Abstract
The comprehension of the ecological structure and the functioning of natural and/or impacted lotic ecosystems is the purpose of most studies concerning rivers and streams. This study aimed to investigate the main factors influencing the zooplankton community organization in nine impacted tropical streams of three different sub-basins of the Cinzas River basin, Paraná state, Brazil. We tested the hypothesis that macro-factors such as productivity, current velocity, habitat structure, and stream order and stretches (headwater, middle and mouth), are the main factors structuring the zooplankton community in tropical streams. Zooplankton was represented by 101 taxa, mainly testate amoebae, followed by rotifers, cladocerans and copepods. Results showed that the greatest differences in physical and chemical characteristics and those related to zooplankton community structure were observed among the three sub-basins studied. However, we found that these differences were not related to the environmental heterogeneity, but were rather influenced mainly by spatial factors related to stochasticity in the structuring of planktonic communities. Thus, our results suggest that connectivity between environments within the same sub-basin, associated with random processes driven by dispersal, may determine the existence or not of spatial patterns, among stream order or stretches, and temporal patterns, between seasons, in the community attributes here analyzed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lotic environments function as open hydrological systems, where there is a constant flow from the source to the mouth, promoting alteration in the physical structure over time and space, besides carrying out exchanges with the terrestrial environment in an intense manner (Gomes, Gomes, et al., 2020; Thorp et al., 2006). In such systems, connectivity along the drainage basin is an important parameter if we consider the stretch (source, middle, and mouth) and the order of the streams (1st, 2nd, and 3rd order). Stretch and order influence aspects such as water flows, sediment discharge, organic matter, solutes, and also the biotic communities, which are more susceptible to changes (e.g., movements of organisms) from upstream to downstream of the river network (Wohl, 2017). Some of these parameters are responsible for habitat structuring and can interfere with current velocity and even ecosystem productivity.
Stream ecosystems vary considerably in their structure, due tochannel-specific characteristics, and how they can structure communities, aspects that have been tested by some models. For example, the Stream Zonation Concept (SZC) proposed by Illies and Botosaneanu (1963), defines a series of discontinuous communities along rivers, separated by larger fauna transition zones, caused by changes in the river slope and its flow velocity. In addition to this, the River Continuum Concept (RCC) proposed by Vanotte et al. (1980), defines changes in biotic communities, both in composition and distribution levels, as important bases for studies of longitudinal zonation of aquatic communities in lotic environments, where the environmental characteristics of a given section of a river depend on environmental conditions and geographical positioning (Vanotte et al., 1980; Statzner & Higler, 1985; Ferreira, et al., 2010).
Such models address several features that influence aquatic commnuties in lotic environments. Among them, especially the temperature, substrate discharge and structure, light, and nutrients affect primary productivity and the input of organic matter (Czerniawski, 2013), which directly influence the structuring of aquatic biota (Gutierrez et al., 2020). In addition to these, macrofactors such as productivity, current velocity, habitat structure (environmental heterogeneity) and stream order and stretch (source, middle and mouth) can be important in structuring these communities in tropical streams (Blatterer, 2002). Moreover, lotic environments differ fundamentally in connectivity when compared to lentic ones. One can envisage that dispersal rates among localities are higher in streams and rivers than among lakes and ponds without stream connections (Gomes, Gomes, et al., 2020). Because of this, high connectivity among habitats may lead to higher homogeneity of assemblages inhabiting lotic environments (Heino et al., 2015a). The zooplankton community is an important link in the trophic chain in aquatic environments because it connects predators and primary producers (Gutierrez et al., 2020). They have short reproductive cycles (generally from one week to months) (Lampert & Sommer, 2007) and studies on lotic systems are still scarce and deserve further research efforts (Picapedra et al., 2019).
Given that the reproductive rate of plankton is often lower than its drift downstream, zooplankton-typical organisms cannot develop large populations in environments with high current velocity (Czerniawski & Domagała, 2014). Lotic environments can be unfavorable to zooplankton development due to rapid temperature oscillations, the strength of water currents, which depend on the heterogeneity of the river bed, and other physical and biological factors, such as turbulence (influence on zooplankton filtration, predation, and reproduction) and the concentration of suspended solids, which can limit feeding efficiency, and the erosive effect of the water current (Lair, 2006; Golec-Fialek et al., 2021). Interestingly, these features of lotic systems determine that the zooplankton community in rivers and streams is influenced by a strong input of fauna from other compartments such as benthic and littoral, structuring a community known as riverine zooplankton or potamoplankton (Lansac-Tôha et al., 2009).
Although lower diversity is found in lotic environments, especially in streams, when compared with lentic ones, many species can find suitable conditions for the development of dense populations (Ejsmont-Karabin & Kruk, 1998). The dominance of different groups in zooplankton generally provides a good idea of the hydrodynamic conditions prevailing in the sampled areas, with the dominance of microcrustaceans in areas with longer water residence time, while the dominance of rotifers is an indication of shorter residence times (Lansac-Tôha et al., 2009; Sampaio et al., 2002). On the other hand, an expressive participation of testate amoebae in plankton samples characterazes environments with a greater influence of lotic conditions (Gomes, Gomes, et al., 2020).
In tropical regions, especially in Brazil, studies on the ecology of the zooplankton community in lotic enviromental have increased in recent decades, contributing to a better understanding of the main mechanisms involved in the regulation of abundance, diversity and spatio-temporal patterns (Gomes, Barbosa, et al., 2020; Gomes, Barbosa, et al., 2020; Gomes, Gomes, et al., 2020; Perbiche-Neves et al., 2012; Picapedra et al., 2017; Silva et al., 2021). However, most of the studies have focused on the influence of abiotic conditions or parameters on communities, leaving the understanding of biotic interactions between communities restricted to few studies. For example, Perbiche-Neves et al. (2012) found a negative relationship between water velocity and increased turbidity in zooplankton abundance and richness; on the other hand, the authors demonstrate that tributaries are important for increasing zooplankton richness in the main channel. Also, according to Gomes, Barbosa, et al. (2020), environmental and spatial variables may not have significant effects on the structure of zooplanktonic communities in streams, which may indicate a strong influence of stochastic factors. The aquatic environments in the tropical region can be considered as less unstable than those located in the temperate region environments. In the tropics, atypical and extreme conditions, such as edaphic and climatic conditions, are only rarely observed (Kwok et al., 2007).
Thus, the aim of this study was to investigate the main factors intervening in the organization of the zooplankton community in tropical streams of different sub-basins of the Cinzas River watershed, situated in southern Brazil. Based on RCC, we tested the hypothesis that macro-factors (productivity, current velocity, habitat structure and stream order and stretch (source, middle and mouth) are the main structuring factors of the zooplankton community in tropical streams. More specifically, the following predictions were tested: (i) the abundance of zooplankton organisms responds to the longitudinal gradient and stream order, with higher density at the mouth of streams where the lowest current velocity is recorded, while richness will be more influenced by environmental heterogeneity (i.e., site-by-site environmental variation) in different sections and streams; (ii) These patterns will be more pronounced in the dry season, as a function of greater channel stability and uniqueness of each compartment.
Material and methods
Study area
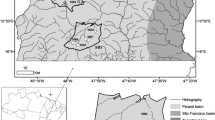
The study was conducted in three watersheds (Água das Araras, Eucalipto and Água da Divisa streams) belonging to the lower stretch of the Cinzas River basin, in the State of Paraná, Brazil (23° 10′ 51″ S, 50° 38′ 49″ W and 23° 9′ 3″ S, 50° 31′ 8″ W). The Cinzas River originates in the center and its mouth is in the northern region of Paraná State. Its basin covers an area of 9658 km2. The three watersheds are located in the geographical region of the third plateau of Paraná, which is characterized by its rugged terrain, with altitude varying between 600 and 700 m. All the environments are within an agricultural area, with a predominance of soybean, corn and wheat crops (Água das Araras stream and Eucalipto) and grapes, vegetables, legumes, citrus and corn (Água da Divisa stream) (Fig. 1).
One stream from the first, second, and third order according to Strahler (1957), was selected for each watershed (SM1), and three sampling points were located along each stream (source, middle course, and lower course or mouth). In this way, sampling was carried out at 27 points through the rainy (March) and dry (September) seasons, during the year 2014, totaling 54 samples. For this purpose, it was assumed that each sampling point, from streams of the same magnitude, would act as a replicate. All streams are located in rural areas, and some stretches have been heavily modified by anthropic activity, such as removal of riparian vegetation, flow control, and channelization.
Sampling
Zooplankton community
The zooplankton community was sampled by filtering the water collected from the subsurface of the environments, using a conical net with 68 µm mesh, with the aid of a 20L graduated bucket. Between 50 and 300L of water were filtered according to the characteristics of each sampling site. The collected material was put in 250 ml plastic bottles and preserved in 4% formaldehyde solution buffered with calcium borate (CaCO3). In the laboratory, the samples were processed according to Wetzel and Likens (2001) and Bicudo and Bicudo (2006), where each sample was concentrated to a volume of 50 ml. After homogenization and with the aid of a common optical microscope (Bioptika) and Sedgewick-Rafter chamber, 5 ml of each sample was analyzed. Identification of zooplankton organisms was performed, whenever possible, at the species level, based on morphological characteristics and with the help of specialized bibliography (Koste, 1978; Reid, 1985; Elmoor-Loureiro, 1997; Souza, 2008; Paggi, 1995; Siemensma, 2021).
Environmental Variables
For each sampling point, water was collected from the subsurface through a 5L plastic jug, for the determination of the physical and chemical variables: Ammoniacal Nitrogen, Nitrite, Total Dissolved Phosphorus, Reactive Phosphate (P-ortho) and chlorophyll-a, following the protocols described in APHA (2017). With the help of a Hanna HI 9828 multiparameter probe, environmental variables such as pH, electrical conductivity, dissolved oxygen, oxygen saturation (%), total dissolved solids and water temperature were measured. Water transparency was measured using a Secchi disk. The width and depth of each sampled site were also measured. Current velocity was estimated using a General Oceanics flow meter. We also gathered data from substrate type and macro-structures such as percentage of leaves, branches, trunks, and rocks, using a visual inspection of a square of 0.25 m2 in triplicates. All environemental variables used have been shown to influence zooplankton richness, abundance and composition (Lansac-Tôha et al., 2009).
Data analysis
We calculated the specific richness, through the total sum of species, and the abundance of organisms, expressed in individuals per cubic meter (ind.m-3), by the formula:
where N = n° organisms counted in the sample; VCo = concentrated volume; VCt = volume counted; VF = filtered volume in m3.
To evaluate prediction i), an analysis of variance (two-way ANOVA) was performed for zooplankton richness and abundance values in the different stream sections (source, middle, and mouth) and among stream orders (1st, 2nd, and 3rd order). In addition, a Pearson correlation analysis was performed to verify whether habitat heterogeneity would be the main regulating factor for zooplankton species richness. The values of habitat heterogeneity were obtained using the Shannon diversity index (Shannon & Weaver, 1949), calculated for the different habitat descriptor variables (substrate type and macro-structures such as: percentage of leaves, branches, trunks, rocks). Before running these analyses we checked for homogeneity and normality assumptions, and no data transformation was needed.
To evaluate the prediction (ii), an analysis of variance (one-way ANOVA) between the two periods (dry and rainy) of the study was performed to verify if the observed patterns would be more pronounced in the dry period.
A Redundancy Analysis (RDA) was performed using the site-by-site abundance matrix of zooplankton species and the site-by-site matrix of environmental variables, including flow, temperature, pH, electrical conductivity, dissolved oxygen, dissolved phosphorus, ammonia, total dissolved solids (TDS), and chlorophyll-a. We checked the multicollinearity with Variance Inflation Factor (VIF) and variables with VIF values higher than 10 were excluded from the analysis. In this case, conductivity and TDS showed collinearity and the latter was excluded.
We also performed a partial redundancy analysis to verify the unique contribution of environmental, spatial and stretch of the river components and their interactions. The spatial component was computed using distance-based Moran’s eigenvector maps (dbMEM,) from a geographic distance matrix. In order to minimize discrepant effects between variables, we used Hellinger transformation for biologic data, while abiotic data were previously standardized. All analyses were performed in the R program version 4.1 (2021), using the “stats” package to calculate the ANOVAs, “vegan” package for performing the RDA and partial RDA, “adespatial” to compute the MEMs and “ggplot2” for visualization. All results were tested at a significance level of p < 0.05.
Results
Taxonomic composition, species richness and abundance of zooplankton
A total of 101 taxa belonging to four taxonomic groups were recorded, with testaceous protozoa (51) standing out, followed by Rotifera (31), Cladocera (12) and Copepoda (7) (SM 2). Testate amoebae were represented by 7 families, the most species-rich being Arcellinidae, Centropyxidae and Difflugidae, while for rotifers the Brachionidae, with 10 species, and Lecanidae, with 8 species, stood out. The cladocerans were best represented by Daphnidae and Chydoridae, and the copepods by Cyclopidae, especially their young forms (SM 2).
Regarding the species recorded, Centropyxis aculeata, Centropyxis ecornis, Arcella vulgaris, Difflugia cylindrus (2.96%) and Difflugia tuberculata (2.87%) stood out among the testaceans. Among rotifers, the highest frequencies of occurrence were recorded for Lecane bulla, Bdelloidea, Platyonus patulus and Kellicottia bostoniensis. The cladocerans were best represented by Moina minuta, Daphnia gessneri, Ceriodaphnia silvestri, Alona glabra and Coronatella popei. For copepods, the larval forms (nauplii) of Calanoida and Cyclopoida were the most frequent.
The results obtained for species richness showed values between 1 and 25 species, with a mean of 9 species for the rainy season, and between 0 and 24 species, with a mean of 10 species for the dry season. These values were quite variable for the different orders and stream sections. Thus, the results of ANOVAs for total richness and for each zooplankton group did not show significant differences among orders, stretches or seasons, nor for interactions terms (Fig. 2; SM3; SM4). Moreover, Pearson’s correlation analysis (p < 0.05), which tested the relationship between species richness and habitat heterogeneity, showed no significant influence of this variable on the zooplankton community in these streams.
Variation in species richness of zooplankton among orders, stretches and periods of sampling, in tropical streams of southern Brazil. The central lines denote the median value, box denotes 25 and 75 percentiles, whiskers represent respectively the smallest and largest value within 1.5 times interquartile range below and above percentiles
For zooplankton abundance, the recorded values ranged from 30 to 8500 ind.m-3, averaging 1746 ind.m-3 for the rainy season, and from 0 to 3870 ind.m-3, averaging 1112 ind.m-3 for the dry season. When considering the different groups, testate amoebae were the ones that presented the highest mean density (about 60%), followed by rotifers (19%), while copepods represented 17% and cladocerans 4% of the zooplankton abundance. Temporally, a decreasing trend in the number of organisms was observed in the rainy season (Fig. 3b). Despite the observed trends, the results of the ANOVAs, which evaluated the effects of orders, stream stretches (source, middle and mouth) and sampling period on the abundance of the zooplankton community and its individual groups, did not show significant results, as was also observed for the interactions terms (Fig. 3; SM5; SM6).
Abundance of zooplankton among orders, stretches and periods of sampling, in tropical streams of southern Brazil. The central lines denote the median value, box denotes 25 and 75 percentiles, whiskers represent respectively the smallest and largest value within 1.5 times interquartile range below and above percentiles
Abiotic variables, zooplankton and the characterization of micro-basins
The results of the Redundancy Analysis (RDA) explained 31% of the total variability of the data. Furthermore, the percentage of explanation provided by the analysis was significant (p < 0.005). The greatest differences in physical and chemical characteristics and those related to zooplankton community structure were observed among the three sub-basins studied (Fig. 4).
Ordination diagram for the first two axes of the Redundancy Analysis (RDA), according to A the abiotic variables (temperature = Temp; electrical conductivity = Cond; dissolved oxygen = DO; pH; flux; secchi; chlorophyll = Chloro; ammonia = NH3 and total phosphorus = PT) and B the zooplankton species categorized by group (testate amoebae, rotifers, cladocerans and copepods)
Thus, on axis 1, a differentiation is observed between the sub-basin of the Divisa River, characterized by the highest values for conductivity (Cond) total phosphorus (PT), and the sub-basin of the Araras River, where the highest values of chlorophyll-a and current velocity were recorded. On the other hand, axis 2 of this analysis discriminated the samples from these first two sub-basins from those taken from the Eucalipto River sub-basin, characterized by higher orthophosphate values (Fig. 4).
Regarding the species, most of the rotifers were more positively correlated to axis 1, characterizing, in general, the samples from the Divisa River sub-basin, while most of the cladocerans and copepods were negatively correlated to this axis and positively correlated to axis 2, showing their higher abundance in the Eucalipto River sub-basin. In relation to the testate amoebae, these were mostly negatively related to axis 2, thus characterizing most of the samples obtained from the sub-basin of the Araras River (Fig. 4).
Influence of environmental and spatial variables on zooplankton structure
RDA results revealed that the contribution of environmental and spatial factors differed in explaining ciliates community structure between dry and rainy seasons (Fig. 5). According to the explanatory power (adjusted R2), the ciliate communities were more strongly determined by spatial (Spa) than environmental (Env) conditions in the dry season (4% and 1.5%, respectively), while in the rainy season environmental and spatial variables explained the same variation in the ciliates community (5.2% in both dry and rainy seasons). Meanwhile, the gradient of the river stretch component (Grad) showed low contribution in both seasons, especially during the rainy season. Besides, a great amount of variation remained unexplained.
Venn diagrams of partial redundancy analysis (pRDA) results for the dry (left) and rainy (right) seasons. Relative contribution (adjusted R2) of the environmental component (Env), the spatial component (Spa), stretch of river gradient (Grad), the shared components and residuals (R) that explain the variation in ciliates community structure
Discussion
Studies indicate the importance of abiotic and biotic factors at the local level (Dodson & Frey, 2001; Williamson and Reid, 2001), as well as regional processes, for example, at the watershed level (Heino et al., 2015a, 2015b; Gomes, Gomes, et al., 2020; Padovesi-Fonseca, 2021). In this study, the predictions that zooplankton would respond directly to local environmental factors, related to macrofactors determined by the longitudinal gradient of each stream, as well as by stream order and hydrological period, were all refuted. On the other hand, the results of RDA and the Analysis of Partition of Variance suggest the influence of geographic distance on community organization when considering the studied micro-watersheds.
Gomes, Gomes, et al. (2020) observed in a study on stream zooplankton of central Brazil that only the spatial variables were able to influence the composition of the zooplankton community, corroborating other studies that also found space to be the main factor in structuring of zooplanktonic communities (Soininen et al., 2018; Bie et al., 2012; Dejenie et al., 2012). They suggested that absence of connectivity between sampled sites can be a factor for the significant explanation of the spatial component. In this context, our study seems to corroborate this idea because we recorded a greater dissimilarity in community structure when space is analyzed among the micro basins. However, at basin level, the freeflow watercourse allows species to disperse passively, following the direction of the flow, which may increase community similarity due to the mass effects mechanism.
Dispersal allows the colonization of species in environments to which they are best adapted. Fragment dynamics assumes that the physical environment is spatially homogeneous and can be occupied and unoccupied over time according to the rates of colonization and extinction, and by interactions between species and dispersal (Rocha et al., 2020). However, according to Lansac-Tôha et al. (2019) neither species sorting nor dispersal mechanisms may shape aquatic communities. Rather, community structure may display a high degree of stochasticity caused by random colonization and extinction events (Hubbell, 2011). This is likely to be the case of phytoplankton inhabiting lotic systems, where the recurrent instability of the water column, continuous downstream flow and high turbulence may impose limits on their colonization, establishment and development (Bovo-Scomparin et al., 2013; Jati et al., 2017). This may, in turn, lead to unexpected absences at sites that are otherwise environmentally suitable, consequently weakening the action of environmental filtering (Heino et al., 2015a).
These challenging conditions imposed by a high-velocity current strongly limit the establishment of typically planktonic populations, resulting in a specific species composition of such systems. In these environments, there is usually a greater exchange of fauna between the aquatic compartments, which leads to a greater contribution of typically benthic or littoral organisms (Lansac-Tôha et al., 2009). Thus, in well-structured lentic environments, zooplankton is normally dominated by rotifers and microcrustaceans (cladocera and copepods), while in lotic ones it is, testate amoebae that contribute remarkably to zooplanktonic composition (Picapedra et al., 2019).
In fact, testate amoebae formed the most representative group in our study, both in richness and abundance, related mainly to the hydrodynamic characteristics of the environments. The streams analyzed are shallow and present a high-water flow, where interactions between the plankton compartment and the sediment, the preferred habitat of these organisms, are more pronounced, promoting a greater contribution of these organisms in the planktonic samples. Studying the occurrence of testate amoebae in the plankton samples, Velho et al. (2004) observed higher densities in compartments that presented hydrological characteristics similar to lotic environments (high current velocity, shallow and narrow). Other authors, such as Fulone et al. (2008), Portinho et al. (2016), Czerniawski and Kowalska-Góralska (2018), and Sartori et al. (2021), emphasized the importance of current velocity as the main intervening factor in the relative contribution of zooplankton groups in lotic environments.
Of the 20 families belonging to the zooplankton groups studied, Difflugidae, Arcellidae and Centropyxidae (testate amoebae), and Brachionidae, Lecanidae and Euchlanidae (rotifers) were the most representative. The high contribution of testate amoebae, especially of the families Difflugiidae, Arcellidae and Centropyxidae, in continental aquatic environments is well documented (Arrieira et al., 2015; Lansac-Tôha et al., 2014; Picapedra et al., 2019). Regarding rotifers, Brachionidae and Lecanidae are commonly highlighted among the families with more species in several Brazilian aquatic environments (Lansac-Tôha et al., 2004). Among copepods, the predominance of the young state (nauplii and copepodids) has been commonly found in several aquatic environments, including lotic systems (Picapedra et al., 2019; Silva et al., 2021).
The importance of resources in structuring zooplankton communities in streams has generally been neglected, with more attention being paid to the relationships of organisms and physical and chemical water variables (Lair, 2006). Thus, one of the biotic factors that often act on the distribution and abundance of organisms within the aquatic environment is resource availability, especially algal primary productivity, expressed by chlorophyll-a concentration. However, due to the lotic characteristics of the streams studied, as well as their low nutrient concentration, no significant influence of chlorophyll-a on the abundance of the zooplankton community was observed, nor for the different groups of this community. In general, substrate structure affects the interactions between the aquatic environment and organisms, in addition to the availability of nutrients, reducing the primary production of the medium (Czerniawski, 2013).
On the other hand, high-order lotic environments (higher than third order) are more conducive to the development of the planktonic community, mainly due to the greater distance between the banks. This allows greater incidence of light and thus greater primary productivity, which no longer occurs in environments lower than third order, which are narrow and therefore shaded by riparian vegetation (Segovia et al., 2017). Similarly, in the present study we did not observe a significant correlation between the abundance of zooplankton organisms and current velocity. As cited earlier, the presence of tecamebas in the plankton plot is attributed to hydrodynamic processes, such as resuspension from sediment and marginal vegetation (Alves et al., 2012).
The RDA results suggested that the major differences in physical and chemical characteristics, as well as those related to zooplankton community structure, respond to a larger spatial scale, that is, with the main differences observed among the three sub-basins studied.
In synthesis, the results obtained in the present research refute the hypotheses and predictions (i and ii) initially raised. Thus, nether order or stretch stream influenced richness and abundance of zooplankton assemblage, and this absence of influence did not differ between periods. Although zooplankton in lentic environments respond directly to factors such as hydrodynamics and productivity of the environment, in lotic ones, especially those of small size, like the ones analyzed here, it seems that spatial factors related to stochasticity in the structuring of planktonic communities are more important than environmental factors, and thus are the main drivers in the organization of such communities. Thus, our results suggest that connectivity between environments within the same sub-basin, associated with random processes driven by dispersal, determine the existence or not of spatial and temporal patterns in the community attributes analyzed here. However, further studies are needed on the importance of macrofactors in structuring aquatic biotic communities and how they may affect the different metrics of zooplankton diversity.
References
Alves, G. M., Velho, L. F. M., Costa, D. M., & Lansac-Tôha, F. A. (2012). Size structure of testate amoebae (Arcellinida and Euglyphida) in different habitats from a lake in the upper Paraná River floodplain. The European Journal of Protistology, 48, 169–177. https://doi.org/10.1016/j.ejop.2011.10.004
APHA. (2017). Standard methods for the examination of water and wastewater (23rd ed.). American Public Health Association.
Arrieira, R. L., Alves, G. M., Schwind, L. T. F., & Lansac-Tôha, F. A. (2015). Local factors affecting the testate amoebae community (Protozoa: Arcellinida; Euglyphida) in a neotropical floodplain. Journal of Limnology, 74(3), 444–452. https://doi.org/10.4081/jlimnol.2015.1078
Bicudo, C. E. M., & Bicudo, D. (2006). Amostragem em Limnologia. Editora Rima: São Carlos.
Bie, T., Meester, L., Brendonck, K., Martens, B., Goddeeris, D., Ercken, H., Hampel, L., Denys, L., Vanhecke, K., Van Der Gucht, J., Van Wichelen, W., & Vyverman, S. D. (2012). Body size and dispersal mode as key traits determining metacommunity structure of aquatic organisms. Ecology Letters, 15, 740–747. https://doi.org/10.1111/j.1461-0248.2012.01794.x
Blatterer, H. (2002). Some conditions for the distribution and abundance of ciliates (Protozoa) in running waters — Do we really find every species everywhere? Internationale Vereinigung Für Theoretische Und Angewandte Limnologie: Verhandlungen, 28, 1046–1049. https://doi.org/10.1080/03680770.2001.11901877
Bovo-Scomparin, V. M., Train, S., & Rodrigues, L. C. (2013). Influence of reservoirs on phytoplankton dispersion and functional traits: A case study in the Upper Paraná River, Brazil. Hydrobiologia, 702, 115–127. https://doi.org/10.1007/s10750-012-1313-8
Czerniawski, R., & Domagala, J. (2010). Similarities in zooplankton community between River Drawa and its two tributaries (Polish part of River Odra). Hydrobiologia, 638, 137–149. https://doi.org/10.1007/s10750-009-0036-y
Czerniawski, R., & Pilecka-Rapacz, M. (2011). Summer zooplankton in small rivers in relation to selected conditions. Central European Journal of Biology, 6, 659–674. https://doi.org/10.2478/s11535-011-0024-x
Czerniawski, R. (2013). Zooplankton community changes between forest and meadow sections in small headwater streams, NW Poland. Biologia, 68, 448–458. https://doi.org/10.2478/s11756-013-0170-x
Czerniawski, R., & Domagała, J. (2014). Small dams profoundly alter the spatial and temporal composition of zooplankton communities in running waters. International Review of Hydrobiology, 99(4), 300–311. https://doi.org/10.1002/iroh.201301674
Czerniawski, R., & Kowalska-Góralska, M. (2018). Spatial changes in zooplankton communities in a strong human-mediated river ecosystem. PeerJ. https://doi.org/10.7717/peerj.5087
Dejenie, T., Declerck, A. S., Asmelash, T., Risch, S., Mergeay, J., De Bie, T., & Meester, L. (2012). Cladoceran community composition in tropical semi-arid highland reservoirs in Tigray (Northern Ethiopia): A metacommunity perspective applied to young reservoirs. Limnologica, 42, 137–143. https://doi.org/10.1016/j.limno.2011.09.008
Diniz, L. P., Petsch, D. K., & Bonecker, C. C. (2021). Zooplankton β diversity dynamics and metacommunitystructure depend on spatial and temporal scales in a Neotropical floodplain. Freshwater Biology, 66, 1328–1342. https://doi.org/10.1111/fwb.13719
Dodson, S. I., & Frey, D. G. (2001). Cladocera and other Branchiopoda. In: Thorp, J. H., Covich, A. P. (Eds.) Ecology and classificartion of North American Freshwater Invertebrates. Academic Press, San Diego, pp 850–914. https://doi.org/10.1016/B978-012690647-9/50022-3
Ejsmont-Karabin, J., & Kruk, M. (1998). Effects of contrasting land use on free-natation rotifer Communities of stream in Masurian Lake District, Poland. In: Wurdak, E., Wallace, R., Segers, H. (Eds.) Rotifera VIII: A comparative approach. Developments in hydrobiology (Vol. 134). Springer, Dordrecht. https://doi.org/10.1007/978-94-011-4782-8_31
Elmi, D., Webster, D. R., & Fields, D. M. (2021). Response of the copepod Acartia tonsa to the hydrodynamic cues of small-scale, dissipative eddies in turbulence. Journal of Experimental Biology. https://doi.org/10.1242/jeb.237297
Elmoor-Loureiro, L. M. A. (1997). Manual de Identificação de Cladóceros Límnicos do Brasil. Universal.
Ferreira, F. C., Souza, U. P., & Petrere, J. R. M. (2010). Zonação longitudinal da ictiofauna em ambientes lóticos. Boletim Da Sociedade Brasileira De Limnologia, 38, 1–17.
Fulone, L. J., Vieira, L. C. G., Velho, L. F. M., & Lima, A. F. (2008). Influence of depth and rainfall on testate amoebae (Protozoa-Rhizopoda) compositions from two streams in northwestern São Paulo state. Acta Limnologica Brasiliensia, 20(1), 29–34.
Golec-Fialek, C., Lansac-Tôha, F. M., & Bonecker, C. C. (2021). Response of the zooplankton community to extreme hydrological variations in a temporary lake in a neotropical floodplain system. Limnologica, 86, 125834. https://doi.org/10.1016/j.limno.2020.125834
Gomes, A. C. A. M., Gomes, L. F., Roitman, I., Pereira, H. R., Junior, A. F. C., da Costa, E. M. M., Silva, M. L. C., Jacobson, T. K. B. J., Ribeiro, R. J. C., Miranda Filho, R. J., Avila, M. L., & Vieira, L. C. G. (2020). Forest cover influences zooplanktonic communities in Amazonian streams. Aquatic Ecology, 54, 1067–1078. https://doi.org/10.1007/s10452-020-09794-6
Gomes, L. F., Barbosa, J. C., Barbosa, H., Vieira, M. C., & Vieira, L. C. G. (2020). Environmental and spatial influences on stream zooplankton communities of the Brazilian Cerrado. Community Ecology, 21(1), 25–31. https://doi.org/10.1007/s42974-020-00008-5
Gutierrez, M. F., Simões, N. R., Frau, D., Saigo, M., & Licursi, M. (2020). Responses of stream zooplankton diversity metrics to eutrophication and temporal environmental variability in agricultural catchments. Environmental Monitoring and Assessment, 192, 1–17. https://doi.org/10.1007/s10661-020-08766-5
Heino, J., Melo, A. S., & Bini, L. M. (2015a). Recomceptualising the beta adversity-environmental heterogeneity relationship in running water systems. Freshwater Biology, 60, 223–235. https://doi.org/10.1111/fwb.12502
Heino, J., Melo, A. S., Bini, L. M., Altermatt, F., Al-Shami, S. A., Angeler, D. G., et al. (2015b). A comparative analysis reveals weak relationships between ecological factors and beta diversity of stream insect metacommunities at two spatial levels. Ecology and Evolution, 5, 1235–1248. https://doi.org/10.1002/ece3.1439
Hubbell, S. P. (2011). A teoria neutra unificada da biodiversidade e biogeografia (MPB-32). Princeton University Press. https://doi.org/10.1515/9781400837526
Illies, J., & Botosaneanu, L. (1963). Problèmes et méthodes de la classification et de la zonation écologique des eaux courantes, considerées surtout du point de vue faunistique: Avec 18 figures dans le texte et en supplément. Internationale Vereinigung Für Theoretische Und Angewandte Limnologie: Verhandlungen, 12, 1–57. https://doi.org/10.1080/05384680.1963.11903811
Jati, S., Bortolini, J. C., Moresco, G. A., Paula, A. C. M. D., Rodrigues, L. C., Iatskiu, P., Pineda, A., Zanco, B. F., Silva, M. V., & Souza, Y. R. (2017). Phytoplankton community in the last undammed stretch of the Paraná River: Considerations on the distance from the dam. Acta Limnologica Brasiliensia. https://doi.org/10.1590/S2179-975X4017
Kwok, K. W., Leung, K. M., Lui, G. S., Chu, V. K., Lam, P. K., Morritt, D., Maltby, L., Brock, T. C. M., Van den Brink, P. J., Warne, M. S. J., & Crane, M. (2007). Comparison of tropical and temperate freshwater animal species’ acute sensitivities to chemicals: implications for deriving safe extrapolation factors. Integrated Environmental Assessment and Management: an International Journal, 3(1), 49–67. https://doi.org/10.1002/ieam.5630030105
Koste, W. (1978). Rotatoria: Die Rädertiere Mitteleuropas. Ein Bestimmungswerk berg. Von Max Voigt. Überordnung Monogononta. Volume I-II. Gebrüder Borntraaeger, Berlin
Krudglova, A. N. (2008). Planktonic fauna of small salmon rivers in the Kola Peninsula. Inland Water Biology, 1, 8–13. https://doi.org/10.1134/S1995082908030024
Lair, N. (2005). Abiotic against biotic factors: lessons drawn from rotifers in the Middle Loire, a meandering river monitored from 1995 to 2002, during low flow periods. Hydrobiology, 546, 457–472. https://doi.org/10.1007/1-4020-4408-9_48
Lair, N. (2006). A review of regulation mechanisms of metazoan plankton in riverine ecosystems: Aquatic habitat versus biota. River Research and Applications, 22, 567–593. https://doi.org/10.1002/rra.923
Lampert, W., & Sommer, U. (2007). Limnoecology: The ecology of lakes and streams. Oxford University Press.
Lansac-Tôha, F. A., Bonecker, C. C., Velho, L. F. M., Simões, N. R., Dias, J. D., Alves, G. M., & Takahashi, E. M. (2009). Biodiversity of zooplankton communities in the Upper Paraná River floodplain: Interanual variation from long-term studies. Brazilian Journal of Biology, 69, 539–549. https://doi.org/10.1590/S1519-69842009000300009
Lansac-Tôha, F. A., Velho, L. F. M., Costa, D. M., Simões, N. R., & Alves, G. M. (2014). Structure of the testate amoeba community in different habitats in a neotropical floodplain. Brazilian Journal of Biology, 74, 181–190. https://doi.org/10.1590/1519-6984.24912
Lansac-Tôha, F. A., Velho, L. F. M., & Bonecker, C. C. (2004). Composition, species richness and abundance of the zooplankton community. In S. M. Thomaz, A. A. Agostinho, & N. S. Hahn (Eds.), The Upper Paraná River and its floodplain: Physical aspects, ecology and conservation (pp. 145–190). Backhuys Publishers.
Lansac-Tôha, F. M., Heino, J., Quirino, B. A., Moresco, G. A., Zapata, O. E. P., Meira, B. R., Rodrigues, L. C., Jati, S., Lansac-Tôha, F. A., & Velho, L. F. M. (2019). Differently dispersing organism groups show contrasting beta diversity patterns in a dammed subtropical river basin. Science of the Total Environment, 691, 1271–1281. https://doi.org/10.1016/j.scitotenv.2019.07.236
Padovesi-Fonseca, C., Souza Rezende, R., Da Costa, D. F., & Martins-Silva, M. J. (2021). Spatial scales drive zooplankton diversity in savanna Cerrado streams. Community Ecololgy, 1, 1–11. https://doi.org/10.1007/s42974-021-00052-9
Paggi, J. C. (1995). Crustacea Cladocera. In: Lopretto EC, Tell G (Eds.). Ecosistemas de aguas continentales. Metodologías para su estúdio (pp. 909–951). Sur, La Plata.
Perbiche-Neves, G., Serafim-Júnior, M., Portinho, J. L., Shimabukuro, E. M., Ghidini, A. G., & Brito, L. (2012). Effect of atypical rainfall on lotic zooplankton: Comparing downstream of a reservoir and tributaries with free stretches. Tropical Ecology, 53, 149–162.
Picapedra, P. H. S., Fernandes, C., & Lansac-Tôha, F. A. (2017). Zooplankton community in the Upper Parnaíba River (Northeastern, Brazil). Brazilian Journal of Biology, 77, 402–412. https://doi.org/10.1590/1519-6984.20215
Picapedra, P. H. S., Fernandes, C., & Baumgartner, G. (2019). Structure and ecological aspects of zooplankton (Testate amoebae, Rotifera, Cladocera and Copepoda) in highland streams in southern Brazil. Acta Limnologica Brasiliensia. https://doi.org/10.1590/S2179-975X2917
Portinho, J. L., Perbiche-Neves, G., & Nogueira, M. G. (2016). Zooplankton community and tributary effects in free-flowing section downstream a large tropical reservoir. International Review of Hydrobiology, 101, 48–56. https://doi.org/10.1002/iroh.201501798
Pryor, S. C., Barthelmie, R. J., & Kjellström, E. (2005). Potential climate change impact on wind energy resources in northern Europe: Analyses using a regional climate model. Climate Dynamics, 25, 815–835. https://doi.org/10.1007/s00382-005-0072-x
Reid, J. W. (1985). Chave de identificação e lista de referências bibliográficas para as espécies continentais sulamericanas de vida livre da ordem Cyclopoida (Crustacea, Copepoda) (pp. 95–107). São Paulo, Instituto de Biociências da Universidade de São Paulo. https://doi.org/10.11606/issn.2526-3358.bolzoo.1985.122293
Rocha, B. D. S., Souza, C. A. D., Machado, K. B., Vieira, L. C. G., & Nabout, J. C. (2020). The relative influence of the environment, land use, and space on the functional and taxonomic structures of phytoplankton and zooplankton metacommunities in tropical reservoirs. Freshwater Science, 39, 321–333. https://doi.org/10.1086/708949
Sampaio, E. V., Rocha, O., Matsumura-Tundisi, T., & Tundisi, J. G. (2002). Composition and abundance of zooplankton in the limnetic zone of seven reservoirs of the Paranapanema River, Brazil. Brazilian Journal of Biology, 62, 525–545. https://doi.org/10.1590/S1519-69842002000300018
Sartori, M., Martins, B. A., & Perbiche-Neves, G. (2021). A variação da diversidade de microcrustáceos (Cladocera e Copepoda) a jusante de pequenos reservatórios é influenciada por táxons litorâneos. Iheringia, Série Zoológica. https://doi.org/10.1590/1678-4766e2021004
Segovia, B. T., Dias, J. D., Cabral, A. F., Meira, B. R., Lansac-Tôha, F. M., Lansac-Tôha, F. A., Bini, L. M., & Velho, L. F. M. (2017). Common and rare taxa of planktonic ciliates: Influence of flood events and biogeographic patterns in Neotropical floodplains. Microbial Ecology, 74, 522–533. https://doi.org/10.1007/s00248-017-0974-2
Silva, N. J., Lansac-Tôha, F. M., Lansac-Tôha, F. A., Sales, P. C. L., & Rocha, J. R. S. (2021). Beta diversity patterns in zooplankton assemblages from a semiarid river ecosystem. International Review of Hydrobiology, 106, 29–40. https://doi.org/10.1002/iroh.201902018
Shannon, C. E., & Weaver, W. (1949). The mathematical theory of communication. University of Illinois Press.
Siemensma, F. J. (2021). Microworld, world of amoeboid organisms. World-wide electronic publication, Kortenhoef, the Netherlands. https://arcella.nl. Accessed March 04, 2022.
Soininen, J., Heino, J., & Wang, J. (2018). A met-analysis of nestedness and turnover components of beta diversity across organisms and ecosystems. Global Ecology and Biogeography, 27, 96–109. https://doi.org/10.1111/geb.12660
Souza, M. B. G. (2008). Guias das Tecamebas, Bacia do Rio Peruaçu – Minas Gerais. Subsídio para conservação e monitoramento da bacia do Rio São Francisco. UFMG, Belo Horizonte.
Statzner, B., & Higler, B. (1985). Questions and comments on the river continuum concept. Canadian Journal of Fisheries and Aquatic Sciences, 42, 1038–1044. https://doi.org/10.1139/f85-129
Strahler, A. N. (1957). Quantitative analysis of watershed geomorphology. Eos, Transactions American Geophysical Union, 38, 913–920. https://doi.org/10.1029/TR038i006p00913
Thorp, J. H., Thoms, M. C., & Delong, M. D. (2006). The riverine ecosystem synthesis: Biocomplexity in river networks across space and time. River Research and Applications, 22, 123–147. https://doi.org/10.1002/rra.901
Vanotte, R. L., Minshall, G. W., Cummins, K. W., Sedell, J. R., & Cushing, C. E. (1980). The river continuum concept. Canadian Journal of Fisheries and Aquatic Sciences, 37, 130–137. https://doi.org/10.1139/f80-017
Velho, L. F. M., Alves, G. M., Lansac-Tôha, F. A., Bonecker, C. C., & Pereira, D. G. (2004). Testate amoebae Abundance in plankton samples from Paraná state reservoirs. Acta Scientiarum, 26, 415–419. https://doi.org/10.4025/actascibiolsci.v26i4.1522
Wetzel, R. G., & Likens, G. E. (2001). Limnological analyses (2nd ed.). New York: Springer. https://doi.org/10.1007/978-1-4757-3250-4
Willianson, C. E., Reid, C. J. (2001). Copepoda. In: Thorp, J. H., & Covich, A. P. (Eds.) Ecology and classification of North American Freshwater Invertebrates. San Diego. Academic Press. pp 915–924. https://doi.org/10.1016/B978-0-12-690647-9.50028-4
Wohl, E. (2017). Connectivity in rivers. Progress in Physical Geography, 41, 345–362. https://doi.org/10.1177/0309133317714972
Acknowledgements
We thank the Araucária Foundation for funding and granting the scholarship; Nupélia/PEA/UEM and UENP for the logistical support for the execution of this work; the CI trainees of GERCOL/LaMoCEQ for the help during the collections. We also thank to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for research scholarships for Lansac-Tôha, F. M, and Bianca Ramos de Meira and support the research developed by Velho, L. F. M., and the Cesumar Institute of Science, Technology, and Innovation (ICETI) for granted the researcher Velho, L. F. M. On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Aggio, C.E.G., Oliveira, F.R., Progênio, M. et al. The zooplankton of tropical streams: is it determinism or stochasticity that drives the spatial and temporal patterns in community structure?. COMMUNITY ECOLOGY 23, 219–229 (2022). https://doi.org/10.1007/s42974-022-00099-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42974-022-00099-2