Abstract
Background and aims
Plant growth-promoting rhizobacteria (PGPR) have garnered interest in agriculture due to their ability to influence the growth and production of host plants. ATP-binding cassette (ABC) transporters play important roles in plant-microbe interactions by modulating plant root exudation. The present study aimed to provide a more precise understanding of the mechanism and specificity of the interaction between PGPR and host plants.
Methods
In the present study, the effects of interactions between a PGPR strain, Bacillus cereus AR156, and Arabidopsis thaliana wild type (Col-0) or its ABC transporter mutants on plant growth have been studied.
Results
B. cereus AR156 promoted the shoot growth of Col-0 and Atabcg30 but repressed the growth of Atabcc5. Bacterial volatiles and secretion promoted the shoot growth of Col-0 and Atabcg30 but had no effect on Atabcc5. We also found that root exudates of Col-0 induced the expression of B. cereus AR156 genes related to siderophore and chitinase production; while root exudates of Atabcc5 inhibited the expression level of those genes. Further analysis of root exudates revealed that amino acids, organic acids, and sugars were significantly less abundant in Atabcc5 when compared to Col-0.
Conclusions
Our findings highlight that both host plant and PGPR play active roles in the outcome of the plant-microbe interaction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant growth-promoting rhizobacteria (PGPR) were initially characterized due to their ability to stimulate the growth of plants in several ways via solubilizing nutrients such as phosphorus and iron, fixing atmospheric nitrogen, and producing phytohormones when grown in association with plant roots (Gray and Smith 2005; Idris et al. 2007; Kloepper and Schroth 1978). In addition to plant growth promotion, PGPRs also exhibit biological control traits including secretion of antibiotics, competition for nutrients, production of lytic enzymes, and induction of systemic resistance in plants (Chet 1990; Chet and Inbar 1994; Loon and Bakker 2006; Weller 1988). Furthermore, an increasing number of studies have shown that PGPRs play important roles in conferring plant tolerance to abiotic stresses such as drought and salinity (Malhotra and Srivastava 2009; Mayak et al. 2004; Wang et al. 2012). Bacillus strains from species such as B. subtilis, B. cereus, B. licheniformis, B. pumilus, B. amyloliquefaciens, B. polymyxa, and B. megaterium have been reported to successfully colonize the roots and rhizosphere of several plants such as tomato, banana, canola, wheat, apple, red pepper, and Arabidopsis resulting in promotion of plant growth and yield, disease resistance, drought tolerance, and heavy metal remediation (Chen et al. 2013; Joo and Chang 2005; Karlidag et al. 2007; Khalid et al. 2004; Mayak et al. 2004; Sgroy et al. 2009). A large body of knowledge has shown the advantages of PGPRs; however, the shortcomings of PGPRs including poor survival rate (Acea et al. 1988) and inconsistent efficacy (Labuschagne et al. 2011) should not be overlooked. Few reports have attempted to comprehend the reasons for the variable effects of PGPRs, showing that these organisms could be highly specific to plant species, cultivars, and genotypes (Figueiredo et al. 2011; Gupta et al. 2000; Lucy et al. 2004; Siddiqui and Shaukat 2003). In addition, the presence of competing microbes and soil-factors such as temperature, water content, oxygen, and pH influence the effect of PGPR on host plants (Dutta and Podile 2010; Frans et al. 2007; Hrynkiewicz et al. 2010).
It is well documented that plant root exudates play key roles in the mediation of plant-microbe interactions in the rhizosphere (Badri et al. 2008, 2009; Chaparro et al. 2013). The composition of plant root exudates is determined by plant species, cultivar, developmental stage, and numerous environmental factors including soil type, temperature, humidity, pH, and nutrient availability (Bais et al. 2006; Brimecombe et al. 2001; Nicholas 2007; Rovira 1969). Root exudates are also responsible for biological control by eliciting microbial biofilm formation on the root surfaces which is now widely recognized as a form of biological control of plant pathogens by weakening pathogens’ competition for nutrients and space (Bais et al. 2004; Chen et al. 2012; Davey and O’Toole 2000). A previous study reported that L-malic acid found in the root exudates of tomato strongly stimulated biofilm formation ex planta of B. subtilis (Chen et al. 2012); thus promoting its biocontrol of the bacterial disease caused by Ralstonia solanacearum (Chen et al. 2013).
ATP-binding cassette (ABC) transporters encompass a large protein family and play key roles in the transportation of compounds in plant cells both extracellularly over the plasma membrane and intracellularly into the vacuoles (Kang et al. 2010; Rea 2007; Yazaki 2005; Yazaki et al. 2009). ABC transporters are important for plant root exudation and play key roles during interaction with rhizosphere organisms (Badri et al. 2008, 2009; Loyola-Vargas et al. 2007; Sugiyama et al. 2007). For instance, the ABC transporter mutant Atabcg30 secreted more phenolic compounds than sugars in their root exudates compared to the wild type which modified the rhizosphere microbial community (Badri et al. 2009). ABC transporters also play a role in plant-fungus symbiont interactions by acting as a cellular strigolactone exporter in the case of PDR1 in Petunia hybrida which serves to regulate the development of arbuscular mycorrhizae and axillary branches (Kretzschmar et al. 2012). Atabcg30 belongs to the pleiotropic drug resistance protein (PDR) subfamily which is involved in exporting antifungal compounds, in disease resistance, and in heavy metal tolerance (Fourcroy et al. 2014; Stein et al. 2006). Atabcc5 belongs to the multidrug resistance-associated protein (MRP) and acts as an auxin conjugate transporter as increased auxin levels were found in Atabcc5 plants (Jasinski et al. 2003). It had been documented that Atabcc2 (AtMRP2) contributed to detoxification, vacuolar organic anion transport and chlorophyll degradation (Frelet-Barrand et al. 2008). Moreover, it had been reported that Arabidopsis Atabcg36 (AtPDR8) and Atabcg37 (AtPDR9) had an involvement in regulation of auxin homeostasis and plant development by directly transporting the auxin precursor indole-3-butyric acid (Strader and Bartel 2009; Růžička et al. 2010).
B. cereus AR156 has been previously described as a PGPR which significantly increased biomass and induced systemic resistance in Arabidopsis thaliana against Pseudomonas syringae pv. lycopersici (DC3000) by concurrently activating salicylate- and jasmonate/ethylene-dependent signaling pathways (Niu et al. 2011). Moreover, another study showed that B. cereus AR156 enhanced drought tolerance of cucumber (Wang et al. 2012). In previous works, B. cereus AR156 had the ability to produce siderophore and chitinases in vitro. Siderophores are produced by many PGPRs and have the potential of stimulating plant growth by binding a variety of iron-containing molecules (Ahmed and Holmström 2014; Crowley 2006; Kloepper et al. 1980; Miethke and Marahiel 2007). Chitinases produced in a variety of Bacillus spp. are involved in plant growth promotion (Lee et al. 2005; Sharp 2013) and in biocontrol of plant pathogens and pests (Herrera-Estrella and Chet 1999). In a previous study, B. cereus AR156 functioned as a biocontrol agent against Pseudomonas syringe and Meloidogyne incognita (Niu et al. 2011; Wei et al. 2010). Yet PGPR’s ability to positively influence plant growth and productivity is inconsistent, thus their application in the field does not always produce desired outcomes (Lambert and Joos 1989; Martínez-Viveros et al. 2010). Here, we aimed at getting a better understanding of the specificity of the interaction between PGPR and host plants in order to determine possible reasons that, in some instances, PGPR inoculations are ineffective. We hypothesized that root exudates could play an important role in PGPR - host plant interactions. To achieve this goal, we set up a system by using B. cereus AR156 and ABC transporter mutants of Arabidopsis. ABC transporter mutants of Arabidopsis were selected because they have been shown to be important in plant root exudation and root exudates play a significant role in initiating and maintaining plant-PGPR interactions. In this study, we show how the PGPR affected the plant’s growth due to bacterial secretions and emissions, and how root exudation modulated biochemical determinants in the PGPR that could affect the performance of the host plant.
Materials and methods
Plant materials, bacterial strains, and growth conditions
Seeds of Arabidopsis thaliana wild type Col-0 and ABC-transporter mutants (Atabcg30, Atabcg36, Atabcg37, Atabcc2, and Atabcc5) (Badri et al. 2008, 2009) were surface-sterilized in 2.5 % NaClO for 1 min, washed five times with sterile water and then placed at 4 °C for 4 days to break dormancy. Seeds were planted on Murashige and Skoog medium (MS) (Murashige and Skoog 1962) agar plates containing 1 % sucrose and placed vertically in a growth chamber at 25 °C under a 16/8 h photoperiod.
The PGPRs in the present study were B. cereus AR156 and its kanamycin-resistant mutant B. cereus AR156-Ka. The kanamycin-resistant mutant was used in root colonization studies under soil conditions when kanamycin was used to exclude other bacteria from being accounted (Niu et al. 2011). B. cereus AR156 was grown on Luria-Bertani (LB) (Bertani 1951) agar plates and B. cereus AR156-Ka was grown on LB agar plates supplemented with 200 mg/L of kanamycin at 30 °C for 24 h. A single colony of each strain was transferred to liquid LB medium and incubated at 30 °C, 200 rpm for 24 h. Bacterial cells were centrifuged (8000 × g, 10 min) and re-suspended in sterile Hoagland’s solution.
Bacterial suspension experiment
Seven days old Arabidopsis seedlings were transferred into pots (6 cm × 3.6 cm × 6 cm) containing a mixture of sterile sand and vermiculite (1:1, volume) and were placed in a growth chamber (25 °C, 16/8 h photoperiod, and relative humidity of 80–85 %). Three mL of B. cereus AR156 suspension (2 × 108 CFU/mL) in Hoagland’s solution was inoculated to 14 days-old plants. The same amount of Hoagland’s solution was used as control. There were eight plants in each tray and three trays for each treatment (24 plants in total). Plant shoot weight was recorded at 35 days-old.
To study the dosage effect of B. cereus AR156 on Arabidopsis plant growth, five concentrations of 3 mL bacterial suspensions of B. cereus AR156 (2 × 104 CFU/mL, 2 × 106 CFU/mL, 2 × 107 CFU/mL, 2 × 108 CFU/mL, and 2 × 109 CFU/mL) were inoculated to 14 days-old A. thaliana seedlings. There were eight plants in each tray and three trays for each treatment (24 plants in total). Plant shoot fresh weight and shoot dry weight of Arabidopsis plants was recorded when plants were 35 days-old.
Colonization of B. cereus AR156 in Arabidopsis rhizosphere
The rhizosphere samples of Arabidopsis plants treated with B. cereus AR156-Ka were collected (three plants of each mutant per replication) at 21 day after inoculation (Huang et al. 2015; Niu et al. 2011). One gram of thoroughly mixed rhizosphere samples from three plants was re-suspended in 9 mL of sterile 0.85 % NaCl and was shaken at 200 rpm for 30 min. Serial dilutions of the rhizosphere sample were placed on LB agar plates supplemented with 200 mg/L kanamycin. The number of CFU per gram of rhizosphere soil was recorded after incubation at 30 °C for 24 h. The quantification of colonization of B. cereus AR156 in the rhizosphere of different Arabidopsis mutants was repeated three times.
Infiltration assay
Leaves of 18 days-old Arabidopsis seedlings were infiltrated with bacterial suspension of B. cereus AR156 (2 × 108 CFU/mL) which were suspended in sterile distilled water. Sterile distilled water was used as control. The treated seedlings were covered in plastic wrap to maintain humidity. Disease symptoms were measured at 24 h after inoculation. There were 6 plants in each treatment and 3 leaves of each plant were infiltrated with B. cereus AR156 or sterile water.
Effect of bacterial volatiles on the shoot growth of A. thaliana
The effect of volatiles secreted by B. cereus AR156 on the shoot growth of Col-0 and ABC transporter mutants was studied as described by Ryu et al. (2003) and Huang et al. (2015). Escherichia coli (DH5α) and LB liquid medium were used as negative controls. There were three plates for each treatment and three plants on each plate. Plates were sealed with Parafilm and incubated in a growth chamber (25 °C, continuous light). Plant shoot fresh weight was recorded after 14 days.
Effect of bacterial culture filtrate on the shoot growth of A. thaliana
The effect of bacterial culture filtrate on Col-0 and ABC transporter mutants’ growth was studied. B. cereus AR156 was cultured in 500 mL M9 liquid medium at 30 °C to reach OD600 = 2.0, then adjusted to 2 × 108 CFU/mL and centrifuged at 12,000 rpm for 10 min to collect the supernatant. The supernatant was filtered through 0.2 μm membrane (Cat No. 722–2520, Thermo Scientific) to remove the bacterial cells. Three mL culture filtrate of B. cereus AR156 was applied to 14 days-old seedlings. The same amount of M9 liquid medium was used as control. The plants were placed in a growth chamber at 25 °C under a 16/8 h photoperiod. Each treatment contained 24 plants. Plant shoot fresh weight was measured at 35 days-old.
Root exudate collection
Root exudates were collected according to the methods of Badri et al. (2008; 2009). Seven-d-old Arabidopsis seedlings were transferred to six-well culture plates (Cat No. 08-772-1B, Fischer Co.) with 5 mL liquid MS medium containing 1 % sucrose in each well. The six-well plates were placed on a shaker (25 ± 2 °C, 90 rpm, and 16/8 h photoperiod). When plants reached 18 days-old, they were washed three times with sterile water and transferred into sterile six-well plates containing fresh 5 mL MS liquid medium (without sucrose). The exudates were collected after 3 days following plants’ transfer (plants were 21 day-old). Every treatment had 4 replicates and each replicate contained 12 individual plants. To remove root-border-like cells and root sheathing, root exudates were filtered through 0.45 μm nylon filters (Millipore).
Effect of root exudates on the growth of B. cereus AR156
The effect of root exudates on the growth of B. cereus AR156 was determined by counting the bacterial colonies on LB agar plates. B. cereus AR156 was grown in LB liquid medium at 30 °C to reach OD600 = 2.0, then adjusted to a final density of 1 × 104 CFU/mL. One hundred μL cell suspensions of B. cereus AR156 were mixed with 100 μL root exudates (Col-0, Atabcg30, and Atabcc5) or MS and placed on LB plates and incubated at 30 °C for 18 h. Root exudates were collected as described above. The colony forming units (CFUs) were counted. Data shown were from at least three replicates. The experiment was repeated twice.
Effect of root exudates on gene expression of B. cereus AR156
Root exudates from Atabcc5 and Col-0 were collected as described above. One mL root exudates or MS liquid medium (control) was added into a flask containing 100 mL LB liquid medium (root exudate at a final concentration of 1 % v/v) (Chen et al. 2012). B. cereus AR156 was added to the flask to a final concentration of 1.0 × 104 CFU/mL. Flasks were put in a shaker at 30 °C and 200 rpm. Bacterial cells were collected from three replications at 7 h and 12 h after inoculation. The selected genes were BACI_c19650 (siderophore biosynthesis protein) and chiA (chitinase). Previous studies have shown that B. cereus AR156 produces siderophore and chitinase, which play important roles in promoting plant growth (unpublished data from the Guo’s lab). In addition, genes related to the production of siderophore and chitinase have been found by sequencing the whole genome of B. cereus AR156 (unpublished data). Total RNA of bacterial cells was extracted with the SV Total RNA Isolation System (Promega, Corporation) and cDNA was made with SuperScript® III First-Strand Synthesis System (Invitrogen) according to the manufacturer’s instructions. Semi-quantitative RT-PCR was performed by using gene-specific primers (Table S1). The experiment was repeated twice.
Gas chromatography–mass spectrometry (GC-MS)
Root exudates for GC-MS analysis were collected as described above with some modifications. The exudates were collected after 3 days following plants’ transfer (plants were 21 days-old), freeze dried and derivatized using the standard methoximation and trimethylsilylation procedure (Broeckling et al. 2005). For each replicate (n = 5) 60 mL of exudates from 12 individually grown Arabidopsis plants were collected. Ribitol was used as an internal standard for each sample. GC-MS was carried out at the Samuel Roberts Nobel Foundation. An Agilent 6890 GC coupled with a 5973 MS at a split ratio of 1:1 was used to inject 1 μL of each sample. Separation was achieved using a 60 m DB-5MS (J & W Scientific) at a flow rate of 1 mL/min. Oven was held at 80 °C for two min, ramped at 5 °C/min to a final temperature of 315 °C and held for 12 min.
Data transformation and statistical analyses
One-way analysis of variance (ANOVA) was carried out and followed with Tukey’s HSD (Honestly Significant Difference) test to compare the difference in fresh shoot weight of by Col-0 and mutants by inoculating B. cereus AR156 at different concentrations. The t-test was conducted to compare the difference in bacterial growth and colonization of the treatments and controls. Peak detection and deconvolution for GC-MS data was achieved through the Automated Mass Spectral Deconvolution and Identification System (AMDIS) (Halket et al. 1999). The quantitative peak area values were extracted using metabolomics ion-based extraction algorithm (MET-IDEA) (Broeckling et al. 2005). All compounds obtained via GC-MS analysis (Table S2) were normalized to the ribitol internal standard. For multivariate statistical analysis redundant peaks were removed and peak areas were pareto scaled. Statistical significant differences of identified root exudate compounds between wildtype and Atabcc5 were determined by Bonferroni corrected t-test.
Results
Effect of B. cereus AR156 on plant growth of Arabidopsis wild type and ABC transporter mutants
Shoot fresh weight was measured and recorded at 21 days after inoculation of 2 × 108 CFU/mL B. cereus AR156 (Fig. S1). As shown in Fig. S1A, B. cereus AR156 showed distinct effects on the shoot weight of wild type and ABC transporter mutants. Fresh shoot weight of Col-0 and Atabcg mutants (Atabcg30, Atabcg36, and Atabcg37) significantly increased after the inoculation of B. cereus AR156 exhibiting a higher fresh shoot weight ranging from 0.07 to 0.20 g (16–72 % increase) compared to their respective un-inoculated controls (t-test, p < 0.05). B. cereus AR156 had no effect on the shoot weight of Atabcc2. In contrast, Atabcc5 had 0.19 g lower (56 % decrease) fresh shoot weight than its control (t-test, p < 0.05) (Fig. S1).
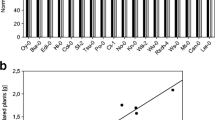
To test the effect of the dosage of B. cereus AR156 on the plat growth, Atabcg30 and Atabcc5 were selected for further experimentation because the shoot weight of Atabcg30 was significantly promoted while the shoot weight of Atabcc5 was significantly repressed compared to their respective controls. As the concentration of B. cereus AR156 increased Col-0 and the mutants showed distinct growth effects (Fig. 1a). The shoot fresh weight of Col-0 significantly increased at 2 × 104 CFU/mL, 2 × 106 CFU/mL, and 2 × 107 CFU/mL (Tukey’s HSD test, p < 0.05) compared to un-inoculated plants. The shoot fresh weight of Col-0 decreased significantly (Tukey’s HSD test, p < 0.05) at concentration 2 × 109 CFU/mL in comparison with plants treated at 2 × 104 CFU/mL, 2 × 106 CFU/mL, and 2 × 107 CFU/mL. B. cereus AR156 increased the shoot fresh weight of Atabcg30 mutant plants at all concentrations. Moreover, there were significant increases of shoot fresh weight at 2 × 104 CFU/mL and 2 × 106 CFU/mL compared to the control plants (Tukey’s HSD test, p < 0.05) (Fig. 1a). In contrast, in Atabcc5 the shoot fresh weight decreased significantly in comparison with control plants as the concentrations of B. cereus AR156 increased to 2 × 108 CFU/mL and 2 × 109 CFU/mL (Tukey’s HSD test, p < 0.05) (Fig. 1a); all other concentrations of B. cereus AR156 did not have an effect on shoot dry weight compared to the control.
Effects of different concentrations of AR156 on shoot fresh weight biomass (a) and shoot dry weight (b) of 35 days-old Atabcg30, Atabcc5 and Col-0, and colonization of AR156 in the rhizosphere of those plants (c). Two week old plants were inoculated with cell suspensions of five concentrations of AR156: 2 × 106 CFU/mL (Six), 2 × 107 CFU/mL (Seven), 2 × 108 CFU/mL (Eight), and 2 × 109 CFU/mL (Nine) re-suspended in Hoagland’s solution; Hoagland’s solution alone was used as a control. Letters indicate statistically significant differences between the treatments of a given plant type with different concentrations of AR156 (Tukey’s honest significance test; p < 0.05)
The shoot dry weight of all plants was not completely consistent with the shoot fresh weight (Fig. 1b). No differences between treatments were seen in Col-0. In Atabcg30, B. cereus AR156 significantly increased the dry biomass at concentrations 2 × 106 CFU/mL, 2 × 108 CFU/mL, and 2 × 109 CFU/mL (Tukey’s HSD test, p < 0.05) (Fig. 1b). The shoot dry weight of Atabcc5 significantly decreased by B. cereus AR156 at concentrations of 2 × 108 CFU/mL and 2 × 109 CFU/mL (Tukey’s HSD test, p < 0.05) (Fig. 1b).
Colonization of B. cereus AR156 in the rhizosphere of Col-0, Atabcg30, and Atabcc5
The colonization of B. cereus AR156 in the rhizospheres of Col-0, Atabcg30, and Atabcc5 was monitored at 21 days post-treatment (Fig. 1c). It was observed that B. cereus AR156 colonized the rhizosphere of Arabidopsis wild type and the two mutants at all five inoculation concentrations. Mutant Atabcc5 had significantly (Tukey’s HSD test, p < 0.05) more B. cereus AR156 cells in the rhizosphere when compared to mutant Atabcg30 and Col-0 at the inoculation concentrations of 2 × 104 CFU/mL, 2 × 106 CFU/mL, and 2 × 107 CFU/mL. Furthermore, both mutants Atabcg30 and Atabcc5 exhibited more B. cereus AR156 colonization in the rhizosphere compared to Col-0 at the inoculation concentrations of 2 × 108 CFU/mL and 2 × 109 CFU/mL (Tukey’s HSD test, p < 0.05) (Fig. 1c).
B. cereus AR156 is not pathogenic to Arabidopsis plants
As an explanation for the inhibition of the growth of Atabcc5, we hypothesized a possible pathogenic effect of B. cereus AR156 towards Atabcc5. To test this possibility, we infiltrated the leaves of 18 days-old seedlings with B. cereus AR156 at 2 × 108 CFU/mL. After 24 h of treatment, there were no disease symptoms on the leaves of both Atabcc5 and Col-0 (Fig. S2). We kept the plants under the same conditions for another three days but no disease symptoms appeared (data not shown).
Effect of bacterial volatile compounds on shoot weight of Col-0, Atabcg30, and Atabcc5
The involvement of B. cereus AR156 volatile compounds on plant growth promotion was tested by using partitioned media plates. B. cereus AR156 showed no effect on Atabcc5 shoot weight compared to the controls (LB and E. coli) (Fig. 2a). However, the volatiles of B. cereus AR156 had a positive effect on Atabcg30 growth as fresh weight significantly increased. The plant fresh weight increased 0.02 g (54 % increase) than that of control plants (Fig. 2a; t-test, p < 0.05).
Effects of AR156 volatile compounds (a) and culture filtrate (b) on Arabidopsis shoot fresh weight. a Shoot fresh weight of plants was measured after 14 days of inoculation of AR156 on the other side of the plate. The controls used were LB medium and E. coli. b Plant shoot fresh weight was measured when the seedlings were 35 days- old. The asterisks above the bars indicate significance relative to the control at p < 0.05level (t-test)
Effect of bacterial culture filtrate on shoot weight of Col-0, Atabcg30, and Atabcc5
The effect of bacterial culture filtrate was tested on Col-0, Atabcg30, and Atabcc5 plants (Fig. 2b). The bacterial culture filtrate of B. cereus AR156 had a significant positive effect on the shoot weight of Atabcg30 from 0.18 to 0.26 g (43.3 % increase) while that of Col-0 increased from 0.17 to 0.26 g (48.2 % increase) (Fig. 2b; t-test, p < 0.05). However, the bacterial culture filtrate did not show significant effect on the growth of Atabcc5 (Fig. 2b).
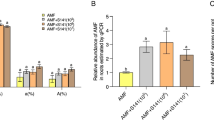
Effect of Arabidopsis Col-0 and mutants’ root exudates on the growth of B. cereus AR156
Root exudates of Col-0, Atabcg30, and Atabcc5 plants were collected from 21 days-old seedlings and applied to B. cereus AR156 to determine the effect of root exudates from Col-0 and different Arabidopsis mutants on the growth of B. cereus AR156 by counting CFUs on LB agar plates. As shown in Fig. 3, root exudates of Col-0, Atabcg30, and Atabcc5 significantly (t-test, p < 0.05) promoted the growth of B. cereus AR156 when compared to the control (MS media alone) (Fig. 3).
Effect of root exudates on gene expression of B. cereus AR156
Here we assessed the effect of root exudates of Col-0 and Atabcc5 on the expression of genes of B. cereus AR156 related to the production of BACI_c19650 (siderophore biosynthesis protein) and chiA (chitinase). As shown in Fig. 4, the transcript level of BACI_c19650 was induced by the addition of Col-0 root exudates at 7 h post treatment. Furthermore, at 12 h post treatment, the expression level of both genes were induced by the addition of Col-0 root exudates and the control (MS) while no expression was observed in the treatment of root exudates of Atabcc5.
Analysis of root exudates of Col-0 and Atabcc5 by GC-MS
Principal component analysis (PCA) revealed that the compounds detected in the root exudates of Col-0 and Atabcc5 by GC-MS were significantly different from each other (Fig. 5). GC-MS analyses identified 537 unique features within Atabcc5 root exudates and of these 89 features were annotated (Table S2). Furthermore it was observed that Atabcc5 secreted significantly (p < 0.05) less organic acids (malic acid, oxalic acid, fumaric acid, trihydroxybutyric acid, p-hydroxy benzoic acid, succinic acid, propionic acid, and citric acid) when compared to Col-0. Similarly, Atabcc5 had significantly (p < 0.05) lower levels of the sugars ribose and D-galactose as well as many amino acids (phenylalanine, valine, serine, tyrosine, proline, threonine, isoleucine, methionine, leucine, alanine, lysine, asparagine, and glycine) in the root exudates.
Discussion
The positive effect of PGPR on host plants is correlated with the colonization of these microbes in the rhizosphere (Knauth et al. 2005; Orr et al. 2011), and by the ability of these bacteria to express functions such as phosphate solubilization (Ramaekers et al. 2010; Richardson and Simpson 2011), and plant protection (Frapolli et al. 2010; Ryan et al. 2004). However, there is limited information on the mechanisms that govern the specificity of the interaction between PGPR and plants. In the present study, we aimed at understanding this specificity.
Effect of B. cereus AR156 on plant is plant type-specific
We observed that B. cereus AR156 promoted the shoot weight of Col-0 and Atabcg30, which was not surprising since the same result has been shown in other plants such as cucumber, tomato, and pepper (Niu et al. 2011; Zhou et al. 2014). It has been reported that B. cereus stimulates plant growth through the release of phytohormones (Dawwam et al. 2013), volatile compounds such as dimethyl disulfide (Huang et al. 2012), and peptidoglycan (Peterson et al. 2006).. Different possibilities were considered and tested to get insights on the mechanisms used by B. cereus AR156 to reduce the shoot weight of Atabcc5. First, we tested whether the negative effect of the bacterium was concentration-dependent. The effect of B. cereus AR156 on the shoot weight of Col-0 and Atabcc5 was consistent with a previous study showing that high concentration of a mixture of B. subtilis and B. cereus decreased the shoot weight and yield of pepper (Zhou et al. 2014). We found that B. cereus AR156 suppressed the shoot weight of Col-0 and Atabcc5 at concentrations 109 CFU/ mL and 107 CFU/ mL or greater, respectively. In comparison, B. cereus AR156 increased the shoot weight of Atabcg30 at concentrations. This indicates that the effect of B. cereus AR156 on Col-0, Atabcg30, and Atabcc5 is plant type-specific.
It had been reported that rhizosphere microbes are able to enhance plant hydration and nutritional status (Aliasgharzad et al. 2006; Azcón et al. 2013; Paul and Lade 2014). Our results showed that B. cereus AR156 had a positive effect on the fresh weight of plants compared to the effect on the shoot dry weight, we suggest that this is likely due to the ability of B. cereus AR156 to improve water uptake by the plants; however, additional studies examining the impact of B. cereus AR156 on Arabidopsis root biomass and plant-water relations are needed.
Some B. cereus strains are food-borne pathogens (Stenfors Arnesen et al. 2008) but some of them are approved to be PGPRs to plants and were used for biocontrol of some plant diseases (Almaghrabi et al. 2013; Niu et al. 2011). Our infiltration assay confirmed that B. cereus AR156 was not pathogenic to Atabcc5 or Col-0.
It is well known that ABC-transporters play important roles in the movement of different compounds both in and out of the cell (Kang et al. 2010; Yazaki 2005). Atabcc5 is an ion channel regulator in guard cells controlling stomata movement and the mutant Atabcc5 is insensitive to abscisic acid (ABA) resulting in partial stomatal closure (Gaedeke et al. 2001). This information indicates that Atabcc5 is incapable of detecting some compounds. The volatiles and secretions of B. cereus AR156 increased the shoot growth of Col-0 and Atabcg30 but not the shoot growth of Atabcc5. These combined facts suggest that Atabcc5 might lack the ability to sense and respond to the secretions of B. cereus AR156.
Effect of root exudates on PGPR
Root exudates contain many kinds of compounds including sugars, organic acids, amino acids, phenolic compounds, and some secondary metabolites (Bais et al. 2006), which are used as substrates for bacteria (Campbell and Greaves 1990). ABC transporters are strongly tied to root exudation (Badri et al. 2008, 2009). Recently, it was reported that root exudates from tobacco induced changes in exopolysaccharides and lipid-packing in the cell surface of B. cereus, and that these changes had a positive effect on bacterial colonization (Dutta et al. 2013). In the current study, we found that root exudates of Col-0, Atabcg30, and Atabcc5 significantly promoted the growth of B. cereus AR156 when compared to MS media alone. The differences in root exudate composition may explain the observation that there was more bacterial colonization on mutants Atabcg30 and Atabcc5 compared to Col-0. For example, it has been reported that Atabcg30 showed increased phenolic compounds and decreased sugars in its root exudates which caused a change in the rhizosphere microbial community increasing the abundance of certain PGPRs (Badri et al. 2009). Moreover, phenolic compounds greatly influence the soil microbial community (Badri et al. 2013). Additionally, bacterial auxin produced in the rhizosphere is able to loosen plant cell walls resulting in increased plant root exudation (Glick 2012). This increase in root exudation could result in the enhanced bacterial colonization that we observed with Atabcg30 and Atabcc5. Interestingly, bacterial colonization was the same in the treatments with higher B. cereus AR156 concentrations (2 × 108 CFU/mL and 2 × 109 CFU/mL) suggesting that the rhizosphere has an upper limit of B. cereus AR156 colonization that it can support.
The expression of certain gene encoding functions in PGPR such as Pseudomonas, Azospirillum, and Bacillus that benefit plants has been shown to be regulated by root exudation (Chen et al. 2012; Notz et al. 2001; Prigent-Combaret et al. 2008; Přikryl and Vančura 1980; Somers et al. 2005; Zakharova et al. 2000). Components of root exudates such as sugars (Notz et al. 2001), defense or development involved compounds for plants such as 2, 4-diacetylphloroglucinol (DAPG) and pyoluteorin (PLT) (de Werra et al. 2011), amino acids (Li and Glick 2001; Malhotra and Srivastava 2006; Rothballer et al. 2005), vitamins, and organic acids (Keshav Prasad Shukla et al. 2011) could either up or down regulate the expression level of genes encoding antifungal compounds and indole acetic acids by the beneficial bacteria. In our studies, we determined the influence of root exudates on the expression of siderophore biosynthesis protein gene BACI_c19650 and chitinase gene chiA of B. cereus AR156. These genes were selected because B. cereus AR156 has the ability to produce siderophore and chitinase. Here, we found that the siderophore biosynthesis protein gene BACI_c19650 was induced by the root exudates of Col-0, but less induced by root exudates of Atabcc5. Thus, indicating this as one of the mechanisms used by B. cereus AR156 to facilitate the growth of Col-0. Organic acids can enhance the amount of siderophore produced by bacteria (Sayyed et al. 2010). Another study showed that amino acids are co-exuded with siderophore in plants (Fan et al. 1997). Our metabolomics analysis (GC-MS) of the root exudates of Atabcc5 revealed that significantly less organic acids (malic acid, oxalic acid, fumaric acid, trihydroxybutyric acid, p-hydroxy benzoic acid, succinic acid, propionic acid, and citric acid) and amino acids were exuded when compared to Col-0. Accordingly, we speculate that decreased secretion of organic acids and amino acids by Atabcc5 inhibits the production of siderophores by B. cereus AR156. Subsequently, this lack of siderophore functioning could have resulted in shoot growth repression of Atabcc5. In the present study, we found the chitinase gene chiA was up-regulated by root exudates of Col-0 compared to those of Atabcc5 12 h after treatment. This result implies that one mechanism of plant growth promotion by B. cereus AR156 could be the production of chitinase which can promote plant growth and protect plants against fungal pathogens (Kim et al. 2005; Lee et al. 2005; Sharp 2013). In summary, our results indicate that root exudates of different mutants of Arabidopsis have the potential to differentially regulate the expression of genes related to plant growth promotion and biological control in B. cereus AR156.
The interaction between root exudates and PGPR leads to distinct plant growth promotion
Transcription level analyses of Atabcg30 roots revealed that the expression of genes involved in biosynthesis and transport of secondary metabolites were induced compared to Col-0 (Badri et al. 2009). Our results showed that the shoot growth of Atabcg30 was enhanced by the secretions of B. cereus AR156 compared to Col-0 and Atabcc5, indicating that the secretions of B. cereus AR156 that induce plant growth could be potentially transported in Atabcg30. Additionally, our metabolomics analysis revealed that Atabcc5 exudes significantly lower amounts of certain compounds (organic acid, sugars, and amino acids) into the rhizosphere compared to Col-0, which may be indicative of a diminished ability to transport compounds into and out of the rhizosphere. The shoot growth of Atabcc5, on the other hand, could not be promoted by secretions of B. cereus AR156 which suggests that Atabcc5 might be important in sensing and transporting these bacterial secretions. Another possible reason for the growth repression on Atabcc5 could be due to the auxin and auxin-like substrates produced by B. cereus AR156 in the rhizosphere that in addition with the increased levels of auxin produced by the mutant might have produced a toxic effect that negatively impacted the mutants’ growth (Pilet and Saugy 1987). For example, one study reported that a Bacillus strain OSU-142 promoted growth and yield of apricot, yet had negative effects on the growth of raspberry potentially due to the high amounts of auxin or secondary metabolites produced by OSU-142 (Orhan et al. 2006). We also speculate that Atabcc5 plays a role in the transport of substances produced by PGPR that could be involved in plant growth. Further experimentation is needed to warrant the validity of these speculations.
In summary, the distinct plant growth promotion effects by PGPR are related to both the host and PGPR interactions which mutually regulated secretions of bioactive compounds. The volatile and secretions from PGPR have the ability to promote plant growth, but this effect is affected by mutations; likewise, plant exudates can modulate bacterial growth and colonization as well as beneficial gene expression of PGPR.
References
Acea MJ, Moore CR, Alexander M (1988) Survival and growth of bacteria introduced into soil. Soil Biol Biochem 20:509–515. doi:10.1016/0038-0717(88)90066-1
Ahmed E, Holmström SJM (2014) Siderophores in environmental research: roles and applications. Microb Biotechnol 7:196–208. doi:10.1111/1751-7915.12117
Aliasgharzad N, Neyshabouri M, Salimi G (2006) Effects of arbuscular mycorrhizal fungi and Bradyrhizobium japonicum on drought stress of soybean. Biologia 61:S324–S328. doi:10.2478/s11756-006-0182-x
Almaghrabi OA, Massoud SI, Abdelmoneim TS (2013) Influence of inoculation with plant growth promoting rhizobacteria (PGPR) on tomato plant growth and nematode reproduction under greenhouse conditions. Saudi J Biol Sci 20:57–61. doi:10.1016/j.sjbs.2012.10.004
Azcón R, Medina A, Aroca R, Ruiz-Lozano JM (2013) Abiotic stress remediation by the arbuscular mycorrhizal symbiosis and rhizosphere bacteria/yeast Interactions. Mol Microb Ecol Rhizosphere. doi:10.1002/9781118297674.ch93, John Wiley & Sons, Inc
Badri DV, Loyola-Vargas VM, Broeckling CD, De-la-Peña C, Jasinski M, Santelia D, Martinoia E, Sumner LW, Banta LM, Stermitz F, Vivanco JM (2008) Altered profile of secondary metabolites in the root exudates of Arabidopsis ATP-Binding cassette transporter mutants. Plant Physiol 146:762–771. doi:10.1104/pp. 107.109587
Badri DV, Quintana N, El Kassis EG, Kim HK, Choi YH, Sugiyama A, Verpoorte R, Martinoia E, Manter DK, Vivanco JM (2009) An ABC transporter mutation alters root exudation of phytochemicals that provoke an overhaul of natural soil microbiota. Plant Physiol 151:2006–2017. doi:10.1104/pp. 109.147462
Badri DV, Chaparro JM, Zhang RF, Shen QR, Vivanco JM (2013) Application of natural blends of phytochemicals derived from the root exudates of Arabidopsis to the soil reveal that phenolic-related compounds predominantly modulate the soil microbiome. J Biol Chem 288:10. doi:10.1074/jbc.M112.433300
Bais HP, Fall R, Vivanco JM (2004) Biocontrol of Bacillus subtilis against infection of Arabidopsis roots by Pseudomonas syringae is facilitated by biofilm formation and surfactin production. Plant Physiol 134:307–319. doi:10.1104/pp. 103.028712
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol 57:233–266. doi:10.1146/annurev.arplant.57.032905.105159
Bertani G (1951) Studies on Lysogenesis I.: the mode of phage liberation by Lysogenic Escherichia coli. J. Bacteriol. 62:293–300
Brimecombe M, Leij F, Lynch J (2001) Nematode community structure as a sensitive indicator of microbial perturbations induced by a genetically modified Pseudomonas fluorescens strain. Biol Fertil Soils 34:270–275. doi:10.1007/s003740100412
Broeckling CD, Huhman DV, Farag MA, Smith JT, May GD, Mendes P, Dixon RA, Sumner LW (2005) Metabolic profiling of Medicago truncatula cell cultures reveals the effects of biotic and abiotic elicitors on metabolism. J Exp Bot 56:323–336. doi:10.1093/jxb/eri058
Campbell RG, Greaves MP (1990) Anatomy and community structure of the rhizosphere. In: Lynch JM (ed) The rhizosphere. Wiley, Chichester
Chaparro JM, Badri DV, Bakker MG, Sugiyama A, Manter DK, Vivanco JM (2013) Root exudation of phytochemicals in Arabidopsis follows specific patterns that are developmentally programmed and correlate with soil microbial functions. PLoS ONE 8, e55731. doi:10.1371/journal.pone.0055731
Chen Y, Cao S, Chai Y, Clardy J, Kolter R, Guo JH, Losick R (2012) A Bacillus subtilis sensor kinase involved in triggering biofilm formation on the roots of tomato plants. Mol Microbiol 85:418–430. doi:10.1111/j.1365-2958.2012.08109.x
Chen Y, Yan F, Chai Y, Liu H, Kolter R, Losick R, Guo JH (2013) Biocontrol of tomato wilt disease by Bacillus subtilis isolates from natural environments depends on conserved genes mediating biofilm formation. Environ Microbiol 15:848–864. doi:10.1111/j.1462-2920.2012.02860.x
Chet I (1990) Biological control of soil-borne plant pathogens with fungal antagonists in combination with soil treatments. In: Hornby D (ed) Biological control of soil-borne plant pathogens
Chet I, Inbar J (1994) Biological control of fungal pathogens. Appl Biochem Biotechnol 48:37–43. doi:10.1007/BF02825358
Crowley D (2006) Microbial siderophores in the plant rhizosphere. In: Barton L, Abadia J (eds) Iron nutrition in plants and rhizospheric microorganisms. Springer, Netherlands
Davey ME, O’Toole GA (2000) Microbial biofilms: from ecology to molecular genetics. Microbiol Mol Biol Rev 64:847–867. doi:10.1128/MMBR.64.4.847-867.2000
Dawwam GE, Elbeltagy A, Emara HM, Abbas IH, Hassan MM (2013) Beneficial effect of plant growth promoting bacteria isolated from the roots of potato plant. Ann Agric Sci 58:195–201. doi:10.1016/j.aoas.2013.07.007
de Werra P, Huser A, Tabacchi R, Keel C, Maurhofer M (2011) Plant- and microbe-derived compounds affect the expression of genes encoding antifungal compounds in a pseudomonad with biocontrol activity. Appl Environ Microbiol 77:2807–2812. doi:10.1128/aem.01760-10
Dutta S, Podile AR (2010) Plant growth promoting rhizobacteria (PGPR): the bugs to debug the root zone. Crit Rev Microbiol 36:232–244. doi:10.3109/10408411003766806
Dutta S, Rani TS, Podile AR (2013) Root exudate-induced alterations in Bacillus cereus cell wall contribute to root colonization and plant growth promotion. PLoS ONE 8, e78369. doi:10.1371/journal.pone.0078369
Fan TWM, Lane AN, Pedler J, Crowley D, Higashi RM (1997) Comprehensive analysis of organic ligands in whole root exudates using nuclear magnetic resonance and gas chromatography–mass spectrometry. Anal Biochem 251:57–68. doi:10.1006/abio.1997.2235
Figueiredo M, Seldin L, Araujo F, Mariano R (2011) Plant growth promoting rhizobacteria: fundamentals and applications. In: Maheshwari DK (ed) Plant growth and health promoting bacteria. Springer, Berlin Heidelberg
Fourcroy P, Sisó-Terraza P, Sudre D, Savirón M, Reyt G, Gaymard F, Abadía A, Abadia J, Álvarez-Fernández A, Briat J-F (2014) Involvement of the ABCG37 transporter in secretion of scopoletin and derivatives by Arabidopsis roots in response to iron deficiency. New Phytol 201:155–167. doi:10.1111/nph.12471
Frans AAMDL, James ML, Melissa JB (2007) Rhizodeposition and microbial populations. The Rhizosphere. CRC Press
Frapolli M, Défago G, Moënne-Loccoz Y (2010) Denaturing gradient gel electrophoretic analysis of dominant 2,4-diacetylphloroglucinol biosynthetic phlD alleles in fluorescent Pseudomonas from soils suppressive or conducive to black root rot of tobacco. Soil Biol Biochem 42:649–656. doi:10.1016/j.soilbio.2010.01.005
Frelet-Barrand A, Kolukisaoglu HU, Plaza S, Ruffer M, Azevedo L, Hortensteiner S, Marinova K, Weder B, Schulz B, Klein M (2008) Comparative mutant analysis of Arabidopsis ABCC-type ABC transporters: AtMRP2 contributes to detoxification, vacuolar organic anion transport and chlorophyll degradation. Plant Cell Physiol 49:557–569. doi:10.1093/pcp/pcn034
Gaedeke N, Klein M, Kolukisaoglu U, Forestier C, Muller A, Ansorge M, Becker D, Mamnun Y, Kuchler K, Schulz B, Mueller-Roeber B, Martinoia E (2001) The Arabidopsis thaliana ABC transporter AtMRP5 controls root development and stomata movement. EMBO J 20:1875–1887. doi:10.1093/emboj/20.8.1875
Glick BR (2012) Plant growth-promoting bacteria: mechanisms and applications. Scientifica 2012:15. doi:10.6064/2012/963401
Gray EJ, Smith DL (2005) Intracellular and extracellular PGPR: commonalities and distinctions in the plant–bacterium signaling processes. Soil Biol Biochem 37:395–412. doi:10.1016/j.soilbio.2004.08.030
Gupta A, Gopal M, Tilak KV (2000) Mechanism of plant growth promotion by rhizobacteria. Indian J Exp Biol 38:856–862
Halket JM, Przyborowska A, Stein SE, Mallard WG, Down S, Chalmers RA (1999) Deconvolution gas chromatography/mass spectrometry of urinary organic acids – potential for pattern recognition and automated identification of metabolic disorders. Rapid Commun Mass Spectrom 13:279–284. doi:10.1002/(SICI)1097-0231(19990228)13:4<279::AID-RCM478>3.0.CO;2-I
Herrera-Estrella A, Chet I (1999) Chitinases in biological control. EXS 87:171–184
Hrynkiewicz K, Baum C, Leinweber P (2010) Density, metabolic activity, and identity of cultivable rhizosphere bacteria on Salix viminalis in disturbed arable and landfill soils. J Plant Nutr Soil Sci 173:747–756. doi:10.1002/jpln.200900286
Huang CJ, Tsay JF, Chang SY, Yang HP, Wu WS, Chen CY (2012) Dimethyl disulfide is an induced systemic resistance elicitor produced by Bacillus cereus C1L. Pest Manag Sci 68:1306–1310. doi:10.1002/ps.3301
Huang XF, Zhou DM, Guo JH, Manter DK, Reardon KF, Vivanco JM (2015) Bacillus spp. from rainforest soil promote plant growth under limited nitrogen conditions. J Appl Microbiol 118:672–684. doi:10.1111/jam.12720
Idris EE, Iglesias DJ, Talon M, Borriss R (2007) Tryptophan-dependent production of indole-3-acetic acid (IAA) affects level of plant growth promotion by Bacillus amyloliquefaciens FZB42. Mol Plant-Microbes Interact 20:619–626. doi:10.1094/mpmi-20-6-0619
Jasinski M, Ducos E, Martinoia E, Boutry M (2003) The ATP-Binding cassette transporters: structure, function, and gene family comparison between rice and Arabidopsis. Plant Physiol 131:1169–1177. doi:10.1104/pp. 102.014720
Joo HS, Chang CS (2005) Production of protease from a new alkalophilic Bacillus sp. I-312 grown on soybean meal: optimization and some properties. Process Biochem 40:1263–1270. doi:10.1016/j.procbio.2004.05.010
Kang J, Hwang JU, Lee M, Kim YY, Assmann SM, Martinoia E, Lee Y (2010) PDR-type ABC transporter mediates cellular uptake of the phytohormone abscisic acid. Proc Natl Acad Sci 107:2355–2360. doi:10.1073/pnas.0909222107
Karlidag H, Esitken A, Turan M, Sahin F (2007) Effects of root inoculation of plant growth promoting rhizobacteria (PGPR) on yield, growth and nutrient element contents of leaves of apple. Sci Hortic 114:16–20. doi:10.1016/j.scienta.2007.04.013
Keshav Prasad Shukla SS, Singh NK, Singh V, Tiwari K, Singh S (2011) Nature and role of root exudates: efficacy in bioremediation. Afr J Biotechnol 10:9717–9724
Khalid A, Arshad M, Zahir ZA (2004) Screening plant growth-promoting rhizobacteria for improving growth and yield of wheat. J Appl Microbiol 96:473–480. doi:10.1046/j.1365-2672.2003.02161.x
Kim HJ, Chen F, Wang X, Rajapakse NC (2005) Effect of chitosan on the biological properties of sweet basil (Ocimum basilicum L.). J Agric Food Chem 53:3696–3701. doi:10.1021/jf0480804
Kloepper JW, Schroth MN (1978) Plant growth-promoting rhizobacteria on radishes. Proceedings of the 4th International Conference on Plant Pathogenic Bacteria. Station de pathologic Vegetal et Phytobacteriologic, Agners, France
Kloepper J, Leong J, Teintze M, Schroth M (1980) Pseudomonas siderophores: a mechanism explaining disease-suppressive soils. Curr Microbiol 4:317–320. doi:10.1007/BF02602840
Knauth S, Hurek T, Brar D, Reinhold-Hurek B (2005) Influence of different Oryza cultivars on expression of nifH gene pools in roots of rice. Environ Microbiol 7:1725–1733. doi:10.1111/j.1462-2920.2005.00841.x
Kretzschmar T, Kohlen W, Sasse J, Borghi L, Schlegel M, Bachelier JB, Reinhardt D, Bours R, Bouwmeester HJ, Martinoia E (2012) A petunia ABC protein controls strigolactone-dependent symbiotic signalling and branching. Nature 483:341–344. doi:10.1038/nature10873
Labuschagne N, Pretorius T, Idris AH (2011) Plant growth promoting rhizobacteria as biocontrol agents against soil-borne plant diseases. In: Maheshwari DK (ed) Plant growth and health promoting bacteria. Springer, Berlin Heidelberg
Lambert B, Joos H (1989) Fundamental aspects of rhizobacterial plant growth promotion research. Trends Biotechnol 7:215–219. doi:10.1016/0167-7799(89)90107-8
Lee YS, Kim YH, Kim SB (2005) Changes in the respiration, growth, and vitamin C content of soybean sprouts in response to chitosan of different molecular weights. HortSci 40:1333–1335
Li J, Glick BR (2001) Transcriptional regulation of the Enterobacter cloacae UW4 1-aminocyclopropane-1-carboxylate (ACC) deaminase gene (acdS). Can J Microbiol 47:359–367. doi:10.1139/w01-009
Loon LC, Bakker PAHM (2006) Induced systemic resistance as a mechanism of disease suppression by rhizobacteria. In: Siddiqui Z (ed) PGPR: biocontrol and biofertilization. Springer, Netherlands
Loyola-Vargas VM, Broeckling CD, Badri D, Vivanco JM (2007) Effect of transporters on the secretion of phytochemicals by the roots of Arabidopsis thaliana. Planta 225:301–310. doi:10.1007/s00425-006-0349-2
Lucy M, Reed E, Glick B (2004) Applications of free living plant growth-promoting rhizobacteria. Antonie Van Leeuwenhoek 86:1–25. doi:10.1023/B:ANTO.0000024903.10757.6e
Malhotra M, Srivastava S (2006) Targeted engineering of Azospirillum brasilense SM with indole acetamide pathway for indoleacetic acid over-expression. Can J Microbiol 52:1078–1084. doi:10.1139/w06-071
Malhotra M, Srivastava S (2009) Stress-responsive indole-3-acetic acid biosynthesis by Azospirillum brasilense SM and its ability to modulate plant growth. Eur J Soil Biol 45:73–80. doi:10.1016/j.ejsobi.2008.05.006
Martínez-Viveros O, Jorquera MA, Crowley DE, Gajardo G, Mora ML (2010) Mechanisms and practical considerations involved in plant growth promotion by rhizobacteria. J Soil Sci Plant Nutr 10:293–319. doi:10.4067/S0718-95162010000100006
Mayak S, Tirosh T, Glick BR (2004) Plant growth-promoting bacteria that confer resistance to water stress in tomatoes and peppers. Plant Sci 166:525–530. doi:10.1016/j.plantsci.2003.10.025
Miethke M, Marahiel MA (2007) Siderophore-based iron acquisition and pathogen control. Microbiol Mol Biol Rev 71:413–451. doi:10.1128/mmbr.00012-07
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497. doi:10.1111/j.1399-3054.1962.tb08052.x
Nicholas CU (2007) Types, amounts, and possible functions of compounds released into the Rhizosphere by soil-grown plants. The Rhizosphere. CRC Press.
Niu DD, Liu HX, Jiang CH, Wang YP, Wang QY, Jin HL, Guo JH (2011) The plant growth–promoting rhizobacterium Bacillus cereus AR156 induces systemic resistance in Arabidopsis thaliana by simultaneously activating salicylate- and jasmonate/ethylene-dependent signaling pathways. Mol Plant-Microbe Interact 24:533–542. doi:10.1094/MPMI-09-10-0213
Notz R, Maurhofer M, Schnider-Keel U, Duffy B, Haas D, Defago G (2001) Biotic factors affecting expression of the 2,4-Diacetylphloroglucinol biosynthesis gene phlA in Pseudomonas fluorescens biocontrol strain CHA0 in the rhizosphere. Phytopathology 91:873–881. doi:10.1094/phyto.2001.91.9.873
Orhan E, Esitken A, Ercisli S, Turan M, Sahin F (2006) Effects of plant growth promoting rhizobacteria (PGPR) on yield, growth and nutrient contents in organically growing raspberry. Sci Hortic 111:38–43. doi:10.1016/j.scienta.2006.09.002
Orr CH, James A, Leifert C, Cooper JM, Cummings SP (2011) Diversity and activity of free-living nitrogen-fixing bacteria and total bacteria in organic and conventionally managed soils. Appl Environ Microbiol 77:911–919. doi:10.1128/aem.01250-10
Paul D, Lade H (2014) Plant-growth-promoting rhizobacteria to improve crop growth in saline soils: a review. Agron Sustain Dev 34:737–752. doi:10.1007/s13593-014-0233-6
Peterson SB, Dunn AK, Klimowicz AK, Handelsman J (2006) Peptidoglycan from Bacillus cereus mediates commensalism with rhizosphere bacteria from the cytophaga-flavobacterium group. Appl Environ Microbiol 72:5421–5427. doi:10.1128/aem.02928-05
Pilet PE, Saugy M (1987) Effect on root growth of endogenous and applied IAA and ABA: a critical reexamination. Plant Physiol 83:33–38. doi:10.1104/pp. 83.1.33
Prigent-Combaret C, Blaha D, Pothier JF, Vial L, Poirier MA, Wisniewski-Dye F, Moenne-Loccoz Y (2008) Physical organization and phylogenetic analysis of acdR as leucine-responsive regulator of the 1-aminocyclopropane-1-carboxylate deaminase gene acdS in phytobeneficial Azospirillum lipoferum 4B and other Proteobacteria. FEMS Microbiol Ecol 65:202–219. doi:10.1111/j.1574-6941.2008.00474.x
Přikryl Z, Vančura V (1980) Root exudates of plants. Plant Soil 57:69–83. doi:10.1007/bf02139643
Ramaekers L, Remans R, Rao IM, Blair MW, Vanderleyden J (2010) Strategies for improving phosphorus acquisition efficiency of crop plants. Field Crop Res 117:169–176. doi:10.1016/j.fcr.2010.03.001
Rea PA (2007) Plant ATP-binding cassette transporters. Annu Rev Plant Biol 58:347–375. doi:10.1146/annurev.arplant.57.032905.105406
Richardson AE, Simpson RJ (2011) Soil microorganisms mediating phosphorus availability update on microbial phosphorus. Plant Physiol 156:989–996. doi:10.1104/pp. 111.175448
Rothballer M, Schmid M, Fekete A, Hartmann A (2005) Comparative in situ analysis of ipdC-gfpmut3 promoter fusions of Azospirillum brasilense strains Sp7 and Sp245. Environ Microbiol 7:1839–1846. doi:10.1111/j.1462-2920.2005.00848.x
Rovira A (1969) Plant root exudates. Bot Rev 35:35–57. doi:10.1007/BF02859887
Růžička K, Strader LC, Bailly A, Yang H, Blakeslee J, Łangowski Ł, Nejedlá E, Fujita H, Itoh H, Syōno K, Hejátko J, Gray WM, Martinoia E, Geisler M, Bartel B, Murphy AS, Friml J (2010) Arabidopsis PIS1 encodes the ABCG37 transporter of auxinic compounds including the auxin precursor indole-3-butyric acid. Proc Natl Acad Sci U S A 107:10749–10753. doi:10.1073/pnas.1005878107
Ryan AD, Kinkel LL, Schottel JL (2004) Effect of pathogen isolate, potato cultivar, and antagonist strain on potato scab severity and biological control. Biocontrol Sci Tech 14:301–311. doi:10.1080/09583150410001665187
Ryu CM, Farag MA, Hu CH, Reddy MS, Wei HX, Pare PW, Kloepper JW (2003) Bacterial volatiles promote growth in Arabidopsis. Proc Natl Acad Sci 100:4927–4932. doi:10.1073/pnas.0730845100
Sayyed RZ, Gangurde NS, Patel PR, Josh SA, Chincholkar SB (2010) Siderophore production by Alcaligenes faecalis and its application for growth promotion in Arachis hypogaea. Indian J Biotechnol 9:302–307
Sgroy V, Cassán F, Masciarelli O, Papa M, Lagares A, Luna V (2009) Isolation and characterization of endophytic plant growth-promoting (PGPB) or stress homeostasis-regulating (PSHB) bacteria associated to the halophyte Prosopis strombulifera. Appl Microbiol Biotechnol 85:371–381. doi:10.1007/s00253-009-2116-3
Sharp R (2013) A review of the applications of chitin and its derivatives in agriculture to modify plant-microbial interactions and improve crop yields. Agronomy 3:36. doi:10.3390/agronomy3040757
Siddiqui IA, Shaukat SS (2003) Plant species, host age and host genotype effects on Meloidogyne incognita biocontrol by Pseudomonas fluorescens strain CHA0 and its genetically-modified derivatives. J Phytopathol 151:231–238. doi:10.1046/j.1439-0434.2003.00716.x
Somers E, Ptacek D, Gysegom P, Srinivasan M, Vanderleyden J (2005) Azospirillum brasilense produces the auxin-like phenylacetic acid by using the key enzyme for indole-3-acetic acid biosynthesis. Appl Environ Microbiol 71:1803–1810. doi:10.1128/aem.71.4.1803-1810.2005
Stein M, Dittgen J, Sánchez-Rodríguez C, Hou BH, Molina A, Schulze-Lefert P, Lipka V, Somerville S (2006) Arabidopsis PEN3/PDR8, an ATP binding cassette transporter, contributes to nonhost resistance to inappropriate pathogens that enter by direct penetration. Plant Cell Online 18:731–746. doi:10.1105/tpc.105.038372
Stenfors Arnesen LP, Fagerlund A, Granum PE (2008) From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol Rev 32:579–606. doi:10.1111/j.1574-6976.2008.00112.x
Strader LC, Bartel B (2009) The Arabidopsis pleiotropic drug resistance8/ABCG36 ATP binding cassette transporter modulates sensitivity to the auxin precursor indole-3-butyric acid. Plant Cell 21:1992–2007. doi:10.1105/tpc.109.065821
Sugiyama A, Shitan N, Yazaki K (2007) Involvement of a soybean ATP-binding cassette-type transporter in the secretion of genistein, a signal flavonoid in Legume-Rhizobium symbiosis. Plant Physiol 144:2000–2008. doi:10.1104/pp. 107.096727
Wang CJ, Yang W, Wang C, Gu C, Niu DD, Liu HX, Wang YP, Guo JH (2012) Induction of drought tolerance in cucumber plants by a consortium of three plant growth-promoting rhizobacterium strains. PLoS ONE 7, e52565. doi:10.1371/journal.pone.0052565
Wei LH, Xue QY, Wei BQ, Wang YM, Li SM, Chen LF, Guo JH (2010) Screening of antagonistic bacterial strains against Meloidogyne incognita using protease activity. Biocontrol Sci Tech 20:739–750. doi:10.1080/09583151003714109
Weller DM (1988) Biological control of soilborne plant pathogens in the rhizosphere with bacteria. Annu Rev Phytopathol 26:379–407. doi:10.1146/annurev.py.26.090188.002115
Yazaki K (2005) Transporters of secondary metabolites. Curr Opin Plant Biol 8:301–307. doi:10.1016/j.pbi.2005.03.011
Yazaki K, Sasaki K, Tsurumaru Y (2009) Prenylation of aromatic compounds, a key diversification of plant secondary metabolites. Phytochemistry 70:1739–1745. doi:10.1016/j.phytochem.2009.08.023
Zakharova EA, Iosipenko AD, Ignatov VV (2000) Effect of water-soluble vitamins on the production of indole-3-acetic acid by Azospirillum brasilense. Microbiol Res 155:209–214. doi:10.1016/s0944-5013(00)80034-8
Zhou DM, Wang KP, Liu HX, Gu C, Guo JH (2014) Field evaluation of different application methods of the mixture of Bacillus cereus strain AR156 and Bacillus subtilis strain SM21 on pepper growth and disease resistance. Biocontrol Sci Tech 24:1451–1468. doi:10.1080/09583157.2014.945899
Acknowledgments
We thank members of Professors Vivanco’s and Guo’s groups for technical assistance and helpful discussions. We especially thank Prof. Congfeng Song (Nanjing Agricultural University, China) and Dr. Azeddine Driouich (Universite’ de Rouen, France) for helpful suggestions. This work was supported by National Natural Science Foundation of China (No.31471812 and No.31171809) to JG, China Scholarship Council (No. 201206850028) to DZ, and by Colorado State University Agricultural Experiment Station.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Jesus Mercado-Blanco.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
Primers used for amplification of Bacillus cereus AR156 bioactivity genes. (PDF 442 kb)
Table S2
Root exudate data of Col-0 and Atabbc5 analyzed via GC-MS. Data is normalized by the internal standard Ribitol within sample. Field name is as follows [molecular weight (retention time followed by compound identification)]. (XLSX 82 kb)
Fig. S1
Effect of B. cereus AR156 on the shoot growth of 35 d-old Arabidopsis Col-0 and different ABC transporter mutants. (A) Shoot fresh weight was determined at 21 days after inoculation of B. cereus AR156. Asterisks indicate statistically significant differences between the treatments of a given mutants with or without AR156 (t-test; p<0.05). (B)The image represents plants at 21 days after inoculation. (GIF 74 kb)
Fig. S2
AR156 is not pathogenic to Atabcc5 and Col-0. The seedlings at 18 d-old were injected with Bacillus AR156 at a concentration of 2×108 CFU/mL on the leaf and incubated for 24 h. The control was inoculated with water. Each treatment contained 6 plants. (GIF 200 kb)
Rights and permissions
About this article
Cite this article
Zhou, D., Huang, XF., Chaparro, J.M. et al. Root and bacterial secretions regulate the interaction between plants and PGPR leading to distinct plant growth promotion effects. Plant Soil 401, 259–272 (2016). https://doi.org/10.1007/s11104-015-2743-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2743-7