Abstract
Endometriosis is one of the most common gynecological diseases that adversely effects the lives of women. Our previous studies showed that LINC01541 plays a key role in 17β-estradiol (17β-E2)-stimulated endometrial stromal cells (ESCs); however, the mechanism by which LINC01541 exerts if effects requires further elaboration. Here, we report that LINC01541 serves to reduce the bioavailability of miR-506-5p by acting as a molecular sponge. Samples of control endometrial tissue and ectopic endometrial tissue were obtained from 10 healthy volunteers and 18 patients with endometriosis, respectively, and the levels of LINC01541 and miR-506-5p expressions in those tissues were measured. The relationship between LINC01541 and miR-506-5p was verified in 17β-E2-stimulated ESCs. Overexpression or silencing of miR-506-5p in ESCs was performed explore its role in endometriosis, and we also investigated whether WNT inhibitory factor 1 (WIF1) might be a target gene of miR-506-5p. Our results showed that LINC01541 was expressed at low levels and miR-506-5p was expressed at high levels in ectopic tissues. LINC01541 expression was negatively correlated with miR-506-5p expression. We also found that miR-506-5p activated the Wnt/β-catenin pathway by inhibiting WIF1 expression, and thereby induced the proliferation, migration, and invasion of ESCs. Furthermore, silencing of miR-506-5p promoted apoptosis and suppressed the proliferation of 17β-E2-treated ESCs. Overexpression of miR-506-5p could reverse the inhibitory effect of LINC01541 in endometriosis. In summary, this study found that in endometriosis, LINC01541 functions as a ceRNA that modulates the Wnt/β-catenin pathway by decoying miR-506-5p.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Endometriosis is a gynecological disease that presents with symptoms of dysmenorrhea, pelvic pain, pelvic masses, infertility, and malignant-like cellular behavior, and affects 6–10% of women of reproductive age [9]. This estrogen-dependent disorder significantly impacts the quality of life of the affected patients and their partners [26]. Women with endometriosis are at a higher risk than the general female population for developing ovarian cancer, and may also be at an increased risk for breast and other cancers, as well as autoimmune and atopic disorders [6]. Thus, there is an urgent need to develop efficient methods for treating endometriosis. Endometriosis is usually diagnosed by laparoscopy, and mainly treated by surgery [4, 10]. There remains a lack of reliable biomarkers and therapeutic targets that can be used to diagnose and treat endometriosis, respectively, and it remains necessary to further study the molecular biology of endometriosis.
Long non-coding RNAs (lncRNAs) are RNAs that contain > 200 nucleotides, but do not code for a protein [12]. LncRNAs that modulate estrogen, androgen, and progesterone receptor responses have been previously identified, suggesting that lncRNAs play a role in attenuating hormone regulatory networks that affect endometrial function [30]. The role played by lncRNA in endometriosis has gradually received more attention during the past 5 years. In endometriosis, lncRNAs such as H19 [5] and LINC00261 [25] have been proved to be critical components of the disease process. The expression of 1682 lncRNAs has been shown to be altered in the circulating serum of women with endometriosis when compared with healthy women, and 1435 lncRNAs show altered expression in the eutopic endometrium of women with endometriosis when compared with healthy women [31]. LncRNA LOC100505776, also known as LINC01541(NR_038325.1), was identified as the most downregulated lncRNA in ectopic endometrial tissue in a previous study [28]. Our previous in vitro experiments proved that LINC01541 could inhibit the EMT process, metastasis, and VEGFA expression by regulating the Wnt/β-catenin pathway in endometrial stromal cells (ESCs) [18]. An in vitro study showed that 17β-estradiol (17β-E2) promoted the migration and invasion of ESCs, and those effects could be partially reversed by overexpression of LINC01541. However, little is known about how LINC01541 functions in endometriosis. The mechanism by which LINC01541 regulates the Wnt/β-catenin pathway also needs further elaboration.
Numerous studies have reported that lncRNAs function as post-transcriptional regulatory molecules that can interact with microRNAs (miRNAs); that mechanism is known as the “competing endogenous RNA (ceRNA)” hypothesis [24]. As results of fluorescence in situ hybridization (FISH) studies showed that LINC01541 was almost exclusively expressed in cytoplasm, we speculated that LINC01541 might function as a ceRNA. In this study, we calculated its potential interaction with miRNA by using the LncBase v.2 database: (http://carolina.imis.athena-innovation.gr/diana_tools/web/index.php?r=lncbasev2%2Findex), and also explored the mechanism by which LINC01541 regulates the Wnt/β-catenin pathway in endometrial stromal cells (ESCs).
Materials and Methods
Clinical Sample Collection
Eighteen patients with endometriosis and ten healthy volunteers were enrolled in this study that was conducted at the Second Affiliated Hospital of Guangxi Medical University. All procedures involving human participants were performed in accordance with ethical standards of the institutional and/or national research committee, as well as with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Control endometrial tissues and ectopic endometrial tissues were obtained from 18 patients with endometriosis and 10 healthy volunteers, respectively. The menstrual cycles of patients with heterotopia in our study were normal, and those patients were included in this study. All 18 patients with heterotopia were diagnosed as ovarian endometriosis, which was classified as advanced endometriosis. These patients included 7 cases of r-AFS III and 11 cases of r-ASF IV, as classified according to the standards for endometriosis staging, as revised by the American Society of Fertility in 1996 (Table 1). The operations were usually performed at 1 week post-period, and the ectopic endometrial tissue obtained during each operation was preserved in a liquid-nitrogen freezer. Samples of endometrial tissue to be used as normal control tissues were obtained from 10 healthy volunteers with benign hysteromyoma after a hysterectomy. Pathological examinations were performed to ensure that no abnormalities existed in the samples of endometrial tissue before they were used in subsequent experiments. Each patient enrolled in the study provided their written informed consent, and the study protocol was approved by the Ethics Committee of the Second Affiliated Hospital of Guangxi Medical University.
Cell Culture
ESCs were purchased from iCell Bioscience Inc. (Shanghai, China), and cultured in Dulbecco’s modified Eagle’s medium (Hyclone, Logan, Utah, USA) supplemented with 10% heat-inactivated fetal bovine serum (Gibco-BRL, Gaithersburg, MD, USA), 100 U/mL of streptomycin, and 100 U/mL of penicillin (Sigma-Aldrich, St. Louis, MO, USA) in a 5% CO2 atmosphere at 37 °C.
Cell Stimulation and Transfection
As our previous study showed that 17β-E2 increased the migration and invasion capabilities of ESCs, we stimulated the ESCs with 10 nmol/L of 17β-E2 (Sigma-Aldrich) for 48 h to simulate the ESCs found in patients with endometriosis. To increase the expression of LINC01541, the ESCs were transfected with plasmid pCDNA3.1 containing the full-length sequence of LINC01541. Additionally, a miR-506-5p inhibitor and mimics were synthesized by GenePharma (Suzhou, China), and transfected into ESCs to interfere with miR-506-5p expression. All transfections were performed according to instructions provided with a Lipofectamine 2000 Kit (Invitrogen, Carlsbad, CA, USA).
Fluorescence In Situ Hybridization
The in situ expression and subcellular distribution of lncRNAs in ESCs were determined by the fluorescence in situ hybridization (FISH) method performed using a Ribo™ Fluorescent In-Situ Hybridization Kit (#R11060.6; RiboBio, Guangzhou, China) according to the manufacturer’s instructions. LncRNA FISH probes specifically targeting LINC00261 were synthesized by the RiboBio company. Briefly, ESCs on microscope slides were washed twice with PBS, fixed with 4% paraformaldehyde at room temperature for 10 min, and then incubated overnight with 0.5 μM lncRNA FISH probe diluted in hybridization solution at 37 °C in darkness. Next, the ESCs were washed with PBS for 5 min at room temperature, stained with DAPI for 10 min at room temperature, and finally observed with a confocal laser scanning microscope (Leica, Wetzlar, Germany).
Assessment of Cell Viability
Cell viability was assessed according to instructions provided with a Cell Counting Kit-8 (Dojindo, Kumamoto-ken, Japan). Briefly, transfected ESCs were collected and transferred into 96-well flat-bottomed microplates (5 × 103 cells/well) at 0 h, 24 h, 48 h, and 72 h after transfection (3 wells per time point). Next, the ESCs were incubated with 100 μL of CCK8 solution for 4 h at 37 °C; after which, the optical density of each well at 490 nm (OD 490 nm) was recorded by a Microplate Reader (Thermo Scientific, Waltham, MA, USA). Cell viability was calculated and compared based on data from at least three biological replicates.
Flow Cytometry
ESCs were harvested at 48 h after transfection and transferred into 6-well culture plates (5 × 105 cells/well). Next, the cells were incubated with Annexin V/PI solution (Multi Sciences BIOTECH, CO., LTD., Hangzhou, China) for 15 min in the dark. The treated cells were then transferred into flow tubes and the Annexin V-FITC-A/PI-A signal was detected with a BD FACS Calibur™ flow cytometer (BD Biosciences, Franklin, NJ, USA). After a data analysis, a final scatter plot was created.
Cell Migration and Invasion Assays
Cell migration and invasion were both measured with a Transwell system (Corning Corp., Corning, NY, USA). The ESCs (2 × 104 cells) used for cell migration assays were treated and harvested, and then placed into the upper chamber of a Transwell plate and cultured with serum-free medium for 12 h. After culture, the medium was removed and the cells were washed three times with PBS. Subsequently, the lower chamber was filled with 700 μL of medium containing 10% FBS, and the Transwell plate was incubated in a 5% CO2 atmosphere at 37 °C for 48 h. Next, the cells on the lower side of the filter were fixed in 3.8% formaldehyde for 20 min, and then stained with 0.1% crystal violet solution. Finally, the number of cells in six randomly selected microscopic fields was counted under a CX41 microscope (Olympus, Tokyo, Japan). With the exception of a Matrigel insert being placed between each upper and lower Transwell chamber, the cell invasion assays were all performed as described above.
Reverse Transcription-Quantitative Polymerase Chain Reaction
Total RNA was extracted from cells with Trizol reagent (Takara Biotechnology Co., Ltd., Dalian, China), and a 1 μg sample of total RNA was reverse-transcribed to cDNA by using a Bestar™ PCR RT kit (DBI, Ludwigshafen, Germany). RT-qPCR was performed by using Bestar™ qPCR Master Mix (DBI) in an ABI Real time PCR system (Applied Biosystems, Waltham, MA, USA). All gene-specific primers (Table 2) were synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). The RT-qPCR reactions were performed at the following conditions: 95 °C for 10 min, followed by 40 cycles of 95 °C for 10 s and 60 °C for 30 s. The relative abundance of LINC01541 or miR-506-5p was determined by normalization to the level of GAPDH and U6, respectively, and using the 2−ΔΔCt method.
Western Blotting
The total cellular proteins were extracted with a lysis buffer that contained 50 mM Tris (pH 7.6), 150 mM NaCl, 1% TritonX-100, 1% deoxycholate, 0.1% SDS, 1 mM PMSF, and 0.2% aprotinin (Sigma), and the total protein concentration in each extract was determined by using a BCA Protein Assay kit (Beyotime, China). Next, a 20 μg sample of total protein from each extract was separated by SDS-PAGE, and the protein bands were transferred onto a polyvinylidene fluoride membrane, which was subsequently blocked with 5% bovine serum albumin at room temperature for 1 h. After being blocked, the membrane was incubated with anti-E-Cadherin (Abcam, Cambridge, MA, USA, diluted 1:2000), anti-N-Cadherin (Abcam, 1:2000), anti-vimentin (Abcam, 1:2000), anti-β-catenin (Abcam, 1:2000), or anti-WIF1 (Abcam, 1:1000) at 4 °C overnight. GAPDH (Abcam, 1:5000) was used as an internal control. After incubation, the blots were washed with TBST and incubated with a horseradish peroxidase–conjugated secondary antibody (Santa Cruz Biotechnology, Dallas TX, USA, diluted 1:2000) at room temperature for 1 h. Finally, the protein bands were visualized with a Pierce ECL Plus Western Blotting Substrate (Thermo Scientific).
Statistical Analysis
All experiments were performed in triplicate. The data were analyzed by Student’s t test as performed using Prism 7.0 software (GraphPad Software, La Jolla, CA, USA). Results are presented as the mean ± standard deviation (SD).
Results
Negative Correlation Between the Expression of LINC01541 and miR-506-5p in Endometriosis
Our previous study showed that LINC01541 was only slightly expressed in 17β-E2-treated ESCs. In this study, we used the FISH technique to show the location of LINC01541 in ESCs. Our results showed that LINC01541 was almost exclusively expressed in cytoplasm (Fig. 1a), suggesting that of LINC01541 might function as a ceRNA. Subsequently, we used a database (LncBase v.2) to determine the potential for LINC01541 to interact with miRNA, and found that miR-506-5p possesses multiple sequences that are complementary to sequences in LINC01541 (Fig. 1b). In order to verify our hypothesis, we measured the levels of LINC01541 and miR-506-5p expressions in control endometrial tissues and ectopic tissues. Our results showed that LINC01541 expression was significantly reduced, while miR-506-5p expression was significantly increased in ectopic tissues when compared with control endometrial tissues (Fig. 1c, d). Furthermore, LINC01541 expression was negatively correlated with miR-506-5p expression (Fig. 1e). These results suggested that miR-506-5p might be related to the function of LINC01541 in endometriosis.
Low expression of miR-506-5p and high expression of LINC01541 in endometriosis. a The location of LINC01541 in normal control ESCs and 17β-E2-stimulated ESCs was shown by FISH. b The potential binding sites of miR-506-5p and LINC01541 as predicted by the LncBase v.2 database. c, d Expression of LINC01541 and miR-506-5p in control endometrial tissues (Control) and ectopic tissues (EMTS: endometriosis). ***P < 0.001, unpaired student’s t test. e LINC01541 expression was negatively correlated with miR-506-5p expression in ectopic tissues. A Pearson correlation analysis was performed to analyze the data
MiR-506-5p Directly Targeted WIF1
To further delineate the molecular mechanisms of LINC01541, we used TargetScanHuman 7.2 (http://www.targetscan.org/vert_72/) to predict the potential target gene of miR-506-5p. That search identified WIFI as a potential target gene; furthermore, WIFI is thought to play an important role in the regulating the Wnt/β-catenin pathway (Fig. 2a). A double luciferase assay showed that miR-506-5p mimics significantly reduced the amount of luciferase activity, and that effect was reversed by overexpression of LINC01541 in ESCs containing the wild-type 3′UTR, but not in cells containing the mutant-type 3′UTR (Fig. 2b). In addition, results of RT-qPCR and Western blot studies showed a significant under-expression of WIF1 in ectopic tissues (Fig. 2c, f). WIF1 expression was positively correlated with LINC01541 expression, but negatively correlated with miR-506-5p expression (Fig. 2d, e). In vitro studies showed that miR-506-5p was upregulated and WIF1 was downregulated in 17β-E2-treated ESCs, while overexpression of LINC01541 reversed those effects (Fig. 3a). Furthermore, a floating level of WIF1 induced negatively correlated changes in β-catenin expression (Fig. 3b). These results suggest that LINC01541 modulates the Wnt/β-catenin pathway by regulating miR-506-5p/WIF1.
MiR-506-5p directly targeted WIF1. a Potential binding sites of miR-506-5p and WIF1 as predicted by the TargetScanHuman 7.2 data base. b The dual luciferase reporter gene assay system was used to confirm the target gene of WIF1. c Expression of WIF1 in control endometrial tissues (Control) and ectopic tissues (EMTS: endometriosis). d, e WIF1 expression was positively correlated with LINC01541 expression and negatively correlated with miR-506-5p expression in ectopic tissues. f)he levels of WIF1 and β-catenin proteins in control endometrial tissues and ectopic tissues. ***P < 0.001, student’s t test
17β-E2 inhibited WIF1 expression by inducing miR-506-5p expression. a The levels of LINC01541, miR-506-5p, and WIF1 gene transcription in 17β-E2-stimulated and LINC01541 overexpressing ESCs. b Representative bands of WIF1 and β-catenin proteins as shown by Western blotting. ***P < 0.001, student’s t test
MiR-506-5p Promoted the Proliferation and Metastasis of ESCs
To verify the role of miR-506-5p in endometriosis, we forced the overexpression of miR-506-5p in untreated ESCs and knock-down of miR-506-5p expression in 17β-E2-treated ESCs. Overexpression of miR-506-5p reduced WIF1 expression at both the mRNA and protein levels, while β-catenin protein expression was increased (Fig. 4a, b). We also investigated the expression of epithelial-mesenchymal transition markers, and found that E-cadherin expression was reduced, while the levels of N-cadherin and Vimentin expression were increased, indicating that the capability of the cells to metastasize was increased. Conversely, inhibition of miR-506-5p downregulated the high levels of WIF1 expression induced by the 17β-E2 treatment. Expression of WIF1 was also identified by immunofluorescence staining (Fig. 4c). Cell proliferation and metastatic potential were both increased, and cell apoptosis was inhibited by miR-506-5p mimics in untreated ESCs (Fig. 5a–c). Those results showed that overexpression of miR-506-5p inhibited cell proliferation and reduced the metastatic potential of 17β-E2-treated ESCs, indicating that miR-506-5p plays an important role in endometriosis.
MiR-506-5p-inhibited WIF1 expression ESCs were transfected with miR-506-5p mimics and 17β-E2-stimulated ESCs were transfected with a miR-506-5p inhibitor. a Levels of WIF1 and miR-506-5p gene transcription. b Representative bands of WIF1, β-catenin, E-cadherin, N-cadherin, and Vimentin proteins as shown by Western blotting. c Representative images of WIF1 as detected by immunofluorescence assays. ***P < 0.001, student’s t test
MiR-506-5p promoted the proliferation and metastasis of ESCs. a Cell proliferation was measured by the CCK-8 assay. b Apoptosis was measured by flow cytometry. c Representative images and analysis data of migrated and invasive cells as determined by Transwell assays. *P < 0.05, **P < 0.01, ***P < 0.001, vs. Scramber; #P < 0.05, ##P < 0.01, ###P < 0.001, vs. Scramber + 17β-E2, student’s t test
LINC01541 Induced WIF1 Expression by Sponging miR-506-5p
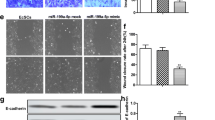
In order to further prove that LINC01541 functions through sponging of miR-506-5p, LINC01541 and miR-506-5p were co-expressed in ESCs. The high expression of WIF1 induced by LINC01541 was reversed by miR-506-5p mimics (Fig. 6a). Co-expression of LINC01541 and miR-506-5p significantly promoted the expression of β-catenin, N-cadherin, and Vimentin, but inhibited E-cadherin expression when compared with the effect produced by overexpression of LINC01541 alone (Fig. 6b). Similarly, cell proliferation and metastatic potential were improved, while apoptosis was inhibited in the co-expression group (Fig. 6c–e). Taken together, these findings suggest that LINC01541 functions as a ceRNA to modulate the Wnt/β-catenin pathway by decoying miR-506-5p in endometriosis.
MiR-506-5p mimics reversed the effect of LINC01541 in ESCs. MiR-506-5p mimics were transfected into LINC01541 overexpressing ESCs. a The levels of WIF1 and miR-506-5p gene transcription. b Representative bands of WIF1, β-catenin, E-cadherin, N-cadherin, and Vimentin as shown by Western blotting. c Cell proliferation was measured by the CCK-8 assay. d Apoptosis was measured by flow cytometry. e Representative images and analysis data of migrated and invasive cells as determined by Transwell assays. *P < 0.05, **P < 0.01, ***P < 0.001, vs. Vector; #P < 0.05, ###P < 0.001, vs. LINC01541, student’s t test
Discussion
Abnormal estrogen metabolism is a major risk factor for endometriosis. Endometriotic lesions produce higher levels of 17β-E2 than the eutopic endometrium of endometriosis patients and control subjects [3]. In our previous work, ESCs were stimulated with 10 nmol/L of 17β-E2 to simulate the internal environment of endometriosis, and the role played by LINC01541 was explored. We found that 17β-E2 significantly promoted the metastasis and invasiveness of ESCs, while LINC01541 exerted an inhibitory effect in endometriosis [18]. In this current study, we focused on how LINC01541 modulates the Wnt/β-catenin pathway. First, we observed the location of LINC01541 in 17β-E2-stimulated ESCs. Studies indicated that 17β-E2 promotes the growth of ESCs, which is considered a major risk factor for the development and progression of endometriosis [1, 13]. A previous study found that 17β-E2 promotes cell proliferation in endometriosis by decreasing PTEN expresssion via a NFκB-dependent pathway, [8] that in vitro model might be useful for studying the molecular pathologic characteristics of endometriosis. Our results showed that LINC01541 was almost exclusively expressed in cytoplasm; therefore, we speculated tha LINC01541 might function as a ceRNA. A previous study reported that H19 lncRNA mediates the 17β-E2-induced proliferation of breast cancer cells [29], and another study found that H19 lncRNA functions to reduce the bioavailability of microRNA let-7 by acting as a molecular sponge [5]. Apparently, lncRNAs are able function as a ceRNA in endometriosis, and thereby mediate the proliferation, apoptosis, and invasion of ESCs. In this study, we found a negative correlation between LINC01541 expression and miR-506-5p expression in ectopic tissues. Results of dual luciferase reporter assays and other in vitro experiments proved that LINC01541 regulates WIF1 expression by sponging miR-506-5p.
Initially, we used the LncBase v.2 database (http://carolina.imis.athena-innovation.gr/diana_tools/web/index.php?r=lncbasev2%2Findex) to screen for miRNAs that might serve as a molecular sponge for LINC01541 based on the criteria that the evaluation score was > 0.9 points and the miRNA had previously been reported. Afterwards, miR-205-3p, miR-147a, miR-506-5p, and miR-432-3p were selected for qPCR assays based on their cellular functions. However, when stimulated with 17 β-E2 in vitro, only one miRNA (miR-506-5p) showed a significant increase in its expression level, while no statistically significant changes were observed for the other miRNAs. Therefore, miR-506-5p was used for our subsequent studies. There have been numerous reports concerning the roles played by miRNAs in endometriosis [17, 19, 20]; however, no investigator has studied the role of miR-506-5p in endometriosis. Only one report in 2015 indicated that miRNA-506 inhibits the proliferation and invasion of gastric cancer cells by directly targeting Yap1 [11]. In our previous study, we found that LINC01541 could inhibit the EMT process, the metastasis of ESCs, and VEGFA expression by regulating the Wnt/β-catenin pathway [18]. Therefore, target genes of mir-506 were initially thought to be involved in the Wnt/β-catenin signaling pathway. In addition, previous evidence indicated that WIF1 was involved in regulating the Wnt/β-catenin signaling pathway [7, 15]. Based on that information, we performed a dual luciferase reporter gene assay, and those results, along with the negative correlation between miR-506 and WIf1 expression, suggested that WIF1 was a target gene of miR-506. We also found that miR-506-5p could promote the proliferation, migration, and invasion of ESCs. Overexpression of miR-506-5p in ESCs inhibited the expression of WIF1 and induced activation of the Wnt/β-catenin signaling pathway.
WIF1 inhibits activation of the Wnt/β-catenin signaling pathway by associating with Wnt proteins [23]. Mediation of the Wnt/β-catenin signaling pathway by WIFI plays an important role in many deseases, such as breast cancer [16], cervical cancer [22], and arthritis [27]. Transcription of the WIF1 gene is mainly regulated by methylation of its promoter region, [21] and the post-transcriptional regulation of WIF1 protein expression can be attributed to miRNAs. MiRNA-552 promotes the progression of osteosarcoma [2] and hepatocellular carcinoma [14] by downregulating WIF1 expression. Furthermore, miR-181c contributes to cisplatin resistance in non-small cell lung cancer cells by regulating WIF1 [32]. In endometriosis, we found that WIF1 expression was regulated by miR-506-5p, and the latter was inhibited by LINC01541. The differential expression of WIF1 in control endometrial tissues vs. ectopic endometrial tissues may have important implications for developing new targeted drugs for treating endometriosis. One limitation of this study is that the endometrial tissues were obtained from patients with benign hysteromyoma after hysterectomy, because it is difficult to obtain eutopic endometrial tissues from patients during the operation. Experiments with eutopic endometrium from patients with endometriosis need to be conducted in order to obtain more conclusive results in the future.
In summary, LINC01541 expression was negatively correlated with miR-506-5p expression in ectopic tissues. Silencing of miR-506-5p promoted apoptosis and suppressed the proliferation of 17β-E2-treated ESCs. Overexpression of miR-506-5p activated the Wnt/β-catenin pathway by inhibiting the expression of WIF1, which subsequently induced the proliferation, migration, and invasion of ESCs. LINC01541 functions as a ceRNA to modulate the Wnt/β-catenin pathway by decoying miR-506-5p in endometriosis.
References
Andrade SS, Azevedo ADC, Monasterio ICG, Paredes-Gamero EJ, Gonçalves GA, Bonetti TC, et al. 17β-Estradiol and steady-state concentrations of H2O2: antiapoptotic effect in endometrial cells from patients with endometriosis. Free Radical Biology Medicine. 2013;60:63–72.
Cai W, Xu Y, Yin J, Zuo W, Su Z. miR-552-5p facilitates osteosarcoma cell proliferation and metastasis by targeting WIF1. Exp Ther Med. 2019;17:3781–8.
Delvoux B, Groothuis P, D’Hooghe T, Kyama C, Dunselman G, Romano A. Increased production of 17beta-estradiol in endometriosis lesions is the result of impaired metabolism. J Clin Endocrinol Metab. 2009;94:876–83.
Dunselman GAJ, Vermeulen N, Becker C, et al. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29:400–12.
Ghazal S, Mckinnon B, Zhou J, et al. H19 lncRNA alters stromal cell growth via IGF signaling in the endometrium of women with endometriosis. Embo Molecular Medicine. 2015;7:996–1003.
Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–99.
Guo M, Zhang X, Wang G, Sun J, Jiang Z, Khadarian K, et al. miR-603 promotes glioma cell growth via Wnt/beta-catenin pathway by inhibiting WIF1 and CTNNBIP1. Cancer Lett. 2015;360:76–86.
Hui Z, Zhao X, Shu L, et al. 17βE 2 promotes cell proliferation in endometriosis by decreasing PTEN via NFκB-dependent pathway. Molecular Cellular Endocrinology. 2010;317:31–43.
Ilangavan K, Kalu E. High prevalence of endometriosis in infertile women with normal ovulation and normospermic partners. Fertility & Sterility. 2009;92:68–74.
Janssen EB, Rijkers AC, Hoppenbrouwers K, et al. Prevalence of endometriosis diagnosed by laparoscopy in adolescents with dysmenorrhea or chronic pelvic pain: a systematic review. Hum Reprod Update. 2013;19:570–82.
Jun D, Wan L, Xiaojun X, et al. MicroRNA-506 inhibits gastric cancer proliferation and invasion by directly targeting Yap1. Tumour Biol. 2015;36:6823–31.
Kapranov P, Cheng J, Dike S, Nix DA, Duttagupta R, Willingham AT, et al. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science. 2007;316:1484–8.
Khan KN, Kitajima M, Inoue T, Fujishita A, Nakashima M, Masuzaki H. 17β-estradiol and lipopolysaccharide additively promote pelvic inflammation and growth of endometriosis. Reprod Sci. 2015;22:585–94.
Li C, Wang Z, Chen S, et al. MicroRNA-552 promotes hepatocellular carcinoma progression by downregulating WIF1. Int J Mol Med. 2018;42:3309–17.
Liang J, Chen C, Liu H, et al. Gossypol promotes Wnt/beta-catenin signaling through WIF1 in ovariectomy-induced osteoporosis. Biomed Res Int. 2019;2019:8745487.
Lingbao A, Qian T, Sheng Z, et al. Inactivation of Wnt inhibitory factor-1 (WIF1) expression by epigenetic silencing is a common event in breast cancer. Carcinogenesis. 2006;27:1341–8.
Maged AM, Deeb WS, Amir AE, et al. Diagnostic accuracy of serum miR-122 and miR-199a in women with endometriosis. Int J Gynaecol Obstet. 2017;141:14–9.
Mai H, Wei Y, Yin Y, Huang S, Lin H, Liao Y, et al. LINC01541 overexpression attenuates the 17beta-estradiol-induced migration and invasion capabilities of endometrial stromal cells. Syst Biol Reprod Med. 2019;65:214–22.
Okamoto M, Nasu K, Abe W, Aoyagi Y, Kawano Y, Kai K, et al. Enhanced miR-210 expression promotes the pathogenesis of endometriosis through activation of signal transducer and activator of transcription 3. Hum Reprod. 2015;30:632–41.
Pateisky P, Pils D, Szabo L, Kuessel L, Husslein H, Schmitz A, et al. hsa-miRNA-154-5p expression in plasma of endometriosis patients is a potential diagnostic marker for the disease. Reprod BioMed Online. 2018;37:449–66.
Pei L, Shen JK, Hornicek FJ, et al. Wnt inhibitory factor 1 (WIF1) methylation and its association with clinical prognosis in patients with chondrosarcoma. Sci Rep. 2017;7:1580.
Ramachandran I, Thavathiru E, Ramalingam S, Natarajan G, Mills WK, Benbrook DM, et al. Wnt inhibitory factor 1 induces apoptosis and inhibits cervical cancer growth, invasion and angiogenesis in vivo. Oncogene. 2012;31:2725–37.
Rong W, Zhai Y, Leung JY, et al. WIF-1 is a downstream target of β-catenin/TCF and acts as a negative feedback regulator of Wnt signaling. Cancer Research. 2004;64:1112.
Ruixi L, Ruobai S, Hicks GR, et al. Arabidopsis ribosomal proteins control vacuole trafficking and developmental programs through the regulation of lipid metabolism. PNAS. 2014;2014:89–98.
Sha L, Huang L, Luo X, Bao J, Gao L, Pan Q, et al. Long non-coding RNA LINC00261 inhibits cell growth and migration in endometriosis. Journal of Obstetrics Gynaecology Research. 2017;43:1563–9.
Simoens S, Dunselman G, Dirksen C, Hummelshoj L, Bokor A, Brandes I, et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod. 2012;27:1292–9.
Stock M, Böhm C, Scholtysek C, Englbrecht M, Fürnrohr BG, Klinger P, et al. Wnt inhibitory factor 1 deficiency uncouples cartilage and bone destruction in tumor necrosis factor α–mediated experimental arthritis. Arthritis Rheumatism. 2013;65:2310–22.
Sun PR, Jia SZ, Lin H, Leng JH, Lang JH. Genome-wide profiling of long noncoding ribonucleic acid expression patterns in ovarian endometriosis by microarray. Fertility & Sterility. 2014;101:1038–46.
Sun H, Wang G, Peng Y, et al. H19 lncRNA mediates 17beta-estradiol-induced cell proliferation in MCF-7 breast cancer cells. Oncol Rep. 2015;33:3045–52.
Taylor DH, Chu ET, Spektor R, et al. Long non-coding RNA regulation of reproduction and development. Molecular Reproduction Development. 2016;82:932–56.
Wang WT, Sun YM, Huang W, He B, Zhao YN, Chen YQ. Genome-wide long non-coding RNA analysis identified circulating LncRNAs as novel non-invasive diagnostic biomarkers for gynecological disease. Sci Rep. 2016;6:23343.
Zhang H, Hu B, Wang Z, et al. miR-181c contributes to cisplatin resistance in non-small cell lung cancer cells by targeting Wnt inhibition factor 1. Cancer Chemotherapy Pharmacology. 2017;80:1–12.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures involving human participants were performed in accordance with ethical standards of the institutional and/or national research committee, as well as with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Conflict of Interest
All authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mai, H., Xu, H., Lin, H. et al. LINC01541 Functions as a ceRNA to Modulate the Wnt/β-Catenin Pathway by Decoying miR-506-5p in Endometriosis. Reprod. Sci. 28, 665–674 (2021). https://doi.org/10.1007/s43032-020-00295-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-020-00295-3