Abstract
Liquor from pretreatment of sugarcane bagasse is a potential substrate for multiple purposes due to the high concentration of residual sugars. Nevertheless, several potentially toxic byproducts are also present. However, a few microorganisms are able to overcome this toxicity by growing on these liquors. Twenty-five filamentous fungi were evaluated in submerged cultivation, but none was able to grow using liquor at a concentration of 100% as the liquid medium. However, six fungi were selected for enzyme induction after being grown in diluted liquor at 50% (v/v) using two feed pulses. Induction experiments were performed using 1% untreated and pretreated sugarcane bagasse. FPase and xylanase activities were detected for all six fungi in submerged cultivation, whereas β-glucosidase was observed in four fungi. The highest xylanase activity (28.8 IU mL−1) was at 72 h for T. harzianum P49P11 using pretreated-SCB as an inducer. This work showed a successful alternative for the final destination of liquor residue as substrate for fungi cultivation prior to enzyme production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
An important concept related to efficient processing of renewable feedstock into bio-based products is the “biomass biorefinery”, which aims to convert lignocellulosic biomass into intermediate outputs (cellulose, hemicellulose, lignin) to be processed into a spectrum of products and bioenergy (Cherubini et al. 2009). In this context, a biorefinery must maximize biomass use, generating energy and chemicals of interest with minimum gas emissions and waste (Jong and Jungmeier 2015). For cellulosic ethanol production (2G ethanol) from sugarcane bagasse, pretreatment is practically compulsory to reduce crystallinity of cellulose and open up the overall structure of biomass by depolymerization and solubilization of hemicelluloses (Alvira et al. 2010).
Pretreatment characteristics should include: low cost, possibility to use on an industrial scale, effectiveness for a wide range of lignocellulosic materials, minimum requirements of preparation and handling prior to the process itself, complete recovery of the lignocellulosic components in usable form, and providing a cellulose fraction that can be enzymatically converted to glucose at a high rate (Kumar and Sharma 2017).
Several pretreatment approaches have been studied along the last decades for biomass deconstruction. Among them, the hydrothermal pretreatment has been one of the main pretreatments of low-cost performed without chemicals, which greatly improves the cellulose digestibility through the use of water at high temperature (160–200 °C) for several minutes in order to solubilize hemicellulose and, perhaps, lignin (Kim et al. 2009, 2011, 2013). Other pretreatments have been developed recently as emerging approaches, such as ozonolysis. This chemical pretreatment uses ozone to promote a selective delignification due to its high specificity of reaction for lignin radicals. Some advantages of ozonolysis include the easy generation of ozone gas, a simplified processing operated at atmospheric pressure and room temperature, moderate cost of production and no generation of wastewater (Barros et al. 2013; Gitifar et al. 2013; Kumar and Sharma, 2017; Panneerselvam et al. 2013; Perrone et al. 2016; Travaini et al. 2013).
The generation of liquor after pretreatment of sugarcane bagasse is common in many processes. These liquors generally contain residual concentrations of sugars, mainly xylose and/or xylooligosaccharides from xylan depolymerization. These liquors can be used as a substrate for enzyme production. However, several byproducts with high toxicity for microorganism growth are potentially formed during the pretreatments using high temperatures, such as furan degradation products of carbohydrates. Other byproducts may be released from lignin. These include phenolic compounds, for example. Considering these components in liquor, its application becomes limited for biological conversion.
To overcome the effects of lignocellulose-derived inhibitors and lignin, different detoxification methods have been evaluated for transformation of inhibitors into inactivated compounds or reduce their concentration. These include physical, chemical and biological methods that differ significantly related to effects on hydrolysate chemistry and fermentability (Jönsson et al. 2013).
Biological detoxification involves the use of microorganisms and/or their enzymes to decrease the inhibitory effects of degradation compounds (Alvira et al. 2010). In comparison to physicochemical detoxification processes, biological detoxification methods are advantageous. For example lower energy requirements, because they take place at milder reaction conditions, no need of chemical additives and fewer side-reactions (Parawira and Tekere 2011). Among different microorganisms, Trichoderma and Aspergillus have the ability to remove different inhibitory compounds (Fillat et al. 2017). In the literature, Delabona et al. (2016) used liquor from hydrothermal pretreatment of sugarcane bagasse as a substrate for growth and induction of Trichoderma harzianum. Michelin and Polizeli (2012) used liquor from hydrothermal pretreatment of wheat straw to produce xylanase by Aspergillus ochraceus. Robl et al. (2015) used liquor from hydrothermal pretreatment of sugarcane bagasse to produce xylanase with Aspergillus niger.
Most studies found in the literature on liquor inhibition have had a focus on the alcoholic fermenting microorganisms while strategies using liquor for filamentous fungi growth and inducing “on-site” enzymes for degrading biomass so far have received relatively little attention. Therefore, the aim of this study was to evaluate filamentous fungi capable of metabolizing liquor as a substrate in pre-culture medium and sequentially produce cellulolytic enzymes using sugarcane bagasse as a low-cost inducer in submerged cultivation.
Materials and methods
Substrate and pretreatments
Liquor from ozonolysis and hydrothermal process pretreatments was collected as a liquid byproduct of sugarcane bagasse (SCB). Ozonolysis pretreatment was performed at room temperature and atmospheric pressure. The moisture level of 25 g of dry untreated-SCB was adjusted to 50% (w/v) with distilled water. Then, ozonolysis was conducted in a fixed bed glass column (2.7 × 50 cm) kept under saturated O3 gas (flux of 32 mg min−1) for 60 min (Travaini et al. 2013). Ozone was produced from atmospheric air by the Corona process (Radast 10C, Ozoxi-Ozonio). Hydrothermal pretreatment was carried out in batches with 3.5 g of dry ozonized-SCB mixed in distilled water at a concentration of 10% solids (w/v), placed in a metal column (2.2 × 13.5 cm). In a sand bath, the temperature was raised to 190 °C and kept for 15 min (Kim et al. 2009; Ko et al. 2015). After cooling, the pretreated material was vacuum filtered using Whatman No. 1 filter paper to separate the pretreated solids from liquor. The initial pH of liquor was quite acid (around 2.0) and required adjustment to pH 5.0, prior to fungal cultivations.
Liquor composition was determined by HPLC analysis (Dionex Ultimate 3000, equipped with an Aminex HPX-87 H 300 mm × 7.8 mm × 9 μm column, at 50 °C, 0.5 mL min−1 flow rate, mobile phase 0.005 M H2SO4, Shodex RI detector at 40 °C, 50 μL injection volume) as described (Robl et al. 2015).
Microorganisms
Twenty-five filamentous fungi from the CTBE (National Laboratory of Science and Technology of Bioethanol) culture collection were submitted to a preliminary screening in order to evaluate the ability to grow in the described liquor without performing procedures for detoxification of residue. Only six microorganism isolates were capable of developing mycelial growth using two pulses of liquor at 50% (v/v) in pre-culture medium. None was able to grow in 100% liquor, even when salts and peptone were added to the medium (data not shown). Amounts of acetic acid, carboxylic acids, furfural, hydroxylmethylfurfural and soluble lignin are found in higher concentration in liquor and are known to be inhibitors and to have negative effects on microorganism growth (Robl et al. 2015; Klinke et al. 2004).
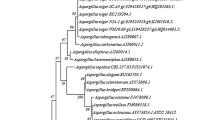
Among the fungi, one fungus (code P49P11) was identified as Trichoderma harzianum (Delabona et al. 2012), and the other five fungi were ascribed to wild-type cultures and encoded as MC08, 826, 839, 840, and 848. Stock cultures of each fungus were prepared by inoculating a piece of mycelial block (20 × 20 mm) on the surface of PDA (potato dextrose agar) plates and incubated for 5–7 days at 29 ± 1 °C and kept at 4 °C for 20 days (Fig. 1).
Pre-culture preparation
The mycelial block (20 × 20 mm) of each fungus was inoculated in 15 mL of pre-culture medium, composed of a low amount of glucose (4 g.L−1), peptone (1 g L−1), urea (0.3 g L−1), and salts (2.0 g L−1 KH2PO4, 1.4 g L−1 (NH4)2SO4, 0.4 g L−1 CaCl2·2H2O, 0.3 g L−1 MgSO4, 5 mg L−1 FeSO4, 1.6 mg L−1 MnSO4, 1.4 mg L−1 ZnSO4, 2.0 mg L−1 CoCl2), adapted from the Mandels and Weber formulation (Delabona et al. 2016). The broth was adjusted to initial pH 5.2 followed by sterilization through 0.22 µm membrane filters. After fungal inoculation, pre-culture media were incubated at 29 ± 1 °C in an orbital shaker (150 rpm) for pellet formation before starting the addition of liquor as substrate. Then, five milliliters of liquor at a concentration of 50% (v/v) was added at 96 h and at 120 h of cultivation as pulse feeding substrate. All the strains were kept under cultivation conditions to promote hyphae growth in the presence of liquor at 50% (v/v) until the carbon source was almost exhausted. Total reducing sugars were monitored along the cultivation by the DNS (3,5-dinitrosalicylic acid) method (Miller 1959). Thus, a final volume of 25 mL of each flask containing mycelia pellets was obtained as inoculum for the next step.
Induction procedures
The composition of the induction medium was the same as the pre-culture medium, except the carbon source. Two types of SCB were applied as substrates for enzyme induction: untreated and pretreated-SCB (ozonolysis and hydrothermal pretreatments), both added at a concentration of 10 g L−1, separately. The induction media were adjusted to pH 5.2 and autoclaved (15 min at 121 °C). The inoculation was performed using 25 mL of pre-cultured media in 225 mL of fresh induction medium (10% v/v inoculum ratio). Flasks were incubated in an orbital shaker at 200 rpm, 29 ± 1 °C for 144 h in duplicate for induction assays. Analyses of enzyme activities, including FPase (filter paper assay) for cellulase, xylanase (birchwood xylan 0.5%), and β-glucosidase (ρNPG 1 mM) were carried out using traditional methodologies adapted to a microplate scale (reduced scale by a factor of ten), as previously described (Delabona et al. 2016).
Results and discussion
Liquor composition characterization
The chemical composition of acid liquor was essentially a mixture of three main residual components from pretreated sugarcane bagasse: (1) sugars released from carbohydrate polymer breakdown, especially those from xylan, which were solubilized and hydrolyzed at the high temperatures of hydrothermal conditions; (2) phenolic compounds released due to delignification promoted by ozone gas and, later, solubilized to the liquid fraction in the hydrothermal step; and (3) the byproducts from carbohydrate degradation, such as organic and furanic acids, as side effects from the pretreatments. The composition of liquor is presented in Table 1. Total reducing sugars accounted for around 20 g L−1, and xylose was the most concentrated. Only monomeric carbohydrates were quantified instead of oligomers. Generally, liquors from hydrothermal pretreatments present more xylooligosaccharides than xylose, as observed by Robl et al. (2015). However, the absence of oligomers in this liquor is suggested to be associated with the acidic condition resulting from the combination of ozonolysis prior to the hydrothermal pretreatment step, which potentially promoted its hydrolysis to monomeric carbohydrates. Also, it was noted that the monomer concentration from this liquor corresponded to a complete conversion of hemicellulose compounds. Michelin et al. (2016) performed similar hydrothermal pretreatment conditions for sugarcane bagasse at 180 °C and 200 °C for 30 min. However, at 200 °C the amount of oligomers was decreased but the amount of monomers was increased. The severity of ozonolysis pretreatment applied in this work was similar to the 200 °C treatment of Michelin and Polizeli (2016), but instead of 30 min it was for 15 min. Michelin and Polizeli (2016) and this work resulted in a similar liquor composition, with totals of 21.5 g L−1 and 21.3 g L−1, respectively. But the difference was clearly observed between the amount of oligomers and monomers. Another difference was the ozonolysis step applied previously to the biomass. Also, it is known that oligomers are obtained in milder conditions of pretreatment. Thus, Michelin and Polizeli (2016) obtained a higher amount of XOS and low concentration of xylose compared to liquor from this work, which presented a very low concentration of oligomers because almost all the xylan available in the biomass was released as monomers (xylose, arabinose, acetic acid) or degradation product (furfural).
Use of acid liquor for xylose assimilation by filamentous fungi
The chemical characterization of liquor showed that the byproduct still contains a concentration of sugars capable of supporting microbial cultivation as substrate. Initially, the liquor was assessed at different concentrations in order to check the possibility of growing strains in 100% of crude liquor, regarding the presence of inhibitors and minimized by dilutions. However, none of the liquor dilutions evaluated allowed the fungal growth (data not shown), probably due to the high complexity of the components, including furfural, organic acids, and soluble phenols, besides the sugars. To mitigate this effect at the beginning of the cultivation, a two-step strategy was used starting without liquor (only glucose as carbon source), followed by sequential feeds of liquor at 50% (v/v) into the pre-culture medium. This strategy was successful for six fungi. The use of glucose as the first substrate promoted the pellet formation at 96 h (Fig. 2a), then the liquor was added and it became a homogeneous suspension of dispersed mycelia (Fig. 2b).
The carbon source is directly related to the morphology of filamentous fungi that influences the rheology of the culture medium. The pre-culture broth with dispersed mycelia increased the viscosity of the medium, and interfered in the production of biomass and metabolites such as proteins, antibiotics, organic acids, polysaccharides, pigments, alkaloids and mycotoxins (Callow and Ju 2012, 2007). Also, the morphological growth forms of filamentous fungi have a significant effect on the rheological properties in submerged cultivation, related to excretion of different metabolites. The growth of dispersed mycelia is effectively equivalent to unicellular cells with a homogeneous distribution of biomass, substrate, and products. On the other hand, pellet formation has major drawbacks because the oxygen concentration in the center of the pellet drops, restricting cell growth (El-Enshasy 2007).
Figure 3 shows the total reducing sugars consumption of fungi using two liquor feeding strategies in pre-culture medium. For the six fungi isolates, glucose was totally consumed by 96 h of cultivation, as expected. For the first pulse containing liquor at 50% (v/v), the total reducing sugars were quickly consumed in 24 h. However, when the second pulse of liquor at 50% (v/v) was added, the time demanded for metabolization ranged between 48 and 72 h among the different strains. It suggested distinct physiological responses to potential accumulation of toxic compounds on the biomass production.
The strategy of two liquor pulses has been used for many years to produce cellulolytic fungal enzymes and this operational mode is known to minimize catabolite repression. In principle, the use of this cultivation strategy should also help to mitigate the effects of inhibitors in the liquor. Responses observed in this study agreed with this effect, because other efforts of continuous cultivation (without liquor pulses) were unsuccessful for the same fungi growth.
Enzymes induction by sugarcane bagasse
The possibility of inducing the synthesis of cellulolytic enzymes under submerged fermentation using SCB as a sole substrate has been proven by other authors in the literature (Cunha et al. 2012; Delabona et al. 2012). Based on this, the six selected strains were cultivated in 1% (w/v) of both untreated-SCB and pretreated-SCB in order to assess FPase, β-glucosidase, and xylanase activities. In general, pretreated-SCB demonstrated a higher capability of improving enzyme synthesis when compared to titers from induction using untreated-SCB. The natural recalcitrance of the cellular wall of SCB represents the main challenge for enzymes to access their substrates, resulting in lower activities due to a restricted surface area of catalysis (Payne et al. 2015). Aguiar et al. (2018) sucessufully demonstrated that SCB pretreatment is an essential step for the complete SCB hydrolysis and facilitates the access to cellulolytic enzymes.
The results of FPase activity obtained for the strains under induction with pretreated and untreated-SCB are presented in Fig. 4.
The strain code 840 presented the highest cellulolytic activity (0.45 ± 0.02 FPU mL−1) using filter paper as substrate at 144 h of fermentation. The FPase activity increment during the fermentation showed the enhanced activity of this fungus to metabolize and degrade compounds in biomass. Another three isolates (T. harzianum P49P11; MC08 and 826) produced around 0.30 ± 0.01 FPU mL−1 at 144 h. Delabona et al. (2013) found that FPase activity could be improved up to three-fold by increasing the carbon source concentration from 2 to 3% during submerged cultivation of T. harzianum P49P11.
The strains 839 and 848 presented the lowest FPase activities during the fermentation. None of the strains was able to use substrate without pretreatment for FPase determination. However, it is important to note that FPase activity may provide an incomplete evaluation of the hydrolysis potential of enzyme extract because filter paper differs substantially from biomass waste in terms of its structure and chemical composition. On the other hand, this traditional method proposed by Ghose in 1987 is widely adopted as an estimate of total cellulase activity because the degradation of paper requires the action of endo- or exoglucanase, and β-glucosidase. Thus, FPase is a well-accepted evaluation, but it may lead to unrepresentative data and, perhaps, artificially low hydrolysis results. Complementary analysis of Avicelase and CMCase can be performed for a complete evaluation of hydrolysis potential for a crude enzyme extract.
Figure 5 shows the β-glucosidase activity from six fungal strains evaluated in submerged fermentation. Only three strains (848, P49P11, 840) presented relevant β-glucosidase production using pretreated-SCB as an inducer. The 840 strain showed the highest β-glucosidase activity (3.46 ± 0.38 IU mL−1) at 144 h. Also, T. harzianum strain P49P11 achieved a higher β-glucosidase production of 3.12 ± 0.09 IU mL−1 at 96 h.
An enzyme production that provides greater productivity offers an alternative option for the on-site production. Therefore, the production of β-glucosidase by P49P11 and the 840 strain showed consistent induction for the carbon source and strategy tested in comparison to Robl et al. (2015). Maximal β-glucosidase production using a strain of A. niger in optimized media composed of pretreated-SCB and soybean bran was 1.16 IU mL−1 (Robl et al. 2015). Aspergillus sp. is known worldwide for its good ability to produce β-glucosidase.
Figure 6 displays xylanase activity over time in submerged fermentation. All strains were able to produce xylanases, but the xylanase activity peak was observed at 72 h (28.80 ± 1.77 IU mL−1) for the P49P11 strain using pretreated-SCB as an inducer. Several studies have suggested that T. harzianum is a potential producer for hydrolytic enzymes (Delabona et al. 2012, 2016). Lee et al. (2018) studied the profile of fungal growth and production of xylanase by a fungus isolated from decayed wood (DWA1) in submerged cultivation using sugarcane bagasse and palm kernel cake as substrate/support and obtained a maximum of 452.3 IU mL−1 of xylanase in 72 h of fermentation. Also, Delabona et al. (2016) obtained 67.00 ± 0.6 IU mL−1 of xylanase after 120 h of cultivation with T. harzianum P49P11 using 10 g L−1 of glucose as an initial carbon source, followed by 10 g L−1 of delignified steam-exploded bagasse addition. Robl et al. (2015) studied endophytic fungi using liquor from hydrothermal pretreatment of sugarcane bagasse. The best strain (Aspergillus niger DR02) produced 458 U mL−1 of xylanase for constant fed-batch mode cultivation.
The MC08 strain can also produce high xylanase activity levels at 144 h of fermentation (24.40 ± 1.83 IU mL−1). For other four strains (826, 839, 840 and 848) xylanase activity was lower than 15 IU mL−1. Differently from other activities, the production of xylanases was also inducted by untreated-SCB, reaching around 25 IU mL−1 with T. harzianum at 120 h of cultivation. This result was close to the peak of production observed using pretreated-SCB as an inducer and it may be explained by the composition of each bagasse. The availability of substrate for xylanases is quite different between them. For pretreated-SCB, there was a low amount of residual xylan (less than 4%) in the solid substrate after the pretreatments. On the other hand, the untreated-SCB had a much greater amount of xylan, but the substrate was inaccessible due to tge natural recalcitrance of biomass without pretreatment, as explained previously. A similar result has been also observed when evaluating commercial cellulolytic enzymatic hydrolysis of untreated and chemical pretreated SCB (Aguiar et al. 2018). From this point of view, both samples of bagasse were capable of inducing a moderate production of xylanase in submerged cultivation.
Conclusions
The liquor resulted from ozonolysis and hydrothermal pretreatments of sugarcane bagasse present a high amount of xylose free of xylo-oligosaccharides, which allows assimilation as a primary substrate for fungi growth. However, the presence of byproducts, including furfural, HMF, and low molecular weight phenolics arising from lignin fragmentation required a fed-batch strategy to reduce their toxicity. A successful pre-cultivation starting with low concentration of glucose, followed by two pulses of 50% diluted liquor allowed the growth of the six filamentous fungi, which demonstrated tolerance to inhibitory compounds and were also able to produce cellulolytic enzymes under induction. Therefore, the use of xylose-rich liquor is suggested as a low-cost substrate for hyphae growth prior to enzyme production, and pretreated-SCB is suggested to be an inducer for the production of enzymes, including cellulases and xylanase.
References
Aguiar MM, Pietrobon VC, Pupo MMS, Torres NH, Américo JHP, Salazar-Banda GR, Silva DP, Monteiro RTR, Ferreira LFR (2018) Evaluation of commercial cellulolytic enzymes for sugarcane bagasse hydrolysis. Cell Chem Technol 52:695–699
Alvira P, Tomás-Pejó E, Ballesteros M, Negro MJ (2010) Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour Technol 101:4851–4861
Barros RDROD, Paredes RDS, Endo T, Bon EPDS, Lee S-H (2013) Association of wet disk milling and ozonolysis as pretreatment for enzymatic saccharification of sugarcane bagasse and straw. Bioresour Technol 136:288–294
Callow NV, Ju L (2012) Promoting pellet growth of Trichoderma reesei Rut C30 by surfactants for easy separation and enhanced cellulase production. Enzyme Microb Technol 50:311–317
Cherubini F, Jungmeier G, Wellisch M, Willke T, Skiadas I, Van Ree R, Jong E (2009) Toward a common classification approach for biorefinery systems. Model Anal Biorefinery Classif 3:534–546
Cunha FM, Esperança MN, Zangirolami TC, Badino AC, Farinas CS (2012) Sequential solid-state and submerged cultivation of Aspergillus niger on sugarcane bagasse for the production of cellulase. Bioresour Technol 112:270–274
da Delabona SP, Farinas CS, da Silva MR, Azzoni SF, da Pradella JGC (2012) Use of a new Trichoderma harzianum strain isolated from the Amazon rainforest with pretreated sugar cane bagasse for on-site cellulase production. Bioresour Technol 107:517–521
da Delabona SP, Sanchez C, Juliana D, Geraldo J (2013) Experimental mixture design as a tool to enhance glycosyl hydrolases production by a new Trichoderma harzianum P49P11 strain cultivated under controlled bioreactor submerged fermentation. Bioresour Technol 132:401–405
da Delabona SP, Lima DJ, Robl D, Rabelo SC, Farinas CS, da Pradella JGC (2016) Enhanced cellulase production by Trichoderma harzianum by cultivation on glycerol followed by induction on cellulosic substrates. J Ind Microbiol Biotechnol 43:617–626
De Jong E, Jungmeier G (2015) Biorefinery concepts in comparison to petrochemical refineries. Elsevier, Amsterdam, pp 3–33
El-Enshasy HA (2007) Filamentous fungal cultures—process characteristics, products, and applications. In: Yang S-T (ed) Bioprocessing for value-added products from renewable resources. Elsevier Press, Amsterdam, pp 225–261
Fillat U, Ibarra D, Moreno AD, Tomas-pejo E (2017) Laccases as a potential tool for the efficient conversion of Lignocellulosic biomass: a review. Fermentation 3:1–30
Gitifar V, EslamLoueyan R, Sarshar M (2013) Bioresource technology experimental study and neural network modeling of sugarcane bagasse pretreatment with H2SO4 and O3 for cellulosic material conversion to sugar. Bioresour Technol 148:47–52
Jönsson LJ, Alriksson B, Nilvebrant N-O (2013) Bioconversion of lignocellulose: inhibitors and detoxification. Biotechnol Biofuels 6:16
Kim Y, Mosier NS, Ladisch MR (2009) Enzymatic digestion of liquid hot water pretreated hybrid poplar. Biotechnol Prog 25:340–348
Kim Y, Ximenes E, Mosier NS, Ladisch MR (2011) Soluble inhibitors/deactivators of cellulase enzymes from lignocellulosic biomass. Enzyme Microb Technol 48:408–415
Kim Y, Kreke T, Hendrickson R, Parenti J, Ladisch MR (2013) Fractionation of cellulase and fermentation inhibitors from steam pretreated mixed hardwood. Bioresour Technol 135:30–38
Klinke HB, Thomsen AB, Ahring BK (2004) Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Appl Microbiol Biotechnol 66:10–26
Ko JK, Ximenes E, Kim Y, Ladisch MR (2015) Adsorption of enzyme onto lignins of liquid hot water pretreated hardwoods. Biotechnol Bioeng 112:447–456
Kumar AK, Sharma S (2017) Recent updates on different methods of pretreatment of lignocellulosic feedstocks : a review. Bioresour Bioprocess 4(7):1–19
Lee SH, Lim V, Lee CK (2018) Newly isolate highly potential xylanase producer strain from various environmental sources. Biocatal Agric Biotechnol 16:669–676
Michelin M, Polizeli MDLTM (2012) Xylanase and β-xylosidase production by Aspergillus ochraceus: new perspectives for the application of wheat straw autohydrolysis liquor. Appl Biochem Biotechnol 166:336–347
Michelin M, Ximenes E, Polizeli MLTM, Ladisch MR (2016) Effect of phenolic compounds from pretreated sugarcane bagasse on cellulolytic and hemicellulolytic activities. Bioresour Technol 199:275–278
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Panneerselvam A, Sharma-Shivappa RR, Kolar P, Ranney T, Peretti S (2013) Potential of ozonolysis as a pretreatment for energy grasses. Bioresour Technol 148:242–248
Parawira W, Tekere M (2011) Biotechnological strategies to overcome inhibitors in lignocellulose hydrolysates for ethanol production: review. Crit Rev Biotechnol 31(1):20–31
Payne CM, Knott BC, Mayes HB, Hansson H, Himmel ME, Sandgren M, Ståhlberg J, Beckham GT (2015) Fungal cellulases. Chem Rev 115:1308–1448
Perrone OM, Colombari FM, Rossi JS, Moretti MMS, Bordignon SE, da Nunes CC, Gomes E, Boscolo M, Da Silva R (2016) Ozonolysis combined with ultrasound as a pretreatment of sugarcane bagasse: effect on the enzymatic saccharification and the physical and chemical characteristics of the substrate. Bioresour Technol 218:69–76
Robl D, da Silva PD, dos Costa PS, da Lima DJS, Rabelo SC, Pimentel IC, Büchli F, Squina FM, Padilla G, da Pradella JGC (2015) Xylanase production by endophytic Aspergillus niger using pentose-rich hydrothermal liquor from sugarcane bagasse. Biocatal Biotransform 33:175–187
Travaini R, Otero MDM, Coca M, Da-Silva R, Bolado S (2013) Sugarcane bagasse ozonolysis pretreatment: effect on enzymatic digestibility and inhibitory compound formation. Bioresour Technol 133C:332–339
Acknowledgements
The authors would like to thank the technical staff of the National Laboratory of Science and Technology of Bioethanol (CTBE) and acknowledge the financial support provided by the Brazilian agency CAPES (finance code 001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bordignon, S.E., da Silva Delabona, P., Lima, D. et al. Induction of fungal cellulolytic enzymes using sugarcane bagasse and xylose-rich liquor as substrates. Braz. J. Chem. Eng. 37, 443–450 (2020). https://doi.org/10.1007/s43153-020-00055-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43153-020-00055-5