Abstract
Bacterial infections are serious complications after orthopaedic implant surgery. Staphylococci, with Staphylococcus epidermidis as a leading species, are the prevalent and most important species involved in orthopaedic implant-related infections. The biofilm mode of growth of these bacteria on an implant surface protects the organisms from the host’s immune system and from antibiotic therapy. Therapeutic agents that disintegrate the biofilm matrix would release planktonic cells into the environment and therefore allow antibiotics to eliminate the bacteria. An addition of a biofilm-degrading agent to a solution used for washing–draining procedures of infected orthopaedic implants would greatly improve the efficiency of the procedure and thus help to avoid the removal of the implant. We have previously shown that the extracellular staphylococcal matrix consists of a poly-N-acetylglucosamine (PNAG), extracellular teichoic acids (TAs) and protein components. In this study, we accessed the sensitivity of pre-formed biofilms of five clinical staphylococcal strains associated with orthopaedic prosthesis infections and with known compositions of the biofilm matrix to periodate, Pectinex Ultra SP, proteinase K, trypsin, pancreatin and dispersin B, an enzyme with a PNAG-hydrolysing activity. We also tested the effect of these agents on the purified carbohydrate components of staphylococcal biofilms, PNAG and TA. We found that the enzymatic detachment of staphylococcal biofilms depends on the nature of their constituents and varies between the clinical isolates. We suggest that a treatment with dispersin B followed by a protease (proteinase K or trypsin) could be capable to eradicate biofilms of a variety of staphylococcal strains on inert surfaces.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Staphylococci are responsible for more than 1 million serious hospital-acquired infections per year (Projan and Novick 1997). Staphylococcus epidermidis, an important member of human skin and mucous membrane microflora, as well as other coagulase-negative staphylococci (CoNS) are the major cause of infections of various indwelling medical devices, including prosthetic cardiac valves, intra-ocular lenses, catheters and orthopaedic prostheses (Götz 2002). Bacterial infections represent one of the most serious and devastating complications after orthopaedic implant surgery. Treatment for chronic infection usually requires removal of the prosthesis, cleaning the bone interface and new arthroplasty (Lortat-Jacob et al. 2002). It often results in long periods of hospitalisation, morbidity, severe functional impairment and increased mortality.
The pathogenesis of foreign-body-associated infections of CoNS and particularly of S. epidermidis is related to their ability to grow as an adherent biofilm (Mack et al. 1999). The biofilms consist of cells encased in a self-synthesized polymeric matrix that holds them together and firmly attaches the bacterial mass to the underlying surface (Sutherland 2001). Bacterial biofilm infections are particularly problematic because biofilms impair the penetration of antibiotics, prevent normal immune responses and increase the difficulty of eradicating biofilm infections. Biofilm cells are capable of persisting in the presence of antimicrobials at concentrations that are 1,000-fold higher than those necessary to eradicate a planktonic population (Cerca et al. 2005; Hamilton 2002). This is believed to be caused by the physical protection by the biofilm matrix or by an altered physiology of the bacterial cells in the biofilm mode of growth (Fux et al. 2005).
Poly-N-acetylglucosamine (PNAG) was initially defined by its biological properties as the polysaccharide intercellular adhesin. PNAG plays a key role in biofilm formation and accumulation (Mack et al. 1996; Maira-Litran et al. 2004) and protects the pathogen from the innate host defence (Vuong et al. 2004). The icaADBC locus, encoding the biosynthesis of PNAG (Heilmann et al. 1996), is widespread in clinical staphylococcal isolates (Galdbart et al. 2000; McKenney et al. 2000). However, a significant percentage of ica negative strains were found by different researchers in S. epidermidis populations isolated from foreign-body infections (Ziebuhr et al. 1997; Frebourg et al. 2000; Rohde et al. 2005).
The enzymatic detachment of biofilms seems to be closely related to their chemical composition. Periodate (HIO4 or NaIO4) was shown to release biofilms of PNAG-producing Escherichia coli (Wang et al. 2004) and PNAG-producing S. epidermidis 1457 (Rohde et al. 2005). An enzyme with a PNAG-hydrolysing activity dispersin B has been recently isolated from the oral pathogen Actinobacillus actinomycetemcomitans (Kaplan et al. 2003, 2004a,b). It was later shown that dispersin B endolytically hydrolysed the glycosidic linkages of poly-β-(1,6)-GlcNAc (Itoh et al. 2005). This enzyme was able to rapidly remove the biofilms produced by the four clinical strains of S. epidermidis isolated from the surfaces of the infected intravenous catheters (Kaplan et al. 2004a,b).
In our recent studies, we investigated the chemical composition of the biofilm of the model biofilm-producing strain S. epidermidis RP62A (Sadovskaya et al. 2005) and 15 other clinical strains of Staphylococcus aureus and CoNS associated with orthopaedic prosthesis infections. In the model strain, biofilm was composed of PNAG, extracellular teichoic acid (TA) and protein components. Some of the clinical strains produced biofilm with the composition similar to that of the model strain, with various amounts of PNAG (Sadovskaya et al. 2006). Biofilms of the strains producing substantial amount of PNAG were detached by dispersin B while being unsusceptible to proteinase K treatment. Several biofilm-forming strains did not produce detectable amounts of PNAG, and their biofilms contained mainly extracellular TA and proteins (Kogan et al. 2006; Sadovskaya et al. 2006). Biofilms of these strains were partially dispersed by proteinase K (Kogan et al. 2006).
A mixture of a carbohydrolase, a proteolytic enzyme and a surfactant is recommended for efficient biofilm removal (Johansen 1996). Pectinex Ultra SP (PUS), a multicomponent enzyme preparation containing protease activity and a wide range of carbohydrolases, was able to remove biofilms of S. epidermidis and S. aureus without any significant bactericidal activity. S. aureus biofilm appeared to be most sensitive to PUS (Johansen et al. 1997). The conclusion has been drawn that the activity of PUS was “mainly a degradation of extracellular polysaccharides,” but this postulate was not proven experimentally. It remains unclear what components of staphylococcal biofilm are sensitive to this enzymatic preparation. Pancreatin, a mixture of digestive enzymes from porcine pancreas with proteolytic, amylase and lipase activities, was also recommended for biofilm removal (Marion et al. 2005).
The purpose of the present study was to assess the susceptibility of biofilms of staphylococcal strains related to orthopaedic prosthesis infections and with known composition of the extracellular biofilm matrix to a wider range of agents and enzymatic preparations (periodate, dispersin B, PUS, proteinase K, trypsin and pancreatin) and to compare the efficiency of different biofilm-degrading agents with the chemical composition of the biofilms of these strains. We have also examined the effect of some of these agents on the purified carbohydrate components of staphylococcal biofilms, PNAG and TA, and tested the proteolytic activities on crude biofilm extracts.
Materials and methods
Bacterial strains and culture conditions
S. epidermidis RP62A (ATCC 35984) was kindly provided by Prof. Gerald Pier (Harvard Medical School, Boston, MA). The clinical isolates of the S. epidermidis strains 5 and 444, S. aureus 383 and Staphylococcus lugdunensis 47 and 18a (Table 1) were selected out of the strains collected from the infected patients hospitalised in the Mignot Hospital of Versailles, France (Chokr et al. 2006; Eleaume and Jabbouri 2004).
Cells were grown statically at 37°C for 24 h in 200 μl tryptic soy broth (TSB; Becton Dickinson, Le Pont de Claix, France) in wells of 96-well polystyrene tissue-culture-treated microtitre plate (Nunc, Roskilde, Denmark) or in 60 ml TSB in tissue-culture-treated Petri dishes (Greiner Bio). All cultures were preliminarily inoculated with 2% (v/v) of 16 h pre-culture grown aerobically in the same medium.
Quantitative biofilm assay
The inoculum for biofilm growth was prepared by dilution of overnight cultures 1:100 into the fresh TSB medium. The wells of a 96-well polystyrene tissue-culture-treated Nunclon microtitre plate (Nunc) were filled with 200-μl aliquots of inoculum, and the plate was incubated for 24 h at 37°C without shaking. The biofilms were washed three times with 200 μl of 0.9% NaCl, stained with 200 μl of 5% safranin (AES Laboratoire, Combourg, France) for 5 min and washed again with 0.9% NaCl. One hundred microlitres of water per wells was added, and the optical density measured using a μQuant microtitre plate reader (Bio-Tek Instruments, Winooski, USA) set to 492 nm. For each experiment, background staining was corrected by subtracting the safranin bound to non-inoculated controls. At least eight replicates were conducted for each sample, and each experiment was performed at least twice.
Enzymes and enzymatic preparations
A. actinomycetemcomitans dispersin B was purified as previously described (Kaplan et al. 2003). The purified enzyme had a specific activity of 970 U/mg of protein, where 1 U of enzyme activity was defined as the amount of enzyme needed to hydrolyse 1 μmol of 4-nitrophenyl-β-d-N-acetylglucosaminide to 4-nitrophenol and N-acetylglucosamine per minute at pH 4.5 at 25°C in 50 mM sodium phosphate buffer–100 mM NaCl (Kaplan et al. 2004a,b). PUS-L was purchased from Novozymes; proteinase K, trypsin and pancreatin were purchased from Sigma.
Microtitre plate biofilm detachment assay
Biofilm detachment assays were carried out essentially as described in Kaplan (2004a). Briefly, the biofilms were washed with 200 μl of 0.9% NaCl and then treated for 2 h at 37°C with 100 μl of 10 mM sodium periodate in 50 mM sodium acetate buffer (pH 4.5), 100 μl of proteinase K at 1 mg ml−1 in 20 mM Tris (pH 7.5)–100 mM NaCl, 100 μl of trypsin at 1 mg ml−1 in 20 mM Tris (pH 7.5)–100 mM NaCl–10 mM ethylenediamine tetraacetic acid, 100 μl of PUS (PUS-L) at 4% in 50 mM sodium acetate buffer (pH 4.5)–NaCl 100 mM, 100 μl of pancreatin at 1% in 50 mM phosphate-buffered saline (PBS; pH 7.5), or 100 μl of 40 μg ml−1 of dispersin B in 50 mM PBS (pH 5.8). Control wells were filled with appropriate buffers. After the treatment, the biofilms were washed with 200 μl of 0.9% NaCl, dried for 45 min at 55°C and stained with 5% safranin as described above. The biofilm detachment assays were performed at least twice, in eight wells, with similar results.
Preparation of purified PNAG, cell wall TAs and a crude biofilm extract
PNAG was prepared from a crude biofilm extract of S. epidermidis 5 from our collection as described previously (Sadovskaya et al. 2006).
The cell wall (CW) TAs from strains S. aureus MN8m (Vinogradov et al. 2006) and S. lugdunensis 18a were prepared by trichloroacetic acid (TCA) extraction (Sadovskaya et al. 2004). For the preparation of the crude biofilm extract, S. aureus 383 was grown statically in 150-mm-diameter, tissue-culture-treated, polystyrene Petri dishes (Corning no. 430199) containing 60 ml of the medium. The biofilms were washed with 0.9% (v/v) NaCl, scraped from the surface using a cell scraper, suspended in 0.9% (v/v) NaCl and sonicated twice for 20 s on ice using an Ikasonic sonicator (IKA Labortechnik) set to 40% amplitude and 40% duty cycle. Cells were removed by centrifugation; the supernatant was further clarified by centrifugation (8,700 × g, 10 min, 4°C), dialysed and freeze-dried. The amount of protein in the crude extract was measured by using a BioRad colorimetric assay, based on the Bradford dye-binding procedure (Bradford 1976) using bovine albumin (Sigma) as a standard.
Enzymatic treatments of purified PNAG and TA
Purified CW TAs (3–5 mg) were treated with the enzyme preparations for 3 h or overnight in the appropriate buffers. Enzyme concentrations were the same as for the Microtitre plate biofilm detachment assays. At the end of the incubation, enzymes were inactivated by heating at 100°C for 5 min. In control experiments, the substrate and the enzyme were mixed and immediately heated at 100°C for 5 min. The insoluble material was removed by centrifugation, and the samples were analysed by gel filtration chromatography on a G-50 column (1 × 40 cm). For these experiments, we have chosen the CW TAs substituted with GlcNAc; thus, their depolymerisation could be followed by screening the fractions (1.6 ml) for aminosugars (Enghofer and Kress 1979). Pancreatine and PUS, desalted on Sephadex G-50 in sodium acetate buffer, and dispersin B were negative in this assay (data not shown).
PNAG (5–6 mg) was solubilised in 50 μl of 5 M HCl or 1 ml of 10% NH4OH, diluted with 1 ml of water and desalted on a PD10 cartridge (Amersham Biosciences) equilibrated in an appropriate buffer. PNAG-containing fractions were pooled and separated in two equal parts, for the enzymatic treatment and control experiments. Enzymatic treatments were performed at 37°C overnight. After the incubation, enzymes were inactivated by heating at 100°C for 5 min. In control experiments, the substrate and the enzyme were mixed and immediately heated at 100°C for 5 min. Enzyme concentrations were the same as for the Microtitre plate biofilm detachment assays. After heating, the insoluble material was removed by centrifugation (12,000 rpm 10 min at 4°C), and the clear supernatants were analysed by gel filtration chromatography on a G-50 column (1 × 40 cm), and the elution profiles were obtained as described above for the CW TAs. Similarly, PNAG was treated with 10 mM NaIO4 in 50 mM sodium acetate buffer at room temperature overnight. The control experiment was incubated at the same conditions without the addition of NaIO4.
Protein degradation after enzymatic digestion of the crude biofilm extract
Lyophilised crude extract of the biofilm of S. aureus 383 was dissolved in 50 mM sodium acetate buffer (pH 4.5)–NaCl 100 mM at 1.5 mg ml−1 (substrate solution). Twenty microlitres of PUS in 1 ml of buffer (enzyme solution) was added, and the mixture was incubated at 37°C for 3 h with shaking. To take into account the autodigestion of the enzymatic preparation, in a control experiment, the substrate and enzyme solutions were incubated separately at 37°C for 3 h with shaking and mixed at the end of the incubation. The enzyme was deactivated by heating at 100°C for 5 min, and the mixture was cooled down and clarified by centrifugation. Protein digestion was assayed by the measurement of free amino groups using a trinitrobenzesulfonic acid (TNBS) assay (Lee 1978) with the subsequent colorimetric detection at 420 nm. One millimolar solution of glycine (ICN Biomedicals) was used as a standard. Proteinase K digestion was performed similarly in 20 mM Tris (pH 7.5)–100 mM NaCl buffer and with the enzyme concentration of 0.1 mg ml−1. The results were expressed in micromolar of liberated –NH2 groups (glycine equivalents) per 1 mg of protein.
General and analytical methods
Neutral and aminosugars were detected colorimetrically using phenol–sulfuric acid (Dubois et al. 1956) and Elson–Morgan (Enghofer and Kress 1979) assays, respectively. Gel filtration chromatography was carried out on Sephadex G-50 columns (1.6 × 80 and 1 × 40 cm, Amersham Biosciences) irrigated with water.
Results
Choice of bacterial strains
Five clinical staphylococcal strains (Table 1) with the known composition of their extracellular biofilm matrix were chosen for this study. The model strain S. epidermidis RP62A and the clinical strains S. epidermidis 5 and 444 were previously shown to produce a considerable amount of PNAG, and S. epidermidis 5 is considered as a PNAG overproducer (Sadovskaya et al. 2006). S. aureus 383 and S. lugdunensis 47 (Kogan et al. 2006) formed biofilms without a detectable amount of PNAG. The biofilm of S. lugdunensis 18a was shown to contain PNAG in very small amounts.
Preparation of crude biofilm extract and purified components of biofilm
Crude biofilm extract was prepared from a large-scale biofilm culture of S. aureus 383, using a previously developed mild procedure that releases cell-bound extracellular polymers with minimal cell lysis (Sadovskaya et al. 2006). PNAG was prepared from a crude biofilm extract of S. epidermidis 5. The TAs used for enzymatic studies were prepared by TCA extraction and gel filtration chromatography, as described previously (Sadovskaya et al. 2004), from strains S. aureus MN8m (Vinogradov et al. 2006) and S. lugdunensis 18a.
Effect of dispersin B and periodate
As expected, dispersin B efficiently disrupted the biofilm of the strains S. epidermidis RP62A, 5 and 444 (Fig. 1a–c), known to contain substantial amount of PNAG. Dispersin B was not efficient on the strains not producing PNAG—S. lugdunensis 18a, 47 and S. aureus 383 (Fig. 1d–f). Sodium periodate had a similar effect but surprisingly did not seem to disrupt the biofilm of S. epidermidis 5 (Fig. 1b), although this strain is a strong PNAG producer (Sadovskaya et al. 2006). To explain this result, it should be taken into account that sodium periodate modifies the polymeric chain of the PNAG by oxidizing the carbons bearing vicinal hydroxyl groups and cleaving the C3–C4 bonds of the GlcNAc residue. Additional mild acidic hydrolysis is required to further depolymerise the PNAG macromolecules. Therefore, a simple treatment with periodate with following washings might not be sufficient to disrupt the thick PNAG-rich biofilm of S. epidermidis 5.
Biofilm detachment of S. epidermidis RP62A (a) and the clinical staphylococcal strains S. epidermidis 5 (b), S. epidermidis 444 (c), S. lugdunensis 18a (d), S.lugdunensis 47 (e) and S. aureus 383 (f) by dispersin B (Dsp B), sodium periodate (NaIO 4), Pectinex Ultra SP (PUS), proteinase K (Prot K), trypsine (Tryp) and pancreatine (Pancr). N No treatment. Histograms corresponding to biofilms containing an important amount of PNAG are shown in dark grey
The effect of dispersin B and periodate on purified staphylococcal PNAG was verified. PNAG isolated from the biofilm of S. epidermidis 5 was incubated with dispersin B or periodate under the conditions used for disruption of biofilm. After the reaction, the product was subjected to gel permeation chromatography on a G-50 column, and its chromatographic profile was compared to that of intact PNAG. As expected, the treatment with dispersin B led to depolymerisation of PNAG (Fig. 2). Periodate treatment of PNAG, while significantly lowering glucosamine content, did not lead to its depolymerisation (data not shown). Our results confirm therefore that depolymerisation of PNAG results in the efficient removal of biofilms in which PNAG is a major component. Treatment with periodate had a more limited effect on the PNAG-containing biofilms.
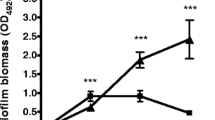
Effect of dispersin B on purified PNAG (“Materials and methods”). The elution profile of the intact PNAG (control experiment) is shown with filled circles and the one of the dispersin B-treated PNAG with empty circles. Void (V o) and total (V t) volumes of the column are shown with arrows
Effect of proteolytic enzymes
Proteinase K and trypsin are proteolytic enzymes with different substrate specificity: proteinase K endolytically cleaves the peptide bonds of aliphatic, aromatic or hydrophobic amino acids, whereas trypsin is specific for the peptide bonds of lysine and arginine. Both proteases were inefficient for the removal of PNAG-rich biofilms of S. epidermidis strains RP62A and 5 (Fig. 1a,b) but efficiently removed the biofilm of S. aureus 383 (Fig. 1f). In the case of S. lugdunensis 47 and 18a (Fig. 1d,e), proteinase K was more efficient than trypsin. Interestingly, trypsin was also able to partially remove the biofilm of S. epidermidis 444 (Fig. 1c), indicating that protein components, as well as PNAG, play an important role in the stabilisation of the biofilm of this strain. The different effect of the two proteolytic enzymes on the biofilms of different staphylococcal strains may indicate that their biofilms are composed of different types of proteins, which may vary from one strain to another.
Effect of pancreatin
Pancreatin is a mixture of digestive enzymes from the porcine pancreas with proteolytic, amylase and lipase activities. Pancreatin was able to completely remove biofilms of staphylococcal strains producing no or very little PNAG—S. aureus 383, S. lugdunensis 47 and 18a (Fig. 1). When the activity of pancreatin was tested on the purified TAs of different origins, no depolymerisation was detected (Fig. 3a,b). Similarly, purified PNAG was not depolymerised by pancreatin (data not shown). We therefore concluded that the ability of this enzyme preparation to remove staphylococcal biofilms was due to its proteolytic activity.
Effect of PUS
PUS very efficiently removed the biofilm of S. aureus 383 (Fig. 1f). Surprisingly, it had very little or no effect on the biofilms of the other four strains tested (Fig. 1). As in the case of pancreatin, no depolymerisation of PNAG or TA by PUS was detected (data not shown), indicating that the effect of PUS on the biofilm of this strain was probably due to its proteolytic activity. To verify that, we tested the effect of PUS on the crude extracellular extract of the strain S. aureus 383 and followed the depolymerisation of proteins with the TNBS assay for the free amino groups, as described in “Materials and methods.” The incubation with PUS resulted in the degradation of proteins in the biofilm extract, the effect being similar to one of proteinase K (12 and 20 μM of –NH2 groups liberated per 1 mg of protein because of PUS and proteinase K treatment, respectively).
Discussion
The interest of the scientific community to the enzymatic degradations of bacterial biofilms of different origins remains very high, as biofilm formation is a cause of industrial, environmental and medical problems in many areas. Different enzymes or enzyme mixtures were recommended for elimination of a biofilm in general, often with no reference to chemical composition of the biofilm of specific bacterial species.
In orthopaedic surgery, bacterial biofilm-related infections represent one of the most serious complications involving prosthetic devices and have a huge impact in terms of morbidity, mortality and medical costs (Campoccia et al. 2006). Treatment of these infections usually requires appropriate surgical intervention combined with a prolonged course of antimicrobial therapy (Trampuz and Zimmerli 2005). In certain cases of infection, washing–draining procedures of the infected device with solutions containing antibiotics are employed to maintain the implant if possible. The use of an agent that would disintegrate the bacterial biofilm, release the planktonic cells into environment and therefore allow the appropriate antibiotic to eliminate infection would greatly improve the efficiency of this medical procedure. Complete elimination of biofilm could thus help to avoid the removal of the orthopaedic implant.
The purpose of the present study was to search for enzymes capable to specifically degrade the constituents of the extracellular staphylococcal matrix. These enzymes could be further used in clinical procedures for the treatment of orthopaedic implant-associated infections.
In our previous studies, we investigated the chemical composition of extracellular matrix of the biofilms of staphylococcal strains widely representing the orthopaedic implant-associated infections. After analyzing the chemical composition of in vitro grown biofilm of 15 clinical isolates, we established that these strains could be separated into two major groups. The first group included strains producing biofilm with important amount of PNAG. The second, larger group consisted of strains producing biofilms with small amount or without PNAG. Biofilms of all strains studied contained proteins and TAs (Kogan et al. 2006; Sadovskaya et al. 2006). The proportion of these constituents seems to vary among the strains and depends on the growth conditions (unpublished data).
To test different enzymes and enzymatic preparations capable to degrade the biofilms with different compositions, we chose five clinical strains with known composition of their extracellular biofilm matrix (Table 1) and tested the sensitivity of their biofilms to dispersin B, periodate, proteinase K, trypsin, PUS and pancreatin. Our results show that dispersin B is an efficient degrading agent for the biofilm of staphylococcal strains producing biofilms with important amounts of PNAG. The biofilm of these strains is generally unsusceptible to treatments with proteases. On the contrary, treatment with proteases gives satisfactory results for biofilm removal of strains producing biofilms without PNAG. The hydrolytic activity of the dispersin B and proteinase K on biofilm components was confirmed by the direct action of these enzymes on PNAG and the protein fraction of biofilms, respectively.
The heterogeneity of the biofilm matrix limits the potential of the monocompound enzyme, and the use of two or several successive treatments may be necessary for a sufficient degradation of biofilms produced by clinical staphylococcal strains. Thus, a treatment with dispersin B followed by a protease (proteinase K or trypsin) could be capable of eradicating biofilms of a variety of staphylococcal strains on inert surfaces. Unfortunately, none of the enzymes tested in this study was able to depolymerise the TAs, an important component of staphylococcal biofilm. Finding of an enzyme able to specifically degrade the TA could favorably complement the action of the dispersin B and a protease. Other enzymatic preparations, such as PUS or pancreatin, might be efficient in degrading biofilms of certain strains that do not produce PNAG because, most probably, of their proteolytic activity. The use of these unpurified preparations, however, cannot be recommended for the treatment of infections caused by a diversity of clinical strains.
A possible inflammatory effect or allergic reactions of an enzymatic treatment might limit the use of these enzymes in the treatment of infected prosthesis in situ. In vivo experiment with an animal model of an infected medical implant and toxicity studies would shed further light on the joined action of dispersin B and proteases for the treatment of biofilm-related infections.
References
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Campoccia D, Montanaro L, Arciola CR (2006) The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials 27:2331–2339
Cerca N, Martins S, Cerca F, Jefferson KK, Pier GB, Oliveira R, Azeredo J (2005) Comparative assessment of antibiotic susceptibility of coagulase-negative staphylococci in biofilm versus planktonic culture as assessed by bacterial enumeration or rapid XTT colorimetry. J Antimicrob Chemother 56:331–336
Chokr A, Watier D, Eleaume H, Pangon B, Ghnassia JC, Mack D, Jabbouri S (2006) Correlation between biofilm formation and production of polysaccharide intercellular adhesin in clinical isolates of coagulase-negative staphylococci. Int J Med Microbiol 296:381–388
Dubois M, Gilles KA, Hamilton JF, Rebers PA, Smyth F (1956) Colorimetric methods for determination of sugars and related substances. Anal Biochem 28:350–356
Eleaume H, Jabbouri S (2004) Comparison of two standardisation methods in real-time quantitative RT-PCR to follow Staphylococcus aureus genes expression during in vitro growth. J Microbiol Methods 59:363–370
Enghofer E, Kress H (1979) An evaluation of the Morgan–Elson assay for 2-amino-2-deoxy sugars. Carbohydr Res 76:233–238
Frebourg NB, Lefebvre S, Baert S, Lemeland JF (2000) PCR-based assay for discrimination between invasive and contaminating Staphylococcus epidermidis strains. J Clin Microbiol 38:877–880
Fux CA, Costerton JW, Stewart PS, Stoodley P (2005) Survival strategies of infectious biofilms. Trends Microbiol 13:34–40
Galdbart JO, Allignet J, Tung HS, Ryden C, El Solh N (2000) Screening for Staphylococcus epidermidis markers discriminating between skin–flora strains and those responsible for infections of joint prostheses. J Infect Dis 182:351–355
Götz F (2002) Staphylococcus and biofilms. Mol Microbiol 43:1367–1378
Hamilton MA (2002) Testing antimicrobials against biofilm bacteria. J AOAC Int 85:479–485
Heilmann C, Schweitzer O, Gerke C, Vanittanakom N, Mack D, Götz F (1996) Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol Microbiol 20:1083–1091
Itoh Y, Wang X, Hinnebusch BJ, Preston JF 3rd, Romeo T (2005) Depolymerization of β-1,6-N-acetyl-d-glucosamine disrupts the integrity of diverse bacterial biofilms. J Bacteriol 187:382–387
Johansen C (1996) A method for enzymatic treatment of biofilm. PCT. N. N. A/S. Denmark
Johansen C, Falholt P, Gram L (1997) Enzymatic removal and disinfection of bacterial biofilms. Appl Environ Microbiol 63:3724–3728
Kaplan JB, Ragunath C, Ramasubbu N, Fine DH (2003) Detachment of Actinobacillus actinomycetemcomitans biofilm cells by an endogenous beta-hexosaminidase activity. J Bacteriol 185:4693–4698
Kaplan JB, Ragunath C, Velliyagounder K, Fine DH, Ramasubbu N (2004a) Enzymatic detachment of Staphylococcus epidermidis biofilms. Antimicrob Agents Chemother 48:2633–2636
Kaplan JB, Velliyagounder K, Ragunath C, Rohde H, Mack D, Knobloch JK, Ramasubbu N (2004b) Genes involved in the synthesis and degradation of matrix polysaccharide in Actinobacillus actinomycetemcomitans and Actinobacillus pleuropneumoniae biofilms. J Bacteriol 186:8213–8220
Kogan G, Sadovskaya I, Chaignon P, Chokr A, Jabbouri S (2006) Biofilms of clinical strains of Staphylococcus that do not contain polysaccharide intercellular adhesin. FEMS Microbiol Lett 255:11–16
Lee YC (1978) Synthesis of some cluster glycosides suitable for attachment to proteins or solid matrices. Carbohydr Res 67:509–514
Lortat-Jacob A, Desplaces N, Gaudias J, Dacquet V, Dupon M, Carsenti H, Dellamonica P (2002) Secondary infection of joint implants: diagnostic criteria, treatment and prevention. Rev Chir Orthop Repar Appar Mot 88:51–61
Mack D, Fischer W, Krokotsch A, Leopold K, Hartmann R, Egge H, Laufs R (1996) The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear β-1,6-linked glucosaminoglycan: purification and structural analysis. J Bacteriol 178:175–183
Mack D, Riedewald J, Rohde H, Magnus T, Feucht HH, Elsner HA, Laufs R, Rupp ME (1999) Essential functional role of the polysaccharide intercellular adhesin of Staphylococcus epidermidis in hemagglutination. Infect Immun 67:1004–1008
Maira-Litran T, Kropec A, Goldmann D, Pier GB (2004) Biologic properties and vaccine potential of the staphylococcal poly-N-acetyl glucosamine surface polysaccharide. Vaccine 22:872–879
Marion K, Pasmore M, Freney J, Delawari E, Renaud F, Costerton JW, Traeger J (2005) A new procedure allowing the complete removal and prevention of hemodialysis biofilms. Blood Purif 23:339–348
McKenney D, Pouliot K, Wang Y, Murthy V, Ulrich M, Döring G, Lee JC, Goldmann DA, Pier GB (2000) Vaccine potential of poly-1-6 β-d-N-succinylglucosamine, an immunoprotective surface polysaccharide of Staphylococcus aureus and Staphylococcus epidermidis. J Biotechnol 83:37–44
Projan SJ, Novick RP (1997) The molecular basis of pathogenicity. In: Crossley GL (ed) The staphylococci in human disease. Churchill Livingstone, New York, NY, pp 55–82
Rohde H, Burdelski C, Bartscht K, Hussain M, Buck F, Horstkotte MA, Knobloch JK-M, Heilmann C, Herrmann M, Mack D (2005) Induction of Staphylococcus epidermidis biofilm formation via proteolytic processing of the accumulation-associated protein by staphylococcal and host proteases. Mol Microbiol 55:1883–1895
Sadovskaya I, Vinogradov E, Li J, Jabbouri S (2004) Structural elucidation of the extracellular and cell-wall teichoic acids of Staphylococcus epidermidis RP62A, a reference biofilm-positive strain. Carbohydr Res 339:1467–1473
Sadovskaya I, Vinogradov E, Flahaut S, Kogan G, Jabbouri S (2005) Extracellular carbohydrate-containing polymers of a model biofilm-producing strain Staphylococcus epidermidis RP62A. Infect Immun 73:3007–3017
Sadovskaya I, Chaignon P, Kogan G, Chokr A, Vinogradov E, Jabbouri S (2006) Carbohydrate-containing components of biofilms produced in vitro by some staphylococcal strains related to orthopaedic prosthesis infections. FEMS Immunol Med Microbiol 47:75–82
Sutherland IW (2001) Biofilm exopolysaccharides: a strong and sticky framework. Microbiology 147:3–9
Trampuz A, Zimmerli W (2005) New strategies for the treatment of infections associated with prosthetic joints. Curr Opin Investig Drugs 6:185–190
Vinogradov E, Sadovskaya I, Li J, Jabbouri S (2006) Structural elucidation of the extracellular and cell-wall teichoic acids of Staphylococcus aureus MN8m, a biofilm forming strain. Carbohydr Res 341:738–743
Vuong C, Voyich JM, Fischer ER, Braughton KR, Whitney AR, DeLeo FR, Otto M (2004) Polysaccharide intercellular adhesin (PIA) protects Staphylococcus epidermidis against major components of the human innate immune system. Cell Microbiol 6:269–275
Wang X, Preston JF 3rd, Romeo T (2004) The pgaABCD locus of Escherichia coli promotes the synthesis of a polysaccharide adhesin required for biofilm formation. J Bacteriol 186:2724–2734
Ziebuhr W, Heilmann C, Gotz F, Meyer P, Wilms K, Straube E, Hacker J (1997) Detection of the intercellular adhesion gene cluster (ica) and phase variation in Staphylococcus epidermidis blood culture strains and mucosal isolates. Infect Immun 65:890–896
Acknowledgements
This work was supported by ANVAR and the CPER of the Nord-Pas de Calais. We thank Prof. G. Pier, Channing Laboratory, Brigham and Women’s Hospital, Boston, MA, for providing the bacterial strain S. aureus MN8m. We thank Prof. P. Hardouin for his support, and Aurélie Fontaine and Audrey Keunebrock for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chaignon, P., Sadovskaya, I., Ragunah, C. et al. Susceptibility of staphylococcal biofilms to enzymatic treatments depends on their chemical composition. Appl Microbiol Biotechnol 75, 125–132 (2007). https://doi.org/10.1007/s00253-006-0790-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-006-0790-y