Abstract
The assembly of microorganisms over a surface and their ability to develop resistance against available antibiotics are major concerns of interest. To survive against harsh environmental conditions including known antibiotics, the microorganisms form a unique structure, referred to as biofilm. The mechanism of biofilm formation is triggered and regulated by quorum sensing, hostile environmental conditions, nutrient availability, hydrodynamic conditions, cell-to-cell communication, signaling cascades, and secondary messengers. Antibiotic resistance, escape of microbes from the body’s immune system, recalcitrant infections, biofilm-associated deaths, and food spoilage are some of the problems associated with microbial biofilms which pose a threat to humans, veterinary, and food processing sectors. In this review, we focus in detail on biofilm formation, its architecture, composition, genes and signaling cascades involved, and multifold antibiotic resistance exhibited by microorganisms dwelling within biofilms. We also highlight different physical, chemical, and biological biofilm control strategies including those based on plant products. So, this review aims at providing researchers the knowledge regarding recent advances on the mechanisms involved in biofilm formation at the molecular level as well as the emergent method used to get rid of antibiotic-resistant and life-threatening biofilms.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microorganisms can live in free form or in a consortium of different or same species, called biofilm. Biofilms are an ordered and arranged group of microorganisms living within an extracellular polymeric substance (EPS) matrix produced by them and are adhered to each other on living or non-living surfaces and show variations in terms of growth rate and gene expression when compared to their planktonic form [1,2,3]. To develop a relationship with the host, to show resistance towards hostile external conditions, and to cope with the known antibiotics and other environmental cues, the microorganisms have evolved to form a protective cover around themselves [4]. Biofilm formation contributes towards the development of antibiotic resistance and the formation of persistent cells which are responsible for the unmanageable persistence of microbial infections [5]. Biofilms have various pathological manifestations and exist almost everywhere, inhabiting medical implants, living tissues, water channels, pipes, hospital floors, food processing units, and other biotic and abiotic surfaces [6, 7]. Changes in phenotype and gene expressions accompanied by the resistance to known antibiotics, metabolic activity and growth rate reduction, and production of virulence-associated factors are some features of biofilm-associated microorganisms [1, 8]. As per the reports of the National Institutes of Health (NIH), about 65% and 80% of microbial and chronic infections, respectively, are caused by microbial biofilms, infecting both tissues and medically implanted devices. Breast implants, ventricular shunts, tissue fillers, ventricular-assisted devices, contact lenses, catheters, joint prostheses, urinary catheters, orthopedic implants, pacemakers, mechanical heart valves, defibrillator, vascular grafts, endotracheal tubes, voice prostheses, etc. are some examples of medically implanted devices often infected by microbial biofilms [2]. Some of the tissue-related infections caused by microbial biofilms include periodontitis, osteomyelitis, lung infection in cystic fibrosis, endocarditis, dental plaque, chronic tonsillitis, chronic laryngitis, chronic wounds, and biliary and urinary tract infections [9]. As per the reports of the Centers for Disease Control, 2007, there were about 1.7 million hospital-acquired infections, more than 0.5 million associated deaths, and an economic burden of about US$11,000 million to cure biofilm-associated infections [10]. Furthermore, different sectors of the food industry, viz. poultry, dairy, ready-to-eat, aquaculture, etc., are severely affected by biofilm-producing microorganisms resulting in food spoilage, disease outbreaks, and deaths [11]. So, keeping in view the prevalence of biofilm-associated microorganisms and inefficiency of current antibiotics, the situation requires a transition towards the formation of non-toxic and potent antibiofilm agents targeting signaling pathways regulating quorum sensing (QS), EPS synthesis, biofilm-related genes, microbial motility, adhesion, dispersion, and many more [12,13,14]. The recent novel antibiofilm approaches include the use of ultrashort antimicrobial peptide nanoparticles [15], host defense peptides (HDPs) [16], surface-active organosilane biocide-Goldshield (GS5) [17], biofilm-specific peptides [18], smart antibiofilm surfaces [19], nanoelements (NEs) [20], and poly(ether urethane) (PEU) films for disposal of antibiofilm agents like gallium (Ga) or zinc (Zn) [21].
Biofilm formation: surface colonization
Biofilm formation is a multi-step and complex process that involves the transition of bacteria from free-swimming planktonic form to biofilm-making sessile form. The whole process of formation is influenced by external conditions like temperature, pH, gravitational forces, hydrodynamic forces, Brownian movements, nature of the inhabiting surfaces, quorum sensing, secondary messengers, and other signaling molecules as well [2, 22]. As shown in Fig. 1, different stages of biofilm formation can be divided into four major steps [23].
Steps of biofilm formation. The multi-step biofilm formation starts with the attachment of planktonic microorganisms to the surfaces which is sub-divided into reversible and irreversible attachment followed by microbial division to form microcolonies. Microcolonies undergo maturation characterized by specific composition, shape, and architecture followed by dispersion of biofilm to repeat the cycle. A mature biofilm is a heterogeneous mixture of planktonic (green flagellated), sessile (green), persistent (brown), dead (black) cells, water channels, and different types of signaling and stabilizing molecules like acyl-homoserine lactones (AHL), lipids, polysaccharides, proteins, extracellular DNA (eDNA)
Attachment: a surface-sensing step
The process of biofilm formation is triggered with the adherence of planktonic microorganisms to surfaces and, thus, considered as an important stage to develop the free-flowing microorganisms into an assembled community structure [24]. During the initial stage of biofilm formation, microorganisms are loosely and reversibly attached to surfaces and this stage is characterized by the presence of polarly attached microorganisms to the surfaces. Thereafter, microorganisms change the orientation to lay flat on the surfaces and go for irreversible attachment which develops resistance to many physical factors hindering biofilm formation [25]. Bis-(3ʹ-5ʹ)-cyclic dimeric guanosine monophosphate (c-di-GMP) is an intracellular signaling molecule that plays a vital role in early events of biofilm formation by restricting flagella-mediated swimming motility and increasing biofilm matrix production [26]. The c-di-GMP concentration increases with each attachment/detachment event due to Pil-Chp surface–sensing system present on microbial surfaces. So, early events of biofilm formation involve the conversion of surface naïve planktonic cells (bacteria that exhibit low concentration of c-di-GMP and have not encountered surfaces initially) to surface sentient planktonic cells (bacteria that exhibit a high concentration of c-di-GMP and have encountered surfaces initially) and irreversible attachment of cells to surfaces leading to biofilm formation [27].
Growth or microcolony formation
Soon after the successful adhesion of microorganisms to the surfaces, the adhered microorganisms start multiplication and aggregation within self-produced EPS leading to the microcolony formation in presence of a high concentration of c-di-GMP. Flagella and type IV pili-mediated motilities are important for interactions between microorganisms and surfaces, and cell–cell aggregations to form microcolonies, respectively [28].
Maturation
EPS plays a crucial role in biofilm maturation as it helps in microbial attachment to surfaces, stabilizing the 3-D structure of the biofilm, grouping cells together, protecting from various stresses like host immune system response, antimicrobials, oxidative damage, and metallic cations, and also encapsulating signaling molecules required for quorum sensing, metabolic products, and enzymes [29]. A mature biofilm may acquire a “mushroom” or “tower” shape structure in which microorganisms are arranged as per aero-tolerance and metabolism rate [28]. Otto [30] demonstrated the role of surfactants and modulins in Staphylococcal biofilm maturation through quorum sensing–mediated mechanisms. A mature biofilm is a three-layered structure: inner regulating layer, middle microbial basement layer, and outer layer inhabited by the planktonic form of microorganisms that are ready to exit the biofilm [22].
Dispersion
Finally, matured biofilm ruptures actively (motility and EPS degradation–dependent dispersion) or passively (physical factors like liquid flow-dependent dispersion) to disperse the microorganisms to start a new cycle of biofilm formation. Some factors which are mainly responsible for the dispersion of matured biofilm include outgrown population, intense competition, lack of nutrients [28], enzyme action that causes alginate digestion in Pseudomonas spp. [31], and variation in environmental conditions like temperature, oxygen deficiency, and metabolite accumulation as well as upregulation of genes responsible for cell motility and EPS degradation, and downregulation of genes important for polysaccharide and fimbriae synthesis [32].
Composition of biofilm: an amalgam of complexity, heterogeneity, and variability
Biofilm is a heterogeneous structure comprising mainly of microbial cells (10–25%) and self-produced EPS matrix (75–90%) as shown in Table 1 [33]. Furthermore, in a heterogeneous biofilm, the interstitial voids or water channels of biofilm are required to separate microcolonies from each other [34]. EPS forms a scaffold that holds the biofilm together and, thus, helps in cell-to-cell communication and provides adhesion and cohesion forces required for biofilm formation. EPS helps in nutrient cycling, maintaining the availability of deoxyribonucleic acid (DNA) for horizontal gene transfer (HGT), and acts as a protective barrier against oxidizing biocides, antibiotics, ultraviolet radiations, desiccation, and host immune defense system [35].
The main constituents of EPS could be categorized as follows.
Polysaccharides
Most of the polysaccharides are heterogeneous while some are homogeneous as well like cellulose, sucrose-derived fructans, and glucans [36]. Various interactions like van der Waals interactions, electrostatic attractive and repulsive forces, ionic attractive forces, and hydrogen bonds promote interaction of polysaccharides with themselves or with proteins and ions required for maintaining structure and stability of biofilm matrix [37]. In Pseudomonas aeruginosa, three exopolysaccharides, namely Pel, Psl, and alginate, contribute predominantly to biofilm formation and maintaining biofilm architecture [38]. The role of the polysaccharides is to act as a molecular glue required for bacterial adhesion to each other and to biotic and abiotic surfaces for colonization besides playing a protective role against the immune system, and other external stresses [39].
Extracellular proteins
Extracellular proteins in the biofilm matrix are the amalgam of secreted extracellular proteins, protein subunits of cell appendages such as pili and flagella, cell surface adhesions, and outer membrane vesicle proteins. They interact with exopolysaccharides and nucleic acid components, thus help in biofilm matrix stabilization, surface colonization, and maintaining integrity and architecture of biofilm [40]. Some proteins help in biofilm matrix degradation and dispersal like proteases that dissolve matrix proteins [41], glycosyl hydrolase dispersin B that degrade polysaccharides [42], and DNases that break extracellular nucleic acids [43, 44]. Jiao et al. [45] reported a significant difference between the proteome composition of EPS and that of individual cell fraction and found a pronounced concentration of protein peptidases, cell wall, and polysaccharide metabolism enzymes, disulfide-isomerases, and chaperones like cold shock and DNA binding proteins in EPS matrix. Toyofuku et al. [46] demonstrated that about 30% of EPS matrix proteins were membrane proteins which were found in outer membrane vesicles (OMVs) while some portion of proteins were derived from lysed cells and secreted proteins in P. aeruginosa.
Extracellular DNA
Extracellular DNA (eDNA) is one of the key constituents of the EPS matrix which is important for microbial aggregation within a biofilm. The mechanism of origin of eDNA is diverse as it is released through bacterial secretion systems, cell death because of phages, autolysis, quorum sensing–regulated DNA release, or maybe found in association with DNA-containing OMVs [47,48,49,50,51]. In the case of human infections like cystic fibrosis (CF), the eDNA in P. aeruginosa is derived from human polymorphonuclear leukocytes (PMNs) which approach the site to fight against infection [52]. eDNA guides motility, provides structural stability, and chelates pathogenicity and antibiotic resistance enhancing cations [53,54,55]. eDNA also plays a comprehensive role in cell adhesion, maintaining matrix structural integrity, HGT, and protection from host immune system and antibiotics [56]. Wilton et al. [50] reported that eDNA is responsible for matrix acidification which contributes towards increased resistance of P. aeruginosa against antibiotics. Also, Harmsen et al. [57] found the role of eDNA in attachment and biofilm formation in Listeria monocytogenes strains.
Surfactants and lipids
Some species like Rhodococcus spp. produce hydrophobic EPS and adhere to Teflon and colonize waxy surfaces [58]. Ron and Rosenberg [59] reported the role of biosurfactants in the binding of heavy metals and the production of virulence factors. Some lipids with surface-active properties available in the EPS matrix are surfactin, emulsan, and viscosin. They increase the availability of hydrophobic substances by dispersing them out [36]. Rhamnolipids, an important class of surfactants studied in P. aeruginosa, initiate microcolony formation, help in shaping biofilm, and facilitate biofilm dispersion as well [60, 61].
Water
Water is regarded as the largest component of the EPS matrix of biofilm. It keeps the biofilm hydrated and protects it from desiccation even during environmental water content fluctuations [36]. The flow and maintenance of essential nutrients within a biofilm are attributed to the amount of water available [62].
Architecture of biofilm: the structural and stabilizing parameter of biofilm
The antibiotic resistance and other functional features of a biofilm are related to biofilm structure, the shape of a matrix, and the 3-D arrangement of microorganisms [63]. The heterogeneous local environmental conditions within biofilm influence gene expression and other metabolic activities of biofilm-forming cells [64, 65]. The water channels and pore-less highly packed cells are the two main components of biofilm architecture [66]. The structural knowledge about biofilms is of greater importance to know about the survival and behavioral strategies of biofilms. Bridier et al. [64] monitored the variability of biofilm structure and formation by analyzing some specific biofilm parameters, viz. substratum coverage, bio-volume, roughness, and thickness, and found significant intra- and inter-species variability. By using confocal laser scanning microscopy (CLSM), they found a marked difference in biofilm structures in terms of dispersion, roughness, and aggregation and analyzed that Escherichia coli and L. monocytogenes strains formed rough biofilms with variable thickness, while Salmonella enterica produced small scattered cell clusters. Also, Staphylococcus aureus strains show variability in the structure of biofilms as some produced flat and densely packed structures while others formed scattered ones. Similarly, P. aeruginosa and Enterococcus faecalis formed mushroom-shaped, and flat and compact biofilms, respectively, suggesting that the biofilm architecture differs from microbe to microbe. Environmental factors, cell-to-cell communication, and secondary messengers like cAMP and c-di-GMP shape biofilms to provide microorganisms better adaptability to the local environment [29]. Some other important factors which influence the biofilm architecture are nutrient abundance, hydrodynamic conditions, bacterial motility, cationic and anionic concentration, and availability of proteins and exopolysaccharides within a biofilm. The exopolysaccharides in E. coli [67] and Vibrio cholerae [68] help in three-dimensional biofilm formation. Alginate, a well-studied exopolysaccharide in P. aeruginosa, helps in forming and maintaining biofilm architecture [69]. The secreted protein TasA and exopolysaccharide in Bacillus subtilis biofilm matrix are important for forming fruiting-body-like biofilm and maintenance of matrix integrity [70]. Biofilm architecture is also altered by acetyl groups, the common substituents of exopolysaccharides, as they are believed to be responsible for increased adhesive and cohesive properties of biofilm [36].
Proteins involved in maintaining architecture and stability of biofilm
McCourt et al. [71] described the role of surface binding fibronectin-binding proteins FnBPA and FnBPB in biofilm formation of the methicillin-resistant S. aureus (MRSA) strain LAC. They found that both FnBPA and FnBPB help in bacterial aggregation, thus assist in the initial attachment of bacteria on the surfaces. Liang et al. [72] carried out a study on the new cell surface protein BapA1 which is important for adhesion and biofilm formation through BapA1-mediated cell–cell interactions. It contains nine putative pilin isopeptide linker domains required for aggregation of pilus in various Gram-positive bacteria like Streptococcus parasanguinis. They reported that the mutant generated by deletion of 3′ portion of the bapA1 gene lacks a cell wall–sorting signal important for cell surface fibril formation, thereby inhibiting biofilm formation and autoaggregation of bacteria. Eukaryotic microorganisms like fungi also have been reported to form a biofilm to resist environmental ques. Manfiolli et al. [73] reported that mutants of mitogen-activated protein kinase (MAPK) (MpkA, MpkC, and SakA) decrease the adherence of Aspergillus fumigatus to plates in in vitro conditions. They also analyzed the impact of A. fumigatus protein phosphatase PphA on biofilm formation and found that ΔpphA strain cell wall has less chitin, more β-(1, 3)-glycans, more susceptible to cell wall-damaging agents, less adhesion, and biofilm formation. Streptococcus mutans is responsible for dental caries as it forms a biofilm in the form of dental plaque. S. mutans secrete comCDE code peptide signal molecule called a competence-stimulating peptide (CSP) that senses cell density and environmental stresses that directly influence biofilm formation [74]. Ye et al. [75] reported the role of outer membrane protein W (OmpW) in biofilm formation along with survivability of Cronobacter sakazakii under salt stress conditions and found that both survival rates and biofilm formation increase with the synthesis of OmpW. Polysaccharide intercellular adhesin (PIA) involved in bacterial cell attachment is replaced by protein components in several virulent S. aureus strains. The suhB gene is important for the production of extracellular amyloid fibers. Overexpression of staphylococcal SuhB (SaSuhB) in E. coli produces extracellular macroscopic fibers made of recombinant SaSuhB protein which helps in cell adhesion as the fibers are sticky [76]. Arenas and Tommassen [77] discussed the adhesion of Neisseria meningitidis through a complex network of eDNA and positively charged surface-exposed proteins. Similarly, Bandekar et al. [78] discussed the role of chromosome I encoded VC0395_0300 protein (Sebox3) of V. cholerae in biofilm formation by synthesizing c-di-GMP from guanosine-5ʹ-triphosphate (GTP). Gordonii surface protein B (GspB) in Streptococcus gordonii [79], accessory Sec system SecY2A2 (serine-rich repeat surface protein, PsrP) in Streptococcus pneumoniae [80], and phosphoenolpyruvate phosphotransferase system (PTS) in Klebsiella pneumoniae [81] play important roles in biofilm formation by enhancing bacterial aggregation, substrate binding, and extracellular matrix production, respectively. These examples give an insight that the expression of different proteins plays an important role in stabilizing and maintaining the architecture of biofilm.
Signaling cascades and genes involved in biofilm formation
Extracellular quorum sensing and intracellular cyclic dinucleotide signaling cascades are important in biofilm formation. It has been found that the two cascades may converge and regulate each other during biofilm formation, thus enhance biofilm formation synergistically [82]. Quorum sensing is the intercellular communication that senses cellular density through signaling molecules like N-acyl-homoserine lactones (AHL), the autoinducing peptide (AIP), and autoinducer-2 (AI-2) in Gram-negative, Gram-positive, and both, respectively, and plays a vital role in biofilm formation [83]. Four QS systems are reported in P. aeruginosa, viz. las, rhl, PQS, and integrated QS (IQS). They follow the hierarchical order; thus, these cascades regulate each other through QS-related genes and other transcription factors [84]. c-di-GMP could regulate quorum sensing by affecting the expression of autoinducer synthases; likewise, quorum sensing regulates the level of c-di-GMP by influencing genes encoding proteins having phosphodiesterases A (PDEA) and diguanylate cyclase (DGC) activities in Thermotoga maritima [85]. Li et al. [86] discussed the importance of glycolysis and gluconeogenesis in biofilm formation as they concluded that glycolysis pathway genes were downregulated and gluconeogenesis genes were upregulated during attachment while glycolysis pathway genes were upregulated and gluconeogenesis genes were downregulated during the maturation period of biofilm formation. They also discussed the involvement of cAMP-PKA and MAPK signaling pathways and Mga1, Phd1, Sok2, and Ash1 genes in the biofilm formation of Saccharomyces cerevisiae. In another study, it was demonstrated that mutation in PA0240-encoding putative porin proteins, PA3710-encoding putative alcohol dehydrogenase, and PA3782-encoding putative AraC-like transcriptional regulator downregulates biofilm development of P. aeruginosa as analyzed through in vivo expression technology system (IVET) [87]. Manfiolli et al. [73] studied signal transduction pathways necessary for adhesion and biofilm formation of A. fumigatus suggesting that MAP kinases like SakA, MpkA, and MpkC play a significant role in biofilm formation by enhancing the adhesion and extracellular matrix (ECM) production. Furthermore, phosphatase null mutants, ΔptcB and ΔsitA, were found to degrade cell wall, thus concluded that phosphatases maintain cellular integrity and regulate phosphorylation of MpkA and SakA. Generally, initial microbial adherence and multiplication within the host depend on host environmental signals/factors like pH, temperature, insulin, steroid hormones, monoamines, and vitamin K that mimic and act as exogenous quorum signaling compounds [88]. Horng et al. [81] demonstrated the role of phosphoenolpyruvate and PTS on enhancing biofilm formation in K. pneumoniae. They found an enzyme II complex, a homolog of PTS: KPN00353–KPN00352–KPN00351, and reported that expression of KPN00353–KPN00352–KPN00351 genes releases putative enzyme II complex in PTS that increases synthesis of capsular polysaccharide and eDNA necessary for biofilm formation. ARO1 gene of Candida albicans is important for the production of a multifunctional enzyme which in turn is required for the synthesis of aromatic amino acids through the shikimate pathway. ARO1 gene knockdown effects on biofilm formation in C. albicans were studied by Yeh et al. [89] and it was found that it is involved in cell wall biogenesis and maintaining integrity through activation of Mkc1 signaling cascade. Chambers and Sauer [90] discussed the role of small non-coding RNAs (sRNAs) like ArcZ targeting CsgD in E. coli and Salmonella typhimurium [91], RsmY and RsmZ targeting RsmA in P. aeruginosa [92], and Qrr1–4 targeting AphA in V. cholerae [93] in biofilm formation in response to environmental factors by regulating the transition of planktonic to sessile form. In a polymicrobial association of A. fumigatus and P. aeruginosa, Zheng et al. [94] discussed the transition in A. fumigatus from vegetative growth to conidiation by phenazine-derived metabolites synthesized by P. aeruginosa through NapA oxidative stress signaling cascade. Autoinducer-2 (AI-2) upregulates quorum sensing in Staphylococcus epidermidis by enhancing transcription levels of ica operon and bhp (a biofilm-associated protein along with icaR). Thus, AI-2 enhances quorum sensing and biofilm formation through ica- and bhp-dependent mechanisms [95]. Wotanis et al. [96] discussed the major role of the NspS/MbaA signaling complex in V. cholerae biofilm formation. Polyamines secreted by Vibrionales as environmental signaling molecules regulate biofilm formation in V. cholera positively. NspS/MbaA signaling cascade detects extracellular polyamine norspermidine which binds to NspS periplasmic binding protein, restricts MbaA’s phosphodiesterase activity, and increases levels of second messenger c-di-GMP which is known to enhance biofilm formation.
Antibiotic resistance of biofilm: an adaptive and strategic success of microorganisms
The increase of antibiotic resistance of microorganisms throughout the world is a worrying matter for humans, veterinary, food, and other sectors [97]. Biofilms are resistant to antibiotics and disinfectants and impervious to phagocytosis, and tolerate the body’s immune system mainly because of self-produced EPS [98]. A multi-layer defense system is constituted in biofilm by the formation of persister cells, development of adaptive stress responses, very less antibiotic penetration, limited nutrition, less growth and metabolic activity [99], and inactivation of antimicrobials within the components of the EPS matrix [100]. Ju et al. [101] reported about a 32,768 times increase in antibiotic resistance in the biofilm phenotype Salmonella serovar Dublin as compared to their planktonic form. Hu et al. [102] demonstrated biofilm formation and subsequent increased antibiotic resistance of foodborne Clostridium perfringens. Guo et al. [103] compared the abundance of antibiotic-resistant genes (ARGs) in naturally occurring biofilms in comparison to associated sediment and water samples and detected a high frequency of ARGs, viz. sul1, sul2, tetA, and tetW, in biofilms as compared to sediment and water samples. Aslantaş and Demir [104] studied biofilm formation and antibiotic resistance of S. aureus isolates from subclinical bovine mastitis cases and reported overexpression of adhesion and biofilm-related genes along with resistance to β-lactam antibiotics. The factors which contribute towards antibiotic resistance of biofilm include restricted antimicrobial penetration, antibiotic-modifying enzymes in matrix, eDNA, hypoxia, reduced growth, variability in physiology, oxidative stress and amino acid starvation responses, efflux pumps, quorum sensing, persister cells, HGT, high mutation rate, and colony variants [100]. Some factors are discussed in detail in the next section.
Low metabolic activity, slow growth, and antibiotic resistance
It has been reported that there is an oxygen gradient within a biofilm. The concentration of oxygen near the surface of biofilm is highest and declines towards the center, creating almost anaerobic conditions in the center [105]. Totani et al. [106] reported that low oxygen condition promotes while normoxia hinders biofilm formation. There is a similar stratification in metabolic activity, growth, and protein synthesis in biofilms with a high rate at the surface and no or very less rate in the center resulting in less penetration and consumption of antibiotics in the biofilm [107]. The phenotypic diversity of microorganisms within biofilm due to depleted nutrients, oxygen, and other responses promotes asynchronous growth and differential gene expression that leads to drug tolerance through regulation of genes important for DNA repair, lipid biosynthesis, toxin efflux, and ion sequestration [9, 108].
Impact of horizontal gene transfer on resistance development
Biofilm acts as a reservoir of antibiotic resistance genes (ARGs, the resistome) which are found to be responsible for providing antibiotic resistance to pathogens through HGT [109] conjugation [110, 111], transformation [112], and transduction [113, 114]. HGT acclimates bacteria to the changing environment and favors biofilm formation and resistance to antibiotics [115]. HGT gives rise to new variants without inducing mutation in the variant [116]. Fan et al. [117] discussed that HGT maintains structural stability, integrity, and robustness of microbial communities coexisting together. HGT contributes towards several traits including pathogenicity and antibiotic resistance in E. faecalis responsible for persistent endodontic infection [118].
Persister cells and antibiotic resistance
Biofilms are characterized by the presence of persister cells—a specialized phenotype of bacterial cells that neither grow normally nor die even in the presence of potent antibiotics. Persister cells are regarded as dormant variants of regular cells [119]. Persister cells within the biofilm can survive the high dose of antibiotic treatment as well as the immune defense system. But upon reduction of the antibiotics, persister cells can repopulate in the biofilm [120]. Persister cells are genetically similar but physiologically different from parent cells. They are formed under environmental stress conditions within the biofilm and show special characteristics, viz. they are metabolically inert, show slow growth and replication, regulate the toxin-antitoxin system, show ineffectiveness towards antibiotics, upregulate phosphate metabolism, and enhance anti-oxidative and DNA repair system [121]. Antibiotics kill planktonic cells and some proportion of biofilm cells, decreasing their population, but show ineffectiveness towards the persister cells. As a result, antibiotic-resistant persister cells reproduce, disperse out of biofilms, and form new biofilms [122].
Role of eDNA in antibiotic resistance
Colonization of bacteria on surfaces releases proteins, exopolysaccharides, and eDNA which confer stability and structural integrity and promote proper nutrient distribution to the forming biofilm [123]. eDNA is produced by cell lysis and active secretions and has been reported to promote microbial adhesion, inhibit antimicrobial diffusion, and chelate cations [56] besides suppressing innate immune response [124]. It has been reported that eDNA contributes to cation gradients, genomic DNA release, and inducible antibiotic resistance. Mulcahy et al. [125] demonstrated the induction of eDNA-mediated antibiotic resistance in P. aeruginosa biofilm by regulating PhoPQ and PmrAB cationic antimicrobial peptide resistance operon PA3552–PA3559 system. One of the reported mechanisms is that eDNA attaches and chelates positively charged aminoglycosides and antimicrobial peptides as proved by Chiang et al. [126] in P. aeruginosa biofilms. Jakubovics and Burgess [127] discussed the role of eDNA in the promotion of bacterial adhesion, maintaining structural integrity, the evolution of bacteria through genetic recombination and HGT, shielding against antimicrobials, and serving as a source of phosphorous.
Efflux pumps and antibiotic resistance
Efflux pumps are proteinaceous active transporters embedded within cytoplasmic membranes. The resistance-nodulation-division family (RND), the multidrug and toxic compound extrusion family (MATE), the small multidrug resistance family (SMR), the major facilitator superfamily (MF), and the ATP-binding cassette family (ABC) are different classes of efflux pumps reported in bacteria by Kumar and Schweizer [128] and Singh et al. [129]. Efflux pumps induce antibiotic resistance to microorganisms by pushing intracellular toxins including antibiotics away from intracellular targets back into extracellular space [100]. Although efflux pumps are active in planktonic bacteria as well, they are upregulated in biofilms leading to multidrug resistance (MDR) [28, 129]. Several efflux pump genes and their overexpression in the biofilm have been reported like efflux pump gene PA1874–1877 in P. aeruginosa [130]; the overexpressed RND efflux pumps like BCAL1672-1676 (RND-3) provide biofilm resistance against tobramycin and ciprofloxacin while BCAM0925-0927 (RND-8) and BCAM1945-1947 (RND-9) protect biofilms from tobramycin in Burkholderia cepacia [131].
Role of antibiotic-modifying enzymes of matrix on resistance development
Another important mechanism for antibiotic resistance is an enzymatic modification of antibiotics to a non-toxic form within EPS. Such enzymes enhance virulence and induce resistance against antibiotics, thus regarded as exotoxins. Lyases, group transferases, hydrolases, and redox enzymes are the reported classes of antibiotic-modifying enzymes [132]. They modify and, thus, inactivate antibiotics by either cleaving chemical bonds necessary for the functioning of enzymes or restricting the binding of antibiotics to specific targets [133]. β-Lactamase secreted by K. pneumoniae biofilms destroys ampicillin and inhibits it from targeting cells [134]. In infected cystic fibrosis lung, over-synthesis of AmpC cephalosporinase has a specific role in providing antibiotic resistance to P. aeruginosa [135].
Escape of biofilm cells from immune system: an evading success
An immediate response is established in the body through immune cells, receptors, and several humoral factors like mannose-binding lectins, and antibodies of innate immunity upon infection [136]. Microbial biofilms are reported to escape the body’s immune system. S. aureus biofilms may evade host immunity by macrophage dysfunction [124], reduce phagocytosis by leukocytes [137], and diminish antibody-mediated phagocytosis because of EPS matrix [138]. A 25% reduction of oxidative burst response of PMNs has been found in P. aeruginosa in biofilm when compared to their planktonic form [139]. Alginate (an exopolysaccharide) and rhamnolipids (glycolipids) have been found to protect the biofilm of P. aeruginosa from leukocyte phagocytosis [140, 141]. Mature biofilms of C. albicans are resistant to monocyte phagocytosis, thus show an immunosuppressive effect [142]. By secreting biofilm promoting compounds like exopolysaccharides, proteins, and other peptides, S. epidermidis regulates host immune responses and escapes getting killed [143]. Rhamnolipids and alginate produced by P. aeruginosa help in polymorphonuclear leukocyte necrosis and in evading macrophages, respectively [144]. Modulation in the deposition of immunoglobulin G (IgG) on the bacterial surfaces and activation of complement protein C3b by S. epidermidis provides resistance to phagocytosis, thus get escaped from the immune system and form biofilm [123, 145].
Biofilm control strategies: convenient and advanced methods to counter-attack biofilm menace
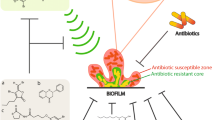
Biofilm is highly resistant to conventional antibiotics, so there is an urgent requirement to develop alternative potent therapeutic solutions to overcome the problem and improve healthcare, food safety, and other industrial sectors [11]. Chen et al. [146] classified the antibiofilm approaches into two broad categories: (a) targeting the process of biofilm formation and (b) replacing material of the substrate. The first category uses small molecules like anti-virulence compounds, e.g., CCG-203592 and CCG-205363 [147], antibiofilm compound 1 (ABC-1)—a novel benzimidazole molecule [148], and metal ion chelators including calcium chelators, e.g., trisodium citrate (TSC) and ethylene glycol tetraacetic acid (EGTA) [149]. The antibiofilm approaches also employ matrix-inhibiting enzymes like DNase I [150], proteinase K, and trypsin [151]. The second category is based on the replacement of substrate material, including medical devices, by biofilm resistant ones like using bactericidal and anti-adhesion coatings. Han et al. [152] used acidic electrolyzed water (AEW) to remove pathogenic foodborne biofilms, and they found that AEW triggers EPS disruption by deforming aromatic rings in tyrosine and phenylalanine and carbohydrate C–O–C bond. van Tilburg Bernardes et al. [153] reviewed some recent approaches for biofilm inhibition, eradication, and dispersion, viz. use of antibiofilm peptides like peptide 1018 [154], bacteriophage therapy [33] like anti-E. faecalis and Enterococcus faecium phage (EFDG1) [155], and molecules that inhibit virulence and quorum sensing signals like pilicides [156], and dihydrosventrin (DHS) [157]. Sadekuzzaman et al. [158] discussed various strategies for fighting against biofilms which include natural products like plant extracts, honey, essential oils, cumin oil, and cinnamon oil; bacteriophage; quorum sensing inhibitors; nanotechnologies like metal nanoparticles and micro- and nanoemulsions; biofilm inhibiting enzymes like deoxyribonuclease 1, lactonase, lyase, lysostaphin (LS), and α-amylase; photodynamic therapy; biosurfactants; bacteriocin; ultrasonic treatment; bioelectric approach; and some particular antibiofilm agents like capsular polysaccharides, catheter lock solution, diethylamine NONOate diethylammonium, molsidomine, xylitol, gallium, chitosan, and povidone-iodine (PVP). Gomes et al. [159] discussed the combinatorial effect of chemical (sodium hypochlorite) and mechanical stress (shear effect) against biofilms of Acinetobacter calcoaceticus and Stenotrophomonas maltophilia in drinking water distribution systems (DWDS). Pires et al. [160] reviewed the strategy of using phages with antimicrobials phage-antibiotic synergy (PAS) and their significant impact on biofilm removal. For example, the synergistic effect of using T4 phage and cefotaxime together to remove biofilm of E. coli ATCC 11,303 has been demonstrated [161]. Lactic acid bacteria (LAB) of genera Lactobacillus, Enterococcus, Lactococcus, and Streptococcus have been used successfully against the biofilms of Salmonella spp. in poultry [162]. In the meat industry, cetyltrimethylammonium bromide (CTAB) and cellulase synergistically can remove mature biofilms of Salmonella spp. efficiently [163]. Aqueous and saline extracts of Moringa oleifera Lam. (drumstick tree) seeds have been used efficiently in eradicating biofilms of Staphylococcus spp. obtained from dairy effluents [164]. Leary et al. [165] analyzed the effect of different biofilm-eradicating and sterilization treatments (autoclave, sonication, saline scrub, 4% chlorhexidine (CHC) scrub) in different combinations and found a significant antibiofilm effect of autoclaving and CHC scrub combination against S. aureus and S. epidermidis biofilms from orthopedic implant materials. As biofilm formation is prominent on both biotic and abiotic surfaces like living tissues, medical devices, and food processing surfaces, it becomes important to modify the surfaces and or use inert materials to inhibit biofilm formation. Ficai and Ficai [166] mentioned different means of enhancement of antibiofilm activity through surface modifications like (a) developing new materials such as metals and alloys, polymers, ceramics, and composites, (b) modification of surfaces by physical means like reducing surface roughness by temperature curing, and (c) modification of surfaces by chemical means like antibiofilm agent immobilization, use of quaternary ammonium salts, chlorhexidine, nanoparticles, and surfactants. Biofilm formation was inhibited significantly by nanoporous (15–100 nm) anodic alumina surfaces in E. coli, L. monocytogenes, S. aureus, and S. epidermidis indicating the applications of these surfaces in the healthcare and food industry [167]. Topographic silica coatings reduced C. albicans biofilm formation as it was found that less biofilm formation occurred when the particle size of silica was in the range of 0.5–2.0 μm as compared to particle size in the range of 4.0–8.0 μm [168]. Gkana et al. [169] mentioned the potential use of organosilane nanoparticles as anti-adhesion and antibiofilm surface agents against various foodborne pathogens. Some novel and innovative surface modifications employed to inhibit biofilm formation include auranofin releasing antibacterial and antibiofilm polyurethane catheter coating [170], polyurethane/Hypericum perforatum extract (PHPE) composite [171], and fluoro-modified polypropylene films like polypropylene polyheptaflourobutyl methacrylate film (PP-PHFBM) [172]. In a recent advancement, the microbiota has been used for the eradication of microbial biofilms. Glatthardt et al. [173] reported antibiofilm activity of the bioactive molecules present in commensal S. epidermidis cell-free conditioned media (CFCM) against Staphylococcus aureus clinical isolates. It was demonstrated that S. epidermidis CFCM demonstrated a significant reduction in biofilm formation and enhanced disruption of established biofilms as well. Many approaches have been studied against biofilm formation and eradication; the plant-based approach is gaining much attention due to the presence of numerous active molecules which may be an alternative to antibiotics. The different current strategies to inhibit or eradicate the process of biofilm formation are represented in Fig. 2.
Plant product–based approach
From ancient times, plants are known to have medicinal value and, therefore, have been used to treat different diseases or in the preservation of food from spoilage. The antimicrobial activities of different plants have been explored exponentially to date. But now the scientists have a keen interest to find out the plant products which can effectively control biofilm formation and/or virulence factors of pathogenic microorganisms rather than killing directly [174, 175]. Several plant extracts, essential oils, and plant-based nanoformulations have been studied extensively to combat the biofilm-related problems, yet many plant products remain unexplored. Figure 3 shows different ways the plants are being used effectively against biofilm.
Plant-based antibiofilm strategies. The figure mentions the different ways plants are being used nowadays to inhibit, reduce, or eradicate biofilm formation. As indicated in the figure, the plants act as inhibitors of quorum sensing (QS), biofilm-related enzymes, extracellular polymeric substance (EPS) matrix synthesis, virulence factors, secondary messengers, signaling cascades, biofilm promoting genes, and other biofilm-related factors. From the figure, it is clear that plants could be used effectively against microbial biofilms
We know that plants like humans and animals come across pathogenic microorganisms frequently, so plants have developed a sophisticated biochemical system to defend themselves from microbial attacks. Plants act as a reservoir of metabolites broadly classified as phenolic compounds, phenolic acids, flavonoids, and terpenes that execute various pharmacological activities like antiviral, antifungal, anti-parasitic, antioxidant, antitumor, antibacterial, and anti-inflammatory. Recent studies suggest that these metabolites are effective against pathogenic biofilm-forming microorganisms even at sub-MIC concentrations [176]. As mentioned earlier, several factors play a vital role in biofilm formation and progression, so we will discuss the role of phytochemicals in the suppression, inhibition, or eradication of microbial biofilms through different mechanisms.
As discussed earlier, QS is a cell density–dependent phenomenon that controls the pathogenicity or virulence, expression of genes important for biofilm formation, and regulation of various physiological activities in most pathogenic bacteria. This change in bacterial phenotype and other physiological characters are responsible for providing the resistance of the microorganisms against antibiotic compounds. Thus, inhibition of QS could be a better option to inhibit biofilm formation as it does not affect bacterial growth, rather it suppresses the synthesis of QS molecules required for enhancing pathogenesis [177]. In this context, several studies have demonstrated that phytochemicals could be used as QS inhibitors because of their stability, effectiveness, and harmless nature. So, phytochemicals as QS inhibitors could be used for biofilm inhibition, dispersal, and eradication without exerting selective pressures on bacteria to develop resistance which is not possible in the case of known antibiotics [178, 179]. Some known QS inhibitors obtained from plants are farnesol, cinnamaldehyde, resveratrol, vanillin, naringin, tannic acid, curcumin, ellagic acid, quercetin, kaempferol, etc. [180]. Since metals play a crucial role in parthenogenesis, virulence, and maintenance of biofilm, the use of phytochemicals as metal-chelators would be an effective tool to minimize biofilm formation at sub-lethal concentrations. Lin et al. [181] demonstrated the use of 1, 2, 3, 4, 6-penta-O-galloyl-b-d-glucopyranose (PGG) as an iron-chelating agent for the inhibition of biofilm formation in S. aureus. A calcium-chelating phytochemical, alizarin, was studied by Lee et al. [182] to demonstrate its role in inhibiting biofilm formation in S. aureus by quenching calcium ions. Plant extracts and/or phytochemicals employ different mechanisms to inhibit biofilm formation. Some of the studies demonstrated the role of medicinal plants to inhibit or reduce the production of biofilm which include the following: inhibition of virulence factors and other regulatory genes (vicR, relA, brpA, and comDE) by Kaffir lime leaf extract in S. mutans [183]; curcumin from Curcuma longa inhibited elastase/protease activity, pyocyanin biosynthesis, production of acyl-homoserine lactone (HSL), and downregulated QS genes in P. aeruginosa [184]; reduction of swimming, swarming, and twitching motility of Yersinia enterocolitica by naringin from orange extract [185]; decreasing the adhesive capability of S. aureus to abiotic surfaces by taxodione derivative obtained from Salvia austriaca [186]; reducing levels of a secondary messenger, bis-(3′-5′)-cyclic dimeric guanosine monophosphate synthesis, by Zingiber officinale crude extract in P. aeruginosa [187]; reducing the synthesis of pyocyanin and 2-heptyl-3-hydroxy-4(1H)-quinolone by cinnamon bark oil in P. aeruginosa and E. coli [188]; breakdown and reduction of EPS by AgNPs and extract of Heliotropium crispum nanoformulation against P. aeruginosa and Acinetobacter baumannii [189]; decreasing hydrophobicity index of E. faecalis and Aeromonas hydrophila by AgNPs and Momordica charantia fruit extract nanoformulations [190]; decrease in hydrophobicity, glucan synthesis, and cell-to-cell adhesion of S. mutans by Emblica officinalis extract [191]; reduction of EPS synthesis by decreasing production of glycosyltransferase by Achyranthes aspera L. extract in S. mutans [192]; attenuation of QS and QS-related virulence factors of P. aeruginosa by Cuphea carthagenensis extract [178]; preventing expression of QS-regulated genes LasIR and RhlIR in P. aeruginosa PAO1 by iberin isolated from Armoracia rusticana extract [177, 193]. Syzygium cumini– and Psidium guajava L.–based silver nanoparticles are some other plant-based nanoformulations that have been reported to exhibit potent antimicrobial and antibiofilm efficiencies [194, 195].
About 70% of the population in India and a good percentage of the population in other developing countries, in the range of 40% (Columbia) to 90% (Ethiopia), rely on the traditional medicinal system as a curative approach or for improving health conditions [196]. Plants have been used in the traditional systems to cure various health issues since time immemorial and are regarded as the important source of new drugs. The use of plants as traditional and complementary medicine has gained interest as a safe alternative to maintain health and cure diseases [197]. The development of resistance of pathogenic microorganisms towards known antibiotics is pushing the researchers to introduce novel and efficient antibiofilm therapy to deal with the biofilm menace. The use of plants and/or phytochemicals as antibiofilm agents is advantageous over known antibiotics in a way that they are less expensive, have less chance of side effects, and are readily available [198, 199]. According to Kim Lewis et al. [200], the plant-derived compounds have less chance to induce resistance in microorganisms due to the reason that plants may use a different chemical strategy for the control of microbial infections, perhaps to decrease the selective pressure for developing antibiotic resistance. But, phytochemicals may also inhibit growth, in which case there would be no such advantage over known antibiotics. Besides, plants are believed to have evolved with the mechanisms of synthesizing QS-interrupting molecules for quorum quenching to treat microbial infections involved in biofilm formation [201].
Conclusion and future perspective
Over time, different strategies have been developed to inhibit the planktonic growth of microorganisms. But the rise of antibiotic ineffectiveness, multidrug-resistant microorganisms, and recalcitrant infections directed researchers to understand different aspects of microbial growth and resistance to environmental cues. Most chronic infections are associated with microbial biofilms due to their potentiality to resist the known antibiotics and survive even in harsh environmental conditions. Our knowledge regarding biofilms has increased progressively since they were noticed and defined. The major achievements include elucidation of mechanisms of biofilm formation at the molecular level, the role of secondary messengers, homoserine lactones, secreted proteins, eDNA, and other metabolites in the regulation of biofilm-related genes, and maintaining the structural integrity of biofilm. The impact of various parameters affecting biofilm formation and maintenance has been studied extensively like the effect of metal ions, environmental cues, and other physiological parameters on the development and maturation of biofilms, and the importance of EPS in nutrient cycling, gene transfer, and protection against antibiotics and immune system. Furthermore, the discovery of methods and mechanisms employed by biofilms to overcome potent antibiotics and the use of ecofriendly biological, physical, and chemical methods to destabilize the biofilm communities are also worth mentioning.
Although people are trying to combat the problems created by biofilms, we have not yet come up with any novel antibiofilm strategy. We should focus on the strategies which are efficient, ecofriendly, persistent, and cost-effective as well. In this regard, researchers are trying to develop potent antibiofilm agents from natural products and/or an amalgam of phytochemicals with other physical, chemical, or biological methods to show synergistic effect and do not contribute towards the enhancement of microbial resistance. In addition to this, development of standardized antibiofilm protocols, the requirement of in vivo validations, and further understanding of mechanisms, signaling cascades, gene regulation, and involvement of signaling molecules including secondary messengers, etc. in the establishment, development, maturation, and dispersal of biofilms are the need of the hour.
Data availability
Not applicable.
Code availability
Not applicable.
References
Gupta P, Sarkar S, Das B, Bhattacharjee S, Tribedi P (2016) Biofilm, pathogenesis and prevention—a journey to break the wall: a review. Arch Microbiol 198:1–15. https://doi.org/10.1007/s00203-015-1148-6
Jamal M, Tasneem U, Hussain T, Saadia Andleeb S (2015) Bacterial biofilm: its composition, formation and role in human infections. Res Rev J Microbiol Biotechnol 4:1–15
Lohse MB, Gulati M, Johnson AD, Nobile CJ (2018) Development and regulation of single-and multi-species Candida albicans biofilms. Nat Rev Microbiol 16:19. https://doi.org/10.1038/nrmicro.2017.107
Castiblanco LF, Sundin GW (2016) New insights on molecular regulation of biofilm formation in plant-associated bacteria. J Integr Plant Biol 58:362–372. https://doi.org/10.1111/jipb.12428
Pang Z, Raudonis R, Glick BR, Lin TJ, Cheng Z (2018) Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Biotechnol Adv 37:177–192. https://doi.org/10.1016/j.biotechadv.2018.11.013
Donlan RM (2002) Biofilms: microbial life on surfaces. Emerging Infect Dis 8:881. https://doi.org/10.3201/2Feid0809.020063
Donelli G, Vuotto C (2014) Biofilm-based infections in long-term care facilities. Future Microbiol 9:175–188. https://doi.org/10.2217/fmb.13.149
Sharma BK, Saha A, Rahaman L, Bhattacharjee S, Tribedi P (2015) Silver inhibits the biofilm formation of Pseudomonas aeruginosa. Adv Appl Microbiol 5:677–685. https://doi.org/10.4236/aim.2015.510070
Rather MA, Gupta K, Bardhan P, Borah M, Sarkar A, Eldiehy KS et al (2021) Microbial biofilm: a matter of grave concern for human health and food industry. J Basic Microbiol 61:380–395. https://doi.org/10.1002/jobm.202000678
Brinkman CL, Schmidt-Malan SM, Karau MJ, Greenwood-Quaintance K, Hassett DJ, Mandrekar JN, Patel R (2016) Exposure of bacterial biofilms to electrical current leads to cell death mediated in part by reactive oxygen species. PLoS ONE 11(12):e0168595. https://doi.org/10.1371/journal.pone.0168595
Giaouris EE, Simões MV (2018) Pathogenic biofilm formation in the food industry and alternative control strategies. Foodborne Diseases. Elsevier, pp. 309–377. https://doi.org/10.1016/B978-0-12-811444-5.00011-7.
Li XH, Lee JH (2017) Antibiofilm agents: a new perspective for antimicrobial strategy. J Microbiol 55:753–766. https://doi.org/10.1007/s12275-017-7274-x
Parrino B, Schillaci D, Carnevale I, Giovannetti E, Diana P, Cirrincione G et al (2018) Synthetic small molecules as anti-biofilm agents in the struggle against antibiotic resistance. Eur J Med Chem 161:154–178. https://doi.org/10.1016/j.ejmech.2018.10.036
Roy R, Tiwari M, Donelli G, Tiwari V (2018) Strategies for combating bacterial biofilms: a focus on anti-biofilm agents and their mechanisms of action. Virulence 9:522–554. https://doi.org/10.1007/s12275-017-7274-x
Almaaytah A, Mohammed GK, Abualhaijaa A, Al-Balas Q (2017) Development of novel ultrashort antimicrobial peptide nanoparticles with potent antimicrobial and antibiofilm activities against multidrug-resistant bacteria. Drug Des Devel Ther 11:3159–3170. https://doi.org/10.2147/2FDDDT.S147450
de la Fuente-Núñez C, Cardoso MH, de Souza CE, Franco OL, Hancock RE (2016) Synthetic antibiofilm peptides. Biochim Biophys Acta 1858:1061–1069. https://doi.org/10.1016/j.bbamem.2015.12.015
Murray J, Muruko T, Gill CI, Kearney MP, Farren D, Scott MG et al (2017) Evaluation of bactericidal and anti-biofilm properties of a novel surface-active organosilane biocide against healthcare associated pathogens and Pseudomonas aeruginosa biofilm. PLoS ONE 12:e0182624. https://doi.org/10.1371/journal.pone.0182624
Dostert M, Belanger CR, Hancock RE (2018) Design and assessment of anti-biofilm peptides: steps toward clinical application. J Innate Immun 1-12https://doi.org/10.1159/000491497
Li X, Wu B, Chen H, Nan K, Jin Y, Sun L, Wang B (2018) Recent developments in smart antibacterial surfaces to inhibit biofilm formation and bacterial infections. J Mater Chem 6:4274–4292. https://doi.org/10.1039/C8TB01245H
Paula AJ, Koo H (2017) Nanosized building blocks for customizing novel antibiofilm approaches. J Dent Res 96:128–136. https://doi.org/10.1177/2F0022034516679397
Ma H, Darmawan ET, Zhang M, Zhang L, Bryers JD (2013) Development of a poly (ether urethane) system for the controlled release of two novel anti-biofilm agents based on gallium or zinc and its efficacy to prevent bacterial biofilm formation. J Control Release 172:1035–1044. https://doi.org/10.1016/j.jconrel.2013.10.005
Zhao X, Zhao F, Wang J, Zhong N (2017) Biofilm formation and control strategies of foodborne pathogens: food safety perspectives. RSC Adv 7:36670–36683. https://doi.org/10.1039/C7RA02497E
Chandki R, Banthia P, Banthia R (2011) Biofilms: a microbial home. J Indian Soc Periodontol 15:111. https://doi.org/10.4103/2F0972-124X.84377
Haggag W (2010). The role of biofilm exopolysaccharides on biocontrol of plant diseases. Biopolymers. IntechOpen, pp. 271–284 https://doi.org/10.5772/10266.
Banerjee P, Singh M, Sharma V (2015) Biofilm formation: a comprehensive review. Int J Pharm Res Health Sci 3:556–560
Römling U, Galperin MY, Gomelsky M (2013) Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol Mol Biol Rev 77:1–52. https://doi.org/10.1128/MMBR.00043-12
Armbruster CR, Parsek MR (2018) New insight into the early stages of biofilm formation. Proc Natl Acad Sci USA 115:4317–4319. https://doi.org/10.1073/pnas.1804084115
Rabin N, Zheng Y, Opoku-Temeng C, Du Y, Bonsu E, Sintim H (2015) Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med Chem 7:493–512. https://doi.org/10.4155/fmc.15.6
Toyofuku M, Inaba T, Kiyokawa T, Obana N, Yawata Y, Nomura N (2016) Environmental factors that shape biofilm formation. Biosci Biotechnol Biochem 80:7–12. https://doi.org/10.1080/09168451.2015.1058701
Otto M (2013) Staphylococcal infections: mechanisms of biofilm maturation and detachment as critical determinants of pathogenicity. Annu Rev Med 64:175–188. https://doi.org/10.1146/annurev-med-042711-140023
Costerton JW, Stewart PS, Greenberg E (1999) Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322. https://doi.org/10.1126/science.284.5418.1318
McDougald D, Rice SA, Barraud N, Steinberg PD, Kjelleberg S (2012) Should we stay or should we go: mechanisms and ecological consequences for biofilm dispersal. Nat Rev Microbiol 10:39. https://doi.org/10.1038/nrmicro2695
Lu TK, Collins JJ (2007) Dispersing biofilms with engineered enzymatic bacteriophage. Proc Natl Acad Sci USA 104:11197–11202. https://doi.org/10.1073/pnas.0704624104
Evans LV (2003) Biofilms: recent advances in their study and control, 1st edn. CRC Press
Flemming HC, Wingender J, Griebe T, Mayer C (2000) Physico-chemical properties of biofilms. Biofilms: recent advances in their study and control, CRC press, pp. 19–34.
Flemming HC, Wingender J (2010) The biofilm matrix. Nat Rev Microbiol 8:623. https://doi.org/10.1038/nrmicro2415
Lembre P, Lorentz C, Di Martino P (2012) Exopolysaccharides of the biofilm matrix: a complex biophysical world. The complex world of polysaccharides. InTech., pp. 371–392. https://doi.org/10.5772/51213.
Rehm BH (2010) Bacterial polymers: biosynthesis, modifications and applications. Nat Rev Microbiol 8:578. https://doi.org/10.1038/nrmicro2354
Limoli DH, Jones CJ, Wozniak DJ (2015) Bacterial extracellular polysaccharides in biofilm formation and function. Microbiol.Spectr. 3https://doi.org/10.1128/microbiolspec.MB-0011-2014
Fong JN, Yildiz FH (2015) Biofilm matrix proteins. Microbiol.Spectr. 3https://doi.org/10.1038/nrmicro2415
Martí M, Trotonda MP, Tormo-Más MÁ, Vergara-Irigaray M, Cheung AL, Lasa I, Penadés JR (2010) Extracellular proteases inhibit protein-dependent biofilm formation in Staphylococcus aureus. Microbes Infect 12:55–64. https://doi.org/10.1016/j.micinf.2009.10.005
Kaplan JB, Ragunath C, Ramasubbu N, Fine DH (2003) Detachment of Actinobacillus actinomycetemcomitans biofilm cells by an endogenous β-hexosaminidase activity. J Bacteriol 185:4693–4698. https://doi.org/10.1128/JB.185.16.4693-4698.2003
Mann EE, Rice KC, Boles BR, Endres JL, Ranjit D, Chandramohan L, Tsang LH, Smeltzer MS, Horswill AR, Bayles KW (2009) Modulation of eDNA release and degradation affects Staphylococcus aureus biofilm maturation. PLoS ONE 4:e5822. https://doi.org/10.1371/journal.pone.0005822
Nijland R, Hall MJ, Burgess JG (2010) Dispersal of biofilms by secreted, matrix degrading, bacterial DNase. PLoS ONE 5:e15668. https://doi.org/10.1371/journal.pone.0015668
Jiao Y, D’haeseleer P, Dill BD, Shah M, VerBerkmoes NC, Hettich RL, Banfield JF, Thelen MP (2011) Identification of biofilm matrix-associated proteins from an acid mine drainage microbial community. Appl Environ Microbiol 77:5230–5237. https://doi.org/10.1128/AEM.03005-10
Toyofuku M, Roschitzki B, Riedel K, Eberl L (2012) Identification of proteins associated with the Pseudomonas aeruginosa biofilm extracellular matrix. J Proteome Res 11:4906–4915. https://doi.org/10.1021/pr300395j
Allesen-Holm M, Barken KB, Yang L, Klausen M, Webb JS, Kjelleberg S, Molin S, Givskov M, Tolker-Nielsen T (2006) A characterization of DNA release in Pseudomonas aeruginosa cultures and biofilms. Mol Microbiol 59:1114–1128. https://doi.org/10.1111/j.1365-2958.2005.05008.x
Montanaro L, Poggi A, Visai L, Ravaioli S, Campoccia D, Speziale P, Arciola CR (2011) Extracellular DNA in biofilms. Int J Artif Organs 34:824–831. https://doi.org/10.5301/2Fijao.5000051
Vorkapic D, Pressler K, Schild S (2016) Multifaceted roles of extracellular DNA in bacterial physiology. Curr Genet 62:71–79. https://doi.org/10.1007/s00294-015-0514-x
Wilton M, Charron-Mazenod L, Moore R, Lewenza S (2016) Extracellular DNA acidifies biofilms and induces aminoglycoside resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother 60:544–553. https://doi.org/10.1128/AAC.01650-15
Ibáñez de Aldecoa AL, Zafra O, González-Pastor JE (2017) Mechanisms and regulation of extracellular DNA release and its biological roles in microbial communities. Front Microbiol 8:1390. https://doi.org/10.3389/fmicb.2017.01390
Lethem M, James SL, Marriott C, Burke JF (1990) The origin of DNA associated with mucus glycoproteins in cystic fibrosis sputum. Eur Respir J 3:19–23
Marshall KC, Stout R, Mitchell R (1971) Mechanism of the initial events in the sorption of marine bacteria to surfaces. Microbiology 68:337–348. https://doi.org/10.1099/00221287-68-3-337
van Loosdrecht MC, Lyklema J, Norde W, Zehnder AJ (1989) Bacterial adhesion: a physicochemical approach. Microb Ecol 17:1–15. https://doi.org/10.1007/BF02025589
Rijnaarts HH, Norde W, Bouwer EJ, Lyklema J, Zehnder AJ (1995) Reversibility and mechanism of bacterial adhesion. Colloids Surf B Biointerfaces 4:5–22. https://doi.org/10.1016/0927-7765(94)01146-V
Okshevsky M, Meyer RL (2015) The role of extracellular DNA in the establishment, maintenance and perpetuation of bacterial biofilms. Crit Rev Microbiol 41:341–352. https://doi.org/10.3109/1040841X.2013.841639
Harmsen M, Lappann M, Knøchel S, Molin S (2010) Role of extracellular DNA during biofilm formation by Listeria monocytogenes. Appl Environ Microbiol 76:2271–2279. https://doi.org/10.1128/AEM.02361-09
Neu TR, Poralla K (1988) An amphiphilic polysaccharide from an adhesive Rhodococcus strain. FEMS Microbiol Lett 49:389–392. https://doi.org/10.1016/j.jep.2012.08.045
Ron EZ, Rosenberg E (2001) Natural roles of biosurfactants. Appl Environ Microbiol 3:229–236. https://doi.org/10.1046/j.1462-2920.2001.00190.x
Boles BR, Thoendel M, Singh PK (2004) Self-generated diversity produces “insurance effects” in biofilm communities. Proc Natl Acad Sci USA 101:16630–16635. https://doi.org/10.1073/pnas.0407460101
Kjelleberg S, Givskov M (2007) The biofilm mode of life: mechanisms and adaptations, first ed. Horizon Scientific Press.
Sutherland IW (2001) The biofilm matrix–an immobilized but dynamic microbial environment. Trends Microbiol 9:222–227. https://doi.org/10.1016/S0966-842X(01)02012-1
Lewis K (2001) Riddle of biofilm resistance. Antimicrob Agents Chemother 45:999–1007. https://doi.org/10.1128/AAC.45.4.999-1007.2001
Bridier A, Dubois-Brissonnet F, Boubetra A, Thomas V, Briandet R (2010) The biofilm architecture of sixty opportunistic pathogens deciphered using a high throughput CLSM method. J Microbiol Methods 82:64–70. https://doi.org/10.1016/j.mimet.2010.04.006
Rani SA, Pitts B, Beyenal H, Veluchamy RA, Lewandowski Z, Davison WM, Buckingham-Meyer K, Stewart PS (2007) Spatial patterns of DNA replication, protein synthesis, and oxygen concentration within bacterial biofilms reveal diverse physiological states. J Bacteriol 189:4223–4233. https://doi.org/10.1128/JB.00107-07
Lear G, Lewis GD (2012) Microbial biofilms: current research and applications. Horizon Scientific Press.
Danese PN, Pratt LA, Kolter R (2000) Exopolysaccharide production is required for development of Escherichia coli K-12 biofilm architecture. J Bacteriol 182:3593–3596. https://doi.org/10.1128/JB.182.12.3593-3596.2000
Watnick PI, Kolter R (1999) Steps in the development of a Vibrio cholerae El Tor biofilm. Mol Microbiol 34:586–595. https://doi.org/10.1046/j.1365-2958.1999.01624.x
Tielen P, Strathmann M, Jaeger KE, Flemming HC, Wingender J (2005) Alginate acetylation influences initial surface colonization by mucoid Pseudomonas aeruginosa. Microbiol Res 160:165–176. https://doi.org/10.1016/j.micres.2004.11.003
Branda SS, Chu F, Kearns DB, Losick R, Kolter R (2006) A major protein component of the Bacillus subtilis biofilm matrix. Mol Microbiol 59:1229–1238. https://doi.org/10.1111/j.1365-2958.2005.05020.x
McCourt J, O’Halloran DP, McCarthy H, O’Gara JP, Geoghegan JA (2014) Fibronectin-binding proteins are required for biofilm formation by community-associated methicillin-resistant Staphylococcus aureus strain LAC. FEMS Microbiol Lett 353:157–164. https://doi.org/10.1111/1574-6968.12424
Liang X, Chen YY, Ruiz T, Wu H (2011) New cell surface protein involved in biofilm formation by Streptococcus parasanguinis. Infect Immun 79:3239–3248. https://doi.org/10.1128/IAI.00029-11
Manfiolli AO, Dos Reis TF, de Assis LJ, de Castro PA, Silva LP, Hori JI, Walker LA, Munro CA, Rajendran R, Ramage G, Goldman GH (2018) Mitogen activated protein kinases (MAPK) and protein phosphatases are involved in Aspergillus fumigatus adhesion and biofilm formation. Cell Surf 1:43–56. https://doi.org/10.1016/j.tcsw.2018.03.002
Matsumoto-Nakano M (2018) Role of Streptococcus mutans surface proteins for biofilm formation. Jpn Dent Sci Rev 54:22–29. https://doi.org/10.1016/j.jdsr.2017.08.002
Ye Y, Ling N, Gao J, Zhang X, Zhang M, Tong L, Zeng H, Zhang J, Wu Q (2018) Roles of outer membrane protein W (OmpW) on survival, morphology, and biofilm formation under NaCl stresses in Cronobacter sakazakii. J Dairy Sci 101:3844–3850. https://doi.org/10.3168/jds.2017-13791
Dutta A, Bhattacharyya S, Kundu A, Dutta D, Das AK (2016) Macroscopic amyloid fiber formation by staphylococcal biofilm associated SuhB protein. Biophys Chem 217:32–41. https://doi.org/10.1016/j.bpc.2016.07.006
Arenas J, Tommassen J (2017) Meningococcal biofilm formation: let’s stick together. Trends Microbiol 25:113–124. https://doi.org/10.1016/j.tim.2016.09.005
Bandekar D, Chouhan OP, Mohapatra S, Hazra M, Hazra S, Biswas S (2017) Putative protein VC0395_0300 from Vibrio cholerae is a diguanylate cyclase with a role in biofilm formation. Microbiol Res 202:61–70. https://doi.org/10.1016/j.micres.2017.05.003
Kim AR, Ahn KB, Kim HY, Seo HS, Yun CH, Han SH (2016) Serine-rich repeat adhesin gordonii surface protein B is important for Streptococcus gordonii biofilm formation. J Endod 42:1767–1772. https://doi.org/10.1016/j.joen.2016.08.016
Bandara M, Skehel JM, Kadioglu A, Collinson I, Nobbs AH, Blocker AJ, Jenkinson HF (2017) The accessory Sec system (SecY2A2) in Streptococcus pneumoniae is involved in export of pneumolysin toxin, adhesion and biofilm formation. Microbes Infect 19:402–412. https://doi.org/10.1016/j.micinf.2017.04.003
Horng YT, Wang CJ, Chung WT, Chao HJ, Chen YY, Soo PC (2018) Phosphoenolpyruvate phosphotransferase system components positively regulate Klebsiella biofilm formation. J Microbiol Immunol Infect 51:174–183. https://doi.org/10.1016/j.jmii.2017.01.007
Camilli A, Bassler BL (2006) Bacterial small-molecule signaling pathways. Science 311:1113–1116. https://doi.org/10.1126/science.1121357
Kalia VC (2013) Quorum sensing inhibitors: an overview. Biotechnol Adv 31:224–245. https://doi.org/10.1016/j.biotechadv.2012.10.004
Lee K, Yoon SS (2017) Pseudomonas aeruginosa biofilm, a programmed bacterial life for fitness. J Microbiol Biotechnol 27:1053–1064. https://doi.org/10.4014/jmb.1611.11056
Johnson MR, Montero CI, Conners SB, Shockley KR, Bridger SL, Kelly RM (2005) Population density-dependent regulation of exopolysaccharide formation in the hyperthermophilic bacterium Thermotoga maritima. Mol Microbiol 55:664–674. https://doi.org/10.1111/j.1365-2958.2004.04419.x
Li Z, Chen Y, Liu D, Zhao N, Cheng H, Ren H, Guo T, Niu H, Zhuang W, Wu J, Ying H (2015) Involvement of glycolysis/gluconeogenesis and signaling regulatory pathways in Saccharomyces cerevisiae biofilms during fermentation. Front Microbiol 6:139. https://doi.org/10.3389/fmicb.2015.00139
Finelli A, Gallant CV, Jarvi K, Burrows LL (2003) Use of in-biofilm expression technology to identify genes involved in Pseudomonas aeruginosa biofilm development. J Bacteriol 185:2700–2710. https://doi.org/10.1128/JB.185.9.2700-2710.2003
Feraco D, Blaha M, Khan S, Green JM, Plotkin BJ (2016) Host environmental signals and effects on biofilm formation. Microb Pathog 99:253–263. https://doi.org/10.1016/j.micpath.2016.08.015
Yeh YC, Wang HY, Lan CY (2018) Candida albicans Aro1 affects cell wall integrity, biofilm formation and virulence. J Microbiol Immunol Infect. https://doi.org/10.1016/j.jmii.2018.04.002
Chambers JR, Sauer K (2013) Small RNAs and their role in biofilm formation. Trends Microbiol 21:39–49. https://doi.org/10.1016/j.tim.2012.10.008
Monteiro C, Papenfort K, Hentrich K, Ahmad I, Le Guyon S, Reimann R, Grantcharova N, Römling U (2012) Hfq and Hfq-dependent small RNAs are major contributors to multicellular development in Salmonella enterica serovar Typhimurium. RNA Biol 9:489–502. https://doi.org/10.4161/rna.19682
Mikkelsen H, Sivaneson M, Filloux A (2011) Key two-component regulatory systems that control biofilm formation in Pseudomonas aeruginosa. Appl Environ Microbiol 13:1666–1681. https://doi.org/10.1111/j.1462-2920.2011.02495.x
Shao Y, Bassler BL (2012) Quorum-sensing non-coding small RNAs use unique pairing regions to differentially control mRNA targets. Mol Microbiol 83:599–611. https://doi.org/10.1111/j.1365-2958.2011.07959.x
Zheng H, Kim J, Liew M, Yan JK, Herrera O, Bok JW, Kelleher NL, Keller NP, Wang Y (2015) Redox metabolites signal polymicrobial biofilm development via the NapA oxidative stress cascade in Aspergillus. Curr Biol 25:29–37. https://doi.org/10.1016/j.cub.2014.11.018
Xue T, Ni J, Shang F, Chen X, Zhang M (2015) Autoinducer-2 increases biofilm formation via an ica-and bhp-dependent manner in Staphylococcus epidermidis RP62A. Microbes Infect 17:345–352. https://doi.org/10.1016/j.micinf.2015.01.003
Wotanis CK, Brennan WP III, Angotti AD, Villa EA, Zayner JP, Mozina AN, Rutkovsky AC, Sobe RC, Bond WG, Karatan E (2017) Relative contributions of norspermidine synthesis and signaling pathways to the regulation of Vibrio cholerae biofilm formation. PLoS ONE 12:e0186291. https://doi.org/10.1371/journal.pone.0186291
Xie T, Wu Q, Zhang J, Xu X, Cheng J (2017) Comparison of Vibrio parahaemolyticus isolates from aquatic products and clinical by antibiotic susceptibility, virulence, and molecular characterisation. Food Control 71:315–321. https://doi.org/10.1016/j.foodcont.2016.06.046
Høiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O (2010) Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents 35:322–332. https://doi.org/10.1016/j.ijantimicag.2009.12.011
Stewart PS (2002) Mechanisms of antibiotic resistance in bacterial biofilms. J Med Microbiol 292:107–113. https://doi.org/10.1078/1438-4221-00196
Hall CW, Mah TF (2017) Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol Lett 41:276–301. https://doi.org/10.1093/femsre/fux010
Ju X, Li J, Zhu M, Lu Z, Lv F, Zhu X, Bie X (2018) Effect of the luxS gene on biofilm formation and antibiotic resistance by Salmonella serovar Dublin. Food Res Int 107:385–393. https://doi.org/10.1016/j.foodres.2018.02.039
Hu WS, Kim H, Koo OK (2018) Molecular genotyping, biofilm formation and antibiotic resistance of enterotoxigenic Clostridium perfringens isolated from meat supplied to school cafeterias in South Korea. Anaerobe 52:115–121. https://doi.org/10.1016/j.anaerobe.2018.06.011
Guo XP, Yang Y, Lu DP, Niu ZS, Feng JN, Chen YR, Tou FY, Garner E, Xu J, Liu M, Hochella MF Jr (2018) Biofilms as a sink for antibiotic resistance genes (ARGs) in the Yangtze Estuary. Water Res 129:277–286. https://doi.org/10.1016/j.watres.2017.11.029
Aslantaş Ö, Demir C (2016) Investigation of the antibiotic resistance and biofilm-forming ability of Staphylococcus aureus from subclinical bovine mastitis cases. J Dairy Sci 99:8607–8613. https://doi.org/10.3168/jds.2016-11310
Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM (1995) Microbial biofilms. Annu Rev Microbiol 49:711–745. https://doi.org/10.1146/annurev.mi.49.100195.003431
Totani T, Nishiuchi Y, Tateishi Y, Yoshida Y, Kitanaka H, Niki M, Kaneko Y, Matsumoto S (2017) Effects of nutritional and ambient oxygen condition on biofilm formation in Mycobacterium avium subsp. hominissuis via altered glycolipid expression. Sci Rep 7:41775. https://doi.org/10.1038/srep41775
Werner E, Roe F, Bugnicourt A, Franklin MJ, Heydorn A, Molin S, Pitts B, Stewart PS (2004) Stratified growth in Pseudomonas aeruginosa biofilms. Appl Environ Microbiol 70:6188–6196. https://doi.org/10.1128/AEM.70.10.6188-6196.2004
Fux CA, Costerton JW, Stewart PS, Stoodley P (2005) Survival strategies of infectious biofilms. Trends Microbiol 13:34–40. https://doi.org/10.1016/j.tim.2004.11.010
von Wintersdorff CJ, Penders J, van Niekerk JM, Mills ND, Majumder S, van Alphen LB, Savelkoul PH, Wolffs PF (2016) Dissemination of antimicrobial resistance in microbial ecosystems through horizontal gene transfer. Front Microbiol 7:173. https://doi.org/10.3389/fmicb.2016.00173
Smillie C, Garcillán-Barcia MP, Francia MV, Rocha EP, de la Cruz F (2010) Mobility of plasmids. Microbiol Mol Biol Rev 74:434–452. https://doi.org/10.1128/MMBR.00020-10
Guglielmini J, de La Cruz F, Rocha EP (2012) Evolution of conjugation and type IV secretion systems. Mol Biol Evol 30:315–331. https://doi.org/10.1093/molbev/mss221
Charpentier X, Polard P, Claverys JP (2012) Induction of competence for genetic transformation by antibiotics: convergent evolution of stress responses in distant bacterial species lacking SOS? Curr Opin Microbiol 15:570–576. https://doi.org/10.1016/j.mib.2012.08.001
Mazaheri Nezhad Fard R, Barton MD, Heuzenroeder MW (2011) Bacteriophage-mediated transduction of antibiotic resistance in Enterococci. Lett Appl Microbiol 52:559–564. https://doi.org/10.1111/j.1472-765X.2011.03043.x
Billard-Pomares T, Fouteau S, Jacquet ME, Roche D, Barbe V, Castellanos M, Bouet JY, Cruveiller S, Médigue C, Blanco J, Clermont O (2014) Characterization of a P1-like bacteriophage carrying an SHV-2 extended-spectrum β-lactamase from an Escherichia coli strain. Antimicrob Agents Chemother 58:6550–6557. https://doi.org/10.1128/AAC.03183-14
Madsen JS, Burmølle M, Hansen LH, Sørensen SJ (2012) The interconnection between biofilm formation and horizontal gene transfer. FEMS Immunol Med Microbiol 65:183–195. https://doi.org/10.1111/j.1574-695X.2012.00960.x
Kaplan T (2014) The role of horizontal gene transfer in antibiotic resistance. Eukaryon 10:80–81
Fan Y, Xiao Y, Momeni B, Liu YY (2018) Horizontal gene transfer can help maintain the equilibrium of microbial communities. J Theor Biol 454:53–59. https://doi.org/10.1016/j.jtbi.2018.05.036
Jain H, Mulay S, Mullany P (2016) Persistence of endodontic infection and Enterococcus faecalis: role of horizontal gene transfer. Gene Reports 5:112–116. https://doi.org/10.1016/j.genrep.2016.09.010
Keren I, Kaldalu N, Spoering A, Wang Y, Lewis K (2004) Persister cells and tolerance to antimicrobials. FEMS Microbiol Lett 230:13–18. https://doi.org/10.1016/S0378-1097(03)00856-5
Lewis K (2010) Persister cells, dormancy and infectious disease. Nat Rev Microbiol 5:48–56. https://doi.org/10.1038/nrmicro1557
Lewis K (2010) Persister cells. Annu Rev Microbiol 64:357–372. https://doi.org/10.1146/annurev.micro.112408.134306
Zhang Y (2014) Persisters, persistent infections and the Yin-Yang model. Emerg Microbes Infect 3:e3. https://doi.org/10.1038/emi.2014.3
Kumar A, Alam A, Rani M, Ehtesham NZ, Hasnain SE (2017) Biofilms: survival and defense strategy for pathogens. Int J Med Microbiol 307:481–489. https://doi.org/10.1016/j.ijmm.2017.09.016
Thurlow LR, Hanke ML, Fritz T, Angle A, Aldrich A, Williams SH, Engebretsen IL, Bayles KW, Horswill AR, Kielian T (2011) Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J Immunol 186:6585–6596. https://doi.org/10.4049/jimmunol.1002794
Mulcahy H, Charron-Mazenod L, Lewenza S (2008) Extracellular DNA chelates cations and induces antibiotic resistance in Pseudomonas aeruginosa biofilms. PLoS Pathog 4(11):e1000213. https://doi.org/10.1371/journal.ppat.1000213
Chiang WC, Nilsson M, Jensen PØ, Høiby N, Nielsen TE, Givskov M, Tolker-Nielsen T (2013) Extracellular DNA shields against aminoglycosides in Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother 57:2352–2361. https://doi.org/10.1128/AAC.00001-13
Jakubovics NS, Burgess JG (2015) Extracellular DNA in oral microbial biofilms. Microbes Infect 17:531–537. https://doi.org/10.1016/j.micinf.2015.03.015
Kumar A, Schweizer HP (2005) Bacterial resistance to antibiotics: active efflux and reduced uptake. Adv Drug Deliv Rev 57:1486–1513. https://doi.org/10.1016/j.addr.2005.04.004
Singh S, Singh SK, Chowdhury I, Singh R (2017) Understanding the mechanism of bacterial biofilms resistance to antimicrobial agents. Open Microbiol J 11:53. https://doi.org/10.2174/2F1874285801711010053
Zhang L, Mah TF (2008) Involvement of a novel efflux system in biofilm-specific resistance to antibiotics. J Bacteriol 190:4447–4452. https://doi.org/10.1128/JB.01655-07
Buroni S, Matthijs N, Spadaro F, Van Acker H, Scoffone VC, Pasca MR, Riccardi G, Coenye T (2014) Differential role of RND efflux pumps in antimicrobial drug resistance of sessile and planktonic Burkholderia cenocepacia cells. Antimicrob Agents Chemother 58:7424–7429. https://doi.org/10.1128/AAC.03800-14
Wright GD (2005) Bacterial resistance to antibiotics: enzymatic degradation and modification. Antimicrob Agents Chemother 57:1451–1470. https://doi.org/10.1016/j.addr.2005.04.002
Schroeder M, Brooks BD, Brooks AE (2017) The complex relationship between virulence and antibiotic resistance. Genes (Basel) 8:39. https://doi.org/10.3390/genes8010039
Anderl JN, Franklin MJ, Stewart PS (2000) Role of antibiotic penetration limitation in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob Agents Chemother 4:1818–1824. https://doi.org/10.1128/AAC.44.7.1818-1824.2000
Giwercman B, Lambert PA, Rosdahl VT, Shand GH, Heiby N (1990) Rapid emergence of resistance in Pseudomonas aeruginosa in cystic fibrosis patients due to in-vivo selection of stable partially derepressed β-lactamase producing strains. J Antimicrob Chemother 26:247–259. https://doi.org/10.1093/jac/26.2.247
Roilides E, Simitsopoulou M, Katragkou A, Walsh TJ (2015) How biofilms evade host defenses. Microbiol Spectr 3https://doi.org/10.1128/microbiolspec.MB-0012-2014
Leid JG, Shirtliff ME, Costerton JW, Stoodley P (2002) Human leukocytes adhere to, penetrate, and respond to Staphylococcus aureus biofilms. Infect Immun 70:6339–6345. https://doi.org/10.1128/IAI.70.11.6339-6345.2002
Cerca N, Jefferson KK, Oliveira R, Pier GB, Azeredo J (2006) Comparative antibody-mediated phagocytosis of Staphylococcus epidermidis cells grown in a biofilm or in the planktonic state. Infect Immun 74:4849–4855. https://doi.org/10.1128/IAI.00230-06
Jensen ET, Kharazmi A, Lam K, Costerton JW, Høiby N (1990) Human polymorphonuclear leukocyte response to Pseudomonas aeruginosa grown in biofilms. Infect Immun 58:2383–2385
Jensen PØ, Bjarnsholt T, Phipps R, Rasmussen TB, Calum H, Christoffersen L, Moser C, Williams P, Pressler T, Givskov M, Høiby N (2007) Rapid necrotic killing of polymorphonuclear leukocytes is caused by quorum-sensing-controlled production of rhamnolipid by Pseudomonas aeruginosa. Microbiology 153:1329–1338. https://doi.org/10.1099/mic.0.2006/003863-0
Alhede M, Bjarnsholt T, Givskov M, Alhede M (2014) Pseudomonas aeruginosa biofilms: mechanisms of immune evasion. Advances in applied microbiology. Elsevier, pp. 1–40. https://doi.org/10.1016/B978-0-12-800262-9.00001-9.
Chandra J, McCormick TS, Imamura Y, Mukherjee PK, Ghannoum MA (2007) Interaction of Candida albicans with adherent human peripheral blood mononuclear cells increases C. albicans biofilm formation and results in differential expression of pro-and anti-inflammatory cytokines. Infect Immun 75:2612–2620. https://doi.org/10.1128/IAI.01841-06
Le KY, Park MD, Otto M (2018) Immune evasion mechanisms of Staphylococcus epidermidis biofilm infection. Front Microbiol 9:359. https://doi.org/10.3389/fmicb.2018.00359
Van Gennip M, Christensen LD, Alhede M, Phipps R, Jensen PØ, Christophersen L, Pamp SJ, Moser C, Mikkelsen PJ, Koh AY, TOLKER-NIELSEN TI, (2009) Inactivation of the rhlA gene in Pseudomonas aeruginosa prevents rhamnolipid production, disabling the protection against polymorphonuclear leukocytes. APMIS 117:537–546. https://doi.org/10.1111/j.1600-0463.2009.02466.x
Kristian SA, Birkenstock TA, Sauder U, Mack D, Götz F, Landmann R (2008) Biofilm formation induces C3a release and protects Staphylococcus epidermidis from IgG and complement deposition and from neutrophil-dependent killing. J Infect Dis 197:1028–1035. https://doi.org/10.1086/528992
Chen M, Yu Q, Sun H (2013) Novel strategies for the prevention and treatment of biofilm related infections. Int J Mol Sci 14:18488–18501. https://doi.org/10.3390/ijms140918488
Ma Y, Xu Y, Yestrepsky BD, Sorenson RJ, Chen M, Larsen SD, Sun H (2012) Novel inhibitors of Staphylococcus aureus virulence gene expression and biofilm formation. PLoS ONE 7:e47255. https://doi.org/10.1016/j.indcrop.2015.10.055
Sambanthamoorthy K, Gokhale AA, Lao W, Parashar V, Neiditch MB, Semmelhack MF, Lee I, Waters CM (2011) Identification of a novel benzimidazole that inhibits bacterial biofilm formation in a broad-spectrum manner. Antimicrob Agents Chemother 55:4369–4378. https://doi.org/10.1128/AAC.00583-11
Abraham NM, Lamlertthon S, Fowler VG, Jefferson KK (2012) Chelating agents exert distinct effects on biofilm formation in Staphylococcus aureus depending on strain background: role for clumping factor. B J Med Microbiol 61:1062–1070. https://doi.org/10.1099/2Fjmm.0.040758-0
Izano EA, Amarante MA, Kher WB, Kaplan JB (2008) Differential roles of poly-N-acetylglucosamine surface polysaccharide and extracellular DNA in Staphylococcus aureus and Staphylococcus epidermidis biofilms. Appl Environ Microbiol 74:470–476. https://doi.org/10.1128/AEM.02073-07
Chaignon P, Sadovskaya I, Ragunah C, Ramasubbu N, Kaplan JB, Jabbouri S (2007) Susceptibility of staphylococcal biofilms to enzymatic treatments depends on their chemical composition. Appl Microbiol Biotechnol 75:125–132. https://doi.org/10.1007/s00253-006-0790-y
Han Q, Song X, Zhang Z, Fu J, Wang X, Malakar PK, Liu H, Pan Y, Zhao Y (2017) Removal of foodborne pathogen biofilms by acidic electrolyzed water. Front Microbiol 8:988. https://doi.org/10.3389/fmicb.2017.00988
van Tilburg BE, Lewenza S, Reckseidler-Zenteno S (2015) Current research approaches to target biofilm infections. Postdoc J 3:36. https://doi.org/10.2174/092986709787909640
De la Fuente-Núñez C, Reffuveille F, Haney EF, Straus SK, Hancock RE (2014) Broad-spectrum anti-biofilm peptide that targets a cellular stress response. PLoS Pathog 10:e1004152. https://doi.org/10.1371/journal.ppat.1004152
Khalifa L, Brosh Y, Gelman D, Coppenhagen-Glazer S, Beyth S, Poradosu-Cohen R, Que YA, Beyth N, Hazan R (2015) Targeting Enterococcus faecalis biofilms with phage therapy. Appl Environ Microbiol 81:2696–2705. https://doi.org/10.1128/AEM.00096-15
Svensson A, Larsson A, Emtenäs H, Hedenström M, Fex T, Hultgren SJ, Pinkner JS, Almqvist F, Kihlberg J (2001) Design and evaluation of pilicides: potential novel antibacterial agents directed against uropathogenic Escherichia coli. ChemBioChem 2:915–918. https://doi.org/10.1002/1439-7633(20011203)2:12%3C915::AIDCBIC915%3E3.0.CO;2-M
Robert III W, EricáBallard T (2008) Inhibition and dispersion of proteobacterial biofilms. Chem. Commun. (Camb.), 1698-1700https://doi.org/10.1039/B719802G
Sadekuzzaman M, Yang S, Mizan MF, Ha SD (2015) Current and recent advanced strategies for combating biofilms. Compr Rev Food Sci Food Saf 14:491–509. https://doi.org/10.1111/1541-4337.12144
Gomes IB, Lemos M, Mathieu L, Simões M, Simões LC (2018) The action of chemical and mechanical stresses on single and dual species biofilm removal of drinking water bacteria. Sci Total Environ 631:987–993. https://doi.org/10.1016/j.scitotenv.2018.03.042
Pires DP, Melo LD, Boas DV, Sillankorva S, Azeredo J (2017) Phage therapy as an alternative or complementary strategy to prevent and control biofilm-related infections. Curr Opin Microbiol 39:48–56. https://doi.org/10.1016/j.mib.2017.09.004
Ryan EM, Alkawareek MY, Donnelly RF, Gilmore BF (2012) Synergistic phage-antibiotic combinations for the control of Escherichia coli biofilms in vitro. FEMS Immunol Med Microbiol 65:395–398. https://doi.org/10.1111/j.1574-695X.2012.00977.x
Merino L, Procura F, Trejo FM, Bueno DJ, Golowczyc MA (2017) Biofilm formation by Salmonella sp. in the poultry industry: detection, control and eradication strategies. Food Res Int. https://doi.org/10.1016/j.foodres.2017.11.024
Wang H, Wang H, Xing T, Wu N, Xu X, Zhou G (2016) Removal of Salmonella biofilm formed under meat processing environment by surfactant in combination with bio-enzyme. LWT - Food Sci Technol 66:298–304. https://doi.org/10.1016/j.lwt.2015.10.049
de Oliveira AM, da Silva FM, de Abreu Filho BA, Gomes RG, Bergamasco R (2018) Inhibition and removal of staphylococcal biofilms using Moringa oleifera Lam. aqueous and saline extracts. J Environ Chem Eng 6:2011–2016. https://doi.org/10.1016/j.jece.2018.02.043
Leary JT, Werger MM, Broach WH, Shaw LN, Santoni BG, Bernasek TL, Lyons ST (2017) Complete eradication of biofilm from orthopedic materials. J Arthroplasty 32:2513–2518. https://doi.org/10.1016/j.arth.2017.03.050
Ficai D, Ficai A (2017) Prevention of biofilm formation by material modification. In Biofilms and Implantable Medical Devices (pp. 159–180). Woodhead Publishing. Chapter 7.
Feng G, Cheng Y, Wang SY, Borca-Tasciuc DA, Worobo RW, Moraru CI (2015) Bacterial attachment and biofilm formation on surfaces are reduced by small-diameter nanoscale pores: how small is small enough? NPJ Biofilms Microbiomes 1:15022. https://doi.org/10.1038/npjbiofilms.2015.22
Lagree K, Mon HH, Mitchell AP, Ducker WA (2018) Impact of surface topography on biofilm formation by Candida albicans. PLoS One 13:e0197925. https://doi.org/10.1371/journal.pone.0197925
Gkana EN, Doulgeraki AI, Chorianopoulos NG, Nychas GJ (2017) Anti-adhesion and anti-biofilm potential of organosilane nanoparticles against foodborne pathogens. Front Microbiol 8:1295. https://doi.org/10.3389/fmicb.2017.01295
Liu H, Shukla S, Vera-González N, Tharmaligam N, Mylonakis E, Fuchs BB, Shukla A (2019) Auranofin releasing antibacterial and antibiofilm polyurethane intravascular catheter coatings. Front Cell Infect Microbiol 9:37. https://doi.org/10.3389/fcimb.2019.00037
Nazlı O, Baygar T, Dönmez ÇE, Dere Ö, Uysal Aİ, Aksözek A, Işık C, Aktürk S (2019) Antimicrobial and antibiofilm activity of polyurethane/Hypericum perforatum extract (PHPE) composite. Bioorg Chem 82:224–228. https://doi.org/10.1016/j.bioorg.2018.08.017
Laitman I, Natan M, Banin E, Margel S (2014) Synthesis and characterization of fluoro-modified polypropylene films for inhibition of biofilm formation. Colloids Surf B Biointerfaces 115:8–14. https://doi.org/10.1016/j.colsurfb.2013.11.027
Glatthardt T, de Mello Campos JC, Chamon RC, de Sá Coimbra TF, de Almeida Rocha G, de Melo MAF, Ferreira RBR. (2020) Small molecules produced by commensal Staphylococcus epidermidis disrupt formation of biofilms by Staphylococcus aureus. Appl. Environ. Microbiol. 86(5) https://doi.org/10.1128/AEM.02539-19.
Ahmad I, Husain FM, Maheshwari M, Zahin M (2014) Medicinal plants and phytocompounds: a potential source of novel antibiofilm agents Antibiofilm Agents. Springer, Berlin Heidelberg, pp 205–232. https://doi.org/10.1007/978-3-642-53833-9_10
Song X, Xia YX, He ZD, Zhang HJ (2018) A review of natural products with anti-biofilm activity. Curr Org Chem 22:789–817. https://doi.org/10.2174/1385272821666170620110041
Koh CL, Sam CK, Yin WF, Tan LY, Krishnan T, Chong YM, Chan KG (2013) Plant-derived natural products as sources of anti-quorum sensing compounds. Sensors 13:6217–6228. https://doi.org/10.3390/s130506217
Borges A, Abreu AC, Dias C, Saavedra MJ, Borges F, Simões M (2016) New perspectives on the use of phytochemicals as an emergent strategy to control bacterial infections including biofilms. Molecules 21:877. https://doi.org/10.3390/molecules21070877
Rather MA, Gupta K, Mandal M. (2020) Inhibition of biofilm and quorum sensing-regulated virulence factors in Pseudomonas aeruginosa by Cuphea carthagenensis (Jacq.) JF Macbr. leaf extract: an in vitro study. J. Ethnopharmacol. 113699. https://doi.org/10.1016/j.jep.2020.113699.
Gupta K, Singh SP, Manhar AK, Saikia D, Namsa ND, Konwar BK, Mandal M (2019) Inhibition of Staphylococcus aureus and Pseudomonas aeruginosa biofilm and virulence by active fraction of Syzygium cumini (L.) Skeels leaf extract: in-vitro and in silico studies. Indian J Microbiol 59(1):13–21. https://doi.org/10.1007/s12088-018-0770-9
Bouyahya A, Dakka N, Et-Touys A, Abrini J, Bakri Y (2017) Medicinal plant products targeting quorum sensing for combating bacterial infections. Asian Pac J Trop Med 10:729–743. https://doi.org/10.1016/j.apjtm.2017.07.021
Lin MH, Shu JC, Huang HY, Cheng YC (2012) Involvement of iron in biofilm formation by Staphylococcus aureus. PLoS ONE 7:e34388. https://doi.org/10.1371/journal.pone.0034388
Lee JH, Kim YG, Ryu SY, Lee J (2016) Calcium-chelating alizarin and other anthraquinones inhibit biofilm formation and the hemolytic activity of Staphylococcus aureus. Sci Rep 6:19267. https://doi.org/10.1038/srep19267
Kooltheat N, Kamuthachad L, Anthapanya M, Samakchan N, Sranujit RP, Potup P, Ferrante A, Usuwanthim K (2016) Kaffir lime leaves extract inhibits biofilm formation by Streptococcus mutans. Nutr J 32:486–490. https://doi.org/10.1016/j.nut.2015.10.010
Rudrappa T, Bais HP (2008) Curcumin, a known phenolic from Curcuma longa, attenuates the virulence of Pseudomonas aeruginosa PAO1 in whole plant and animal pathogenicity models. J Agric Food Chem 56:1955–1962. https://doi.org/10.1021/jf072591j
Truchado P, Giménez-Bastida JA, Larrosa M, Castro-Ibáñez I, Espín JC, Tomás-Barberán FA, García-Conesa MT, Allende A (2012) Inhibition of quorum sensing (QS) in Yersinia enterocolitica by an orange extract rich in glycosylated flavanones. J Agric Food Chem 60:8885–8894. https://doi.org/10.1021/jf301365a
Wysokińska H, Różalski M, Budzyńska A, Więckowska-Szakiel M, Sadowska B, Paszkiewicz M, Kisiel W, Różalska B (2012) Antimicrobial and anti-biofilm properties of new taxodione derivative from hairy roots of Salvia austriaca. Phytomedicine 19:1285–1297. https://doi.org/10.1016/j.phymed.2012.07.016
Kim HS, Park HD (2013) Ginger extract inhibits biofilm formation by Pseudomonas aeruginosa PA14. PLoS ONE 8:e76106. https://doi.org/10.1371/journal.pone.0076106
Kim YG, Lee JH, Kim SI, Baek KH, Lee J (2015) Cinnamon bark oil and its components inhibit biofilm formation and toxin production. Int J Food Microbiol 195:30–39. https://doi.org/10.1016/j.ijfoodmicro.2014.11.028
Khan F, Hashmi MU, Khalid N, Hayat MQ, Ikram A, Janjua HA (2016) Controlled assembly of silver nano-fluid in Heliotropium crispum extract: a potent anti-biofilm and bactericidal formulation. Appl Surf Sci 387:317–331. https://doi.org/10.1016/j.apsusc.2016.05.133
Malaikozhundan B, Vaseeharan B, Vijayakumar S, Sudhakaran R, Gobi N, Shanthini G (2016) Antibacterial and antibiofilm assessment of Momordica charantia fruit extract coated silver nanoparticle. Biocatal Agric Biotechnol 8:189–196. https://doi.org/10.1016/j.bcab.2016.09.007
Hasan S, Danishuddin M, Adil M, Singh K, Verma PK, Khan AU (2012) Efficacy of E. officinalis on the cariogenic properties of Streptococcus mutans: a novel and alternative approach to suppress quorum-sensing mechanism. PLoS One 7:e40319. https://doi.org/10.1371/journal.pone.0040319
Murugan K, Sekar K, Sangeetha S, Ranjitha S, Sohaibani SA (2013) Antibiofilm and quorum sensing inhibitory activity of Achyranthes aspera on cariogenic Streptococcus mutans: an in vitro and in silico study. Pharm Biol 51:728–736. https://doi.org/10.3109/13880209.2013.764330
Jakobsen TH, Bragason SK, Phipps RK, Christensen LD, van Gennip M, Alhede M, Skindersoe M, Larsen TO, Høiby N, Bjarnsholt T, Givskov M (2012) Food as a source for quorum sensing inhibitors: iberin from horseradish revealed as a quorum sensing inhibitor of Pseudomonas aeruginosa. Appl Environ Microbiol 78:2410–2421. https://doi.org/10.1128/AEM.05992-11
Gupta K, Hazarika SN, Saikia D, Namsa ND, Mandal M (2014) One step green synthesis and anti-microbial and anti-biofilm properties of Psidium guajava L. leaf extract-mediated silver nanoparticles. Mater Lett 125:67–70. https://doi.org/10.1016/j.matlet.2014.03.134
Gupta K, Barua S, Hazarika SN, Manhar AK, Nath D, Karak N, Mandal M (2014) Green silver nanoparticles: enhanced antimicrobial and antibiofilm activity with effects on DNA replication and cell cytotoxicity. RSC Adv 4(95):52845–52855. https://doi.org/10.1039/C4RA08791G
Sen S, Chakraborty R (2017) Revival, modernization and integration of Indian traditional herbal medicine in clinical practice: importance, challenges and future. J Tradit Complement Med 7:234–244. https://doi.org/10.1016/j.jtcme.2016.05.006
Karunamoorthi K, Jegajeevanram K, Vijayalakshmi J, Mengistie E (2013) Traditional medicinal plants: a source of phytotherapeutic modality in resource-constrained health care settings. Evid Based Complement Alternat Med 18:67–74. https://doi.org/10.1177/2F2156587212460241
Chung PY, Toh YS (2014) Anti-biofilm agents: recent breakthrough against multi-drug resistant Staphylococcus aureus. Pathog Dis 70:231–239. https://doi.org/10.1111/2049-632X.12141
Borges AJ, Saavedra M, Simoes M (2015) Insights on antimicrobial resistance, biofilms and the use of phytochemicals as new antimicrobial agents. Curr Med Chem 22(21):2590–2614
Lewis K, Ausubel FM (2006) Prospects for plant-derived antibacterials. Nat Biotechnol 24(12):1504–1507. https://doi.org/10.1038/nbt1206-1504
Namasivayam SK, Vivek JM (2016) Anti quorum sensing activities of medicinal plant extracts against quorum sensing mediated virulence factors of Pseudomonas aeruginosa. Pharm Lett 8:412–423. https://doi.org/10.4103/JMAU.JMAU_10_18
Funding
Financial support was provided by DBT via the DBT NER Twinning Programme vide letter No. BT/PR16149/NER/95/85/ 2015 dated January 19, 2017, and Tezpur University via memo No. DoRD/RIG/10–73/1362-A dated 19/02/2019.
Author information
Authors and Affiliations
Contributions
Muzamil Ahmad Rather had the idea of the article and drafted it; Muzamil Ahmad Rather and Dr. Kuldeep Gupta did the literature search and data analysis; and the work was critically revised by Prof. Manabendra Mandal.
Corresponding author
Ethics declarations
Ethics approval
No human participants and/or animals were used in the study.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Responsible Editor: Carla Taddei
Highlights
• Biofilm is a safe and antibiotic-resistant home to microorganisms.
• Biofilm formation is a complex and quorum sensing–dependent process.
• Polysaccharides, proteins, eDNA, etc. are the architecture and stability determinants of biofilm.
• Recent advances in physical, chemical, and biological antibiofilm strategies.
Rights and permissions
About this article
Cite this article
Rather, M.A., Gupta, K. & Mandal, M. Microbial biofilm: formation, architecture, antibiotic resistance, and control strategies. Braz J Microbiol 52, 1701–1718 (2021). https://doi.org/10.1007/s42770-021-00624-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-021-00624-x