Abstract
The use of glycerol obtained as an intermediate of the biodiesel manufacturing process as carbon source for microbial growth is a potential alternative strategy for the production of enzymes and other high-value bioproducts. This work evaluates the production of cellulase enzymes using glycerol for high cell density growth of Trichoderma harzianum followed by induction with a cellulosic material. Firstly, the influence of the carbon source used in the pre-culture step was investigated in terms of total protein secretion and fungal morphology. Enzymatic productivity was then determined for cultivation strategies using different types and concentrations of carbon source, as well as different feeding procedures (batch and fed-batch). The best strategy for cellulase production was then further studied on a larger scale using a stirred tank bioreactor. The proposed strategy for cellulase production, using glycerol to achieve high cell density growth followed by induction with pretreated sugarcane bagasse, achieved enzymatic activities up to 2.27 ± 0.37 FPU/mL, 106.40 ± 8.87 IU/mL, and 9.04 ± 0.39 IU/mL of cellulase, xylanase, and β-glucosidase, respectively. These values were 2 times higher when compared to the control experiments using glucose instead of glycerol. This novel strategy proved to be a promising approach for improving cellulolytic enzymes production, and could potentially contribute to adding value to biomass within the biofuels sector.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Considerable research effort has been focused on developing efficient processes for producing the enzymes needed to produce second generation ethanol. Among the potential alternatives, the use of glycerol as a carbon source for microbial growth is being evaluated as a strategy for the production of enzymes [33] and other high value bioproducts [8, 13, 19, 31, 37]. This offers an economic advantage, because large volumes of glycerol are generated as a co-product in the production of biodiesel, with 10 lbs of crude glycerol produced for every 100 lbs of biodiesel [7]. According to Yang et al. [38], the global biodiesel market is estimated to reach 37 billion gallons by 2016, with average annual growth of 42 %, which means that about 4 billion gallons of raw glycerol will be produced. Flooding of the market with glycerol originating from biodiesel production has led to a dramatic decrease in the price of glycerol [23]. Hence, there is great interest in the development of processes to convert this low-value glycerol into high-value products by means of biological or chemical routes. Biological conversion can offer the opportunity to synthesize a wide range of products with diverse uses, and an especially interesting application of glycerol involves the production of cellulases.
Cellulases are a group of enzymes mainly consisting of endoglucanases, cellobiohydrolases (exoglucanase), and β-glucosidase, which act synergistically in the conversion of cellulose to glucose [39]. The production efficiency of cellulases depends on the choice of microorganism (considering yield and productivity), the carbon and nitrogen sources (substrates), and the cultivation conditions (temperature, aeration, and pH, amongst others). It is generally conceived that glucose is a primary carbon source for the growth of filamentous fungi, and that protein secretion and biomass production depend on the choice of carbon source. However, in the case of cellulolytic fungi such as Trichoderma reesei and Aspergillus niger, the use of glucose as carbon source leads to a strong repression of cellulase gene transcription, by means of a mechanism known as catabolite repression [26]. On the other hand, glycerol has been found to be a highly assimilable carbon source for Trichoderma growth, without promoting cellulase repression [17].
In previous work [9] we described the cultivation of Trichoderma harzianum P49P11 for cellulase production using different insoluble (sugarcane bagasse) and soluble (fructooligosaccharide, sucrose, lactose, and glycerol) carbon sources, and their binary combinations. Good cellulase induction was achieved with all the insoluble cellulosic carbon sources tested, while the use of glycerol and lactose as carbon sources resulted in very low cellulase induction, although total protein secretion was similar for all the carbon sources tested. A potential strategy therefore seemed to be the use of glycerol alone for the initial microbial biomass growth, which could then be followed by the addition of a more suitable cellulase inducer. The selection of a carbon source able to enhance fungal growth without leading to catabolite formation is highly desirable, because a high concentration of microbial biomass is required in cultivations in order to maximize volumetric enzyme productivity and attain high titers. Therefore, the use of high cell density cultivation is required in order to improve cellulases production. To the best of our knowledge, there has been no work concerning the evaluation of such a strategy for improving cellulase production by T. harzianum. Florencio, Cunha, Badino and Farinas [14] compared the production of cellulolytic enzymes of T. harzianum P49P11 and T. reesei Rut-C30 using steam explosion-pretreated sugarcane bagasse by submerged fermentation. In this study the T. harzianum P49P11 showed superior capacities for β-glucosidase (26.1 ± 5 IU/L), Avicelase (53.0 ± 16 IU/L) and FPase (21.2 ± 4 FPU/L) than T. reesei Rut C30 (6.3 ± 0 IU/L of β-glucosidase; 9.1 ± 1 IU/L of Avicelase and 5.2 ± 0 FPU/L of FPase). The production of cellulolytic enzymes was also maximum for a T. harzianum strain isolated from the Brazilian Cerrado when compared with T. reesei Rut-C30 (ATCC 56765) grown on in natura sugarcane bagasse [4].
Aspergillus niger is known worldwide for its ability to produce an extensive range of xylanases and β-glucosidase [36]. Robl et al. [28] screened an endophytic A. niger DR02 and obtained maximum xylanase activity (4.5 IU/mL) and β-glucosidase (1.16 IU/mL) in 120 h of incubation by submerged fermentation using pretreated delignified sugar cane bagasse plus soybean bran as carbon source. The production of xylanase and β-glucosidase for T. harzianum P49P11 using the same substrate was 84.40 and 8.33 IU/mL, respectively [10].
This work investigates the influence of different substrates selected as carbon sources for either growth or induction during the production of biomass and cellulase by T. harzianum. Larger-scale cultivations in a stirred tank bioreactor were carried out under the selected conditions. The combination of the use of glycerol for high cell density growth followed by cellulase induction using pretreated sugarcane bagasse is proposed as a novel strategy for improving cellulase production.
Methods
Microorganism
The T. harzianum P49P11 strain used in this work was a wild-type strain isolated from the Amazon rainforest [11], kept at the Embrapa Food Technology microorganism collection (Rio de Janeiro, Brazil) under strain number BRMCTAA 115. The fungus was stored at 4 °C on slants of potato dextrose agar (PDA) (Difco, Detroit, USA). The PDA plates were subsequently incubated at 29 °C for 7 days.
Pretreated sugarcane bagasse
Three different pretreated sugarcane bagasses were used: steam explosion pretreated (SB), delignified steam-exploded (DSB), and hydrothermally pretreated (HB). SB was pretreated by steam explosion at 200 °C for 15 min, and for DSB the same procedure was used, followed by delignification (100 g/L pulp concentration, 10 g/L NaOH, 30 °C, 2 h), as described by Rocha et al. [29]. HB was prepared according to Santucci et al. [30] using a solid/liquid ratio of 1:10 (w/w), in a 2 L high-pressure reactor (Model 4848, Parr) kept at 190 °C for 12 min.
The chemical compositions of the pretreated bagasses were determined according to the National Renewable Energy Laboratory (NREL) method [32]. The samples were hydrolyzed with H2SO4 (72 %, w/w) for 1 h at 30 °C in a thermostatic bath, followed by dilution to 4 % (w/w) acid concentration and autoclaving at 121 °C for 1 h in pressure-resistant glass tubes. After filtration, the insoluble lignin content was determined gravimetrically (with correction for the ash content), while soluble lignin in the filtrate was determined by ultraviolet absorbance at 280 nm. Carbohydrates, organic acids, and furanic aldehydes were determined by high performance liquid chromatography (HPLC).
In addition, the dried and milled pretreated bagasses were combusted in a furnace at 575 °C and the remaining ash was measured. Table 1 summarizes the contents of cellulose, hemicelluloses, lignin, and ash in the SB, DSB, and HB materials.
Enzyme production
In the present work, enzyme production was divided into two steps: (a) a pre-culture in a carbon source, followed by (b) induction with the DSB pretreated sugarcane bagasse.
Selection of pre-culture conditions
A conidia suspension, prepared by adding 20 mL of sterilized distilled water and Tween 80 to the grown PDA plates, was transferred to Erlenmeyer flasks containing 180 mL of pre-culture medium (adapted from Mandels and Weber [21]). The composition of the medium was as follows: 1 mL Tween 80; 0.3 g/L urea; 2.0 g/L KH2PO4; 1.4 g/L (NH4)2SO4; 0.4 g/L CaCl2·2H2O; 0.3 g/L·MgSO4·7H2O; 1.0 g/L proteose peptone; 5.0 mg/L FeSO4·7H2O; 1.6 mg/L MnSO4·4H2O; 1.4 mg/L ZnSO4·7H2O; 2.0 mg/L CoCl2·6H2O; and the carbon source (as described below). Unless stated, 50 mM potassium phthalate buffer was employed in order to mitigate the effect of pH fluctuations on enzyme production. The initial pH was adjusted to 5.0 and the pre-culture medium was sterilized at 121 °C for 30 min, followed by incubation for 96 h at 29 °C in a rotary shaker operated at 200 rpm.
The following carbon sources were used to identify the best pre-culture, in terms of the maximum amount of total protein excreted [6]: lactose (10 g/L) and glycerol (10 g/L) as carbon sources for growth; sucrose (10 g/L), 10, 40, and 80 % (v/v) of the filtrate fractions obtained after hydrothermal pretreatment of sugarcane bagasse, and fructooligosaccharide (FOS, 10 g/L) (Nutraflora®, Corn Products, Brazil) as carbon sources for both growth and induction; Celufloc™ 200 (10 g/L) and the three types of pretreated sugarcane bagasse (SB, DSB, and HB, at 10 g/L each) as carbon sources for induction.
Selection of the cellulase induction strategy
After the 48 h pre-culture period, a 10 % (v/v) volume (prepared as described above) was inoculated into 200 mL of culture medium in 500 mL Erlenmeyer flasks. The composition of the induction medium was the same as that of the pre-culture medium, except for the type of carbon source (specified below). The flasks were incubated at 29 °C in a rotary shaker at 200 rpm for a total period of 120 h. Two control conditions and five different cultivation strategies were tested, as follows the Table 2.
Microscopy observations
Microscopy observations of the fermentation broth were carried out using an Eclipse E100 microscope (Nikon, Tokyo, Japan) with a DS-U3 digital camera controller and NIS-Elements software. Sterile 20 μL aliquots were collected after 72 h from pre-cultures cultivated using glucose and glycerol. The images were acquired after focusing with a 40×/0.65 NA objective lens.
High cell density bioreactor cultivations
A 110 mL volume of the pre-culture obtained using 20 g/L glycerol was transferred to a BioFlo 115 fermenter (New Brunswick Scientific Co., USA) equipped with automatic control of temperature, pH, agitation rate (Rushton impeller), and aeration rate. The working volume of the fermenter was 1.1 L, and the temperature was maintained at 29 °C. The medium used was the same as that employed for the pre-culture, except that the carbon source was delignified steam-exploded sugarcane bagasse (DSB) (30 g/L, batch culture). The aeration rate was adjusted so that the dissolved O2 level in the culture medium did not drop below 30 % of air saturation. The pH was controlled at pre-set values using either 0.4 M H2SO4 or aqueous NH4OH solution (1:3). Foaming was controlled by the manual addition of previously sterilized polypropylene glycol antifoaming agent (P2000, Dow Chemical, Brazil). The antifoaming agent was added in a proportion of 1 mL per liter, at the beginning of each cultivation. Samples were periodically removed, centrifuged at 10,000g for 20 min (at 4 °C), and measurements made of the enzyme activities (as described in “High cell density bioreactor cultivations”).
Analytical methods
Filter paper activity (FPase) was determined as described by Ghose [15], with modifications to reduce the scale of the procedure by a factor of ten. Reducing sugars were measured by the DNS method, using glucose as standard. The activity of β-glucosidase was measured at pH 4.8 using p-nitrophenol-β-d-glucopyranoside (pNPG) (Sigma-Aldrich, USA). The assay was carried out using 20 µL of diluted centrifugation supernatant and 80 µL of 1 mM pNPG, diluted in 50 mM citrate buffer (pH 4.8), and the mixture was incubated for 10 min at 50 °C. The reaction was stopped by adding 100 µL of 1 M Na2CO3, and the absorbance was measured at 400 nm. The xylanase activity was determined employing 50 μL of 0.5 % birchwood xylan, 40 μL of 50 mM sodium citrate buffer (pH 4.8), and 10 μL of enzyme extract (supernatant from the centrifuged culture). After 10 min of incubation at 50 °C, the reaction was stopped by adding 100 μL of DNS reagent, the measurement was made at 540 nm, and the standard curve was constructed with xylose.
Total protein in the centrifuged supernatant was measured using micro plates with Bio-Rad protein assay reagent (Bio-Rad Laboratories, USA), employing a procedure based on the Bradford method [6]. Bovine serum albumin was used as standard.
The biomass concentrations in the pre-cultures used for the bioreactor fermentations were determined with 50 mL of culture medium, which was centrifuged in a 50 mL Falcon tube (10,000 rpm, 20 min, 10 °C), followed by removal of the supernatant using a pipette. The biomass was weighed after drying overnight at 80 °C.
Results and discussion
Effect of the carbon source used in the pre-culture step
The effect of the carbon source used in the pre-culture step on the time course of the total amount of protein secreted by T. harzianum is shown in Fig. 1. In this set of cultivations, 11 different carbon sources were investigated in order to select the best carbon source to achieve high microbial growth.
Effect of carbon source on T. harzianum pre-culture regarding total protein secretion using a steam-exploded (SB), delignified steam-exploded (DSB) hydrothermally pretreated bagasse (HB) and Celufloc™ 200 for induction, b lactose and glycerol for growth, and c sucrose, 10, 40 and 80 % (v/v) hydrothermal pretreatment of sugarcane bagasse liquor and fructooligosaccharide (FOS) for growth and induction. (Concentration of tested carbon source was 10 g/L excepted for the liquor)
Figure 1a shows the effect on total protein secretion by T. harzianum using carbon sources considered to be cellulase inducers (SB, HB, DSB, and Celufloc™ 200) [9]. In this group, the best carbon source for total protein production was DSB (7.15 mg/L h of total protein), followed by Celufloc™ 200 (6.01 mg/L h). The use of pretreated bagasses SB and HB resulted in similar values, of 4.75 and 5.66 mg/L h, respectively.
The effects on total protein secretion of the use of glycerol and lactose as carbon sources in the pre-culture step are shown in Fig. 1b. Glycerol was the best carbon source for protein secretion, providing a faster production rate (7.5 mg/L h at 72 h) and greater secretion (676.70 mg/L of total protein after 96 h of cultivation). Similar behavior has been reported previously for the use of glycerol as carbon source for the production of phytase, an important industrial enzyme used as an additive in feeds for swine, poultry, and fish [33]. It was found that glycerol outperformed glucose and methanol as carbon source, in terms of both protein expression and cell growth, corroborating the results obtained in the present study.
Lactose is known to be a carbon and energy source for cellulases production, and is a good inducer of protein synthesis by T. reesei, but its use did not improve total protein secretion by T. harzianum P49P11 (3.64 mg/L h). This finding was in agreement with the value reported by Delabona et al. [10] (3.02 mg/L h) for a submerged fermentation pre-culture employing lactose and the same fungal strain.
Figure 1c presents the protein secretion results for different carbon sources used for both growth and induction: sucrose, the liquor from the hydrothermal pretreatment of sugarcane bagasse, at concentrations of 10, 40, and 80 % (v/v) (liquor), and fructooligosaccharide (FOS). For all of these soluble carbohydrate sources, protein secretion showed little variation during the first 60 h (sucrose: 5.58 g/L h; liquor 10 %: 6.03 g/L h; liquor 40 %: 5.96 g/L h; liquor 80 %: 5.0 g/L h; FOS: 5.24 g/L h). When liquor 80 % was used as the carbon source, the total protein decreased after 72 h, until the end of the cultivation and low micelial growth was observated on the microscopy (data no shown). This decrease in total protein was probably due to the high contents of inhibitors such as acetic acid (1.47 ± 0.18 g/L), formic acid (0.23 ± 0.10 g/L), furfural (1.05 ± 0.06 g/L), and soluble lignin (3.15 ± 0.49 g/L). The same liquor was used for xylanase production by A. niger DR02 fed-batch cultivation using different concentration of the liquor [22]. The authors obtained decreased cell growth when high liquor concentrations were used, suggesting possible negative effects of toxic compounds present in the liquor. However, lower concentrations of liquor (10 and 40 % v/v) appeared to induce protein synthesis. The hemicelluloses are decomposed into soluble products, including xylooligomers and xylose, which can be used as substrates for the production of proteins such as xylanases. The liquor that was used in this study contained very high levels of xylooligosaccharides and free xylose (10 and 5 g/L, respectively). The pre-culture using sucrose showed the same protein secretion rate obtained with liquor 40 % (5.96 g/L h).
Microbial growth is normally associated with exponential increases in biomass when conditions are favorable for growth and when nutrients are not limiting. In recent work, protein secretion was shown to be correlated with FPase activity, and maximum values of xylanase and β-glucosidase were obtained for cultivation of Penicillium echinulatum using Celufloc or DSB as carbon source [25]. The relation between protein secretion and the growth profile of the microorganism is therefore a key consideration for increased enzyme production. Hence, the carbon source used in the growth medium could be one of the main factors affecting enzyme/protein production during a fermentation process. The results showed that when glycerol was used in the pre-culture for T. harzianum growth, protein secretion was favored, indicating that this could be a potential way of increasing fungal biomass and consequently enzyme production during fermentation.
Effect of carbon source on the morphology of T. harzianum
The morphology of filamentous fungi plays an important role in the secretion of enzymes and structural proteins. It has been reported that protein secretion in filamentous fungi mainly occurs at the tips of growing hyphae, and that conditions favoring greater numbers of active tips should improve protein yield [24].
Figure 2 shows the morphology of T. harzianum grown using 10 g/L glycerol (a) and 10 g/L glucose + 10 g/L Celufloc™ (1:1) (b) as carbon sources in the pre-culture step on minimal medium. The use of glycerol resulted in long hyphae and a greater number of mycelia tips, so higher protein secretion was expected. In contrast, use of the glucose and Celufloc™ medium resulted in a greater number of spores and fewer hyphae tips, indicative of lower protein secretion. The findings of the macroscopic morphological analysis were in good agreement with the results obtained for the pre-culture step (Fig. 1), where glycerol was shown to be the best carbon source for protein secretion (676.70 mg/L at 96 h). The use of Celufloc™ for fungal growth also resulted in good protein secretion (577.39 mg/L at 96 h). However, the addition of glucose to the pre-culture medium stimulated sporulation and resulted in the production of only a small amount of mycelium, which restricted protein secretion.
Effect of different carbon source on mycelial morphology. Mycelial morphology generated by growing T. harzianum in glycerol 10 g/L (a), and 10 g/L glucose + 10 g/L Celufloc™ (1:1) b at peak enzyme activity (72 h) on a rotary shaker (temperature of 29 °C, an agitation of 200 rpm and a culture pH of 5.0)
Ahamed et al. [1] described the way in which fungal morphology influenced the volumetric cellulase productivity of T. reesei cultivated in media containing lactose and lactobionic acid, and reported that enzyme production depended on the number of tips per hypha. Similar results were obtained by Amanullah et al. [2], who found a correlation between hyphae tip activity and the specific amyloglucosidase productivity of Aspergillus oryzae.
Proteins are packaged within vesicles that are transported to the cell surface, where they fuse with the plasma membrane, releasing their contents to the periplasmic space; the proteins may then remain within the periplasmic space, be retained at the mature cell wall, or pass through the cell wall into the medium [1]. With an increase in hyphae length, the number of vesicles produced is likely to increase, explaining the relationship between hyphae growth rate and length, while the excess vesicles are used for the production of new branches, explaining the exponential increase in the number of tips at constant hyphae growth volume [24].
There are several mathematical models that can be used to describe fungal growth Wangt al. [35], although few are able to simultaneously consider the influence of morphology on the formation of products such as proteins. In this study, the microscopy evaluation showed that growth in glycerol resulted in larger cells and provided an increase in tip formation due to protein secretion.
Cellulase induction strategies
Table 3 presents the maximum FPase, xylanase, and β-glucosidase activities achieved by T. harzianum using different cultivation strategies. It was found that the use of glycerol in the pre-culture step, strategies 1–5 (Table 2) resulted in outperformance, compared to the control experiments using glucose, controls 1 and 2 (Table 2), in terms of all the enzyme activities measured, with values up to twofold higher.
When 20 g/L of glucose was used in the pre-culture step (control 2), the results were almost the same as obtained using 10 g/L of glucose (control 1). Catabolite repression by glucose is a major mechanism that can halt the synthesis of cellulase in filamentous fungi [3]. Ilment al. [17] showed that glucose acts as a repressor of cellulase synthesis at the transcriptional level in T. reesei cultivations. On the other hand, it was found that glycerol is a readily available carbon source for the growth of T. reesei and is neutral in terms of cellulase biosynthesis, without either promoting or inhibiting the process [17]. No cellulase mRNAs was detected when T. harzianum P49P11 grown on glycerol (unpublished data) suggesting that glycerol may be considered also a “neutral” carbon source for T. harzianum. Therefore, the use of Strategies 1–5, performed with glycerol as carbon source in the pre-culture step, probably led to higher enzyme activity values because of an absence of repression.
Increasing the glycerol concentration in the pre-culture step from 10 g/L (strategy 1) to 20 g/L (strategy 3) resulted in a further increment in enzymatic activity, compared to the controls (Table 3). However, the difference between these strategies was not substantial, with FPase titers of 1.3 ± 0.2 FPU/mL (strategy 1) and ∼1.6 ± 0.8 FPU/mL (strategy 3). For xylanase and β-glucosidase, an increase of the glycerol concentration in the pre-culture step resulted in an increment in the titers of these enzymes (79.00 ± 0.8 IU/mL and 7.13 ± 0.4 IU/mL respectively, using strategy 1, and 103.00 ± 0.1 and 9.71 ± 0.09 IU/mL, respectively, using strategy 3). For applications such as the enzymatic hydrolysis of sugarcane bagasse, the enzymatic cocktail from strategy 3 would be preferable, due to the higher contents of xylanase and β-glucosidase.
When further additions of 10 g/L DSB were made at 36 and 60 h, using 10 and 20 g/L of glycerol in the pre-culture (fed-batch Strategies 2 and 4, respectively), the enzymatic activities were increased. In the case of strategy 5, using 20 g/L of glycerol in the pre-culture and batch induction with 30 g/L of DSB, the FPase and β-glucosidase activities showed up to twofold increases (1.76 ± 0.03 FPU/mL and 16.5 ± 0.3 IU/mL, respectively). Despite the high cellulose concentration (30 g/L), the use of a rotary shaker for the cultivations provided adequate oxygen and mass transfer, favoring cell growth and the maintenance of activity.
Delabona et al. [10] found that FPase activity could be improved up to threefold by increasing the DSB concentration from 2 to 3 % during submerged cultivation of T. harzianum P49P11 in a stirred tank bioreactor. dos Reis et al. [12] studied the growth of Penicillium echinulatum and obtained higher β-glucosidase activity when a high cellulose concentration was employed (5.8 IU/mL at 168 h of cultivation using 60 g/L of cellulose). Maedaet al. [20] used different feeding strategies to enhance cellulase production by T. reesei RUT C30, and found that the highest cellulase activity (19.07 FPU/mL) was obtained using a strategy with 50 g/L initial cellulose concentration and then feeding at intervals of 6 h (27.13 g for the first feed, followed by progressive decreases of 5 g in subsequent feeds).
The best strategy identified in the shaker flask tests (strategy 5:20 g/L of glycerol in the pre-culture, and batch induction with 30 g/L of DSB as shown Table 3) was evaluated at a larger scale using a bioreactor, in order to validate the previous results and enable use of the procedure in pilot scale trials.
Stirred tank bioreactor cultivations using high cell density
The selected conditions for the pre-culture step were firstly used to identify the time required for the complete consumption of glycerol and attainment of the peak biomass concentration for inoculation of the bioreactor (Fig. 3).
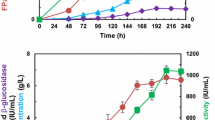
In these experiments, an initial glycerol concentration of 20 g/L was used, and after 72 h, the glycerol was almost exhausted (2.86 g/L). A maximum biomass concentration of 9.09 g/L was achieved at 72 h. In order to ensure a high cell concentration in the induction step, three flasks containing the pre-cultures obtained using the above procedure were mixed after 72 h, resulting in a T. harzianum total cell mass of 5.46 g (dry basis). This biomass was transferred to a bioreactor containing 30.0 g/L of DSB (batch culture), resulting in an initial biomass concentration of approximately 4.5 g/L. The time course of glucohydrolase production during cultivation of T. harzianum in the stirred tank bioreactor, using DSB for cellulase induction, is illustrated in Fig. 4a (FPase activity and protein concentration) and 4b (xylanase and β-glucosidase activities).
FPase and total protein (a), and xylanase and β-glucosidase (b) activities during cultivation of T. harzianum in a stirred tank bioreactor using 5.47 g of biomass and 30 g/L of inoculum media. The results are presented as averages and standard deviations (error bars) for cultivations carried out in duplicate
The experiments revealed that the use of glycerol as carbon source for growth, with initial biomass concentration of ~4.5 g/L, followed by induction with DSB, resulted in the highest production of FPase (2.27 ± 0.37 FPU/mL) and good total protein secretion (2.35 ± 0.1 mg/mL). Uzbas et al. [34] reported a maximum of 45 mg/L of cloned cellulase in their T. reesei QM9414 transformants. Figure 5 summarizes the results of the relationship between the maximum FPAse values and the initial amounts of fungal biomass inoculated in the cultivations carried out under all the different strategies, using shake flasks or the bioreactor.
It was evident that a higher initial biomass concentration resulted in greater FPase activity (Fig. 5). The activities of the other glucohydrolases also benefited from the high cell density protocol. In the bioreactor cultivations, the maximum xylanase and β-glucosidase activities obtained were 106.40 ± 8.87 and 9.04 ± 0.39 IU/mL, respectively (Fig. 4). For the control experiments using 20 g/L of glucose and 20 g/L of Celufloc™ 200 as carbon source in the pre-culture step (instead of glycerol), values of 0.77 ± 0.02 FPU/mL and 0.89 ± 0.01 mg/mL of total protein were obtained. Lower values were also found for the other activities (78.77 ± 5. 35 IU/mL of xylanase and 8.04 ± 1.01 IU/mL of β-glucosidase).
The use of high cell density culture systems makes it possible to achieve high productivity of various metabolites. Maeda et al. [20] evaluated the profile of cellulase production by Penicillium funiculosum, using different inoculum concentrations and with glucose and sugar cane bagasse as substrates. A higher pre-inoculum concentration resulted in increases in enzyme titers and volumetric productivity, with percentage increases of 88.0, 84.0, and 42.0 % for the activities of FPase, Avicelase, and CMCase, respectively.
The goal is to obtain an improved method for cellulases production that provides greater productivity and offers an alternative option for the on-site production of enzymes. According to Himmel et al. [16] and Pradella, Rossell, Scandiffio, Cunha, Pinho and Bonomi [27], productivity of 75 FPU/L h is desirable for the production of cellulases used in industrial processes with cellulose as carbon source. In this study, the calculated FPase production rate was about 37.5 FPU/L h using glycerol in the pre-culture followed by induction with pretreated sugarcane bagasse (DSB). This value is almost twofold higher than obtained earlier for T. harzianum P49P121 (16.8 FPU/L h) in a batch reactor using DSB + sucrose culture medium [9], and about 1.5-fold higher than achieved with a mutant strain of P. echinulatum cultivated in a fed-batch bioreactor using DSB as carbon source (24.3 FPU/L h) [25]. Bigelow et al. [5] reported 3.1 FPU/L h for T. reesei RUT-C30 grown on bagasse pretreated with hot water. Kovács et al. [18] described the production of cellulase by a mutant strain of T. atroviride, using pretreated willow as the carbon source in submerged bioreactor fermentation, and achieved a value of 9.2 FPU/L h. Benoliel, Torres and de Moraes [4] reported 15.4 FPU/L h for the T. harzianum L04 strain grown using in natura sugarcane bagasse. Hence, compared to the values reported in the literature, very high cellulase productivity was obtained here for T. harzianum P49P11 grown using glycerol followed by induction with pretreated sugarcane bagasse.
Glycerol is reported to be a neutral carbon source for T. reesei [17], and the present data seem to suggest this is also true for T. harzianum. Use of a high cell concentration for cellulase production resulted in a higher enzyme titer and faster biosynthesis, which could be explained by a low availability of glucose to the cell population, alleviating catabolite repression and hence increasing cellulase biosynthesis. This promising strategy enables us to propose a two-phase cellulase production process: the first phase is devoted to the production of biomass at a high cell density, using a readily available carbon source that presents low catabolite repression, and this is followed by a second phase with an inductor.
Conclusions
The results described here demonstrate the importance of adding glycerol to cultivation media used for the production of biomass-degrading enzymes, offering an alternative option for on-site enzyme production. The T. harzianum P49P11 strain showed good ability to produce cellulase when grown on glycerol followed by induction with pretreated sugarcane bagasse. This appeared to be due to a greater number of active tips of the mycelia, as well as long hyphae, which increased the protein secretion capacity. This proposed strategy for cellulase production using glycerol for high cell density growth of T. harzianum, followed by induction with pretreated sugarcane bagasse, appears to be a viable means of increasing cellulase production, while at the same time adding value to glycerol, a byproduct of the biodiesel industry.
References
Ahamed A, Vermette P (2009) Effect of culture medium composition on Trichoderma reesei’s morphology and cellulase production. Bioresour Technol 100:5979–5987
Amanullah A, Christensen L, Hansen K, Nienow A, Thomas C (2002) Dependence of morphology on agitation intensity in fed-batch cultures of Aspergillus oryzae and its implications for recombinant protein production. Biotechnol Bioeng 77:815–826
Amore A, Giacobbe S, Faraco V (2013) Regulation of cellulase and hemicellulase gene expression in fungi. Curr Genom 14:230–249. doi:10.2174/1389202911314040002
Benoliel B, Torres FAG, de Moraes LMP (2013) A novel promising Trichoderma harzianum strain for the production of a cellulolytic complex using sugarcane bagasse in natura. SpringerPlus 2:656
Bigelow M, Wyman CE (2002) Cellulase production on bagasse pretreated with hot water. Appl Biochem Biotechnol 98:921–934
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chen Y-H, Walker TH (2011) Biomass and lipid production of heterotrophic microalgae Chlorella protothecoides by using biodiesel-derived crude glycerol. Biotechnol Lett 33:1973–1983
Da Silva GP, Mack M, Contiero J (2009) Glycerol: a promising and abundant carbon source for industrial microbiology. Biotechnol Adv 27:30–39
Delabona Pda S, Farinas CS, da Silva MR, Azzoni SF, Pradella JG (2012) Use of a new Trichoderma harzianum strain isolated from the Amazon rainforest with pretreated sugar cane bagasse for on-site cellulase production. Bioresour Technol 107:517–521. doi:10.1016/j.biortech.2011.12.048
Delabona Pda S, Farinas CS, Lima DJ, Pradella JG (2013) Experimental mixture design as a tool to enhance glycosyl hydrolases production by a new Trichoderma harzianum P49P11 strain cultivated under controlled bioreactor submerged fermentation. Bioresour Technol 132:401–405. doi:10.1016/j.biortech.2012.11.087
Delabona PdS, Pirota RDPB, Codima CA, Tremacoldi CR, Rodrigues A, Farinas CS (2012) Using Amazon forest fungi and agricultural residues as a strategy to produce cellulolytic enzymes. Biomass Bioenergy 37:243–250. doi:10.1016/j.biombioe.2011.12.006
dos Reis L, Fontana RC, Delabona Pda S, da Silva Lima DJ, Camassola M, Pradella JG, Dillon AJ (2013) Increased production of cellulases and xylanases by Penicillium echinulatum S1M29 in batch and fed-batch culture. Bioresour Technol 146:597–603. doi:10.1016/j.biortech.2013.07.124
Fan X, Burton R, Zhou Y (2010) Glycerol (byproduct of biodiesel production) as a source for fuels and chemicals—mini review. Open Fuels Energy Sci J 3:17–22
Florencio C, Cunha F, Badino A, Farinas C (2015) Validation of a novel sequential cultivation method for the production of enzymatic cocktails from trichoderma strains. Appl Biochem Biotechnol 175:1389–1402
Ghose T (1987) Measurement of cellulase activities. Pure Appl Chem 59:257–268
Himmel ME, Ruth MF, Wyman CE (1999) Cellulase for commodity products from cellulosic biomass. Curr Opin Biotechnol 10:358–364
Ilmen M, Saloheimo A, Onnela M-L, Penttilä ME (1997) Regulation of cellulase gene expression in the filamentous fungus Trichoderma reesei. Appl Environ Microbiol 63:1298–1306
Kovács K, Szakacs G, Zacchi G (2009) Comparative enzymatic hydrolysis of pretreated spruce by supernatants, whole fermentation broths and washed mycelia of Trichoderma reesei and Trichoderma atroviride. Bioresour Technol 100:1350–1357
Li C, Lesnik KL, Liu H (2013) Microbial conversion of waste glycerol from biodiesel production into value-added products. Energies 6:4739–4768
Maeda RN, Barcelos CA, Santa Anna LMM, Pereira N (2013) Cellulase production by Penicillium funiculosum and its application in the hydrolysis of sugar cane bagasse for second generation ethanol production by fed batch operation. J Biotechnol 163:38–44
Mandels M, W J (1969) Production of cellulases. Adv Chem Ser 95:391–414
Robl D, da Silva Delabona P, Costa PS, da Silva Lima DJ, Rabelo SC, Pimentel IC, Büchli F, Squina FM, Paddila G, da Cruz Pradella JG (2015) Xylanase production by endophytic Aspergillus niger using pentose-rich hydrothermal liquor from sugarcane bagasse. Biocatal Biotransform 33:175–187. doi:10.3109/10242422.2015.1084296
Naranjo JM, Posada JA, Higuita JC, Cardona CA (2013) Valorization of glycerol through the production of biopolymers: the PHB case using Bacillus megaterium. Bioresour Technol 133:38–44
Peberdy JF (1994) Protein secretion in filamentous fungi—trying to understand a highly productive black box. Trends Biotechnol 12:50–57
Pereira BMP, Alvarez TM, da Silva Delabona P, Dillon AJP, Squina FM, da Cruz Pradella JG (2013) Cellulase on-site production from sugar cane bagasse using Penicillium echinulatum. BioEnergy Res 6:1052–1062
Portnoy T, Margeot A, Linke R, Atanasova L, Fekete E, Sándor E, Hartl L, Karaffa L, Druzhinina IS, Seiboth B (2011) The CRE1 carbon catabolite repressor of the fungus Trichoderma reesei: a master regulator of carbon assimilation. BMC Genom 12:269
Pradella JGC, Rossell CEV, Scandiffio MIG, Cunha MP, Pinho MGO, Bonomi A (2009) Estudo preliminar do custo de produção in house de celulases na biorrefinaria de etanol de segunda geração. Anais do XVII Simpósio Nacional de Bioprocessos
Robl D, Delabona Pda S, Mergel CM, Rojas JD, Costa Pdos S, Pimentel IC, Vicente VA, da Cruz Pradella JG, Padilla G (2013) The capability of endophytic fungi for production of hemicellulases and related enzymes. BMC Biotechnol 13:94. doi:10.1186/1472-6750-13-94
Rocha GJM, Goncalves AR, Oliveira BR, Olivares EG, Rossell CEV (2012) Steam explosion pretreatment reproduction and alkaline delignification reactions performed on a pilot scale with sugarcane bagasse for bioethanol production. Ind Crops Prod 35:274–279. doi:10.1016/j.indcrop.2011.07.010
Santucci BS, Maziero P, Rabelo SC, Curvelo AA, Pimenta MTB (2015) Autohydrolysis of hemicelluloses from sugarcane bagasse during hydrothermal pretreatment: a kinetic assessment. BioEnergy Res 8:1778–1787
Sarma SJ, Brar SK, Sydney EB, Le Bihan Y, Buelna G, Soccol CR (2012) Microbial hydrogen production by bioconversion of crude glycerol: a review. Int J Hydrogen Energy 37:6473–6490
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D (2006) Determination of sugars, byproducts, and degradation products in liquid fraction process samples. Golden, National Renewable Energy Laboratory
Tang S, Boehme L, Lam H, Zhang Z (2009) Pichia pastoris fermentation for phytase production using crude glycerol from biodiesel production as the sole carbon source. Biochem Eng J 43:157–162
Uzbas F, Sezerman U, Hartl L, Kubicek CP, Seiboth B (2012) A homologous production system for Trichoderma reesei secreted proteins in a cellulase-free background. Appl Microbiol Biotechnol 93:1601–1608
Wang L, Ridgway D, Gu T, Moo-Young M (2005) Bioprocessing strategies to improve heterologous protein production in filamentous fungal fermentations. Biotechnol Adv 23:115–129. doi:10.1016/j.biotechadv.2004.11.001
Ward O, Qin W, Dhanjoon J, Ye J, Singh A (2006) Physiology and biotechnology of Aspergillus. Adv Appl Microbiol 58:1–76
Wu H, Karanjikar M, San K-Y (2014) Metabolic engineering of Escherichia coli for efficient free fatty acid production from glycerol. Metab Eng 25:82–91
Yang F, Hanna MA, Sun R (2012) Value-added uses for crude glycerol—a byproduct of biodiesel production. Biotechnol Biofuels 5:1–10
Zhang Y-HP, Himmel ME, Mielenz JR (2006) Outlook for cellulase improvement: screening and selection strategies. Biotechnol Adv 24:452–481
Acknowledgments
The authors would like to thank the technical staff of the National Laboratory of Science and Technology of Bioethanol (CTBE), and acknowledge the financial support provided by the Brazilian agencies CNPq, CAPES and FAPESP.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The financial support provided by the Brazilian agencies CNPq, CAPES and FAPESP and not have any potential Conflicts of interest (financial or non-financial).
This manuscript complies to the Ethical Rules applicable for Journal of Industrial Microbiology and Biotechnology. In this research is not involve human participants and/or animals.
Rights and permissions
About this article
Cite this article
da Silva Delabona, P., Lima, D.J., Robl, D. et al. Enhanced cellulase production by Trichoderma harzianum by cultivation on glycerol followed by induction on cellulosic substrates. J Ind Microbiol Biotechnol 43, 617–626 (2016). https://doi.org/10.1007/s10295-016-1744-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-016-1744-8