Abstract
Zooplankton is an important community in aquatic ecosystems due to its linkage between primary producers and secondary consumers, and by their key role in cycling organic materials. However, a low number of studies were performed in small lotic systems, mainly in savanna Cerrado headwater systems. Therefore, our objective was to investigate α and β diversities of the zooplankton community along 21 headwater streams in relation to micro-basin and sub-basin spatial scales and the water physicochemical parameters. Our hypotheses are that α diversity is negatively related to the increase on spatial scale and β diversity is positively related to the increase on scale. Zooplankton samples were taken using a bucket and filtered 200 L by a 64 µm mesh plankton net and preserved for subsequent identification. Water temperature, dissolved oxygen, pH, electrical conductivity, ions, and anions (nutrients) were measured. A total of 2650 individuals (m−3) from 19 taxa were collected. The most abundant taxa were Cyclopoida (copepodite; 24.4%) and rotifer Tricocerca cylindrica (17.7%). The lower spatial scale was the most important factor driving the α diversity. However, the richness of the zooplankton community did not change among sub-basins and micro-basins. Changes on species composition by nested spatial organization between habitat patches drove the β diversity. The communities from the same sub-basin and micro-basin were more structurally related than those from different basins. Finally, closer points show similar environmental characteristics and electrical conductivity concentration increases the species richness. Therefore, patterns of species diversity are essential to understand community ecology, as well as to provide information for conservation planning on savanna Cerrado streams.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Zooplankton must be considered an essential quality tool for environmental conservation in aquatic systems (Chiba et al., 2018; Rocha, 2020). This is possible by the cosmopolite presence of the zooplankton community in freshwater habitats of tropical systems, mainly in lentic and large lotic systems (Kumar et al., 2011). The usage of zooplankton as a tool in environmental conservation demands understanding the community functioning (Chiba et al., 2018; Padovesi-Fonseca & Rezende, 2017). Many recent studies on the freshwater zooplankton community have been conducted in Brazil (Picapedra et al. 2016, 2019; Gomes et al., 2020). However, few studies were performed in small lotic systems (Brito, 2020; Xiong et al., 2020), mainly in tropical headwater systems (Picapedra et al., 2016, 2019; Gomes et al., 2020), compared to tropical lentic systems (Lopes et al., 2019; Pinese et al., 2015). The zooplankton community of lakes and ponds of the Cerrado, the largest neotropical savanna, are fairly well investigated (Lopes et al., 2014, 2019; Padovesi-Fonseca & Rezende, 2017). Therefore, surveying the fauna in streams of savanna Cerrado systems configure an important strategy to headwaters conservation of aquatic ecosystems (Padovesi-Fonseca et al., 2016).
The nuclear region of this neotropical savanna that is located in the Central Brazilian Plateau was also the divisor between the main drainage basins from Brazil and South America continent (Fonseca et al., 2014). Inland waters of savanna Cerrado tend to exhibit a conspicuous environmental heterogeneity (Feio et al., 2018; Rezende et al., 2014), encompassing a high zooplankton diversity (Gomes et al., 2020; Lopes et al., 2019). The headwaters originated in uplands naturally flow toward the basins, most of the time forming ecological corridors for many species (Bambi et al., 2017; Durães et al., 2016; Rezende et al., 2017). Based on the expected high environmental heterogeneity of the aquatic systems located in uplands and protected areas, their permanent preservation has been a challenge for shelter of endemic and endangered species, revealing a high biodiversity patrimony (Rezende, Biasi, et al., 2019). In this way, studies on zooplankton community composition (mainly by local and regional diversity) and abiotic water conditions are relevant to headwaters conservation in areas of savanna Cerrado.
The local or alpha diversity (α), in a strict sense, may be represented in most common approaches by: (1) richness in species or number of taxa; and (2) the slope measurements by importance-value sequence through the diversity index, in ecological studies (Legendre et al., 2005; Whittaker, 1960). On the other hand, the regional or beta (β) diversity measures the degree of species turnover and the ratio among total and mean number of species per sample to show a spatial variation in species composition among sites (Anderson et al., 2011; Whittaker, 1960). The differences in communities on space or range of habitat are shown by β diversity (Anderson et al., 2011; Whittaker, 1960). Finally, there is also the total, or gamma diversity (γ) (Whittaker, 1960, 1972). From these indexes, the β diversity is an important estimate to understanding the spatial richness patterns of the zooplankton community (Lopes et al., 2014, 2019). The β diversity origin for running waters conceptual framework may occur by two general forms in a diversity–environmental heterogeneity relationship (Heino et al., 2015; Legendre et al., 2005). First, local environmental control (lower spatial scale as riffle sites) within each region unit (higher spatial scale as a basin). Second, relative importance of spatial level, changing the local species composition (i.e., among riffle sites) within each region unit (Heino et al., 2015; Legendre et al., 2005).
Once the aforementioned diversity analysis is scale-based, different patterns and structures are expected (Wiens, 1989). More specifically, the β diversity may increase at a higher spatial scale by: (1) the effect of dispersal limitation; (2) high environmental heterogeneity; (3) the sampling of different regional species’ pools; and (4) a negative relationship between the pairwise similarity in assemblage composition and geographic distance (Cottenie, 2005; Rezende, Biasi, et al., 2019). This organized view of spatial variation among and within stream systems in a hierarchical framework is also known as “riverscapes” (for more see also Frissell et al., 1986).
High transparence and low nutrients in water are the general characteristics of the streams situated in Brazilian savannah (Fonseca et al., 2014). In this way, it is important to investigate the environmental characteristics that drive zooplankton community in tropical systems. Therefore, our goals were to study α and β diversities of the zooplankton community along tropical streams at two spatial scales (micro-basin and sub-basin) and evaluate the effects of water physicochemical variables on zooplankton community structure. Our hypotheses are that (1) α diversity is negatively related to the increase in the spatial scale and, in opposite way, β diversity is positively related to the scale; and (2) stream systems that are more nutrient rich in their environmental gradient will be more similar and would have high species diversity and correlate species.
Methods
Study sites
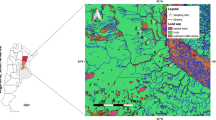
This study was conducted in 21 headwater streams (first to third order) with preserved riparian vegetation, located in three legally protected areas in the Brazilian Federal District (Fig. 1): sub-basin 1 in the Gama and Cabeça-de-Veado Environmental Protection Area (APA), sub-basin 2 in the Águas Emendadas Ecological Station (AE), and the sub-basin 3 in the Brasília National Park (PNB). The distance between the studied areas varies between 10 and 35 km, while distance within areas varies from 0.22 to 17 km.
The streams are located within the climatic area of the Cerrado (tropical savanna, Aw), with a hot and rainy season from October to April and a cold and dry season from May to September. The selected streams were separated into seven catchments, which represent important watercourses that cover the preserved areas of the Federal District. Sampling was undertaken during the dry season, in three streams stretches (20 m apart) per sampling point, between June and August 2015.
Procedures
Physical and chemical parameters of water
A set of seven in situ measurements was obtained in triplicates per stream: depth (m), width (m), temperature (°C), pH (pHmeter PHTEK®, Curitiba, PR, BR), electrical conductivity (μS cm−1; Conductivimeter Quimis®, Diadema, SP, BR), dissolved oxygen (mg L−1; Jenway 970 Dissolved Oxygen Meter, Staffordshire, OSA, UK), and canopy cover [%; digital camera (Nikon D5100) with a 10-mm Fisheye lens (Sigma)]. Water samples were collected to determine chemical concentrations by ion chromatography according to the methods described in APHA (1999).
Zooplankton community
Zooplankton sampling was taken by filtering 100 L of water through 64 μm mesh plankton net and preserved in 4% formalin. All samples are deposited in the freshwater plankton collection at the Laboratory of Limnology, Department of Ecology, University of Brasília. Three 1 mL subsamples were counted in a Sedgewick-Rafter chamber for rotifers. Cladocera and Copepoda were counted under a stereomicroscope at 25 × magnification. Specimens were observed using an optical microscope (200 and 400 × magnification) and identified following specialized bibliography (Koste, 1978; Elmoor-Loureiro et al., 2015).
Estimating α and β diversities
The number of invertebrates’ taxa (identified to the lowest taxonomic level possible) at all sampling sites was used to estimate the α diversity. We estimated the β diversity by implementing a multivariate dispersion method (Anderson et al. 2006). Multivariate dispersion [function “betadiver,” vegan package for R version 2.0.8; (Oksanen et al., 2013a)] estimated the β diversity as sites average dissimilarity (i.e., distance) from the centroid of their group in a multivariate space. The comparison among micro- and sub-basins (βgl) was based on Lennon et al. (2001), as proposed by Koleff et al. (2003). The βgl values depend on the difference in the number of taxa between the two quadrats under consideration and were employed to test whether the other β diversity measures serve to recover patterns in the local number of taxa gradients (Koleff et al., 2003; Lennon et al., 2001).
Statistical analysis
To verify the independence of the sampling points on sub-basin scale, the correlation between community composition and geographic distance was tested using a Mantel test (Oksanen et al., 2013b). The α diversity (number of taxa) in the zooplankton community was examined with a nested analysis of variance (Bailey, 1992) to detect the importance at nested spatial scales: sub-basin and micro-basin (nested into sub-basin). The abiotic variables (water temperature, water flow and concentration of pH, electrical conductivity, oxygen dissolved, TDS, sodium, potassium, calcium, magnesium, nitrate, sulfate, fluoride, chloride, and bromide in water) were also tested by nested analysis of variance among sub-basin and micro-basin. A Permutational Multivariate Analysis of Variance (PerMANOVA) was used (distance matrix of Bray–Curtis, 10,000 permutation and with pseudo-F; adonis function from vegan package for R; Oksanen et al. 2008) to estimate the difference in β diversity (different axes related to the distance from the centroid; function “betadiver” from vegan package for R version 2.0.8) among the scales. In this analysis, we tested the dispersion differences and not the location differences on the multivariate space (Heino et al. 2013). The sum of squares percentage in the nested ANOVA and PerMANOVA analyses was used to determine which accounted for the higher variance among the different scales for α and β diversity (for more see also Anderson, 2001).
The physical and chemical parameters of water (standardized) were filtered by the variance inflation factor (vif function from usdm package for R). Variance inflation factor exclude the highly correlated variables from the set through a stepwise procedure to deal with multicollinearity problems (Dormann et al. 2013). A Redundancy Analysis (RDA) was used to detect variations in community composition along the environmental gradient (standardized) and to identify potential environmental requirements to differentiate the assemblages (Hellinger transformed) (rda function from vegan package for R; Legendre & Legendre, 2012). The statistical significance of the correlation between the environmental features and biotic variables extracted from the RDA was determined by a Monte Carlo test based on 5000 permutations (p < 0.05; envfit function from R). A PerMANOVA was also used (Oksanen et al., 2008) to test the difference of community composition among the micro-basin (1–7).
Results
Physical and chemical parameters of water
In streams of all sub-basins (Table 1), we found low mean values of water temperature (19.8 ± 1.24 °C; standard deviation—s.d.), total dissolved solids (TDS; 4.28 ± 3.36 mg L−1 s.d.), electrical conductivity (9.18 ± 5.76 µS cm2 s.d.), and neutral pH (7.59 ± 0.75 s.d.). On the other hand, we found high mean values of dissolved oxygen (8.59 ± 0.34 mg L−1 s.d.) and water flow (0.65 ± 0.21 m s−1 s.d.). We also measured the concentrations of sodium (0.18 ± 0.03 mg L−1 s.d.), potassium (0.14 ± 0.03 mg L−1 s.d.), calcium (0.98 ± 1.11 mg L−1 s.d.), magnesium (0.81 ± 0.12 mg L−1 s.d.), nitrate (0.02 ± 0.01 mg L−1 s.d.), sulfate (0.09 ± 0.01 mg L−1 s.d.), fluoride (0.01 ± 0.01 mg L−1 s.d.), chloride (0.13 ± 0.03 mg L−1 s.d.), and bromide (0.65 ± 0.21 mg L−1 s.d.).
The water temperature shows difference among micro-basins (F = 6.83; p = 0.002), with highest values in micro-basin 1 and micro-basin 6 compared to others. However, no difference on water temperature among sub-basins (F = 0.02; p = 0.971) was found. The pH differs among micro-basin (F = 14.16; p < 0.001) with highest values in micro-basin 5 and micro-basin 4 compared to the other micro-basins. The pH differs also among sub-basin (F = 9.56; p = 0.002) with highest values in sub-basin 2 compared to others. On the other hand, the concentration of total dissolved solids (F = 1.54; p = 0.248; F = 0.35, p = 0.315), electrical conductivity (F = 1.16; p = 0.341; F = 1.31; p = 0.315), TDS (F = 0.55; p = 0.584; F = 1.03; p = 0.423), water flow (F = 0.18; p = 0.831; F = 0.31, p = 0.864), concentrations of sodium (F = 0.18; p = 0.835; F = 1.19, p = 0.354), potassium (F = 0.16; p = 0.853; F = 0.61, p = 0.667), calcium (F = 1.51; p = 0.255; F = 1.01, p = 0.421), magnesium (F = 1.26; p = 0.313; F = 1.41, p = 0.282), nitrate (F = 0.85; p = 0.446; F = 0.22, p = 0.918), sulfate (F = 0.03; p = 0.962; F = 1.16, p = 0.369), fluoride (F = 2.18; p = 0.151; F = 0.34, p = 0.845), chloride (F = 0.286; p = 0.755; F = 2.47, p = 0.092), and bromide (F = 1.18; p = 0.335; F = 0.077, p = 0.562) did not differ among sub-basins (n = 21; degree of freedom used = 2) and micro-basins (n = 21; degree of freedom used = 4), respectively.
Zooplankton community
A total of 2650 individuals (m−3) from 19 taxa were collected (Table 2). The most abundant taxa were Cyclopoida (copepodites; 24.4%) and rotifer Tricocerca cylindrica (17.7%). The richness ranged from two taxa in sub-basin 2 to 16 taxa in sub-basin 3. Cladocera represented 64.7% of the sampled taxa, followed by Copepoda and Rotifera, with 17.6% each one.
Higher values of Shannon–Wiener and Simpson indexes were found in micro-basins 2, 3, and 7 (Figure SM1, A and B). The Pielou’s Evenness showed higher values in micro-basins 2 and 3, and maximum values in micro-basins 6 and 7 (Figure SM1, C). Sub-basins 1 and 3 showed mean values of Shannon–Wiener and Simpson indexes near to one, while low diversity indexes were obtained in sub-basin 2 (Figure SM2, A and B). Higher values of Pielou’s Evenness were obtained by sub-basin 1 and sub-basin 3 that reached 50% of evenness (Figure SM2, C).
The α and β diversities in spatial scales
The Mantel test between sub-basin 1 and sub-basin 2 (Correlation R = 0.0517; p = 0.318), sub-basin 1 and sub-basin 3 (Correlation R = 0.0894; p = 0.196) and sub-basin 2 and sub-basin 3 (Correlation R = 0.1487; p = 0.143) showed no significant difference. Therefore, no correlation between taxa composition and geographic distance among sampling points on the sub-basin scale was observed by the Mantel test.
Micro-basin level was the most important spatial scale for zooplankton α diversity, compared to sub-basin level and interaction between these two factors. High importance of micro-basin level was observed due to the higher variance explanation in this scale represented on the percentage of sum of squares (Table 3A; Fig. 2). Higher richness values were found in micro-basin 2 and 7 compared to the general average (dotted line in Fig. 2A). Based on the sub-basin level, all values were below the general average (dotted line in Fig. 2B). However, the richness of the zooplankton community did not change among micro-basins, sub-basins, and interaction between these factors (Table 3A).
The α diversity estimated by taxa number (A, C) and β diversity (B, D) of the zooplankton community in the micro-basin (A, B) and sub-basins (C, D) of tropical streams. Average values (bold line), maximum and minimum (bars), third and first quartile (box). If the notches of two plots do not overlap this is ‘strong evidence’ that the two medians differ (for more see also Chambers et al. 1983, p. 62)
The β diversity was mainly explained by the micro-basin level, followed by interaction between factors and sub-basin level (regional scale; Table 3B; Fig. 2). The highest β diversity values were found in micro-basins 1, 2, and 7 compared to the others (Table 3B). On sub-basin level, sub-basin 2 shows the lowest values compared to sub-basin 1 and sub-basin 3 (Table 3B). However, the β diversity of zooplankton community did not change in response to the interaction between these factors (Table 3B).
Abiotic factors that structure the zooplankton community
The RDA (total inertia of 0.802) of the abiotic variables and the community exhibited high explanatory power (70%, inertia of 0.567). Axis 1 of the RDA ordination explained 28% (inertia of 0.223) of the total variance, while axis 2 explained 13% (inertia of 0.107). The structure and composition of zooplankton community change among micro-basin (PerMANOVA, F(6,15) = 1,34; p = 0.047).
Only pH, oxygen dissolved, TDS, and sulfate concentration in water in a stream of micro-basin 2 were correlated positively with axis 1 and 2 (Fig. 3). Chloride concentration in water and Alona ossiani, Thermocyclops sp.(female), Cyclopoida (copepodite) and Calanoida (copepodite), and in streams of micro-basin 4 and 6 were correlated negatively with axis 2 and positively with axis 1 (Fig. 3).
The values of water temperature, water flow and concentration of electrical conductivity, bromide, fluoride, sodium, magnesium and nitrate in water, as also Acroperus tupinambá, Alona cf.guttata, Alona yara, Euchlanis dilatate, Flavalona iheringula, Lecane bulla, Ovalona glabra, and Tricocerca cylindrica were correlated negatively with axis 1 and positively with axis 2 in streams of micro-basin 1, 2, 3, 5, and 7 (Fig. 3).
Alonella clathratula, Bosmina hagmanni, Ceriodaphnia cornuta, Ilyocryptus spinifer, Nicsmirnovius paggi, and Harpacticoida (copepodite) were correlated negatively with axis 1 and 2 in streams of micro-basin 6 (Fig. 3). Finally, electrical conductivity and magnesium concentration in water were statistically significant to structure the composition of zooplankton community extracted from the RDAs vectors (Monte Carlo test; Table 1).
Discussion
The lower spatial scales (micro-basin) seem to drive the local (α) and regional (β) diversities of the zooplankton community. The importance of lower spatial scales may be explained by a sum of two main factors: the passive dispersal of zooplanktonic organisms (Karna et al., 2015; Padial et al., 2014) drive by the connectivity among sampling points (Brito et al., 2020; Durães et al., 2016); and niche characteristics (Lopes et al., 2019; Rezende et al., 2014), such as environmental heterogeneity (Brito et al., 2020; Pinel-Alloul, 1995) and environmental stability by protective function of riparian vegetation (Van Onsem et al., 2010; Xiong et al., 2020). Higher importance of lower spatial scales was also corroborated by other aquatic communities and ecological processes in streams (Graça et al., 2015; Rezende Petrucio et al., 2014; Rezende, Biasi, et al., 2019). This result also highlights the role of zooplankton community as useful bioindicators and a helpful tool for monitoring water quality (Chiba et al., 2018; Kumar et al., 2011).
Zooplankton richness in the studied streams (19 taxa) was lower compared to other tropical lotic systems (ranged from 97 to 170 species in Picapedra et al., 2016, 2019; Gomes et al., 2020). Most taxa found in this study were previously recorded in streams, except by Lecane bulla, Trichocerca cylindrica, Euchlanis dilatate, Bosmina hagmanni (ecological details summarized in https://cladocera.wordpress.com). Low zooplankton richness may be explained by the association of these organisms with backwaters (rare habitat in headwaters streams compared to rivers) and with preference for lentic environments (Pearson & Duggan, 2018). Therefore, due to the zooplankton preference for backwaters, this community shows low densities in lotic environments and is dominated by protozoans, small cladocerans, rotifers, and copepoda (Perbiche-Neves et al., 2012; Picapedra et al., 2019), as also observed in this study. Additionally, the zooplankton community shows high dependence on nutrients dissolved in water (Padovesi-Fonseca & Rezende, 2017). This result is corroborated by the high species richness related to nutrients significantly important for structuring the zooplankton community, mainly by electrical conductivity (8 zooplankton taxa), a nutrient concentration proxy in stream water (Rezende et al., 2014). However, headwater streams of savanna Cerrado present a low concentration of nutrients (Fonseca et al., 2014), explaining the low density, diversity, and richness of the zooplankton community (Muylaert et al., 2010).
The richness of the zooplankton community did not differ among micro-basin and sub-basin. This can be explained by low richness, as also by the lotic characteristics of the streams. The stream’s characteristics naturally are not favorable to establishment of plankton communities, as discussed above. Additionally, the lack of variation on richness of zooplankton may be also explained by: (1) similarity between sampling points by the conservation status of streams that preserve a similar number of habitats (Pinel-Alloul, 1995; Van Onsem et al. 2010), associated with (2) low concentration of nutrients (Fonseca et al. 2014; Rezende, Biasi, et al., 2019) that may limit the number of zooplankton species (Muylaert et al. 2010). The similarity between locations was confirmed by the lack of variation among sites for most of the abiotic variables, mainly in nutrients concentrations, that was corroborated by RDA analysis. These small watercourses are situated in protected areas, in which headwaters are originally protected by a dense riparian vegetation (Feio et al., 2018; Rezende, Biasi, et al., 2019). Under natural conditions, their waters are characterized as oligotrophic, slightly acid and have low concentration of nutrients, total dissolved solids, and electric conductivity (Feio et al., 2018; Fonseca et al., 2014; Quintão et al., 2013; Rezende et al., 2014).
The highest β diversity values were found in sub-basin 1 and sub-basin 3, mainly in micro-basin 1 and micro-basin 2 compared to others. Therefore, the β diversity of the zooplankton community was spatially nested organized, as expected for many aquatic communities (Durães et al., 2016; Heino et al., 2013; Padial et al., 2014; Rezende, Biasi, et al., 2019). In this way, the highest scale, river basin, was the determinant level for the zooplankton community. This can be confirmed by the observations that streams belonging to the Paraná river basin (sub-basin 1 and sub-basin 3) have higher β diversity and community structure compared to streams belonging to the Tocantins river basin (sub-basin 2). Continuing the nested spatial process, the β diversity of the zooplankton community in micro-basin scale was also organized by sub-basin distribution. The nested spatial organization highlights the importance of spatial distribution on change in species composition between habitat patches of the zooplankton community (Gomes et al. 2020; Lopes et al. 2019). In this way, the predominance of headwater in highlands, combined with the rugged terrain, may promote the formation of geographic barriers to zooplankton dispersal. Therefore, the patterns nested in β diversity may be explained by the passive dispersal of zooplanktonic organisms in aquatic systems, that are generally carried by the flow of streams (Picapedra et al. 2016; Wang et al. 2016). If the zooplankton community shows a passive dispersal, the importance of interconnectivity between aquatic systems increases (Karna et al., 2015; Padial et al., 2014), driving the community structure and the spatial extensions (Gomes et al., 2020; Lopes et al., 2019).
Finally, the environmental conditions enable a conspicuous richness (Lopes et al., 2019; Xiong et al., 2020), with tendency of endemism, especially in a pristine condition of savanna Cerrado streams (Gomes et al., 2020; Padovesi-Fonseca & Rezende, 2017). For instance, Phytophilous cladocerans have been evaluated in several wetland areas distributed in central Brazil, and more than a half of them were classified as new or endemic species (Elmoor-Loureiro, 2007; Sousa & Elmoor-Loureiro, 2012). Indeed, the high endemism level of Cladocera in Cerrado’s inland waters was endorsed by many studies, from which most of the species have their distribution range restricted to a small area (Brasil et al., 2019; Torres et al., 2019). In our study, the species with the most restricted distribution range were Acroperus tupinamba, Alona yara, Alona ossiani, Flavalona iheringula, and Ovalona glabra. In addition, it is known that few species and organisms represent the plankton assemblages in low-order streams in Brazilian savanna Cerrado (Medeiros et al., 2019). Also, the stability in the community structure can be related to the observation that a great part of resources in headwaters is originally from the riparian vegetation (Feio et al., 2018; Rezende et al., 2014). The riparian vegetation can be essential for the maintenance of the aquatic biota of savanna Cerrado stream (Cid et al., 2019; de Rezende, Medeiros, et al., 2019; Graça et al., 2015).
Conclusions
The lower level of the spatial scale (micro-basin/microhabitat) was the most sensitive factor driving the α diversity of the zooplankton community. In this way, we support the first hypothesis of a negative relationship between the increase in the scale within a micro-basin and the α diversity of the zooplankton community. The hypothesis of a positive relationship among β diversity and spatial scale was partially supported, once the nested spatial organization explained the change in zooplanktonic species composition between habitat patches. Also, we found that high electrical conductivity concentration in the streams increases the species richness and that closer points, organized at micro-basin scale, show a similar environmental characteristics and correlate species. Thus, patterns of species diversity are essential to understand community ecology, as well as to provide information to conservation planning on savanna Cerrado streams. Finally, based on the peculiar characteristics of these streams which are located in high and protected areas, their permanent preservation should be considered essential to preserve several endemic species, revealing a huge patrimony, as grounded by this study for the central savanna Cerrado streams.
References
Anderson, E. P., Freeman, M. C., & Pringle, C. M. (2006). Ecological consequences of hydropower development in Central America: Impacts of small dams and water diversion on neotropical stream fish assemblages. River Research and Applications, 22, 397–411. https://doi.org/10.1002/rra.899
Anderson, M. J. (2001). A new method for non-parametric multivariate analysis of variance. Austral Ecology, 26, 32–46. https://doi.org/10.1111/j.1442-9993.2001.01070.pp.x
Anderson, M. J., Crist, T. O., Chase, J. M., et al. (2011). Navigating the multiple meanings of beta diversity: A roadmap for the practicing ecologist. Ecology Letters, 14, 19–28. https://doi.org/10.1111/j.1461-0248.2010.01552.x
APHA (1999) Standard methods for the examination of water and wastewater. American Public Health Association, Washington DC.
Bailey, R. C. (1992). Hierarchical analysis of community and habitat structure. Coenoses, 7, 127–135.
Bambi, P., de Souza, R. R., Feio, M. J., et al. (2017). Temporal and spatial patterns in inputs and stock of organic matter in savannah streams of central Brazil. Ecosystems, 20, 757–768. https://doi.org/10.1007/s10021-016-0058-z
Brasil, L. S., Luiza-Andrade, A., Kisaka, T. B., et al. (2019). Cladocera distribution along an environmental gradient on the Cerrado-Amazon ecotone: A preliminary study. Acta Limnologica Brasiliensia, 31, e29. https://doi.org/10.1590/s2179-975x2919
Chambers, J. M., Cleveland, W. S., Kleiner, B., & Tukey, P. A. (1983). Graphical methods for data analysis. Wadsworth & Brooks/Cole.
Chiba, S., Batten, S., Martin, C. S., et al. (2018). Zooplankton monitoring to contribute towards addressing global biodiversity conservation challenges. Journal of Plankton Research, 40, 509–518. https://doi.org/10.1093/plankt/fby030
Cottenie, K. (2005). Integrating environmental and spatial processes in ecological community dynamics. Ecology Letters, 8, 1175–1182. https://doi.org/10.1111/j.1461-0248.2005.00820.x
da Brito, M. T. S., Heino, J., Pozzobom, U. M., & Landeiro, V. L. (2020). Ecological uniqueness and species richness of zooplankton in subtropical floodplain lakes. Aquaic Sciences, 82, 43. https://doi.org/10.1007/s00027-020-0715-3
da Rocha, B. S., de Souza, C. A., Machado, K. B., et al. (2020). The relative influence of the environment, land use, and space on the functional and taxonomic structures of phytoplankton and zooplankton metacommunities in tropical reservoirs. Freshwater Science. https://doi.org/10.1086/708949
Rezende, R. S., Medeiros, A. O., Gonçalves, J. F., et al. (2019). Patterns of litter inputs, hyphomycetes and invertebrates in a Brazilian savanna stream: a process of degradative succession. Journal of Tropical Ecology, 35, 297–307. https://doi.org/10.1017/S0266467419000269
del Cid, C. C., Rezende, R. S., Calor, A. R., et al. (2019). Temporal dynamics of organic matter, hyphomycetes and invertebrate communities in a Brazilian savanna stream. Community Ecology, 20, 301–313. https://doi.org/10.1556/168.2019.20.3.10
Dormann, C. F., Elith, J., Bacher, S., et al. (2013). Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography, 36, 27–46. https://doi.org/10.1111/j.1600-0587.2012.07348.x
dos Picapedra, P. H. S., Fernandes, C., & Baumgartner, G. (2019). Structure and ecological aspects of zooplankton (Testate amoebae, Rotifera, Cladocera and Copepoda) in highland streams in southern Brazil. Acta Limnologica Brasiliensia, 31, e5. https://doi.org/10.1590/s2179-975x2917
Durães, L., Roque, F. O., Siqueira, T., et al. (2016). Simulating the role of connectivity in shaping stream insect metacommunities under colonization cycle dynamics. Ecological Modelling, 334, 19–26. https://doi.org/10.1016/j.ecolmodel.2016.04.020
Elmoor-Loureiro, L. M. A. (2007). Phytophilous cladocerans (Crustacea, Anomopoda and Ctenopoda) from Paranã River Valley, Goiás, Brazil. Revista Brasileira De Zoologia, 24, 344–352.
Elmoor-Loureiro, L. M. A., Mendonça-Galvão, L., Reid, J. W. & Fernandes, L. F. L (2015). Avaliação dos Copépodos (Harpacticoida: Canthocamptidae, Parastenocarididae; Calanoida: Diaptomidae, Temoridae; Cyclopoida: Cyclopidae). In: Pinheiro, M.; Boos, H.. (Org.). Livro Vermelho dos Crustáceos do Brasil: Avaliação 2010–2014. Sociedade Brasileira de Carcinologia, Porto Alegre. p. 113–125.
Feio, M. J., Leite, G. F. M., Rezende, R. S., et al. (2018). Macro-scale (biomes) differences in neotropical stream processes and community structure. Global Ecology and Conservation, 16, e00498. https://doi.org/10.1016/j.gecco.2018.e00498
Fonseca, B. M., de Mendonca-Galvao, L., Padovesi-Fonseca, C., et al. (2014). Nutrient baselines of Cerrado low-order streams: Comparing natural and impacted sites in Central Brazil. Environmental Monitoring and Assessment, 186, 19–33. https://doi.org/10.1007/s10661-013-3351-8
Frissell, C. A., Liss, W. J., Warren, C. E., & Hurley, M. D. (1986). A hierarchical framework for stream habitat classification: Viewing streams in a watershed context. Environmental Management, 10, 199–214.
Gomes, L. F., Barbosa, J. C., de Oliveira, B. H., et al. (2020). Environmental and spatial influences on stream zooplankton communities of the Brazilian Cerrado. Community Ecology. https://doi.org/10.1007/s42974-020-00008-5
Graça, M. A. S., Ferreira, V., Canhoto, C., et al. (2015). A conceptual model of litter breakdown in low order streams. International Review of Hydrobiology, 100, 1–12. https://doi.org/10.1002/iroh.201401757
Heino, J., Grönroos, M., Ilmonen, J., et al. (2013). Environmental heterogeneity and β diversity of stream macroinvertebrate communities at intermediate spatial scales. Freshwater Science, 32, 142–154. https://doi.org/10.1899/12-083.1
Heino, J., Melo, A. S., & Bini, L. M. (2015). Reconceptualising the beta diversity-environmental heterogeneity relationship in running water systems. Freshwater Biology, 60, 223–235. https://doi.org/10.1111/fwb.12502
Karna, O. M., Gronroos, M., Antikainen, H., et al. (2015). Inferring the effects of potential dispersal routes on the metacommunity structure of stream insects: As the crow flies, as the fish swims or as the fox runs? Journal of Animal Ecology, 84, 1342–1353. https://doi.org/10.1111/1365-2656.12397
Koleff, P., Gaston, K. J., & Lennon, J. J. (2003). Measuring beta diversity for presence-absence data. Journal of Animal Ecology, 72, 367–382. https://doi.org/10.2307/3505672
Koste, W. (1978). Rotatoria Die Radertiere Mitteleuropas bergriindet von Max Voigt-Monogononta. Koste W (ed) Gebrlider Borntraeger, Berlin.
Kumar, N. J. L., Das, M., Mukherji, R., & Kumar, R. N. (2011). Assessment of zooplankton diversity of a tropical wetland system. International Journal of Pharmaceutical and Life Sciences, 2, 983–990.
Legendre, P., Borcard, D., & Peres-Neto, P. R. (2005). Analyzing beta diversity: Partitioning the spatial variation of community composition data. Ecological Monographs, 75, 435–450. https://doi.org/10.1890/05-0549
Legendre, P., & Legendre, L. (2012). Numerical ecology (3rd ed.). . Elsevier.
Lennon, J. J., Koleff, P., GreenwooD, J. J. D., & Gaston, K. J. (2001). The geographical structure of British bird distributions: Diversity, spatial turnover and scale. Journal of Animal Ecology, 70, 966–979. https://doi.org/10.1046/j.0021-8790.2001.00563.x
Lopes, P. M., Bini, L. M., Declerck, S. A., et al. (2014). Correlates of zooplankton beta diversity in tropical lake systems. PLoS ONE, 9, e109581–e109581. https://doi.org/10.1371/journal.pone.0109581
Lopes, V. G., Branco, C. W. C., Kozlowsky-Suzuki, B., & Bini, L. M. (2019). Zooplankton temporal beta diversity along the longitudinal axis of a tropical reservoir. Limnology, 20, 121–130. https://doi.org/10.1007/s10201-018-0558-y
Medeiros, Í. L. S., dos Santos, F. A., El-Deir, A. C. A., & de Melo Júnior, M. (2019). A mata ripária influencia a composição e estrutura da comunidade zooplanctônica de poças temporárias? Iheringia. Sér Zool, 109, e2019037. https://doi.org/10.1590/1678-4766e2019037
Muylaert, K., Pérez-Martínez, C., Sánchez-Castillo, P., et al. (2010). Influence of nutrients, submerged macrophytes and zooplankton grazing on phytoplankton biomass and diversity along a latitudinal gradient in Europe. Hydrobiologia, 653, 79–90. https://doi.org/10.1007/s10750-010-0345-1
Oksanen, J., Blanchet, F. G., & Kindt, R., et al. (2013a). Community ecology package: Ordination, diversity and dissimilarities.
Oksanen, J., Blanchet, F. G., Kindt, R., et al. (2013b). Community ecology package: Ordination, diversity and dissimilarities. version 2.0-8
Oksanen, J., Kindt, R., Legendre, P., et al. (2008). Adonis function. In: Vegan: Community ecology package (pp. 15–0). R package version 1.13-1
Padial, A. A., Ceschin, F., Declerck, S. A., et al. (2014). Dispersal ability determines the role of environmental, spatial and temporal drivers of metacommunity structure. PLoS ONE, 9, e111227–e111227. https://doi.org/10.1371/journal.pone.0111227
Padovesi-Fonseca, C., & de Rezende, R. S. (2017). Factors that drive zooplankton diversity in Neo-Tropical Savannah shallow lakes. Acta Limnologica Brasiliensia. https://doi.org/10.1590/s2179-975x1817
Padovesi-Fonseca, C., Saraiva, M. F., & da Fernandes, C. L. S. (2016). First record of cladocerans from the headwaters of the Cerrado-Amazon boundary, central Brazil. Biodiversity, 17, 90–92. https://doi.org/10.1080/14888386.2016.1235510
Pearson, A., & Duggan, I. (2018). A global review of zooplankton species in freshwater aquaculture ponds: What are the risks for invasion? Aquatic Invasions, 13, 311–322. https://doi.org/10.3391/ai.2018.13.3.01
Perbiche-Neves, G., Serafim-Júnior, M., Portinho, J. L., et al. (2012). Effect of atypical rainfall on lotic zooplankton: Comparing downstream of a reservoir and tributaries with free stretches. Journal of Tropical Ecology, 52, 149–162.
Picapedra, P. H. S., Fernandes, C., & Lansac-Tôha, F. A. (2016). Zooplankton community in the Upper Parnaíba River (Northeastern, Brazil). Brazilian Journal of Biology, 77, 402–412. https://doi.org/10.1590/1519-6984.20215
Pinel-Alloul, B. (1995). Spatial heterogeneity as a multiscale characteristic of zooplankton community. Hydrobiologia, 300, 17–42.
Pinese, O. P., Pinese, J. F., & Del Claro, K. (2015). Structure and biodiversity of zooplankton communities in freshwater habitats of a Vereda Wetland Region, Minas Gerais, Brazil. Acta Limnologica Brasiliensia, 27, 275–288. https://doi.org/10.1590/S2179-975X0415
Quintão, J. M. B., Rezende, R. S., & Gonçalves JFJr, . (2013). Microbial effects in leaf breakdown in tropical reservoirs of different trophic status. Freshwater Science, 32, 933–950. https://doi.org/10.1899/12-112.1
Rezende, R., Biasi, C., Mello Pretrucio, M., & Gonçalves Júnior, J. F. (2019). Effects of leaf litter traits on alpha and beta diversities of invertebrate assemblages in a tropical watershed. Ecología Austral, 29, 365–379. https://doi.org/10.25260/EA.19.29.3.0.750
Rezende, R. S., Petrucio, M. M., & Gonçalves, J. F. (2014). The effects of spatial scale on breakdown of leaves in a tropical watershed. PLoS ONE, 9, e97072. https://doi.org/10.1371/journal.pone.0097072
Rezende, R. S., Sales, M. A., Hurbath, F., Roque, N., Gonçalves, J. F., Jr., & Medeiros, A. O. (2017). Effect of plant richness on the dynamics of coarse particulate organic matter in a Brazilian Savannah stream. Limnologica, 63, 57–64. https://doi.org/10.1016/j.limno.2017.02.002
Sousa, F. D. R., & Elmoor-Loureiro, L. M. A. (2012). How many species of cladocerans (Crustacea, Branchiopoda) are found in Brazilian Federal District? Acta Limnologica Brasiliensia, 24, 351–362. https://doi.org/10.1590/S2179-975X2013005000008
Torres, C. R., de Paiva, L. O., Durão, M. S., et al. (2019). Novas ocorrências de cladóceros em ambientes aquáticos do Brasil Central: compreendendo a biodiversidade. Heringeriana, 13, 21–28.
Van Onsem, S., De Backer, S., & Triest, L. (2010). Microhabitat–zooplankton relationship in extensive macrophyte vegetations of eutrophic clear-water ponds. Hydrobiologia, 656, 67–81. https://doi.org/10.1007/s10750-010-0442-1
Wang, L., Chen, Q., Han, R., et al. (2016). Zooplankton community in Yangtze River Estuary and adjacent sea areas after the impoundment of the Three Gorges Reservoir. Annales De Limnologie-International Journal of Limnology, 52, 273–284.
Whittaker, R. H. (1960). Vegetation of the Siskiyou Mountains, Oregon and California. Ecological Monographs, 30, 279–338.
Whittaker, R. H. (1972). Evolution and measurement of species diversity. Taxon, 21, 213–251. https://doi.org/10.2307/1218190
Wiens, J. A. (1989). Spatial scaling in ecology. Functional Ecology, 3, 385–397.
Xiong, W., Huang, X., Chen, Y., et al. (2020). Zooplankton biodiversity monitoring in polluted freshwater ecosystems: A technical review. Environ Sci Ecotech, 1, 100008. https://doi.org/10.1016/j.ese.2019.100008
Acknowledgements
This study is cooperation research of NEL (Nucleous of Limnological Studies) and Aquariparia, both from the University of Brasília, Brazil. RSR is grateful to National Council for Scientific and Technological Development (CNPq) and Chico Mendes Institute for Conservation of Biodiversity (ICMBio) in Projects Number 421288/2017-5 and 405290/2018-7. We thank the support from the Foundation to Support the Research and Innovation of State of Santa Catarina (FAPESC) and the Community University of the Chapecó Region. DMC thanks CAPES (Process: 1690069) for the scholarship on Post-Graduate Program in Zoology of UnB.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Padovesi-Fonseca, C., de Souza Rezende, R., da Costa, D.F. et al. Spatial scales drive zooplankton diversity in savanna Cerrado streams. COMMUNITY ECOLOGY 22, 249–259 (2021). https://doi.org/10.1007/s42974-021-00052-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42974-021-00052-9