kg escalated the anti-convulsant activity of antiepileptic drugs such as carbamazepine, phenytoin, and valproate in maximal electroshock seizure threshold test in mice [18]. Also, it was reported that acute modafinil (22.5, 45, and 90 mg/kg, i.p.) treatment significantly protected against PTZ-induced convulsive behaviors in a dose-dependent manner in mice [17]. Another study by Ozsoy et al. [34], a chronic administration of various doses (1, 2, 4, 45, and 180 mg/kg i.p) of modafinil in PTZ-kindling model of seizure in mice, revealed that modafinil postponed the onset of the first myoclonic jerk and reduced the whole major seizure period between 2 and 180 mg/kg doses but did not impact on seizure onset. These are in accordance with our results, which a single injection of modafinil (80 mg/kg) exerted a considerable anti-convulsant activity in PTZ-induced seizure threshold in mice.
On the other hand, several studies indicated that modafinil axacerbated the seizures incidence. Chen et al. [17] unveiled that modafinil at 180 mg/kg—which shows anti-seizure activity in the MES model—exerted an increase in the the seizure stage in the PTZ-kindling model in mice. In our results, as well, the seizure threshold reduced after treatment with modafinil given at 180 mg/kg in mice.
In clinical reports, 2 of 13 children experienced exacerbation of their seizures with modafinil therapy (346 ± 119 mg/day) for almost 15 months [35]. One person of 27 participants allocated in modafinil group (200-mg total daily dose) for the 11 days was withdrawn for seizure occurrence [36]. Modafinil was discontinued for 6 patients due to seizure exacerbation and 4 patients because of observation of de novo seizure after starting modafinil (about 200 mg/day), in the 10 year review by Astry et al. between 205 patients [19]. It is important to note that in our study, PTZ-induced tonic-clonic seizure threshold was used in NMRI strain mice, but Zolkawska et al. performed their experiments in the maximal electroshock seizure threshold test (sine-wave, 0.2-s stimulus duration, 500 V, 25 mA, and 50 Hz) in male Albino Swiss (BALB/c) mice. However, Chen et al. employed electroshock model (0.4-s stimulus duration and 70 mA) and PTZ-kindling model in male Kunming mice [17, 18]. The apparent discrepancy in modafinil convulsive activity between all above mentioned reports may be explained either by different seizure models or mouse strains applied in experiments. In addition, it can be assumed that time of treatment, doses of injection, duration of consumption may be associated with diverse effects in both human and animal.
Our data demonstrated that nitric oxide mediates both modulatory effects of modafinil on CST induced by PTZ. Nitric oxide is a known modifier of seizure threshold with either anti-convulsant [37,38,39] or pro-convulsant [40,41,42] effects in different seizure paradigms. Many signs suggest that both neuronal and inducible isoforms of nitric oxide synthase take part in several crucial brain processes [43,44,45,46]. This complex and paradox effect of NO has displayed in many previous published articles [37,38,39,40,41,42] and in few articles nitric oxide involved in biphasic activities of some drugs such as D-penicillamine and morphine. In these previous studies, D-penicillamine and morphine exhibited bimodal effects in seizure threshold induced by PTZ in mice, and both anti- and pro-convulsant effects of them reversed by l-NAME intervention. So, reduction of NO level was pro-convulsant in the anti-convulsant while anti-convulsant in the pro-convulsant [6, 24].
Regarding to our results, all NOS inhibitors blocked both pro- and anti-convulsive activity of modafinil, while pretreatment with l-arginine, with no effect on anti-convulsant activity, blocked the pro-convulsant characteristic of modafinil. These caused to assume that overproduction of NO may be more associated with protective effect of modafinil. But surprisingly, after measuring the NO level in mice hippocampi in all groups, data of 80 and 150 mg/kg modafinil-treated animals’ hippocampi unveiled a significant escalation in nitrite levels compared to control animals. So, to substantiate the NO impact on convulsant properties of modafinil needs other experiments and studies like gene expression.
Our experiments explicated the involvement of glutamatergic, and GABAergic systems in the pro-convulsant activity of modafinil. Based on former literatures modafinil increases the glutamate and decreases the GABA release [47,48,49]. Enhancement of glutamate in the synapse activates the ionotropic and metabotropic receptors, which is so important for initializing and spreading of seizure activity [50, 51]. In rodent and human studies of seizures, there is a credit for enhancement of functions and activity of NMDA receptors [52,53,54,55,56]. In addition, Glutamate levels in ventromedial and ventrolateral thalamus, and hippocampal formation of the awake rats are increased by modafinil [47]. As seen in our results, injection of NMDA receptors antagonist (MK-801) leaded to increase in anti-convulsant effect and blockade of pro-convulsant activity of modafinil.
Our examinations revealed that GABA pathway has an important role in pro-convulsant property of modafinil. GABAA receptor is an inhibitory channel activated by agonists like benzodiazepines, augmented the chloride influx, and caused hyperpolarization of the membrane [8]. There are many literatures expressing the involvement of GABA pathway in seizure [57,58,59,60]. Pfluger et al. showed that gamma-decanolactone decreases the convulsive behaviors by modulation of GABA system in seizures induced by picrotoxin, isoniazid, and 4-aminopyridine in mice [57]. Another study revealed that protodioscin has an anti-convulsant effect against pilocarpine-induced convulsions through escalation in GABA levels [58]. Li et al. unveiled that agent SR 57227 lowers the PTZ inhibitory effect on GABA levels in hippocampi of mice in PTZ-induced seizure [59]. Finally, our data exhibited that pretreatment with diazepam may block the inhibitory effect of modafinil on GABA release resulting in potentiation of anti-convulsant activity of modafinil and inhibition of pro-convulsant effect.
We evaluated the involvement of serotonin pathway in anti- and pro-convulsant effects of modafinil. Some literatures expressed that 5-HT3 subtype receptors which are responsible for many pharmacological actions of SSRIs can modulate the seizure threshold induced by PTZ in mice. Moreover, anti- and pro-convulsant properties of citalopram have been observed in some paradigms and 5-HT3 receptors are responsible for bimodal effect on seizure susceptibility in mice [7, 61]. Our research showed that serotonin and 5-HT3 receptors have no impact on both anti- and pro-modulatory properties of modafinil in clonic seizure threshold induced by PTZ in mice.
Conclusions
In conclusion, modafinil at low and high doses expresses anti- and pro-convulsant effects respectively in PTZ-induced clonic seizure threshold in mice. We also revealed that modafinil may exert anti-convulsant effect through nitric oxide system and pro-convulsant activity via glutamate, nitric oxide, and GABA pathways. Finally, serotonin receptors have no role in these features.
References
Koda-Kimble MA, Young LY, Alldredge BK, Corelli RL, Ernst ME, Guglielmo BJ, Jacobson PA, Kradjan WA, Williams BR (2009) Applied therapeutics: the clinical use of drugs, seizure disorders, 2. Lippincott Williams & Wilkins, Philadelphia, pp 1387–1388
Brunton LL, Chabner BA, Knollmann BC (2006) Goodman and Gilman’s the pharmacological basis of therapeutics, pharmacotherapy of the epilepsies, 1. McGraw-Hill, New York, p 583
Hitiris N, Brodie MJ (2006) Modern antiepileptic drugs: guidelines and beyond. Curr Opin Neurol 19:175–180
Vezzani A, Bartfai T, Bianchi M, Rossetti C, French J (2011) Therapeutic potential of new antiinflammatory drugs. Epilepsia 52:67–69
Javadian N, Rahimi N, Javadi-Paydar M, Doustimotlagh AH, Dehpour AR (2016) The modulatory effect of nitric oxide in pro-and anti-convulsive effects of vasopressin in PTZ-induced seizures threshold in mice. Epilepsy Res 126:134–140
Rahimi N, Sadeghzadeh M, Javadi-Paydar M, Heidary MR, Jazaeri F, Dehpour AR (2014) Effects of d-penicillamine on pentylenetetrazole-induced seizures in mice: involvement of nitric oxide/NMDA pathways. Epilepsy Behav 39:42–47
Payandemehr B, Bahremand A, Rahimian R, Ziai P, Amouzegar A, Sharifzadeh M, Dehpour AR (2012) 5-HT3 receptor mediates the dose-dependent effects of citalopram on pentylenetetrazole-induced clonic seizure in mice: Involvement of nitric oxide. Epilepsy Res 101:217–227
Khan GM, Smolders I, Lindekens H, Manil J, Ebinger G, Michotte Y (1999) Effects of diazepam on extracellular brain neurotransmitters in pilocarpine-induced seizures in rats. Eur J pharmacol 373:153–161
Ballon JS, Feifel D (2006) A systematic review of modafinil: potential clinical uses and mechanisms of action. J Clin Psychiatry 67:554–566
Minzenberg MJ, Carter CS (2008) Modafinil: a review of neurochemical actions and effects on cognition. Neuropsychopharmacol: Off Publ Am Coll Neuropsychopharmacol 33:1477–1502
Fone KC, Nutt DJ (2005) Stimulants: use and abuse in the treatment of attention deficit hyperactivity disorder. Curr Opin Pharmacol 5:87–93
Kumar R (2008) Approved and investigational uses of modafinil: an evidence-base review. Drugs 68:1803–1839
Heal DJ, Cheetham SC, Smith SL (2009) The neuropharmacology of ADHD drugs in vivo: insights on efficacy and safety. Neuropharmacology 57:608–618
Kane JM, D’Souza DC, Paktar AA, Youakim JM, Tiller JM, Yang R, Keefe RS (2010) Armodafinil as adjunctive therapy in adults with cognitive deficits associated with schizophrenia: a 4-week, double-blind, placebo-controlled study. J Clin Psychiatry 71:1475–1148
Lindenmayer JP, Nasrallah H, Pucci M, James S, Citrome L (2013) A systematic review of psychostimulant treatment of negative symptoms of schizophrenia: challenges and therapeutic opportunities. Schizophr Res 147:241–252
Peñaloza RA, Sarkar U, Claman DM, Omachi TA (2013) Trends in on-label and off-label modafinil use in a nationally representative sample. JAMA Intern Med 173:704–706
Chen CR, Qu WM, Qiu MH, Xu XH, Yao MH, Urade Y, Haung ZL (2007) Modafinil exerts a dose-dependent antiepileptic effect mediated by adrenergic α1 and histaminergic H1 receptors in mice. Neuropharmacology 53:534–541
Zolkowska D, Andres-mach M, Prisinzano TE, Baumann MH, Luszczki JJ (2015) Modafinil and its metabolites enhance the anticonvulsant action of classical antiepileptic drugs in the mouse maximal electroshock-induced seizure model. Psychopharmacology 232:2463–2479
Artsy E, McCarthy DC, Hurwitz SH, Pavlova MK, Dworetzky BA, Woo Lee J (2012) Use of modafinil in patients with epilepsy. Epilepsy Behav 23:405–408
Gupta R, Gupta LK, Bhattacharya SK (2014) Chronic administration of modafinil induces hyperalgesia in mice: Reversal by l-NG-nitro-arginine methyl ester and 7-nitroindazole. Eur J Pharmacol 736:95–100
Gilbert ME (1988) The NMDA receptor antagonist, MK-801, suppresses limbic kindling and kindled seizures. Brain Res 463:90–99
Waxman EA, Lynch DR (2005) N-methyl-d-aspartate receptor subtypes: multiple roles in excitotoxicity and neurological disease. Neuroscience 11(1):37–49
Mohammad Jafari R, Ghahremani MH, Rahimi N, Shadboorestan A, Rashidian A, Esmaeili J, Ejtemaei Mehr S, Dehpour AR (2018) The anticonvulsant activity and cerebral protection of chronic lithium chloride via NMDA receptor/nitric oxide and phospho-ERK. Brain Res Bull 137:1–9
Homayoun H, Khavandgar S, Namiranian K, Gaskari SA, Dehpour AR (2002) The role of nitric oxide in anticonvulsant and proconvulsant effects of morphine in mice. Epilepsy Res 48:33–41
Szyndler J, Maciejak kolosowskaK, Chmielewska N, Skorzewska A, Daszczuk P, Płaznik A (2017) Altered expression of GABA-A receptor subunits in hippocampus of PTZ-kindled rats. Pharmacol Rep 17:14–21
Gholipour T, Rasouli A, Jabbarzadeh A, Ghazi Nezami B, Riazi K, Sharifzadeh M, Dehpour AR (2009) The interaction of sildenafil with the anticonvulsant effect of diazepam. Eur J Pharmacol 617:79–83
Tecott LH, Sun LM, Akana SF, Strack AM, Lowenstein DH, Dallman MF, Julius D (1995) Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature 374:542–546
Loscher W, Honack D, Fassbender CP, Nolting B (1991) The role of technical, biological and pharmacological factors in the laboratory evaluation of anticonvulsant drugs. III Pentylenetetrazole seizure models. Epilepsy Res 8:171–189
Swinyard EA, Kupferberg HJ (1985) Antiepileptic drugs: detection, quantification, and evaluation. Fed Proc 44:2629–2633
Khavandgar S, Homayoun H, Dehpour AR (2002) The role of nitric oxide in the proconvulsant effect of δ-opioid agonist SNC80 in mice. Neurosci Lett 329:237–239
Hassanipour M, Shirzadian A, Boojar MM, Abkhoo A, Abkhoo A, Delazar S, Amiri S, Rahimi N, Ostadhadi S, Dehpour AR (2016) Possible involvement of nitrergic and opioidergic systems in the modulatory effect of acute chloroquine treatment on pentylenetetrazol induced convulsions in mice. Brain Res Bull 121:124–130
Rahimi N, Hassanipour M, Allahabadi NS, Sabbaghziarani F, Yazdanparast M, Dehpour AR (2018) Cirrhosis induced by bile duct ligation alleviates acetic acid intestinal damages in rats: Involvements of nitrergic and opioidergic systems. Pharmacol Rep 70(3):426–433
Swanson JM, Greenhill LL, Lopez FA, Sedillo A, Earl CQ, Jiang JG, Biederman J (2006) Modafinil film-coated tablets in children and adolescents with attention-deficit/hyperactivity disorder: results of a randomized, double-blind, placebo-controlled, fixed-dose study followed by abrupt discontinuation. J Clin Psychiatr 67:137–147
Ozsoy S, Aydin D, Ekici F (2015) Effects of modafinil on pentylenetetrazol-induced convulsive epilepsy. Bratisl Lek Listy 116:162–166
Ivanenko A, Tauman R, Gozal D (2003) Modafinil in the treatment excessive daytime sleepiness in children. Sleep Med 4:579–582
Jha A, Weintraub A, Allshouse A, Morey C, Cusick C, Kittelson J, Harrison-Felix C, Whiteneck G, Gerber D (2008) A randomized trial of modafinil for the treatment of fatigue and excessive daytime sleepiness in individuals with chronic traumatic brain injury. J Head Trauma Rehabil 23:52–63
Buisson A, Lakhmeche N, Verrecchia C, Plotkine M, Boulu RG (1993) Nitric oxide: an endogenous anticonvulsant substance. Neuroreport 4:444–446
Starr MS, Starr BS (1993) Paradoxical facilitation of pilocarpine-induced seizures in the mouse by MK-801 and the nitric oxide synthesis inhibitor l-NAME. Pharmacol Biochem Behav 45:321–325
Theard MA, Baughman VL, Wang Q, Pelligrino DA, Albrecht RF (1995) The role of nitric oxide in modulating brain activity and blood flow during seizure. Neuroreport 6(6):921–924
Mülsch A, Busse R, Mordvintcev PI, Vanin AF, Nielsen EO, Scheel-Krüger J et al (1994) Nitric oxide promotes seizure activity in kainate-treated rats. Neuroreport 5:2325–2328
Riazi K, Roshanpour M, Rafiei-Tabatabaei N, Homayoun H, Ebrahimi F, Dehpour AR (2006) The proconvulsant effect of sildenafil in mice: role of nitric oxide–cGMP pathway. Br J Pharmacol 147:935–943
Osonoe K, Mori N, Suzuki K, Osonoe M (1994) Antiepileptic effects of inhibitors of nitric oxide synthase examined in pentylenetetrazol-induced seizures in rats. Brain Res 663:338–340
Licinio J, Prolo P, McCann SM, Wong ML (1999) Brain iNOS: current understanding and clinical implications. Mol Med Today 5:225–232
Hassanipour M, Rajai N, Rahimi N, Fatemi I, Jalali M, Akbarian R, Shahabaddini A, Nazari A, Amini-Khoei H, Dehpour AR (2018) Sumatriptan effects on morphine-induced antinociceptive tolerance and physical dependence: The role of nitric oxide. Eur J Pharmacol 2018 835:52–60
Dawson T, Synder Sh (1994) Gases as biological messengers: nitric oxide and carbon monoxide in the brain. J Neurosci 14:5147–5159
Del.Bel EA, Oliveira PR, Oliveira JAC, Mishra PK, Jobe PC, Garcia-Cairasco N (1997) Anticonvulsant and proconvulsant roles of nitric oxide in experimental epilepsy models. Braz J Med Biol Res 30:971–979
Ferraro L, Antonelli T, O’Conner WT, Tanganelli S, Rambert F, Fuxe K (1997) The antinarcoleptic drug modafinil increases glutamate release in thalamic areas and hippocampus. Neuroreport 8:2883–2887
Ferraro L, Antonielli T, O’Connor WT, Tanganelli S, Rambert FA, Fuxe K (1998) the effects of modafinil on striatal, pallidal and nigral GABA and glutamate release in the conscious rat: evidence for a preferential inhibition of striato-pallidal GABA transmission. Neurosci Lett 253:135–138
Ferraro L, Antonelli T, Tanganelli S, O’Connor WT, Perez de la Mora M, Mendez-Franco J, Rambert FA, Fuxe K (1999) The vigilance promoting drug modafinil increases extracellular glutamate levels in the medial preoptic area and the posterior hypothalamus of the conscious rat: prevention by local GABAA receptor blockade. Neuropsychopharmacology 20:346–356
Ronne-Engström E, Hillered L, Flink R, Spännare B, Ungerstedt U, Carlson H (1992) Intracerebral microdialysis of extracellular amino acids in the human epileptic focus. J Cereb Blood Flow Metab 12:873–876
During M, Spencer D (1993) Extracellular hippocampal glutamate and spontaneous seizure in the conscious human brain. Lancet 341:1607–1610
Chapman AG (1998) Glutamate receptors in epilepsy. Prog Brain Res 116:371–383
Chapman AG (2000) Glutamate and epilepsy. J Nutr 130:1043S–1043S5S
Chapman A (1995) Excitatory neurotransmission and antiepileptic drug development: a status report. Recent Adv Epilepsy 6:1–21
Meldrum BS (1995) Excitatory amino acid receptors and their role in epilepsy and cerebral ischemia. Ann N Y Acad Sci 757:492–505
Rogawski MA (1992) The NMDA receptor, NMDA antagonists and epilepsy therapy. Drugs 44:279–292
Pfluger P, Coelho VR, Renger GG, da Silva LL, Maerinez K, Fonseca A, Viau CM, Pereira P (2018) Neuropharmacological profile of gamma-decanolactone on chemically-induced seizure in mice. Cent Nerv Syst Agents Med Chem 11:162–169
Song SH, Fajol A, Chen Y, Ren B, Shi S (2018) Anticonvulsive effects of protodioscin against pilocarpine-induced epilepsy. Eur J Pharmacol 833:237–246
Li B, Wang L, Sun ZH, Zhou Y, Shao D, Zhao J, Song Y, Lv J, Dong X, Liu Ch, Wang P, Zhang X, Cui R (2014) The anticonvulsant effects of SR 57227 on pentylenetetrazole-induced seizure in mice. Plos one 9:1–6
Oakley JC, Cho AR, Cheah CS, Scheuer T, Catteral WA (2013) Synergistic GABA-enhancing therapy against seizures in a mouse model of Dravet Syndrome. J Pharmacol Exp Ther 345:215–224
Gholipour T, Ghasemi M, Riazi K, Ghaffarpour M, Dehpour AR (2010) Seizure susceptibility by alteration through 5-HT3 receptor: Modulation by nitric oxide. Seizure 19:17–22
Acknowledgements
The study was supported by a grant from Experimental Medicine Research Center, Tehran University of Medical Sciences (Grant No. 96-02-30-35219). This work was supported by a grant (96002757) from Iran National Science Foundation (INSF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declares that they have no conflict of interests.
Rights and permissions
About this article
Cite this article
Bahramnjead, E., Kazemi Roodsari, S., Rahimi, N. et al. Effects of Modafinil on Clonic Seizure Threshold Induced by Pentylenetetrazole in Mice: Involvement of Glutamate, Nitric oxide, GABA, and Serotonin Pathways. Neurochem Res 43, 2025–2037 (2018). https://doi.org/10.1007/s11064-018-2623-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-018-2623-7