Abstract
Endometriosis is an estrogen-dependent disease that is characterized by pelvic pain and infertility. MicroRNAs have been shown to implicate in the progression of endometriosis. In our study, we used real-time PCR to evaluate the expression of miR-141-3p in endometrial samples. In addition, western blot analysis was used to assess the expression of Krüppel-like factor 12 (KLF-12). The proliferation and migration of ectopic endometrial stromal cells (ESCs) were determined by MTT assay and Transwell assay, respectively. Cell apoptosis was evaluated using a Cell Death Detection ELISA Plus kit. The results showed that miR-141-3p and KLF-12 were significantly different in paired ectopic and eutopic endometrial samples. miR-141-3p overexpression significantly restrained the proliferation and migration and promoted the apoptosis of ectopic ESCs, whereas a decreased level of miR-141-3p was associated with opposite results. Furthermore, dual-luciferase reporter assay confirmed that KLF-12 was a novel target of miR-141-3p, while it also decreased the effects of miR-141-3p on the proliferation, apoptosis, and migration of ectopic ESCs. Our data suggested that enhanced expression of miR-141-3p suppressed the proliferation and migration of ectopic ESCs and promoted their apoptosis via targeting KLF-12. Our results may provide a novel potential therapeutic target for the treatment of endometriosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endometriosis is a chronic and estrogen-dependent gynecological disorder that is characterized by the presence and growth of functional endometrial-like tissue outside the uterus [8]. Endometriosis can severely decrease the quality of life because of pelvic masses, pelvic pain, dysmenorrhea, infertility, and cancerous lesions [2, 27]. Although the disorder is known to be a benign disease, the migratory and invasive properties of ectopic endometrial stromal cells (ESCs) are similar to those of malignant cancer cells [1]. However, the mechanisms underlying the migration of ectopic ESCs are not well known.
MicroRNAs, well known as small noncoding RNAs with 20–22 nucleotides, regulate the expression of target genes and control a variety of cellular functions, including apoptosis, proliferation, migration, and invasion [13, 21, 34]. Numerous studies suggested that microRNAs are involved in the development of human diseases, including cardiovascular disorders, gynecological diseases, cancer, and inflammatory diseases [20, 28, 33]. Furthermore, abnormal levels of microRNAs have been associated with the progression of several female reproductive tract diseases, including preeclampsia [15], endometrioid endometrial adenocarcinoma [35], uterine leiomyomata [4], ovarian adenocarcinoma [3], and endometriosis [24]. Multiple studies have indicated that miR-141-3p may be decreased in endometriosis [10, 26]. However, the role of miR-141-3p in endometriosis is still largely unknown.
In this work, we explored the roles and potential mechanisms of miR-141-3p in the development of endometriosis. We found that the level of miR-141-3p was significantly decreased in ectopic endometrium, while Krüppel-like factor 12 (KLF-12) was upregulated. Functional experiments indicated that miR-141-3p overexpression was associated with a clear decline in the proliferation and invasion of ectopic ESCs, and it promoted apoptosis of ectopic ESCs. Moreover, KLF-12 is a direct target of miR-141-3p, obviously attenuated the inhibitory effect of miR-141-3p on the proliferation and migration of ectopic ESCs. These findings may provide insight into developing a new therapeutic biomarker for endometriosis treatment.
Materials and methods
Tissue acquisition
Twenty patients with endometriosis at our hospital were selected for collection of paired ectopic (EC) endometrial samples and eutopic (EU) endometrial samples. Control samples were collected from the endometrium of patients with hysteromyoma. All participants had regular periods and had no hormone therapy within 3 months before surgery. Patients with polycystic ovary syndrome or inflammatory were excluded from our study. Each patient signed an informed consent form, and the hospital ethics committee approved this study. Their clinical profiles are shown in Table 1.
Isolation and culture of normal, eutopic, and ectopic ESCs
We isolated primary human endometrial stromal cells according to a previously described method with slight modification [19]. Briefly, the tissues were minced into 1 mm pieces after washing PBS for three times and then digested with 0.1% type II collagenase for 1 h. Cells were centrifuged with 500g for 5 min and then resuspended in Ham’s F12/DMEM (1:1). Cell suspension was passed through 100-μm and 40-μm nylon mesh to separate glandular epithelial cells. After 12 h of adherence, the culture medium was replaced to remove nonadherent glandular epithelial cells and blood cells. After purification, stromal cells were grown in Ham’s F12/DMEM (1:1) including heat-inactivated 10% fetal bovine serum (FBS; Gibco, Grand Island, NY, USA), 1% penicillin/streptomycin, and 0.25 μg/mL amphotericin B. Cells were incubated in a humidified atmosphere consisting of 5% CO2 and 95% air at 37 °C. In order to avoid contamination by epithelial cells, the isolated stromal cells were immune-stained with vimentin- and cytokeratin-specific antibodies.
Construction of adenovirus
The AdMax (Microbix) system was used to generate adenoviruses harboring KLF-12 (NCBI Reference Sequence: NM_007249.4) according to a previously described method. After being packaged, HEK293A cells amplified the viruses, which were then purified and dialyzed with CsCl banding and 10 mmol/L Tris-buffered saline, respectively. The Adeno-X Rapid Titer kit (BD, Franklin Lakes, NJ, USA) was used to measure the titering in HEK293A cells, following the manufacturer’s protocol.
Cell transfection
The ectopic ESCs were respectively transfected with miR-NC (negative control), miR-141-3p mimic, miR-141-3p inhibitor, mutant KLF-12 3′-UTR, wild-type KLF-12 3′-UTR, and Ad-Con or Ad-KLF-12 using Lipofectamine 2000 reagent (Thermo, Waltham, MA, USA) following the manufacturer’s illustration. Afterward, cells were incubated at 37 °C for 24 h.
Quantitative real-time polymerase chain reaction assay
We isolated total RNA, using an RNA Isolation Kit (Takara Biotechnology, Dalian, China) in accordance with the manufacturer’s instructions. cDNA was synthesized by using PrimeScript RT reagent kit (Takara, Tokyo, Japan). Following the manufacturer’s protocols, we measured the expression of miR-141-3p with an ABI 7500 Real-time PCR system (Bio-Rad Laboratories Inc., Hercules, CA, USA), using a TaqMan miRNA assay (Qiagen, Shanghai, China). We chose U6 as the internal control for measuring the expression of miRNA. In addition, we used the ABI 7500 Real-time PCR system to measure the KLF-12 expression through SYBR Green Master Mix (Thermo), with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as the internal control for mRNA expression.
Western blot
We respectively used a total protein extraction kit (Kaiji Biological Inc., Nanjing, China) and the BCA kit (Beyotime, Shanghai, China) to extract the protein and determine the protein concentration. SDS-PAGE was performed to separate the proteins, which were then electrotransferred to polyvinylidene difluoride (PVDF) membranes and incubated with the appropriate primary and secondary antibodies. The optical density (OD) value of the bands was measured by an enhanced ECL kit and a film, while the Image-Pro Plus software (Pierce Biotechnology Inc., Rockford, IL, USA) was used to quantify the protein levels. β-Actin was used for normalization.
Dual-luciferase reporter assay
First, we respectively synthesized the wild-type KLF-12 3′-UTR (KLF12 3′-UTR Wt) and mutant KLF-12 3′-UTR (KLF-12 3′-UTR Mut) of KLF-12 and then cloned them into the pGL3 luciferase reporter vector (Promega, Madison, WI, USA). Then, 24-well plates were used to seed cells until reaching 70% confluence. We subsequently cotransfected cells with KLF12 3′-UTR Wt or KLF-12 3′-UTR Mut and pRL-TK plasmid (Promega), along with 30 nM miR-141-3p mimic, or negative control was transfected into cells using the Lipofectamine 2000. The dual-luciferase reporter assay was performed to analyze Renilla luciferase activity after transfection for 48 h.
MTT assay
Cell viability was evaluated using the MTT assay (Sigma-Aldrich Co., St. Louis, MO, USA). After transfection for 24 h, ectopic ESCs were grown into 96-well plates. Subsequently, 20 μL of MTT solution was mixed into the media and incubated for 4 h, and we then added 200 μL of dimethyl sulfoxide to solubilize the formazan crystals for 15 min. The OD value was determined at 490 nm.
Transwell assay
Adjusting the density of ectopic ESCs to 5 × 103 cells per well, we then seeded the upper Transwell chamber and added 400 μL of serum-free cell culture medium. One milliliter of medium containing 5% FBS was added to the lower chamber, and the cells were incubated for 6 h. Using a microscope (Olympus, Tokyo, Japan) with a × 10 objective, we counted the number of cells that had migrated through the pores in five independent visual fields. Migrated cells were stained with Giemsa dye for observation.
Cell apoptosis analysis
A Cell Death Detection PLUS ELISA (Roche Diagnostics, Indianapolis, IN, USA) was performed to detect DNA fragmentation as a molecular marker of apoptosis. Cells were grown in 96-well plates and transfected with miR-NC, miR-141-3p mimic, or miR-141-3p inhibitor. Cytoplasmic fractions were collected and transferred to streptavidin-coated 96-well plates, followed by incubated with anti-histone antibody and anti-DNA antibody for 2 h. The absorbance of each well was determined at 405 nm using a microplate reader and normalized to the concentration of protein.
Statistical analysis
The SPSS software (ver. 13.0; SPSS, Chicago, IL) was used to analyze all data, which are expressed as means ± SD. One-way analysis of variance followed by the Student–Newman–Keuls test was used to determine significant differences of multiple groups. Statistical significance was defined as P < 0.05.
Results
The level of miR-141-3p is clearly decreased in endometriosis
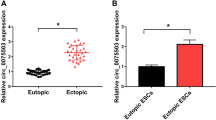
The expression of miR-141-3p in the patients with and without endometriosis was measured by RT-qPCR. We found that miR-141-3p was prominently decreased in ectopic endometrium compared with normal endometrium and eutopic endometrium (P < 0.05, Fig. 1a). In addition, miR-141-3p in ectopic ESCs was significantly lower than that in normal ESCs and eutopic ESCs (P < 0.05, Fig. 1b). Furthermore, we conducted a western blot analysis to determine the expression of KLF-12 in the endometrial samples from patients with and without endometriosis. As shown in Fig. 1c, KLF-12 was clearly increased in ectopic endometrium. Similarly, we observed that KLF-12 was markedly upregulated in ectopic ESCs (P < 0.05, Fig. 1d). These data indicated that the expression of miR-141-3p and KLF-12 was shown as inverse relationship.
The expression of miR-141-3p and KLF-12 in endometrial tissues and ESCs is different. a, b Differential levels of the miR-141-3p in endometrial tissues (a) and ESCs (b) were evaluated by qRT-PCR. c, d Differential expressions of the KLF-12 in endometrial tissues (c) and ESCs (d) were detected by western blot. EN normal endometrium, EU eutopic endometrium, EC ectopic endometrium. *P < 0.05 compared with the normal endometrium or ESC group
Restoration expression of miR-141-3p inhibits the proliferation and migration of ectopic ESCs
To study the effects of miR-141-3p in endometriosis, we transfected the miR-141-3p mimic or miR-141-3p inhibitor into ectopic ESCs and then evaluated the proliferation and migration of ESCs. As evident in Fig. 2a, compared with the miR-NC group, miR-141-3p was significantly elevated in the ESCs transfected with miR-141-3p mimic. The expression of miR-141-3p was prominently reduced after transfection with miR-141-3p inhibitor. As shown in Fig. 2b, compared with the miR-NC group, miR-141-3p overexpression showed a lower growth rate; however, upregulation of miR-141-3p significantly promoted ectopic ESC proliferation. Moreover, the results of Transwell assays indicated that miR-141-3p overexpression substantially reduced the migration of ectopic ESCs, whereas suppression of miR-141-3p had an opposite effect (P < 0.05, Fig. 2c). These results revealed that miR-141-3p impeded the proliferation and migration of ectopic ESCs.
miR-141-3p overexpression inhibited the proliferation and migration of ectopic ESCs. a Ectopic ESCs transfected with the miR-141-3p mimic or inhibitor to overexpress or suppress miR-141-3p expression, respectively. b MTT assay was performed to determine the cell viability of ectopic ESCs after transfection with miR-NC or miR-141-3p mimic or miR-141-3p inhibitor. c Cell migration was measured using Transwell assay. *P < 0.05 compared with the miR-NC group
Enforced expression of miR-141-3p induces apoptosis in ectopic ESCs
The incidence of apoptosis was detected using a Cell Death Detection PLUS ELISA.
Enhanced expression of miR-141-3p significantly promoted apoptosis in the ectopic ESCs, while knockdown of miR-141-3p showed the opposite effect (P < 0.05, Fig. 3a). Besides, Bcl-2 and Bax expression was determined by western blot. As shown in Fig. 3b, miR-141-3p overexpression downregulated the expression of Bcl-2 and raised the expression of Bax, whereas inhibiting the expression of miR-141-3p remarkably elevated Bcl-2 expression and decreased Bax expression. These findings demonstrated that miR-141-3p contributes to the apoptosis of ectopic ESCs.
miR-141-3p promoted ectopic ESCs apoptosis. a Cell apoptosis was examined in ectopic ESCs transfected with miR-141-3p mimic or miR-141-3p inhibitor. b The expression of Bax and Bcl-2 was examined in ectopic ESCs transfected with miR-141-3p mimic or miR-141-3p inhibitor by western blot. *P < 0.05 compared with the miR-NC group
KLF-12 is directly regulated by miR-141-3p
We cotransfected cells with wild-type KLF-12 3′-UTR or mutant KLF-12 3′-UTR along with miR-141-3p mimic or miR-NC and then performed dual-luciferase reporter assay to explore whether KLF-12 is a direct target of miR-141-3p. The predictive binding sites between miR-141-p and KLF-12 are shown in Fig. 4a. We found that the luciferase activity of pGL3-KLF-12-WT was decreased compared with the pGL3-KLF-12-Mut (P < 0.05, Fig. 4b). In addition, the protein level of KLF-12 was detected. The results indicated that miR-141-3p overexpression significantly decreased the protein level of KLF-12, while the miR-141-3p inhibitor-transfected cells had a significantly increased level (P < 0.05, Fig. 4c). These data showed that KLF-12 is a direct target of miR-141-3p.
miR-141-3p directly targeted KLF-12 in ectopic ESCs. a The putative binding sites for miR-141-3p in KLF-12. b Relative luciferase activity in ectopic ESCs cotransfected with miR141-3p or miR-NC, and wild-type or mutant 3′-UTR KLF-12 was measured by dual-luciferase reporter assay. c The expression of KLF-12 in ectopic ESCs transfected with miR-141-3p mimic, or miR-141-3p inhibitor was detected by western blot. *P < 0.05 compared with the miR-NC group
KLF-12 reverses the effects of miR-141-3p on the proliferation and migration of ectopic ESCs
To understand the potential mechanism of miR-141-3p in the regulation of proliferation and migration during endometriosis, the proliferation and migration of ESCs were detected after cotransfection with Ad-KLF-12 and miR-141-3p mimic. MTT assay indicated that miR-141-3p mimic inhibited the proliferation of ectopic ESCs, and this effect was reversed by KLF-12 overexpression (P < 0.05, Fig. 5a). Similarly, the inhibitory role of miR-141-3p in migration was blocked by gain function of KLF-12 (P < 0.05, Fig. 5b).
KLF-12 reversed the roles of miR-141-3p in apoptosis
Furthermore, we evaluated the apoptosis of ectopic ESCs after cotransfection with Ad-KLF-12 and miR-141-3p mimic. As shown in Fig. 6a, overexpression of KLF-12 inhibited the increase in ectopic ESC apoptosis induced by miR-141-3p (P < 0.05). Consistent with these results, overexpression of KLF-12 downregulated Bax expression and raised the level of Bcl-2 in miR-141-3p mimic-transfected ectopic ESCs (P < 0.05, Fig. 6b). These findings support that miR-141-3p facilitated the apoptosis of ectopic ESCs via targeting KLF-12.
KLF-12 attenuates miR-141-3p-induced apoptosis. a Cell apoptosis was examined in ectopic ESCs transfected with miR-141-3p mimic or miR-141-3p mimic and Ad-KLF-12. b The expression of Bax and Bcl-2 was examined in ectopic ESCs transfected with miR-141-3p mimic or miR-141-3p mimic and Ad-KLF-12 by western blot. *P < 0.05 compared with the control group. #P < 0.05 compared with the Ad-Con+miR-141-3p mimic group
Discussion
Aberrant expression of miRs is implicated in the endometriosis [10]. It has been reported that miR-141-3p is decreased in certain kinds of cancer [12, 36, 38]. Recent report revealed that miR-141 was notably decreased in the ectopic endometrium [29]. In this study, we demonstrated that the levels of miR-141-3p were significantly reduced in ectopic endometrium, suggesting that miR-141-3p may be involved in the development of endometriosis.
Previous research showed that the migratory ability of endometriotic cells may play an important role in the development of endometriosis [22]. miR-141-3p plays an integral role in the progression and development of tumors. Recent report showed that overexpression of miR-141-3p promoted cell migration and invasion in cervical cancer [17]. However, Hou et al. reported that miR-141-3p inhibited the proliferation, migration, and invasion of hepatocellular carcinoma cell lines [11]. miR-141-3p served as a tumor suppressor through inhibiting the proliferation and invasion of NSCLC cells [18]. Similarly, miR-141-3p also inhibited the growth and metastasis of papillary thyroid cancer cells [7]. Consistently, our data demonstrated that enforced expression of miR-141 inhibited cell migration and triggered cell apoptosis in ectopic ESCs. However, the opposite trends were observed upon inhibition of miR-141-3p.
miRNAs are known to exert their biological functions through binding to the 3′-UTR of target genes. These targets have been suggested to constitute molecular pathways potentially involved in the development of endometriosis. Members of the Krüppel-like factor (KLF) family of transcription factors have critical roles in regulating cell differentiation, physiologic function, and phenotypic modulation [5, 14, 30, 32]. Genetic studies have demonstrated that KLF-12 plays an important role in embryonic development, tumor genesis, and progress [23, 25, 31]. For example, KLF-12 promoted colorectal cancer growth by regulating early growth response protein 1 [16]. Mounting studies have confirmed that KLF-12 acts as an oncogene in multiple cancers, including gastric cancer [6], colorectal cancer [16], and basal-like breast carcinoma [9]. In addition, a recent study demonstrated that KLF12 acts as a negative regulator during the decidualization of ESCs [12, 30, 37] . In the present study, the results indicated that the protein levels of KLF-12 differed in ectopic versus eutopic endometrial tissue. In addition, we demonstrated that KLF-12 was a direct target of miR-141-3p. Importantly, the results of rescue experiments and functional assays strongly demonstrated that restoration of KLF-12 abolished the inhibitory effects of miR-141-3p on proliferation and migration in ectopic ESCs. Moreover, enforced expression of KLF-12 reversed the increased apoptosis in ectopic ESCs mediated by miR-141-3p overexpression.
In conclusion, we found that miR-141-3p may suppress cell proliferation and migration, promoted apoptosis via targeting KLF-12 in ectopic ESCs. Our study suggests that miR-141-3p may be a potential target in developing treatment against endometriosis.
References
Ahn JH, Choi YS, Choi JH (2015) Leptin promotes human endometriotic cell migration and invasion by up-regulating MMP-2 through the JAK2/STAT3 signaling pathway. Mol Hum Reprod 21(10):792–802
Anger DL, Foster WG (2008) The link between environmental toxicant exposure and endometriosis. Front Biosci 13:1578–1593
Bachmayr-Heyda A, Auer K, Sukhbaatar N, Aust S, Deycmar S, Reiner AT, Polterauer S, Dekan S, Pils D (2016) Small RNAs and the competing endogenous RNA network in high grade serous ovarian cancer tumor spread. Oncotarget 7(26):39640–39653
Chuang TD, Khorram O (2018) Regulation of cell cycle regulatory proteins by microRNAs in uterine leiomyoma. Reprod Sci 1933719118768692
Dang DT, Pevsner J, Yang VW (2000) The biology of the mammalian Kruppel-like family of transcription factors. Int J Biochem Cell Biol 32(11–12):1103–1121
Du Y, Chen Y, Wang F, Gu L (2016) miR-137 plays tumor suppressor roles in gastric cancer cell lines by targeting KLF12 and MYO1C. Tumour Biol 37(10):13557–13569
Fang M, Huang W, Wu X, Gao Y, Ou J, Zhang X, Li Y (2019) MiR-141-3p suppresses tumor growth and metastasis in papillary thyroid cancer via targeting Yin Yang 1. Anat Rec (Hoboken) 302(2):258–268
Giudice LC (2010) Clinical practice. Endometriosis. N Engl J Med 362(25):2389–2398
Guan B, Li Q, Shen L, Rao Q, Wang Y, Zhu Y, Zhou XJ, Li XH (2016) MicroRNA-205 directly targets Kruppel-like factor 12 and is involved in invasion and apoptosis in basal-like breast carcinoma. Int J Oncol 49(2):720–734
Hawkins SM, Creighton CJ, Han DY, Zariff A, Anderson ML, Gunaratne PH, Matzuk MM (2011) Functional microRNA involved in endometriosis. Mol Endocrinol 25(5):821–832
Hou X, Yang L, Jiang X, Liu Z, Li X, Xie S, Li G, Liu J (2019) Role of microRNA-141-3p in the progression and metastasis of hepatocellular carcinoma cell. Int J Biol Macromol 128:331–339
Huang S, Wa Q, Pan J, Peng X, Ren D, Huang Y, Chen X, Tang Y (2017) Downregulation of miR-141-3p promotes bone metastasis via activating NF-kappaB signaling in prostate cancer. J Exp Clin Cancer Res 36(1):173
Jannot G, Simard MJ (2006) Tumour-related microRNAs functions in Caenorhabditis elegans. Oncogene. 25(46):6197–6201
Kaczynski J, Cook T, Urrutia R (2003) Sp1- and Kruppel-like transcription factors. Genome Biol 4(2):206
Khaliq OP, Murugesan S, Moodley J, Mackraj I (2018) Differential expression of miRNAs are associated with the insulin signaling pathway in preeclampsia and gestational hypertension. Clin Exp Hypertens:1–8
Kim SH, Park YY, Cho SN, Margalit O, Wang D, DuBois RN (2016) Kruppel-like factor 12 promotes colorectal cancer growth through early growth response protein 1. PLoS One 11(7):e0159899
Li JH, Zhang Z, Du MZ, Guan YC, Yao JN, Yu HY, Wang BJ, Wang XL, Wu SL, Li Z (2018) microRNA-141-3p fosters the growth, invasion, and tumorigenesis of cervical cancer cells by targeting FOXA2. Arch Biochem Biophys 657:23–30
Li W, Cui Y, Wang D, Wang Y, Wang L (2019) MiR-141-3p functions as a tumor suppressor through directly targeting ZFR in non-small cell lung cancer. Biochem Biophys Res Commun 509(3):647–656
Liu H, Zhang Z, Xiong W, Zhang L, Xiong Y, Li N, He H, Du Y, Liu Y (2017) Hypoxia-inducible factor-1alpha promotes endometrial stromal cells migration and invasion by upregulating autophagy in endometriosis. Reproduction 153(6):809–820
Miron L (2003) Systemic treatment of colorectal cancers—factual standards and perspectives. Rev Med Chir Soc Med Nat Iasi 107(4):752–758
Miska EA (2005) How microRNAs control cell division, differentiation and death. Curr Opin Genet Dev 15(5):563–568
Moggio A, Pittatore G, Cassoni P, Marchino GL, Revelli A, Bussolati B (2012) Sorafenib inhibits growth, migration, and angiogenic potential of ectopic endometrial mesenchymal stem cells derived from patients with endometriosis. Fertil Steril 98(6):1521–30 e2
Nakamura Y, Migita T, Hosoda F, Okada N, Gotoh M, Arai Y, Fukushima M, Ohki M, Miyata S, Takeuchi K, Imoto I, Katai H, Yamaguchi T, Inazawa J, Hirohashi S, Ishikawa Y, Shibata T (2009) Kruppel-like factor 12 plays a significant role in poorly differentiated gastric cancer progression. Int J Cancer 125(8):1859–1867
Nothnick WB (2017) MicroRNAs and endometriosis: distinguishing drivers from passengers in disease pathogenesis. Semin Reprod Med 35(2):173–180
Oates AC, Pratt SJ, Vail B, Yan Y, Ho RK, Johnson SL, Postlethwait JH, Zon LI (2001) The zebrafish klf gene family. Blood 98(6):1792–1801
Ohlsson Teague EM, Van der Hoek KH, Van der Hoek MB, Perry N, Wagaarachchi P, Robertson SA, Print CG, Hull LM (2009) MicroRNA-regulated pathways associated with endometriosis. Mol Endocrinol 23(2):265–275
Painter JN, Anderson CA, Nyholt DR, Macgregor S, Lin J, Lee SH, Lambert A, Zhao ZZ, Roseman F, Guo Q, Gordon SD, Wallace L, Henders AK, Visscher PM, Kraft P, Martin NG, Morris AP, Treloar SA, Kennedy SH, Missmer SA, Montgomery GW, Zondervan KT (2011) Genome-wide association study identifies a locus at 7p15.2 associated with endometriosis. Nat Genet 43(1):51–54
Rapado-Gonzalez O, Majem B, Muinelo-Romay L, Alvarez-Castro A, Santamaria A, Gil-Moreno A, Lopez-Lopez R, Suarez-Cunqueiro MM (2018) Human salivary microRNAs in cancer. J Cancer 9(4):638–649
Rekker K, Saare M, Roost AM, Kaart T, Soritsa D, Karro H, Soritsa A, Simon C, Salumets A, Peters M (2015) Circulating miR-200-family micro-RNAs have altered plasma levels in patients with endometriosis and vary with blood collection time. Fertil Steril 104(4):938–946 e2
Shen X, Hu Y, Jiang Y, Liu H, Zhu L, Jin X, Shan H, Zhen X, Sun L, Yan G, Sun H (2013) Kruppel-like factor 12 negatively regulates human endometrial stromal cell decidualization. Biochem Biophys Res Commun 433(1):11–17
Suda S, Rai T, Sohara E, Sasaki S, Uchida S (2006) Postnatal expression of KLF12 in the inner medullary collecting ducts of kidney and its trans-activation of UT-A1 urea transporter promoter. Biochem Biophys Res Commun 344(1):246–252
Suske G, Bruford E, Philipsen S (2005) Mammalian SP/KLF transcription factors: bring in the family. Genomics 85(5):551–556
Ultimo S, Zauli G, Martelli AM, Vitale M, McCubrey JA, Capitani S, Neri LM (2018) Cardiovascular disease-related miRNAs expression: potential role as biomarkers and effects of training exercise. Oncotarget. 9(24):17238–17254
Vandenboom Ii TG, Li Y, Philip PA, Sarkar FH (2008) MicroRNA and cancer: tiny molecules with major implications. Curr Genomics 9(2):97–109
Wang L, Chen YJ, Xu K, Xu H, Shen XZ, Tu RQ (2014) Circulating microRNAs as a fingerprint for endometrial endometrioid adenocarcinoma. PLoS One 9(10):e110767
Wang N, Li P, Liu W, Lu Z, Feng J, Zeng X, Yang J, Wang Y, Zhao W (2018) miR-141-3p suppresses proliferation and promotes apoptosis by targeting GLI2 in osteosarcoma cells. Oncol Rep 39(2):747–754
Zhang Q, Zhang H, Jiang Y, Xue B, Diao Z, Ding L, Zhen X, Sun H, Yan G, Hu Y (2015) MicroRNA-181a is involved in the regulation of human endometrial stromal cell decidualization by inhibiting Kruppel-like factor 12. Reprod Biol Endocrinol 13(1):23
Zhou X, Wu W, Zeng A, Nie E, Jin X, Yu T, Zhi T, Jiang K, Wang Y, Zhang J, You Y (2017) MicroRNA-141-3p promotes glioma cell growth and temozolomide resistance by directly targeting p53. Oncotarget 8(41):71080–71094
Author information
Authors and Affiliations
Contributions
Yiwei Zhang and Juan Yan conceived and designed the study; Yiwei Zhang, Juan Yan, and Xiaowei Pan conducted experiments and collected the data; Yiwei Zhang and Juan Yan analyzed and interpreted the experiments results; Yiwei Zhang wrote the first draft of the manuscript; Yiwei Zhang, Juan Yan, and Xiaowei Pan revised the paper. All authors contributed to and approved the final manuscript.
Corresponding author
Ethics declarations
The hospital ethics committee approved this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
1. miR-141-3p is obviously decreased in endometriosis.
2. KLF-12 is significantly upregulated in endometriosis.
3. Restoration of miR-141-3p expression in ectopic ESCs inhibits cellular proliferation and migration.
4. Restoration of miR-141-3p expression in ectopic ESCs induces the apoptosis in ectopic ESCs.
5. KLF-12 is directly regulated by miR-141-3p.
6. KLF-12 reverses the miR-141-3p-induced suppression of cell proliferation and migration.
7. KLF-12 inhibits the miR-141-3p-mediated induction of cell apoptosis.
Rights and permissions
About this article
Cite this article
Zhang, Y., Yan, J. & Pan, X. miR-141-3p affects apoptosis and migration of endometrial stromal cells by targeting KLF-12. Pflugers Arch - Eur J Physiol 471, 1055–1063 (2019). https://doi.org/10.1007/s00424-019-02283-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-019-02283-2