Abstract
Purpose
There are several lines of evidence on the protective roles of opioids in gastrointestinal inflammatory conditions. This study aims to distinguish the central and peripheral roles of methadone, a non-selective opioid receptor agonist, in an acute model of ulcerative colitis in male rats.
Methods
Ulcerative colitis was induced by intrarectal administration of acetic acid 4%. Methadone was injected subcutaneously (s.c.), 5 and 10 mg/kg, and intracerebroventricular (i.c.v.), 50 and 300 ng/rat. Opioid antagonists were employed. Methylnaltrexone (MNTX; 5 mg/kg, i.p.), a peripherally acting opioid receptor antagonist, and naltrexone (NTX; 5 mg/kg, i.p. and 10 ng/rat, i.c.v.), a peripherally and centrally acting opioid receptor antagonist were injected before methadone (10 mg/kg, s.c. and or 300 ng/rat, i.c.v.) administration. NTX (5 mg/kg, i.p. and 10 ng/rat, i.c.v.) were administered 30 min prior to administration of methadone (10 mg/kg, s.c. and 300 ng/rat, i.c.v.), respectively. MNTX (5 mg/kg, i.p.) was injected 30 min prior to methadone (10 mg/kg, s.c.). Seventy-two hours following colitis induction, macroscopic and microscopic mucosal lesions, and the colonic levels of tumor necrosis factor-alpha (TNF-α) and interleukin-1β (IL-1β) were determined.
Results
Methadone (300 ng/rat, i.c.v.) and Methadone (5 and 10 mg/kg, s.c.) improved the macroscopic and microscopic scores through opioid receptors. Also, a significant reduction in TNF-α and IL-1β was observed. Peripherally and centrally injected NTX significantly reversed methadone 10 mg/kg s.c. anti-inflammatory effects while MNTX could not completely reverse this effect. Moreover, centrally administered methadone (300 ng/rat) showed the anti-inflammatory effect which was reversed by central administration of NTX (10 ng/rat).
Conclusions
The opioid receptors mainly the central opioid receptors may mediate the protective actions of methadone on the experimental model of inflammatory bowel disease in rat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammatory bowel disease (IBD) is characterized by a chronic inflammatory process in the gut due to leukocyte infiltration and excessive generation of pro-inflammatory cytokines (Fakhraei et al. 2014). Colitis triggers activation of the neuroendocrine system via hypothalamic corticotropin-releasing factor (CRF) pathways in the paraventricular nucleus of the hypothalamus (PVN) involved in the autonomic, behavioral, and neuroendocrine response to inflammation (Porcher et al. 2004; Greenwood-Van Meerveld et al. 2006).
The main therapeutic goals in IBD are control of intestinal inflammation and treatment of the most common clinical symptoms (Baumgart and Sandborn 2007). Diarrhea is considered one of the main symptoms in IBD and its mechanisms are complex and multifactorial. The mainstays of conventional pharmacotherapy for IBD are aminosalicylates and corticosteroids. Immunosuppressive agents and biological response modifiers are alternative therapies. Nevertheless, available drugs are not universally effective and cause considerable adverse effects. As a result, there is a demand for research that leads to the development of new therapeutic approaches (Mousavizadeh et al. 2009).
Opioids are recognized for their anti-nociceptive and anti-diarrheal roles in the gastrointestinal (GI) tract. Opioid receptors and their ligands play important roles in the GI secretory and motor functions. Activation of opioid receptors may modulate several inflammatory mechanisms like mitogen-induced immune mononuclear cells (Eisenstein and Hilburger 1998), splenocyte proliferation (Shahabi et al. 1991), and production of inflammatory and immunomodulatory cytokines (Peterson et al. 1998). Moreover, immune cells have been shown to express different opioid receptors; kappa (κ), mu (μ), and delta (δ) which bind both agonists and antagonists (McCarthy et al. 2001; Janecka et al. 2004). Opioid receptors, which are expressed in immune cells are often the same as or similar to neuronal-type opioid receptors, particularly κ- and δ. Studies also indicated expression of opioid receptors or binding sites on lymphocytes that are selective for morphine (Bidlack 2000). It has been shown that chronic administration of narcotic analgesics significantly reduced immune cell function (Bryant et al. 1987) and opiates inhibited chemokine-induced chemotaxis (Grimm et al. 1998).
In this context, using opioids as therapeutic options in IBD has been widely discussed (Philippe et al. 2003; Zagon and McLaughlin 2011). Surprisingly, anti-inflammatory roles of peripheral opioid agonists in experimental colitis models in mice were demonstrated. Notably, the opioids exert their anti-inflammatory effects mainly through a peripheral route (Philippe et al. 2003). In fact, opioids might modify the inflammatory process through their effects on the synthesis and secretion of pro- and anti-inflammatory cytokines. Increased expression of κ-opioid receptors was reported in the inflamed rat colon induced by trinitrobenzenesulfonic acid (TNBS) (Sengupta et al. 1999) and acetic acid 5% (Burton and Gebhart 1998). In addition, expression of μ- and δ-opioid receptors increased in intestinal inflammation induced by croton oil in mice (Pol et al. 1994).
Interestingly, psycho-immune modulation through the brain–gut axis might have a pivotal role in the pathogenesis of IBD. Several comorbidities, including psychiatric disorders like depression and anxiety have been reported in IBD patients (Bonaz and Bernstein 2013). In this context, nitric oxide (NO)-mediated neuroinflammation might be responsible for the behavioral despair associated with a mouse model of Crohn’s disease (Heydarpour et al. 2016). On the other hand, the central nervous system (CNS) regulates innate immune responses. For example, the neuroendocrine stress response and the sympathetic and parasympathetic nervous systems inhibited innate immune responses (Sternberg 2006).
Evidence for involvement of the cholinergic anti-inflammatory pathway in IBD pathogenesis is well established (Seyedabadi et al. 2018). Acetylcholine (ACh), the main neurotransmitter in the vagus nerve, decreases the production of pro-inflammatory cytokines (Khalifeh et al. 2015; Seyedabadi et al. 2018). All these findings indicate that new strategies for IBD treatment should focus on interventions that both ameliorate IBD psychological comorbidities and potentiate central anti-inflammatory mechanisms. Opioids could be among the potential candidates. They elicit potent anti-inflammatory properties in experimental peripheral and central inflammatory diseases.
Methadone is a synthetic opioid agonist that has a greater penetration through the blood–brain barrier (BBB) rather than other opioids such as morphine (Kafami et al. 2013). Among opioids, the immunomodulatory effect of methadone has been reported in many immune diseases, for example, human immunodeficiency virus (HIV) and diabetes mellitus type 1 (Al-Hashimi et al. 2013). The immunomodulatory effects of methadone have been addressed in neurodegenerative diseases and peripheral inflammatory conditions (Amirshahrokhi et al. 2008; Kafami et al. 2013). Interestingly, in vitro studies demonstrated that methadone activates nicotinic ACh receptors (Pakkanen et al. 2005) and this effect might potentiate CNS-induced anti-inflammatory cascades in the gut.
Accordingly, this study aims to distinguish the effects of central and peripheral administration opioid receptor agonist, methadone on colonic inflammation in acetic acid-induced colitis in rats.
Materials and methods
Animals
Male Wistar rats (6–7 weeks old) weighing 200–250 g were kept for a week prior to study so as to be adapted to the animal room conditions. The animal room was maintained at 22–24 °C with a lighting regimen of 12-hour light/12-hour dark. Rats had free access to standard pelleted chew and water. All the experimental procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978) with the approval of Research and Medical Ethics Committees of Tehran University of Medical Science.
Chemicals
Methadone, naltrexone, methylnaltrexone, and dexamethasone were purchased from Merck co. (Darmstadt, Germany). Xylazine and ketamine were bought from Alfasan co. (Woerden Holland). Acetic acid, formalin solution, and diethyl ether oxide were obtained from Dr. Mojallali Chemical Laboratories (Iran).
Experimental groups
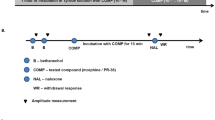
The study period was 72 h and the drugs were started 1 h following the induction of colitis (day one) and continued in two successive days. The rats were divided into 18 groups of 7–9. Two control groups received intrarectal (i.r.) acetic acid (one got s.c. saline and another i.c.v. saline). Two sham groups were administered distilled water intrarectally (one got s.c. saline and another i.c.v. saline). Further, a standard treatment group received dexamethasone (1 mg/kg, i.p.) (Antonioli et al. 2007). Moreover, two IBD groups for methadone (5 and 10 mg/kg, s.c.) and two IBD groups for methadone (50 and 300 ng/rat, i.c.v.) administrations were assigned. To assess which route of the opioid administration is the primary action site of methadone, the most effective dose for each route was determined and their combinations with the antagonists assessed. In this regard, nine groups were randomly selected. Non-selective opioid receptor antagonists, methylnaltrexone (MNTX; 5 mg/kg, ip) and naltrexone (NTX; 5 and 10 mg/kg, i.p. and 10 ng/rat, i.c.v.) were injected individually 30 min before administration of the most effective dose of methadone (10 mg/kg, s.c. and 300 ng/rat, i.c.v.). NTX (10 ng/rat, i.c.v.) was administered before the most effective dose of methadone 300 ng/rat, three distinct IBD groups received the antagonists. Finally, to prove that peripheral administration of methadone exerts its protective effect against colitis by a central mechanism, three groups received intrarectal (i.r.) acetic acid; one of them received NTX (10 ng/rat, i.c.v.) + methadone (10 mg/kg, s.c.), one group received saline (i.c.v.) + methadone (10 mg/kg, s.c.) and another received saline (i.c.v.) + saline (s.c.) (Table 1).
Induction of colitis
Pathological profile of the acute colitis induced by acetic acid might vary from those of the chronic disease. Acetic acid induces a non-specific inflammation similar to chronic models such as trinitrobenzenesulphonic acid. However, this model might have limitations for addressing the cascades that initiate inflammation in humans. On the other hand, excessive oxidative stress, prolonged infiltration of neutrophils and elevated levels of inflammatory mediators (that also occur in acute model), factors with significant pathological roles in human IBD including, allow using acetic acid-induced colitis, a reliable model for screening agents with potential benefits in IBD (Yamada et al. 1991; Elson et al. 1995; Medany et al. 2005; Mahgoub et al. 2005; Perez-Navarro et al. 2005).
The rats fasted for 24 h prior to any intra-colonic studies; however, they always had access to water. Briefly, the rats were anesthetized with a mixture (1:1 v/v; 1 ml/kg body weight) of xylazine 2% (10 mg/kg i.p.) and ketamine 10% (50 mg/kg) (Alfasan Woerden Holland). Colitis was then induced according to the previous methods (Fakhraei et al. 2014; Rahimian et al. 2016). Briefly, a medical-grade polyurethane cannula for enteral feeding (external diameter 2 mm) was inserted into the anus, and the tip advanced 7 cm proximal to the anus verge and 1 ml 4% acetic acid (V/V) (Merck, Darmstadt, Germany) introduced into the colon.
Intracerebroventricular (i.c.v.) injections
Rats were anesthetized by an i.p. injection of a mixture (1:1 v/v; 1 ml/kg body weight) of xylazine 2% (10 mg/kg) and ketamine 10% (50 mg/kg). The rats were placed individually in a stereotaxic apparatus. A midline incision of the scalp was made and the skull carefully cleared from the skin and muscles. After that, a hole was drilled into the skull above the right lateral brain ventricle, according to coordinates obtained from Paxinos and Watson (2007), from the bregma: anterior–posterior = − 0.8 mm, lateral − 1.5 mm and depth − 3.5 mm. Drugs were injected directly into the right lateral ventricle using a 30-gauge needle (Plastic One Inc.), connected to a 10-µl Hamilton syringe by a PF-50 catheter (Intramedic Polyethylene Tubing, Clay Adams, Sparks, MD) filled with saline. A small air bubble (1 µl) was drawn at the distal end of the PE-50 tubing for visual monitoring of the i.c.v. injection. Drugs were administered at a fixed volume of 5 μl (at a constant rate of 2 μl/min) into the right lateral ventricle (Hashemizadeh et al. 2014; Chen et al. 2018).
Macroscopic and histopathologic colon damages
Seventy-two hours following the colitis induction, the rats were euthanized. In an ice bath, the distal colons were cut open and cleansed gently with normal saline. Subsequently, the colons were cut into two similar pieces, one for histopathologic assessment (kept in 5 ml of formalin 10% w/w) and another for analysis of biochemical markers.
After cutting open and cleaning the colon samples, high-quality photos were taken for the macroscopic evaluation. The macroscopic scoring was performed by an independent observer (Morris and Moore 1989; Fakhraei et al. 2014) (Table 2).
For evaluation based on microscopical (histologic) characters, the tissues were fixed in phosphate-buffered formaldehyde, embedded in paraffin and 4-µm sections were prepared. The tissues were stained with hematoxylin and eosin (H&E) and were evaluated by light microscopy and scored in a blinded manner by an expert pathologist using a Zeiss® microscope equipped with an Olympus® color video camera for digital imaging as indicated in Table 3. Each section was then scored for each feature separately by establishing the product of the grade for that feature and the percentage involvement (in a range from 0 to 12 for inflammation and for extent, and in a range from 0 to 16 for regeneration and for crypt damage) (Dieleman et al. 1998).
Cytokine measurements
The colonic levels of tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β) were determined with an enzyme-linked immunosorbent assay (ELISA kit) (Enzo Life Sciences, Lorrach/Germany). The colon was dissected out and homogenized in 50-mmol/L ice-cold potassium phosphate buffer (pH 6.0) containing 0.5% of hexadecyltrimethylammonium bromide. Afterward, the homogenate was centrifuged at 4000 rpm for 20 min at 4 °C and the supernatant was separated and kept at − 80 °C until analysis. Briefly, the wells were pre-coated with a monoclonal antibody serving to trap cytokine molecules in the homogenated specimen. Eventually, the results were expressed as pg/mg of the wet tissue (Fakhraei et al. 2014).
Statistical analysis
All the values are expressed as mean ± standard error using SPSS (version 19.0, Chicago, USA). One-way analysis of variance was employed for analyzing the data, followed by Tukey’s post hoc test for multiple comparisons. Significance was ascribed when P < 0.05.
Results
Mortality rates
The mortality rates are shown in Table 4. As can be observed, sham and methadone (300 ng/rat, i.c.v.) groups did not have any mortality. The mortality rate for control, methadone (10 mg/kg, s.c.); and NTX (5 mg/kg, i.p.) was 40–45%. However, mortality rate of methadone (5 mg/kg, s.c.) and MNTX (5 mg/kg, i.p.) was 33.3%. NTX (5 mg/kg, i.p.) + met (10 mg/kg, s.c.) group had 50% mortality which represented the highest mortality in the animals.
Comparing the effective doses of methadone against colitis and experimental pain: in a study, the analgesic ED50, the effective dose for 50 percent of the group, for methadone s.c. was found to be 2.04 mg/kg (95% confident limit, 1.58–2.63) in the rat (Liu et al. 1983). On the other hand, the median lethal dose (MLD) for methadone was 22.5 (19.3:24.1) mg/kg in the rat (Borron et al. 2002). Besides, NTX was not toxic in any species (rat, dog, and monkey) at the dose of at least 20 mg/kg, which is 20 times higher than recommended clinically (1 mg/kg) (Willette and Barnett 1981).
Macroscopic data
Effect of methadone on acetic acid-induced colitis is illustrated in Fig. 1, according to the macroscopic scores. Control group has higher scores which are very highly significant compared to sham group P < 0.001. In addition, methadone group (50 ng/rat, i.c.v.) is very highly significant compared to the sham group and has a higher score, P < 0.001. Moreover, methadone groups (5 and 10 mg/kg, s.c.), dexamethasone group (1 mg/kg, i.p.) and methadone (300 ng, i.c.v.) show very highly significant differences compared to the control group and have lower scores, P < 0.001.
Figure 2 illustrates the effect of the opioid antagonists on methadone effect in acetic acid-induced colitis, according to macroscopic scores. Figure 2a shows the administration of methadone (10 mg/kg, s.c.) alone or 30 min after administration of the opioid antagonists (MNTX and NTX 5 mg/kg, i.p.). NTX (5 mg/kg, i.p.) significantly reverses methadone 10 mg/kg, s.c. effects (P < 0.05). Figure 2b shows administration of NTX (10 ng/rat, i.c.v.), alone and or 30 min before methadone (300 ng/rat i.c.v.). NTX (10 ng/rat, i.c.v.) highly significantly reverses methadone (300 ng/rat, i.c.v.) effects (P < 0.01). Figure 2c shows administration of NTX (10 ng/rat, i.c.v.) 30 min before methadone (10 mg/kg, s.c.). As can be seen, methadone (10 mg/kg s.c.) has lower macroscopic score which are very highly significant compared with the control group (P < 0.001). Conversely, NTX (10 ng/rat, i.c.v.), highly significantly (P < 0.01) reverses the effect of methadone (10 mg/kg, s.c.).
Effect of opioid antagonists on methadone doses (10 mg/kg, s.c. and 300 ng/rat, i.c.v.) in acetic acid-induced colitis according to macroscopic scores. a Methadone sc and antagonists i.p. administrations. b i.c.v. methadone and antagonists administrations (n = 6–8). *P < 0.05 and ** P < 0.01 significantly different from methadone groups. c NTX (10 ng/rat, i.c.v.) and methadone (10 mg/kg, s.c.) administrations. ***P < 0.001 significantly different from control group. ##P < 0.01 significantly different from methadone group (10 mg/kg, s.c.)
Figure 3 represents the effects of methadone on the amount of TNF-α. Control group is very highly significant from the sham group and has a higher TNF-α level, P < 0.001. Dexamethasone (1 mg/kg, i.p.), methadone (10 mg/kg, s.c.) and methadone (50 and 300 ng/rat, s.c.) groups are very highly significant from the control group (P < 0.001) and have lower levels of TNF-α. Methadone (5 mg/kg, s.c.) is also highly significant from the control group (P < 0.01) and has a lower TNF-α level.
In Fig. 4, the impact of the opioid antagonists on the methadone doses (10 mg/kg and 300 ng/rat) in acetic acid-induced colitis, according to TNF-α levels (n = 6–8) is demonstrated. Figure 4a represents s.c. methadone administrations. NTX and MNTX (5 mg/kg, i.p.) 30 min. before methadone (10 mg/kg, s.c.) increase TNF-α levels compared to methadone 10 mg/kg alone, the differences were highly significant P < 0.01 and very highly significant P < 0.01, respectively. Figure 4b represents i.c.v. methadone administrations. NTX 30 min before methadone 10 ng/rat increases TNF-α level very highly significantly P < 0.001 compared to methadone 10 ng/rat.
Effect of methadone on the amount of IL-1β on acetic acid-induced colitis is represented in Fig. 5 (n = 6–8). Control group is very highly significant from the sham group and has a higher IL-1β level, P < 0.001. In addition, methadone (50 ng/rat, i.c.v.) group is very highly significant from the sham group and has a higher IL-1β level (P < 0.001). Dexamethasone (1 mg/kg, i.p.), methadone (10 mg/kg, s.c.) and methadone (300 ng/rat, i.c.v.) groups are very highly significant from the control group P < 0.001 and have a lower level of IL-1β. Methadone (5 mg/kg, s.c.) also is highly significant from the control group (P < 0.01) and has lower level of IL-1β.
Figure 6 represents impact of opioid antagonists on the optimum methadone doses (10 mg/kg and 300 ng/rat) in acetic acid-induced colitis according to IL-1β levels (n = 6–8). Figure 6a shows s.c. methadone administrations. NTX (5 mg/kg, i.p.) 30 min before methadone (10 mg/kg, s.c.) very highly significantly increases IL-1β level in IBD rats compared to methadone 10 mg/kg, P < 0.001. Figure 6b represents i.c.v. methadone administrations. NTX (10 ng/rat, i.c.v.) 30 min before methadone (300 ng/rat, i.c.v.) very highly significantly increases IL-1β level compared to methadone (300 ng/rat) P < 0.001.
Figure 7 illustrates photographic images of rat colitis colons. Macroscopic examination reveals: mucosal erythema with ulcer and exudate in vehicle-treated group (a), mild erythema without ulcer in dexamethasone 1 mg/kg (b), methadone 10 mg/kg (c), methadone 300 ng/rat (d) groups; erythema with patchy erosions in (MNTX 5 mg/kg + methadone 10 mg/kg) group (e); erythema with ulcer and exudate in (NTX 5 mg/kg + methadone 10 mg/kg) (f) and (NTX 10 ng/rat + methadone 300 ng) (g) groups. Besides, mucosal erythema with ulcer and exudate in (NTX 10 ng/rat, i.c.v. + methadone 10 mg/kg, s.c.) (h) and mild erythema with a restricted small ulcer in (saline i.c.v. + methadone 10 mg/kg, s.c.) (i).
Photographic images of rat colitis colons treated with a vehicle; b dexamethasone 1 mg/kg; c methadone 10 mg/kg; d methadone 300 ng; e MNTX 5 mg/kg + methadone 10 mg/kg; f NTX 5 mg/kg + methadone 10 mg/kg; g NTX 10 ng + methadone 300 ng; h NTX 10 ng + methadone 10 mg/kg; i saline i.c.v. ng + methadone 10 mg/kg
Cross-sections of H&E stained sections of acetic acid-treated colon tissues are illustrated in Fig. 8. A, F, and G show severe (grade 3) transmural inflammation (grade 3) with entire crypt damage (grade 4) and desquamated epithelium in most parts of the specimen (grade 4). There is no evidence of tissue regeneration (grade 4). B, C, and D: moderate inflammation (grade 2) in mucosa and submucosa (grade 2) without crypt damage (grade 0) and almost complete regeneration (grade 1) are seen. E: Moderate mucosal and submucosal inflammations (grade 2) with crypt damage in 2/3 of the basal part (grade 2) are seen. Features of regeneration with crypt depletion (grade 2) are also evident.
Microscopic (histophatologic) features
Table 5 demonstrates the pathologic results of the s.c. methadone route. IBD group has higher inflammation severity and extent which are very highly significant compared to the sham group, P < 0.001. Moreover, crypt damage and percentage of involvement are very highly significantly increased as well, P < 0.001. Regeneration and total index are also raised highly significantly P < 0.01 and very highly significantly P < 0.001, respectively. In comparison to the IBD group, dexamethasone shows highly significantly lower inflammation severity and significantly lower inflammation extent, P < 0.01 and P < 0.05, respectively. It also has significantly lower total index P < 0.05. Methadone 5 mg/kg shows significantly less crypt damage and percentage of involvement P < 0.05. In methadone 10 mg/kg group, inflammation severity decreases significantly and crypt damage decreases very highly significantly, P < 0.05 and P < 0.001, respectively. Moreover, it has a very highly significantly lower total index, P < 0.001. On the other hand, compared to methadone 10 mg/kg group, in methadone [10 mg/kg + MNTX 5 mg/kg] group crypt damage and percentage of involvement have a considerable increase, with a higher total index, P < 0.001, P < 0.001, and P < 0.01, respectively. Similarly, in the group [methadone 10 mg/kg + NTX 5 mg/kg], crypt damage and percentage of involvement have considerable increase, with a higher total index, P < 0.01, P < 0.05 and P < 0.05, respectively.
In addition, the group (NTX 10 ng/rat, i.c.v. + methadone 10 mg/kg, s.c.) has significant increase in inflammation severity P < 0.01, crypt damage P < 0.001 and percentage of involvement P < 0.05 compared to the group (saline i.c.v. + methadone 10 mg/kg). Also, it has a higher total index P < 0.001.
Table 6 demonstrates the pathologic results of i.c.v. methadone route. Control group has very highly significantly higher inflammation severity and extent compared to the sham group, P < 0.001. Moreover, crypt damage, the percentage of involvement are increased very highly significantly as well, P < 0.001. Regeneration and total index are also raised very highly significantly P < 0.001. In comparison to the IBD group, dexamethasone shows considerably lower inflammation severity and extent, P < 0.001 and P < 0.01, respectively. It also has significantly lower crypt damage and total index, P < 0.05. In methadone 300 ng/rat group, the inflammation severity and extent decrease highly significantly P < 0.01. Moreover, it has significantly lower crypt damage, the percentage of involvement, regeneration, and total index, P < 0.001, P < 0.05, P < 0.05 and P < 0.001, respectively. On the other hand, compared to methadone 300 ng/rat group, in the group (methadone 300 ng/rat + NTX 10 ng/rat), crypt damage and the percentage of involvement have very highly significantly increases, P < 0.001 In addition, regeneration and total index rise significantly P < 0.05.
Discussion
The current investigation highlighted a crucial role for the brain–gut axis in the control of intestinal inflammation related to IBD in a rat model. Our results demonstrated that central and peripheral methadone administrations significantly reduced the severity of the ulcerative lesions induced by acid acetic and markedly improved macroscopic and microscopic scores via opioid receptor-dependent mechanisms. Nevertheless, using specific peripheral and central antagonists, our results showed that in the treatment of IBD, methadone acts mainly through the central route.
Virtually consistent with our experiment, two selective peripheral µ-opioid receptor agonists, named DALDA and DAMGO, significantly reduced inflammation in experimental colitis models in mice. They also stated that opioids exert their anti-inflammatory effects mainly through the peripheral route (Philippe et al. 2003). Showing physiologic anti-inflammatory effects of the peripheral opioid receptors in experimental colitis models, this experiment is approximately in line with our study except that the current experiment mostly highlights a role for central opioid receptors in control of a peripherally induced gut inflammation.
There is no doubt that opioid peptides are a link between the neuroendocrine and immune systems, and the immunomodulatory effect of enkephalins may play a significant clinical role in immune-mediated diseases through HPA axis and other parts of the limbic system in the brain (Collins and Verma-Gandhu 2006; Straub et al. 2008). Peripherally mediated immunosuppressive effects of opioids may play a significant role in opioid-induced immunosuppression (Wei et al. 2003). Accumulating evidence supported a role for endogenous opioid peptides such as enkephalins and endorphins in the development and/or perpetuation of inflammation (Rogers and Peterson 2003; Pol and Puig 2004). Chronic oral NTX promoted the mucosal healing in subjects with moderate to severe Crohn’s disease (Smith et al. 2011). On the other hand, low doses of morphine were pro-inflammatory in adjuvant arthritis (Earl et al. 1994).
Opioid therapy is an exciting new development for treating inflammatory diseases such as arthritis, especially since they cause fewer side effects compared to molecules, which act outside the CNS. In this regard, κ-opioid drugs showed powerful anti-inflammatory effects, reducing arthritis severity by as much as 80%, attenuating the disease in a dose-dependent, stereo-selective and antagonist-reversible manner (Walker 2003). As another example, loperamide, a peripherally acting mu (µ)-opioid receptor agonist, commonly used as anti-diarrhea agent (Hanauer 2007). It showed analgesic and anti-inflammatory effects in a similar manner to peripheral endogenous opioids, in peripheral inflamed tissue in a rat model (Hua 2014). Moreover, loperamide showed both central and peripheral anti-nociceptive effects in the formalin test, an inflammatory pain model in rats (Shannon and Lutz 2002).
The vagus nerve (VN) (Bonaz 2007; Meregnani et al. 2011) and HPA axis (Mackner et al. 2011) have mainly been reported as a modulator of the neuro-intestinal inflammatory pathway (Pavlov et al. 2009). Moreover, impairments of the autonomic nervous system (ANS) and lower parasympathetic function have been reported as another etiological pathway in IBD (Pavlov et al. 2009). Neuromodulation as a therapeutic approach opens a new era in the treatment of IBD. Amongst the potential therapeutic neuronal pathways, the VN, based on its anti-inflammatory properties, is the most likely therapeutic target, in particular through VN stimulation. The VN is the longest nerve in the body innervating numerous organs including the GI tract (Bonaz et al. 2017). The VN is the main component of the parasympathetic nervous system and innervates the entire GI tract except for the rectum in the rat (Altschuler et al. 1993).
It is well established that dysregulation of the brain neurotransmitters can affect the peripheral inflammatory conditions such as IBD. Psycho-neuro-endocrine-immune modulation through the brain–gut axis likely has a key role in the pathogenesis of IBD (Taché and Bernstein 2009; Bonaz and Bernstein 2013). Chronic GI inflammation also induced anxiety and behavioral despair in mice which was associated with decreased hippocampal brain-derived neurotrophic factor (BDNF) messenger RNA and increased circulating TNF-α and interferon-α (Bercik et al. 2010). Similarly, the gut–brain communication was observed following induction of IBD in mice and the animals showed behavioral despair (Heydarpour et al. 2016).
In the current study, following central and peripheral administration of methadone, the notable reduction in elevated levels of the pro-inflammatory markers (TNF-α and IL-1β) was observed in IBD rats. During the course of experimental colitis, TNF-α releases and aggravates the tissue damage. Secretion of TNF-α by the epithelial cells may act as a pivotal factor in the pathogenesis of IBD. TNF-α, IL-1β and other inflammatory cytokines secreted by lymphocytes and macrophages in the inflamed intestine can profoundly affect the activation state of mesenchymal cells, thereby amplifying the inflammatory response and probably contributing to fibrosis, one of the most important complications of IBD (Daneshmand et al. 2009). In our investigation, both central and peripheral methadone administrations showed the protective effect on acetic acid-induced colitis. However, it should be noted that this effect of methadone was mediated mainly through the central opioid receptors. The i.c.v. route had more potency due to its significantly lower administered doses, which has shown to markedly diminish methadone drawbacks like cardiac and respiratory suppression (Ricardo Buenaventura et al. 2008; Chugh et al. 2008). This might also explain, at least partly, the difference in the effect of peripherally and centrally given methadone on intestinal lesions and mortality rate. While the previous one was reduced significantly by methadone peripheral injection, the mortality rate in IBD-rat was not affected. In contrast, 300 ng dose of methadone given centrally highly reduced both the mortality rate and the intestinal lesion. Although MNTX as a peripheral opioid antagonist could not significantly reverse the methadone anti-inflammatory effect, the contribution of peripheral mechanisms to the effect of methadone is also probable. Nevertheless, NTX reversed the protective effect of methadone both centrally and peripherally. In consistence with that, investigators have utilized MNTX, a quaternary form of NTX that does not cross the BBB, to separate central from peripheral effects. The compound was able to antagonize most of the immune alterations produced by systemic morphine injection when it was administered intracerebroventricularly, but failed to do the same while administered subcutaneously in rats (Lysle and Coussons-Read 1995; Fecho et al. 1996). In line with that, morphine inhibited carrageenan-induced paw swelling (Gyires et al. 1985) and near-toxic doses of morphine were able to attenuate adjuvant arthritis in rats (Levine et al. 1985; Walker et al. 1996). Moreover, a decrease in serum-free met-enkephalin value was observed in IBD patients (Owczarek et al. 2011). Following methadone treatment, the pathological parameters such as severity and extent of inflammation, crypt damages and, the tissue involvement reduced markedly and tissue regeneration improved. It is worth mentioning that though the scores were not significantly affected, MNTX reversed the methadone-induced decreased level of TNF-α, consequently, the contribution of peripheral mechanisms to the effect of methadone cannot be excluded. Moreover, reversal of methadone’s protective effect by NTX demonstrated that in the treatment of IBD, methadone may act mainly through the central route.
Conclusion
In this study, we showed for the first time that probably the central opioid receptors are mainly involved in anti-inflammatory aspects of methadone in acetic acid-induced colitis and the role of the brain–gut axis is highlighted. Because of diversity in opioid receptors, further studies are crucial to address which types of opioid receptors are involved in beneficial effects of central methadone. Our findings open a new platform to target gut inflammation in IBD-associated comorbidities like memory impairment, depression, and anxiety. Indeed, methadone would be considered as a pharmacophore to design central-acting therapeutic agents to treat IBD. Overall, our findings provide evidence that opioid receptors’ agonists may have the potential to control the gut inflammation and might be new therapeutics.
References
Al-Hashimi M, Scott SW, Thompson JP, Lambert DG (2013) Opioids and immune modulation: more questions than answers. Br J Anaesth 111:80–88
Altschuler SM, Escardo J, Lynn RB, Miselis RR (1993) The central organization of the vagus nerve innervating the colon of the rat. Gastroenterology 104:502–509
Amirshahrokhi K, Dehpour AR, Hadjati J, Sotoudeh M, Ghazi-Khansari M (2008) Methadone ameliorates multiple-low-dose streptozotocin-induced type 1 diabetes in mice. Toxicol Appl Pharmacol 232:119–124
Antonioli L, Fornai M, Colucci R, Ghisu N, Da Settimo F, Natale G, Kastsiuchenka O et al (2007) Inhibition of adenosine deaminase attenuates inflammation in experimental colitis. J Pharmacol Exp Ther 322(2):435–442
Baumgart DC, Sandborn WJ (2007) Inflammatory bowel disease: clinical aspects and established and evolving therapies. The Lancet 369:1641–1657
Bercik P, Verdu EF, Foster JA, Macri J, Potter M, Huang X, Malinowski P et al (2010) Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology 139:2102–2112
Bidlack JM (2000) Detection and function of opioid receptors on cells from the immune system. Clin Diagn Lab Immunol 7(5):719–723
Bonaz B (2007) The cholinergic anti-inflammatory pathway and the gastrointestinal tract. Gastroenterology 133:1370–1373
Bonaz BL, Bernstein CN (2013) Brain–gut interactions in inflammatory bowel disease. Gastroenterology 144:36–49
Bonaz B, Sinniger V, Pellissier S (2017) Vagus nerve stimulation: a new promising therapeutic tool in inflammatory bowel disease. J interna med 282(1):46–63
Borron SW, Monier C, Risede P, Baud FJ (2002) Flunitrazepam variably alters morphine, buprenorphine, and methadone lethality in the rat. Hum Exp Toxico 21(11):599–605
Brown DR, Goldberg LI (1985) The use of quaternary narcotic antagonists in opiate research. Neuropharmacology 24(3):181–191
Bryant HU, Bernton EW, Holaday JW (1987) Immunosuppressive effects of chronic morphine treatment in mice. Life Sci 41:1731–1738
Burton MB, Gebhart GF (1998) Effects of kappa-opioid receptor agonists on responses to colorectal distension in rats with and without acute colonic inflammation. J Pharmacol Exp Ther 285:707–715
Chen W, Taché Y, Marvizón JC (2018) Corticotropin-releasing factor in the brain and blocking spinal descending signals induce hyperalgesia in the latent sensitization model of chronic pain. Neuroscience 381:149–158
Chugh SS, Socoteanu C, Reinier K, Waltz J, Jui J, Gunson K (2008) A community-based evaluation of sudden death associated with therapeutic levels of methadone. Am J Med 121:66–71
Collins S, Verma-Gandhu M (2006) The putative role of endogenous and exogenous opiates in inflammatory bowel disease. Gut 55:756–757
Daneshmand A, Rahimian R, Mohammadi H, Ejtemaee-Mehr S, Tavangar SM, Kelishomi RB, Dehpour AR (2009) Protective effects of lithium on acetic acid-induced colitis in rats. Dig Dis Sci 54:1901–1907
Dieleman LA, Palmen MJ, Akol H, Bloemena E, Peña AS, Meuwissen SG, Van Rees EP (1998) Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol 114:385–391
Earl JR, Claxson AW, Blake DR, Morris CJ (1994) Proinflammatory effects of morphine in the rat adjuvant arthritis model. Int J Tissue React 16:163–170
Eisenstein TK, Hilburger ME (1998) Opioid modulation of immune responses: effects on phagocyte and lymphoid cell populations. J Neuroimmunol 83:36–44
Elson CO, Sartor RB, Tennyson GS, Riddell H (1995) Experimental models of inflammatory bowel disease. Gastroenterology 109:1344–1367
Fakhraei N, Abdolghaffari AH, Delfan B, Abbasi A, Rahimi N, Khansari A, Rahimian R, Dehpour AR (2014) Protective effect of hydroalcoholic olive leaf extract on experimental model of colitis in rat: involvement of nitrergic and opioidergic systems. Phytother Res 28:1367–1373
Fecho K, Maslonek KA, Dykstra LA, Lysle DT (1996) Assessment of the involvement of central nervous system and peripheral opioid receptors in the immunomodulatory effects of acute morphine treatment in rats. J Pharmacol Exp Ther 276:626–636
Greenwood-Van Meerveld B, Johnson AC, Schulkin J, Myers DA (2006) Long-term expression of corticotropin-releasing factor (CRF) in the paraventricular nucleus of the hypothalamus in response to an acute colonic inflammation. Brain Res 1071:91–96
Grimm MC, Ben-Baruch A, Taub DD, Howard OM, Wang JM, Oppenheim JJ (1998) Opiate inhibition of chemokine-induced chemotaxis. Ann N Y Acad Sci 840:9–20
Gyires K, Budavári I, Fürst S, Molnár I (1985) Morphine inhibits the carrageenan-induced oedema and the chemoluminescence of leucocytes stimulated by zymosan. J Pharm Pharmacol 37:100–104
Hanauer SB (2007) The benefits of loperamide in the treatment of patients with IBS or IBD. Introduction. Rev gastroenterol disord 7:S1
Hashemizadeh S, Sardari M, Rezayof A (2014) Basolateral amygdala CB1 cannabinoid receptors mediate nicotine-induced place preference. Prog Neuropsychopharmacol Biol Psychiatry 51:65–71
Heydarpour P, Rahimian R, Fakhfouri G, Khoshkish S, Fakhraei N, Salehi-Sadaghiani M, Wang H, Abbasi A, Dehpour AR, Ghia JE (2016) Behavioral despair associated with a mouse model of Crohn’s disease: role of nitric oxide pathway. Prog Neuropsychopharmacol Biol Psychiatry 64:131–141
Hua S (2014) Development of an effective topical liposomal formulation for localized analgesia and anti-inflammatory actions in the complete Freund’s adjuvant rodent model of acute inflammatory pain. Pain Physician 17:E719–E735
Janecka A, Fichna J, Janecki T (2004) Opioid receptors and their ligands. Curr Top Med Chem 4:1–17
Kafami L, Etesami I, Felfeli M, Enayati N, Ghiaghi R, Aminian A, Dehpour A (2013) Methadone diminishes neuroinflammation and disease severity in EAE through modulating T cell function. J Neuroimmunol 255:39–44
Khalifeh S, Fakhfouri G, Mehr SE, Mousavizadeh K, Dehpour AR, Khodagholi F, Kazmi S, Rahimian R (2015) Beyond the 5-HT3 receptors: a role for α7nACh receptors in neuroprotective aspects of tropisetron. Hum Exp Toxicol 34:922–931
Levine JD, Moskowitz MA, Basbaum AI (1985) The contribution of neurogenic inflammation in experimental arthritis. J Immunol 135(2 Suppl):843s–847s
Liu SJ, Roerig DL, Wang RI (1983) Brain and plasma levels of methadone and their relationships to analgesic activity of methadone in rats. Drug Metab Dispos 11:335–338
Lysle DT, Coussons-Read ME (1995) Mechanisms of conditioned immunomodulation. Int J Immunopharmacol 17:641–647
Mackner LM, Clough-Paabo E, Pajer K, Lourie A, Crandall WV (2011) Psychoneuroimmunologic factors in inflammatory bowel disease. Inflamm Bowel Dis 17:849–857
Mahgoub A, El-Medany A, Mustafa A, Arafah M, Moursi M (2005) Azithromycin and erythromycin ameliorate the extent of colonic damage induced by acetic acid in rats. Toxicol Appl Pharmacol 205:43–52
McCarthy L, Wetzel M, Sliker JK, Eisenstein TK, Rogers TJ (2001) Opioids, opioid receptors, and the immune response. Drug Alcohol Depend 62:111–123
Medany A, Mahgoub A, Mustafa A, Arafa M, Morsi M (2005) The effects of selective cyclooxygenase-2 inhibitors, celcoxib and rofecoxib, on experimental colitis induced by acetic acid in rats. Eur J Pharmacol 507:291–295
Meregnani J, Clarençon D, Vivier M, Peinnequin A, Mouret C, Sinniger V, Picq C, Job A, Canini F, Jacquier-Sarlin M, Bonaz B (2011) Anti-inflammatory effect of vagus nerve stimulation in a rat model of inflammatory bowel disease. Auton Neurosci 160:82–89
Morris DD, Moore JN (1989) Antibody titres to core lipopolysaccharides in horses with gastrointestinal disorders which cause colic. Equine Vet J Suppl7:29–32
Mousavizadeh K, Rahimian R, Fakhfouri G, Aslani FS, Ghafourifar P (2009) Anti-inflammatory effects of 5-HT3 receptor antagonist, tropisetron on experimental colitis in rats. Eur J Clin Invest 39:375–383
Owczarek D, Cibor D, Mach T, Cieśla A, Pierzchała-Koziec K, Sałapa K, Kuśnierz-Cabała B (2011) Met-enkephalins in patients with inflammatory bowel diseases. Adv Med Sci 56:158–164
Pakkanen JS, Nousiainen H, Yli-Kauhaluoma J, Kylänlahti I, Möykkynen T, Korpi ER, Peng JH, Lukas RJ, Ahtee L, Tuominen RK (2005) Methadone increases intracellular calcium in SH-SY5Y and SH-EP1-hα7 cells by activating neuronal nicotinic acetylcholine receptors. J Neurochem 94:1329–1341
Pavlov VA, Parrish WR, Rosas-Ballina M, Ochani M, Puerta M, Ochani K, Chavan S, Al-Abed Y, Tracey KJ (2009) Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain Behav Immun 23:41–45
Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates. Academic Press, San Diego
Perez-Navarro R, Ballester I, Zarzuelo A, S¢anchez de Medina F (2005) Disturbances in epithelial ionic secretion in different experimental models of colitis. Life Sci 76:1489–1501
Peterson PK, Molitor TW, Chao CC (1998) The opioid–cytokine connection. J Neuroimmunol 83:63–69
Philippe D, Dubuquoy L, Groux H, Brun V, Chuoï-Mariot MT, Gaveriaux-Ruff C, Colombel JF, Kieffer BL, Desreumaux P (2003) Anti-inflammatory properties of the μ opioid receptor support its use in the treatment of colon inflammation. J Clin Invest 111:1329–1338
Pol O, Puig MM (2004) Expression of opioid receptors during peripheral inflammation. Curr Top Med Chem 4:51–61
Pol O, Ferrer I, Puig MM (1994) Diarrhea associated with intestinal inflammation increases the potency of mu and delta opioids on the inhibition of gastrointestinal transit in mice. J Pharmacol Exp Ther 270:386–391
Porcher C, Sinniger V, Juhem A, Mouchet P, Bonaz B (2004) Neuronal activity and CRF receptor gene transcription in the brains of rats with colitis. Am J Physiol Gastrointest Liver Physiol 287:G803–G814
Rahimian R, Zirak MR, Keshavarz M, Fakhraei N, Mohammadi-Farani A, Hamdi H, Mousavizadeh K (2016) Involvement of PPARγ in the protective action of tropisetron in an experimental model of ulcerative colitis. Immunopharmacol Immunotoxicol 38:432–440
Ricardo Buenaventura M, Rajive Adlaka M, Nalini Sehgal M (2008) Opioid complications and side effects. Pain Physician 11:S105–S120
Rogers TJ, Peterson PK (2003) Opioid G protein-coupled receptors: signals at the crossroads of inflammation. Trends Immunol 24:116–121
Sengupta JN, Snider A, Su X, Gebhart GF (1999) Effects of kappa opioids in the inflamed rat colon. Pain 79:175–185
Seyedabadi M, Rahimian R, Ghia JE (2018) The role of alpha7 nicotinic acetylcholine receptors in inflammatory bowel disease: involvement of different cellular pathways. Expert Opin Ther Targets 22:161–176
Shahabi NA, Burtness MZ, Sharp BM (1991) N-acetyl-β-endorphin1–31 antagonizes the suppressive effect of β-endorphin1–31 on murine splenocyte proliferation via a naloxone-resistant receptor. Biochem Biophys Res Commun 175:936–942
Shannon HE, Lutz EA (2002) Comparison of the peripheral and central effects of the opioid agonists loperamide and morphine in the formalin test in rats. Neuropharmacology 42:253–261
Smith JP, Bingaman SI, Ruggiero F, Mauger DT, Mukherjee A, McGovern CO, Zagon IS (2011) Therapy with the opioid antagonist naltrexone promotes mucosal healing in active Crohn’s disease: a randomized placebo-controlled trial. Dig Dis Sci 56:2088–2097
Sternberg EM (2006) Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat Rev Immunol 6:318
Straub RH, Wolff C, Fassold A, Hofbauer R, Chover-Gonzalez A, Richards LJ, Jessop DS (2008) Antiinflammatory role of endomorphins in osteoarthritis, rheumatoid arthritis, and adjuvant-induced polyarthritis. Arthritis Rheum 58:456–466
Taché Y, Bernstein CN (2009) Evidence for the role of the brain–gut axis in inflammatory bowel disease: depression as cause and effect? Gastroenterology 136:2058
Walker JS (2003) Anti-Inflammatory effects of opioids. In: Machelska H, Stein C (eds) Immune mechanisms of pain and analgesia. ©Eurekah.com and Kluwer Academic/Plenum Publishers, Dordrecht
Walker JS, Chandler AK, Wilson JL, Binder W, Day RO (1996) Effect of μ-opioids morphine and buprenorphine on the development of adjuvant arthritis in rats. Inflamm Res 45:557–563
Wei G, Moss J, Yuan CS (2003) Opioid-induced immunosuppression: is it centrally mediated or peripherally mediated? Biochem Pharmacol 65:1761–1766
Willette RE, Barnett G (1981) Narcotic antagonists: naltrexone pharmacochemistry and sustained-release preparations. Department of Health and Human Services, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute on Drug Abuse, Division of Research
Yamada T, Zimmerman BJ, Specian RD, Grisham MB (1991) Role of neutrophils in acetic acid-induced colitis in rats. Inflammation 15:399–411
Zagon IS, McLaughlin PJ (2011) Targeting opioid signaling in Crohn’s disease: new therapeutic pathways. Expert Rev Gastroenterol Hepatol 5:555–558
Acknowledgements
The study was supported by a Grant from Tehran University of Medical Sciences, Tehran, Iran (No. 16555-15-04-90).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Fakhraei, N., Javadian, N., Rahimian, R. et al. Involvement of central opioid receptors in protective effects of methadone on experimental colitis in rats. Inflammopharmacol 26, 1399–1413 (2018). https://doi.org/10.1007/s10787-018-0538-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-018-0538-1