Abstract
Oxidative polymerization of phenolic compounds and indolic compounds produces brown or black pigments called Melanins. In microbes, melanin production is generally related with various survival mechanisms. Some of the bacteria like Bacillus thuringiensis, Klebsiella sp., Proteus mirabilis, Pseudomonas aeruginosa, Pseudomonas stutzeri and Rhizobium sp. are reported to produce melanins. They have immense applications in the field of agriculture, cosmetics and pharmaceutical industries due to their photo-protective, UV protective and anti-oxidant activities. The objective of this study was to investigate the effects of different growth media and process parameters on melanin production. The marine bacterial strain Pseudomonas stutzeri HMGM-7 (MTCC 11712) was selected and the effect of different media such as Nutrient Broth (NB), Luria Bertini (LB) broth, Bushnell-Haas broth (BHB) and Trypticase Soy broth (TSB) and various medium components were investigated with a one factor at a time approach. Parameters like shaking frequency, inoculum age, inoculum size, pH and temperature were also investigated in order to obtain the optimum conditions for maximum melanin production. The highest yield of melanin concentration, 0.27 g/L, was obtained in nutrient broth at 32 h. The yield was 1.53 times higher than the melanin obtained before optimization, 0.177 g/L at 48 h. The increase in the productivity of melanin after selection of suitable medium and optimization of process parameters was 128.73 %.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Pigments are colorful chemical compounds which absorbs light in the visible spectrum. The produced color is due to the absorption of energy by a group of molecules known as chromophore which leads to the excitation of an electron. The non-absorbed energy which is reflected or refracted is captured by the eye to generate neural impulses which are then carried to the brain where they could be decoded as a color (Hari 1994). Pigments are of two types, synthetic pigments and natural pigments. They are widely used in clothes, cosmetics, furniture, foods, medicines, and in other products. Based on their structural characteristics, the natural pigments are classified as Tetrapyrrole derivatives, Isoprenoid derivatives, Benzopyran derivatives, Quinones and Melanins (Hari 1994). Melanins are nitrogenous polymeric compounds with indole ring as their monomeric unit but they are not homopolymers. Generally, they are present as a mixture of macromolecules and are responsible for most of the black, brown and gray colorations of plants, animals, and microorganisms. Melanins are classified into three groups, they are; Eumelanins which are black or brown pigments and are widely distributed in vertebrates and invertebrates. It is the most common type of melanin. Pheomelanins which are yellow to red pigments and are found in mammals and birds. And finally allomelanins that are present in fungi, seeds and spores.
The very first report of melanin production was reported in Pseudomonas aeruginosa producing pyomelanin (Osawa et al. 1963) followed by Shewanella colwelliana, Vibrio cholera, and Hyphomonas strain (Kotob et al. 1995; Ruzafa et al. 1995). The melanin synthesis using homogentisic acid as a precursor was first reported in Vibrio cholerae, Hyphomonas species and Shewanella colwelliana (Kotob et al. 1995). The synthesis of melanin and its characterization such as solubility, free radical nature was initially studied in Proteus mirabilis (Agodi et al. 1996). A novel marine bacterium Alteromonas strain MMB-1, was isolated from the Mediterranean Sea and its melanin synthesis ability was studied using l-tyrosine as a precursor (Solano et al. 1997). Melanin production was studied by UV-resistant mutant of Bacillus thuringiensis subsp. Kurstaki and its UV-protection ability for insecticidal crystals was tested (Saxena et al. 2002). The thermo tolerant strains of Bacillus thuringiensis were also reported for melanin production (Ruan et al. 2004). Another important melanin producing bacterium reported was Marinomonas mediterranea, which produces black eumelanin from l-tyrosine (Lucas-Elio et al. 2012). A marine bacterium, Pseudomonas stutzeri HMGM-7 producing considerable amount of melanin in sea water medium without the addition of l-tyrosine was also reported (Ganesh Kumar et al. 2013).
The different properties and functions of melanins are being explored for various applications. The high reactivity of melanin due to the presence of =O, –OH, –NH, and –COOH groups is the reason behind growing interest in melanin research. Melanins have a broad spectrum of biological applications. This includes inhibition of Human Immunodeficiency Virus (HIV) replication, antivenin activity, antimicrobial activity and antioxidant activity. Melanins also have physical and chemical applications. This includes their use in nano particle synthesis, cosmetics and in lenses.
Inspite of so many potential applications, melanins are not used due to non-availability of a sustainable and cost effective method of melanin production. The major sources of melanin are cephalopods, plants and microorganisms. Melanin obtained from microbes has great advantages over melanin from animals and plants. Microorganisms do not cause the problems of seasonal variations and are fast in growth. They also modify themselves according to the medium and conditions provided. The bacterial sources have been used as a main source of melanin with immense applications in the field of agriculture, cosmetics and pharmaceutical industries (Riley 1997), hence its optimization is important for large scale production. The high production cost and high commercial value of melanin has given rise to the need of a demanding research for cheaper production methods. The present study aims to increase the productivity of melanin from an epiphytic bacterium known as Pseudomonas stutzeri HMGM-7 (MTCC 11712). This bacterium was isolated from the branches of sea weed Hypnea musciformis, which released a black extracellular pigment into the medium. The physical parameters and nutritional requirements which plays an important role in the production of melanin were optimized in the present study.
2 Materials and Methods
2.1 Microorganisms and Culture Conditions
Nutrient agar slants and plates were prepared for maintenance of the organism (Pseudomonas stutzeri HMGM-7) obtained from MTCC Chandigarh. Periodical subcultures were done for maintenance of the viability of the strain. Media volume used throughout the experiments was 50 ml in 250 ml Erlenmeyer flasks which was maintained at 37 °C at 150 rpm. Nutrient broth prepared in distilled water was used for shake flask studies.
2.2 Growth Studies
2 % inoculum volume was added to each flask and incubated at 37 °C and observed at two different rpm: 150 and 250. During the incubation period of 72 h, the OD of the samples was measured at 660 nm after every 4 h using appropriate blank. Biomass dry weight for a volume of 28 ml of the culture was accounted for by centrifuging (8000 rpm, 8 min, and 40 °C) and drying the pellet for 8 h at 60 °C in a hot air oven. The supernatant obtained after centrifugation is filter sterilized with 0.45 µm syringe filters and their absorbance is measured at 400 nm to quantify the melanin.
2.3 Optimization of Nutritional Parameters for Melanin Production
All the experiments conducted used a constant media volume of 50 ml in 250 ml Erlenmeyer flasks. NB was used as the basal medium which was incubated at 37 °C and 150 rpm unless otherwise stated. The effect of different parameters on the production of melanin such as pH, temperature, carbon sources, nitrogen sources, trace elements were evaluated by keeping Nutrient broth as the basal medium. The factorial design of experiments known as ‘one factor at a time’ method was applied to improve the reproducibility of the experimental results and to optimize the entire biosynthesis process. The experiments were conducted by varying one factor at a time and keeping the remaining factors constant.
2.4 Effect of Inoculum Age
The effect of age of the inoculum on the melanin production was studied using Nutrient Broth medium by using 6, 12, 18 and 32 h old cultures maintained at 37 °C and 150 rpm.
2.5 Effect of Inoculum Size
0.5, 5, 10, 15 and 20 % inoculum volume were evaluated for melanin production using Nutrient Broth medium at 37 °C and 150 rpm.
2.6 Effect of Shaking Frequency
The shaking frequency was optimized by incubating the Erlenmeyer flasks in incubator shaker at 100, 150, 200 and 250 rpm at 37 °C with a shaking diameter of 25 mm.
2.7 Effect of pH
The optimum pH for the production of melanin was determined by setting initial pH of medium to 4, 5, 6, 7, 8 and 9 by using 0.1 N HCl and 0.1 N NaOH.
2.8 Effect of Temperature
The optimization of temperature for melanin production was carried out by incubating flasks at 30, 35, 37, 40 and 45 °C in incubator shaker.
2.9 Effect of Different Growth Media
Four different growth media, Nutrient Broth (NB), Luria Bertini (LB) Broth, Bushnell-Haas Broth (BHB) and Trypticase Soy Broth (TSB) were studied for melanin production by culturing the microorganism in each of the medium at 37 °C and 150 rpm.
2.10 Effect of Carbon Sources
The effect of various carbon sources was studied by adding each carbon source in the medium at the concentration (2.5 g/L). The carbon sources evaluated were glucose, sucrose, lactose, fructose, starch, xylose, maltose, glycerol and dextrose.
2.11 Effect of Organic Nitrogen Sources
To evaluate the various nitrogen sources for maximum melanin, the production medium was supplemented with each organic nitrogen source at the concentration (1.5 g/L). The organic nitrogen sources tested were peptone, beef extract, yeast extract, and tryptone.
2.12 Effect of l-Tyrosine
To study the effect of l-tyrosine on melanin yield, 1.6 g/L of l-tyrosine was added to TSB and NB and were incubated in an orbital shaker at 150 rpm maintained at 37 °C.
2.13 Extraction and Purification of Melanin
The extraction of melanin was done in accordance with the procedure described for the purification of melanin from the culture of Aspergillus bridgeri (Kumar et al. 2011) with some minor modifications. In short, the medium was centrifuged at 5000 g for 10 min to remove the biomass. The supernatant collected was then treated with 1 M NaOH and then autoclaved at 120 °C for 15–20 min. After autoclaving, the solution was cooled and centrifuged at 5000 g for 10 min to collect the alkylated supernatant which was then acidified to pH 2 by using 1 N HCl, in order to precipitate the melanin. The precipitated melanin was collected by centrifuging at 12,000 g for 20 min and washed with distilled water and evaporated to dryness at room temperature and was stored for further use.
2.14 Characterization Studies
Purified melanin was dissolved in 0.1 N NaOH for UV-visible spectrophotometric analysis. The solution was scanned from 200 to 900 nm. The absorbance was measured by using a double beam UV-visible spectrophotometer (Hitachi, Labomed Inc). The absorption spectrum of the melanin pigment from the Pseudomonas stutzeri HMGM-7 strain was compared with that of standard melanin. For FT-IR analysis, the pigment and standard melanin were scanned between the wavenumber range of 4000–400 cm−1 by using KBr discs with an FT-IR spectrophotometer (IR Prestige, Shimadzu).
3 Results and Discussions
3.1 Effect of Inoculum Age
After evaluating different inoculum age (6, 12, 18, and 32 h) for their melanin production, the 12 h old inoculum gave the highest melanin concentration (197 mg/L) at the 48th h. When 6 h old culture was used, the highest melanin concentration (195.2 mg/L) was attained in the 40th h itself. The maximum biomass yield was obtained for the 32 h old culture (1.434 g/L 12th h) whereas the 6 h old culture managed to attain its maximum biomass in the 22nd h (1.69 g/L). 1.344 and 1.410 g/L were the highest biomass concentrations for cultures that were 12 and 18 h old respectively. The highest melanin obtained in the control medium was 177 mg/L and biomass attained was 1.107 g/L. Increase in inoculum age thus results in increase in biomass whereas reduction inoculum age resulted in increase in melanin production.
3.2 Effect of Inoculum Size
Different inoculum volumes (0.5, 5, 10, 15 and 20 %) were investigated to observe their effect on melanin production. The highest melanin production of 270.9 mg/L (32nd h) was achieved when 10 % inoculum was added to 50 ml of Nutrient Broth medium. Change in inoculum size did not alter the biomass yields, 1.425, 1.4, 1.46, 1.418 and 1.385 g/L being the maximum biomass concentrations obtained at 0.5, 5, 10, 15 and 20 % inoculum sizes respectively. As a result of which, 10 % inoculum volume was selected to be the optimum inoculum volume to be used for further investigations. The melanin production was observed after 10th h. The highest yield of melanin was achieved (270.9 mg/L) at the 32nd h. Thus there was a 53 % increase in melanin productivity.
3.3 Effect of Shaking Frequency on Biomass Production
Nutrient broth medium was used as the growth medium which was maintained at 37 °C and the shaking frequency was varied to study its effect on the melanin production. The maximum biomass production obtained for Pseudomonas stutzeri HMGM-7 was 1.107 g/L at the 8th and 12th h when the organism was allowed to grow for a period of 72 h at 150 rpm. In the medium prepared in sea water without adding l-tyrosine, Ganesh Kumar et al. (2013) obtained maximum biomass production of 2.5 g/L. There was a substantial increase in the biomass at the increased shaking frequency of 250 rpm as compared to 150 rpm.
3.4 Effect of Shaking Frequency on Melanin Production
There was a steady increase in the melanin production till the 48th h when the culture flasks were maintained at 37 °C and 150 rpm, where maximum production of 0.177 g/L was obtained followed by a decline in its productivity by the end of the incubation period. The onset of melanin production was significant only after the 8th h. Ganesh Kumar et al. (2013) obtained the maximum melanin production of 6.7 g/L at the 60th h in the sea-water medium without l-tyrosine supplementation. The melanin yield obtained in this study is comparatively lesser since Nutrient broth medium prepared in distilled water was used instead of sea-water medium which is known to be conducive for marine species like Pseudomonas. Melanin production at 37 °C and 250 rpm increased till the 32nd h, where maximum production of 0.164 g/L was obtained followed by a decline in its productivity by the end of the incubation period. When l-tyrosine was used as a sole carbon and nitrogen source into the melanin production media containing KH2PO4, NaCl and MgSO4·7H2O made in Distilled water by Kurian et al. (2014), Pseudomonas stutzeri Strain BTCZ10 produced 47.47 ± 0.2 μg/mL of melanin. Thus, in present study it was found that increase in shaking frequency from 150 to 250 rpm caused a decrease in melanin productivity.
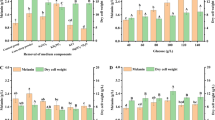
3.5 Effect of Growth Media
Nutrient Broth (NB), Luria Bertini (LB) broth, Bushnell-Haas broth (BHB) and Trypticase Soy broth (TSB) were the four different growth media that were utilized in this study to screen for the medium that produced more amount of melanin. The highest melanin yield, 167.38 mg/L was obtained at the 32nd h in NB, followed by TSB and LB, whereas BHB had very low melanin production (Fig. 1). None of the additional nutrients could affect a significant rise in melanin production when compared to NB alone.
3.6 Melanin Yield Before and After Optimization
The highest yield of melanin concentration, 0.27 g/L, was obtained in nutrient broth at 32 h. The yield was 1.53 times higher than the melanin obtained before optimization, 0.177 g/L at 48 h (Fig. 2). The increase in the productivity of melanin after selection of suitable medium and optimization of process parameters was 128.73 %. The melanin yield obtained can be further enhanced by statistical optimization and evaluating the effect of different combinations of nutrients like carbon and nitrogen sources and trace elements and further scale up of the process can be done.
3.7 UV-Visible Absorption Spectra and FTIR Analysis
The spectral property of the pigment was analyzed to confirm the nature of the pigment. Its UV spectrum was found to be similar to that of synthetic melanin which exhibited absorption peak of maxima between 200 and 300 nm (Fig. 3).
It showed a high degree of similarity, when the main absorption peaks in the FT-IR spectra of the synthetic melanin (Sigma Aldrich) and melanin obtained from Pseudomonas stutzeri HMGM-7 were compared (Fig. 4).
4 Conclusion
Many bacterial sources have been used widely as a major source of melanin in recent years and hence its optimization is important for large scale production. Pseudomonas stutzeri HMGM-7 used in this investigation has a competence to produce melanin under various process conditions and in different growth medium and can prove to be of commercial use for the large scale industrial production. Physical parameters and nutritional requirements often determine the melanin productivity that can be obtained from bacterial sources, and hence these parameters were evaluated in the current study. Pseudomonas stutzeri HMGM-7 was able to produce its highest melanin yield within shorter incubation period (32 h) for most of the studies that were conducted by varying different nutritional and process parameters. The optimum inoculum age and size that produced higher melanin yield was found to be 6 h and 10 % respectively. Nutrient broth along with three different media (TSB, BHB, LB) were evaluated for its melanin production, from which Nutrient broth proved to be the best, 0.27 g/L being the highest melanin yield produced across all the experiments conducted. There was no significant increase in the melanin production when the media was supplemented with additional nutrients.
References
Agodi, A., Stefani, S., Corsaro, C., Campanile, F., Gribaldo, S., Sichel, G.: Study references: of a melanic pigment of Proteus mirabilis. Res. Microbiol. 147(3), 167–174 (1996)
Ganesh Kumar, C., Mongolla, P., Pombala, S., Kamle, A., Joseph, J.: Physicochemical characterization and antioxidant activity of melanin from a novel strain of Aspergillus bridgeri ICTF-201. Lett. Appl. Microbiol. 53(3), 350–358 (2011)
Ganesh Kumar, C., Sahu, N., Narender Reddy, G., Prasad, R.B., Nagesh, N., Kamal, A.: Production of melanin pigment from Pseudomonas stutzeri isolated from red seaweed Hypnea musciformis. Lett. Appl. Microbiol. 57(4), 295–302 (2013)
Hari, R.K., Patel, T.R., Martin, A.M.: An overview of pigment production in biological systems: functions, biosynthesis, and applications in food industry. Food Rev. Int. 10(1), 49–70 (1994)
Kotob, S.I., Coon, S.L., Quintero, E.J., Weiner, R.M.: Homogentisic acid is the primary precursor of melanin synthesis in Vibrio cholerae, a Hyphomonas strain, and Shewanella colwelliana. Appl. Environ. Microbiol. 61(4), 1620–1622 (1995)
Kurian, N.K., Nair, H.P., Bhat, S.G.: Melanin producing Pseudomonas stutzeri BTCZ10 from marine sediment at 96 m depth (Sagar Sampada cruise #305). Int. J. Curr. Biotechnol. 2(5), 6–11 (2014)
Lucas-Elio, P., Goodwin, L., Woyke, T., Pitluck, S., Nolan, M., Kyrpides, N.C., Sanchez-Amat, A.: Complete genome sequence of the melanogenic marine bacterium Marinomonas mediterranea type strain (MMB-1(T)). Stand Genomic Sci. 6(1), 63–73 (2012)
Osawa, S., Yabuuchi, E., Narano, Y., Nakata, M., Kosono, Y., Takashina, K., Tanabe, T.: Pigment production by Pseudomonas aeruginosa on glutamic acid medium and gel filtration of the culture fluid filtrate. Jpn. J. Microbiol. 7, 87–95 (1963)
Riley, P.: Melanin. Int. J. Biochem. Cell Biol. 29 (1997)
Ruan, L., Yu, Z., Fang, B., He, W., Wang, Y., Shen, P.: Melanin pigment formation and increased UV resistance in Bacillus thuringiensis following high temperature induction. Syst. Appl. Microbiol. 27(3), 286–289 (2004)
Ruzafa, C., Sanchez-Amat, A., Solano, F.: Characterization of the melanogenic system in Vibrio cholerae, ATCC 14035. Pigment Cell Res. 8(3), 147–152 (1995)
Saxena, D., Ben-Dov, E., Manasherob, R., Barak, Z., Boussiba, S., Zaritsky, A.: A UV tolerant mutant of Bacillus thuringiensis subsp. kurstaki producing melanin. Curr. Microbiol. 44(1), 25–30 (2002)
Solano, F., Garcia, E., Perez, D., Sanchez-Amat, A.: Isolation and characterization of strain MMB-1 (CECT 4803), a novel melanogenic marine bacterium. Appl. Environ. Microbiol. 63(9), 3499–3506 (1997)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media Singapore
About this chapter
Cite this chapter
Thaira, H., Bhosle, S.S., Balakrishnan, R., Raval, K. (2016). Selection of Medium and Optimization of Process Parameters for Melanin Biosynthesis from Pseudomonas stutzeri HMGM-7. In: B. D., P., Gummadi, S., Vadlani, P. (eds) Biotechnology and Biochemical Engineering. Springer, Singapore. https://doi.org/10.1007/978-981-10-1920-3_1
Download citation
DOI: https://doi.org/10.1007/978-981-10-1920-3_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-1919-7
Online ISBN: 978-981-10-1920-3
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)