Abstract
To evaluate if root architecture changes observed in Arabidopsis thaliana inoculated with Azospirillum argentinense Az39 depend exclusively on the bacterial capacity to produce indole-3-acetic acid (IAA) and plant ability to sense IAA levels. Azospirillum argentinense Az39, A. argentinense Az39 ipdC–, flagellin from A. argentinense Az39, and pure IAA were applied to A. thaliana Col-0 (wild-type) and tir1.1 (a lateral root deficient mutant) seedlings. Inoculation with heat-inactivated A. argentinense Az39 cells and a non-PGPR bacterium (Escherichia coli DH5α) was also tested. The primary root (PR) length, lateral roots (LR) number, and root hair (HR) density were assessed, and the root transcriptome was sequenced (Illumina HiSeq), followed by DEGs and GO term enrichment analyses. Inoculation with both A. argentinense strains resulted in a shorter PR and an increased number of LR and RH. IAA application (0.1 µM) led to a similar root phenotype than inoculation with Az39 (108 CFU mL−1). The addition of 1 µM flagellin, as well as plant exposure to non-lysed A. argentinense Az39 or E. coli DH5α cells, enhanced RH formation. Genes related to auxin signaling were highly expressed in the roots of Az39-inoculated seedlings; genes related to jasmonate and salicylic acid metabolism were highly expressed in the roots of plants inoculated with ipdC − . Root architecture changes in A. thaliana inoculated with A. argentinense Az39 do not depend exclusively on root IAA levels/IAA plant perception. This PGPR induces root morphological changes through both IAA-dependent and IAA-independent mechanisms. Flagellin may be a key molecule involved in IAA-independent mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The interaction between plants and beneficial microorganisms usually results in root architecture changes that improve plant capacity to take up water and nutrients from the soil (Grover et al. 2021). In this regard, it has been largely recognized that the well-known plant growth–promoting rhizobacterium (PGPR) Azospirillum can improve plant growth due to the production of phytohormones such as auxins, mainly indole-3-acetic acid (IAA), as well as cytokinins, gibberellins, abscisic acid, and free polyamines, among many other mechanisms (Cassán et al. 2020 and references therein). Several studies have demonstrated that Azospirillum can alter root architecture by promoting the development and elongation of lateral roots (LR) and root hairs (RH), and inhibiting the elongation of the primary root (PR), thereby presumably increasing the root surface (Cassán et al. 2020; Méndez-Gómez et al. 2020; Rondina et al. 2020; Dubrovsky et al. 1994). These morphological changes at the root level have been mostly related to the bacterial capacity to produce and release IAA (Prinsen et al. 1993; Coniglio et al. 2019); less is known about changes induced by other phytohormones and much less about the role of other bacterial components.
Azospirillum brasilense synthesizes IAA almost exclusively through the indole-3-pyruvic acid-dependent (IPyA) pathway (Costacurta et al. 1994), one of the three tryptophan-dependent pathways described for this genus. The indole-3-pyruvate decarboxylase (IPDC), encoded by the ipdC gene, is a key enzyme in this pathway. Silencing this gene reduces IAA biosynthesis (Malhotra and Srivastava 2008); for this reason, Azospirillum ipdC − mutants constitute a valuable tool for investigating the role of IAA in plant–microbe interactions. In this regard, inoculation experiments with an A. brasilense ipdC − mutant performed by our group suggested that other molecules than IAA might be responsible for some changes in the root system of Azospirillum-inoculated plants (Cassán et al. 2020; Puente et al. 2018).
In the present study, we aimed to evaluate more thoroughly root architecture changes in Arabidopsis thaliana inoculated with Azospirillum argentinense Az39 (formerly A. brasilense Az39; dos Santos Ferreira et al. 2022) and try to link these changes to global gene expression in the roots of inoculated plants. To shed light on the involvement of IAA-dependent and IAA-independent routes in root architecture changes, we included in our assays a new ipdC − mutant of A. argentinense Az39, an experimental A. thaliana line impaired in lateral roots formation, as well as other comparators.
2 Materials and Methods
2.1 Bacterial Strains and Culture Conditions
Azospirillum argentinense Az39 was obtained from Instituto de Microbiología y Zoología Agrícola, INTA-Castelar, Argentina, and reactivated in Luria–Bertani (LB) medium with 2.5 mM CaCl2 and 2.5 mM MgSO4. Routine A. argentinense Az39 multiplication was performed at 30 °C and 120 rpm (orbital shaking) until reaching an optical density at 595 nm (OD595) of 1.2, corresponding to the late exponential growth phase. Aliquots of 100 μl were subsequently transferred to 100-mL flasks containing 25 mL of minimal medium for A. brasilense (MMAB) (Vanstockem et al. 1987) supplemented with 10 mg·l−1 of L-tryptophan (Trp) plus 50 μg·mL−1 of kanamycin (Km) when culturing A. argentinense Az39 ipdC − strain. Escherichia coli DH5α (used as a comparator in a subset of experiments) was grown in LB at 37 °C and 120 rpm under orbital shaking until reaching an OD595 of 1.8 (late exponential growth phase).
2.2 Construction of the A. argentinense Az39 ipdC − Mutant
A new procedure was developed to obtain an ipdC − mutant of A. argentinense Az39. This IAA-deficient mutant was obtained by insertional mutagenesis in the ipdC gene using the conjugative suicide vector pKNOCK-Km, as described by Alexeyev (1999). An internal fragment of the ipdC gene was amplified from the genomic DNA of A. argentinense Az39 by PCR with the primer pairs: Fw_ipdC, 5′-CGGAATTCGCCCGGTCTATCTGGAAATC-3′; Rv_ipdC 5′-CGGAATTCGTCCATGGCGGTGAACAG -3′ introducing EcoRI sites. The PCR product was cloned into pKNOCK-Km and digested with EcoRI by T4 DNA ligase (ThermoFisher Scientific®). The resulting vector pKNOCK-Km-ipdC was introduced into E. coli S17-1/λpir (lysogen for strain S17-1) and then transferred to A. argentinense Az39 by conjugation (Gullett et al. 2017). The transconjugants were selected using solid Luria–Bertani Congo Red (LBCR) culture medium (Molina et al. 2014) containing kanamycin (50 μg·mL−1) and ampicillin (200 μg·mL−1). The plasmid insertion into an appropriate position was confirmed by PCR with the primers Fw_ipdC and pKnock4_Rev: 5′-ATGTAAGCCCACTGCAAGCTA-3′ that is homologous to a specific sequence of the pKNOCK-Km. The ipdC − mutants of A. argentinense Az39 generated by single crossover integration of the plasmids into the genome were named A. argentinense Az39-ipdC − . Further details about the strains and plasmid used are supplied in Table S1.
2.3 Measurement of IAA Production by A. argentinense Strains
An unequivocal methodology combining a one-step solid-phase extraction (SPE) purification method (Torres et al. 2018) with sensitive and selective liquid chromatography–multiple reaction monitoring-mass spectrometry (LC-MRM-MS) (Matsuda et al. 2005) was used to determine IAA production by A. argentinense Az39 and its derivative A. argentinense Az39-ipdC − (onwards referred to as Az39 and ipdC − , respectively). The strains were grown in MMAB or LB to late exponential (OD595 = 1.2–1.4) and stationary phases (OD595 = 1.6–1.8), respectively, centrifuged and filtered to obtain clean supernatants.
UHPLC-MS/MS measurements were performed on Nexera X2 UHPLC (Shimadzu Handels GmbH) coupled with a mass spectrometer MS-8050 (Shimadzu Handels GmbH). Chromatographic separation was performed on an Acquity UPLC BEH C18 (50 × 2.1 mm; 1.7 µm particle size) column (Waters, Milford, MA, USA) with the corresponding pre-column kept at 40 °C. The mobile phase consisted of 0.05% acetic acid in water (component A) and 0.05% acetic acid in methanol (component B). The analytes were separated using a binary gradient starting at 10% of solvent B (0.05% acetic acid in methanol), which increased to 90% of solvent B for 3.5 min, then decreased to 10% of solvent B for the next 0.1 min. The equilibration to the initial conditions took 2.4 min. The flow rate was 0.4 mL·min−1, and the injection volume was 2 µL. The identification of IAA was performed via electrospray ionization in positive MRM mode.
2.4 IAA Bacterial Production/IAA Sensing by A. thaliana Seedlings
In a complementary assay, a DR5::GUS reporter line of A. thaliana was used to assess IAA production by A. argentinense strains and IAA sensing by inoculated plants. The seeds of the DR5::GUS line kindly provided by prof. Guilfoyle (University of Missouri, Columbia, USA) were placed in 96-well microplates containing half-strength MS medium and stratified at 4 °C in the darkness for 4 days. Subsequently, the microplates were placed into a phytotron under controlled conditions (75 rpm, 21 °C, 16-/8-h light/dark, light intensity 100 µmol·m−2·s−1) for 7 days, after which suspensions of Az39 or ipdC − at titers of 108 and 109 CFU mL−1 (prepared in half-strength MS) were added. Also, 5 µM IAA dissolved in 0.1% acetic acid was prepared and added to a third set of A. thaliana seedlings to obtain a positive control. Negative controls were achieved by applying half-strength MS medium with or without 0.1% acetic acid. After 17 h of incubation, the medium was discarded, and the seedlings were directly treated with 150 μl lysis buffer (50 mM sodium phosphate, pH 7.0, 10 mM EDTA, 0.1% Triton X-100) containing 1 mM of 4-methylumbelliferyl glucuronide (4-MUG), and incubated at 37 °C for 90 min. At the end of the incubation period, 50 μl of 1 M Na2CO3 (stop solution) was added to each well, and the fluorescence due to 4-methylumbelliferone (4-MU) formation was measured in a microplate reader (excitation/emission wavelengths 365/460 nm).
2.5 A. argentinense Flagellin Collection
The flagellin from Az39 was obtained according to Elías et al. (2021). Briefly, Az39 was grown in nitrogen-free (NFb) liquid medium at 30 °C and 120 rpm for 24 h, after which cells were collected through centrifugation at 2000 × g for 10 min and washed twice with sterile bi-distilled water. Subsequently, cells were resuspended in 20 mL of 100 mM Tris–HCl buffer pH 7.0, vortexed for 10 min to detach flagella, and centrifuged at 6000 × g at 4 °C for 30 min. The supernatant containing the flagella and other extracellular proteins was recovered and loaded in a 12% (w/v) denaturing polyacrylamide gel for protein electrophoresis, according to standard procedures. The band corresponding to Azospirillum flagellin AzFlap (100 kDa) was excised from the gel, electroeluted in Tris–glycine buffer pH 8.3 at 40 V for 12 h, dialyzed, lyophilized, and resuspended in distilled water to a convenient concentration. The identity of the protein was confirmed by Western blot using an A. brasilense Sp7 anti-flagellin obtained from rabbit (Viruega-Góngora et al. 2020) as the primary antibody and, as the secondary antibody, an alkaline phosphate-conjugated goat anti-rabbit commercial antibody (Sigma-Aldrich, St Louis, MO, USA).
2.6 Plant Material and Growth Conditions
To check the effects of A. argentinense inoculation on Arabidopsis root morphology, Arabidopsis thaliana (L.) ecotype Columbia 0 (Col-0, wild-type) and the A. thaliana mutant tir1.1, deficient in various auxin-regulated growth processes, including lateral root formation (Ruegger et al. 1998), were used.
Arabidopsis seeds were surface sterilized with 95% (v/v) ethanol (5 min) followed by aqueous 2.5% (v/v) sodium hypochlorite solution containing 0.1% (v/v) Triton X-100 (5 min). After washing four times with sterile distilled water, the seeds were stratified in the darkness at 4 °C in plastic tubes containing sterile distilled water. Two days later, the seeds were sown in Petri dishes containing Murashige and Skoog (MS) medium (Murashige and Skoog 1962) supplemented with 0.8% (w/v) agar and a final pH of 5.7. Subsequently, plates were sealed with micropore tape, and seeds were allowed to germinate and grow vertically for 7 days in a growth chamber at 22 ºC, under 16–8-h light–dark photoperiod (intensity of 60 µmol m−1·s−1).
Seven days after sowing, five Arabidopsis seedlings per plate were aseptically transferred to new Petri dishes containing MS medium supplemented with 0.8% (w/v) agar and subjected to the following treatments (in different sets of experiments): inoculation with 108 CFU mL−1 of A. argentinense Az39; inoculation with 108 CFU·mL−1 of A. argentinense Az39 ipdC − ; addition of 0.1 µg·mL−1 IAA; addition of A. argentinense Az39 cells inactivated by previous exposure to 57 °C for 45 min (Az39 φ); inoculation with 108 CFU·mL−1 of E. coli DH5α (no-PGPR, unable to produce IAA) (Table S2); addition of flagellin from A. argentinense Az39 (obtained as described before) at two contrasting concentrations: 200 nM and 1 µM. The A. argentinense titer and the IAA concentration were selected based on preliminary experiments (see next item).
For inoculation assays, bacteria from the late exponential growth phase in MMAB medium were obtained through centrifugation (3900xg, 12 min, 16 °C), washed with 0.85% (w/v) sterile saline, and resuspended in 0.01 M MgSO4. Subsequently, variable volumes (0.02–2.00 mL) of bacterial suspensions (about 109 CFU·mL−1) were mixed with variable volumes (19.98–18.00 mL) of molten MS medium (below 40 °C) to ensure a final titer between 103 and 108 CFU·mL−1, homogeneously distributed in the growing medium.
Before the experiments with A. argentinense Az39 heat-inactivated cells (Az39 φ), a theoretical titer of 109 CFU·mL−1 was verified through the microdroplet method (Puente et al. 2018), using as culture medium LB supplemented with 1.5% agar (w/v) and modified by the addition of Congo red dye (LBRC). The same amount of the 0.01 M MgSO4 solution was added and homogenized in the melted medium to obtain uninoculated control seedlings. Flagellin treatment was achieved by adding 5 µL of Az39 flagellin solution 200 nM on the roots of five A. thaliana Col-0 seedlings per plate, according to results reported for A. argentinense REC3 flagellin by Elías et al. (2021). Also, 1 µM flagellin was evaluated to test dose-dependence effects. All Petri dishes containing Arabidopsis seedlings were kept vertically in a growth chamber at 22 °C, and a light/dark photoperiod of 16/8 h (light intensity of 60 µmol·m−1·s−1) for 5 days until image analysis.
2.7 Preliminary Experiments
Before conducting the assays designed to evaluate the effects of A. argentinense inoculation on the root architecture of Arabidopsis, it was necessary to analyze three relevant points: (1) if A. argentinense could grow or remain viable in the conditions established for A. thaliana growth; (2) which would be the best bacterial concentration to apply; (3) which would be the best IAA concentration to apply (as comparator).
To address point 1, a pure culture of Az39 n (109 CFU mL−1) was sown through the microdroplet method in Petri dishes containing Murashige Skoog (MS) medium supplemented with 1.5% (w/v) agar or LB medium supplemented with 1.5% agar (w/v), modified by the addition of Congo red dye (LBRC) (Molina et al. 2014), and incubated at 24 °C and 37 °C. Colonies were counted after 7 days of incubation.
To address points 2 and 3, dose–response tests using incremental concentrations of bacterial cells (103 to 108 CFU·mL−1) and IAA dissolved in MS medium to reach final concentrations of 0.01; 0.1; 1.0, 5.0; 10.0; 15.0, and 25.0 µg IAA mL−1 were conducted.
2.8 Evaluation of Arabidopsis Root Architecture
Arabidopsis thaliana images were acquired with a Canon PowerShot SX510 HS camera 5 days after transplant. The length of the primary root (PR) and the number of lateral roots (LR) (n = 15) were analyzed by the use of the RootNav software v1.8.1 (Pound et al. 2013). Lateral root density (LRD) was determined by dividing the number of LR by the length of the PR. Root hairs density (RHD) was calculated by using a 25-mm2 representative square area (Adu et al. 2017). To obtain more information about root architecture changes, the root system was divided into three sections: basal, middle, and apical root. These sections were observed using a digital stereoscopic magnifying glass (Motic SMZ-171-TLED-Digital). After flagellin treatments, the roots were microphotographed using an Olympus BX-51 microscope. Data were analyzed using Infostat software (Di Rienzo et al. 2012).
2.9 Gene Expression Analysis in A. thaliana Roots Inoculated with A. argentinense
For these studies, Arabidopsis Col-0 seedlings were inoculated 4 days after initial planting with 2 µL (OD595 = 1) of the corresponding Azospirillum suspension (Az39 or ipdC −) or with 2 µL of saline solution (NaCl 0.9% w/v), as the control.
Root systems were collected 24 h and 7 days post-treatment and flash-frozen in liquid nitrogen. Two biological replicates were collected at each time point, which included at least 50 individual root systems.
Nucleic acids were isolated from root tissue using Invitrogen PureLink Plant RNA Reagent (Thermo Fisher Scientific, Waltham, MA, USA), and DNA contaminants were removed by treating the samples with Ambion TURBO-DNA-free (Thermo Fisher Scientific, Waltham, MA, USA), according to the manufacturer’s protocol. RNA quantity was determined with a Nanodrop spectrophotometer, and RNA quality was checked with an RNA nanochip on the Agilent 2100 bioanalyzer system. Reverse transcription, fragmentation, end repair, adapter ligation, and PCR enrichment were conducted with the NEBNext Ultra II DNA Library Prep Kit (New England Biolabs, Whitby, ON, Canada), as indicated by the manufacturer’s protocols. Library size distribution was determined using a high-sensitivity DNA chip on the Agilent 2100 bioanalyzer system. Library concentration and molarity were determined with the NEBNext Library Quant Kit (New England Biolabs, Whitby, ON, Canada) in conjunction with PerfeCTa qPCR ToughMix (QuantaBio, Beverly, MA, USA) and PicoGreen fluoro-spectrophotometry. Libraries were sent to Genome Québec (Montréal, QC, Canada) for 150 bp single-end sequencing on the Illumina HiSeq 4000 platform.
The raw reads were processed using high-performance computing clusters provided by WestGrid (www.westgrid.ca) and Compute Canada (www.computecanada.ca). The subsequent steps to allow further bioinformatic analyses, including raw reads trimming, alignment to the Arabidopsis thaliana TAIR10 reference genome, gene counts, differential gene expression (DEGs) analysis of RNA-seq data and clustering, Gene Ontology (GO) term enrichment analysis, and plots generation were accomplished as described in Robertson et al. (2022). All RNA sequencing data have been deposited at the Gene Expression Omnibus (GEO): GSE192383.
3 Results
3.1 Azospirillum argentinense Az39 Synthesizes IAA if Tryptophan is Available
The IAA production of A. argentinense Az39 and its derivative ipdC − mutant was analyzed in both LB and MMAB culture media. IAA production by A. argentinense Az39 ipdC − mutant was not detected in any of the culture media evaluated, while A. argentinense Az39 produced 3.45 µg mL−1 of IAA in LB, a rich culture medium expected to supply tryptophan (Trp), the precursor necessary to allow this biosynthesis. Conversely, in the MMAB medium (without the addition of the precursor Trp), no IAA production was detected for any A. argentinense strain (Table S2). These results confirm that the presence of the precursor Trp is essential for IAA biosynthesis by A. argentinense Az39, as demonstrated previously (Puente et al. 2018). Additionally, the inability of the mutant ipdC − to produce IAA was confirmed.
3.2 Flagellin from A. argentinense Az39
The identification of the flagellin from A. argentinense Az39, after denaturing polyacrylamide gel for protein electrophoresis and electroelution, was assessed by Western immunoblotting using a specific rabbit antibody obtained from the purifed polar fagellin of A. brasilense Sp7. A single band of ~ 100 kDa was observed (Fig. S5). This let us infer that the flagellin obtained from the strain Az39 was suitable for the following experiments.
3.3 Corroboration of ipdC − Impairment for IAA Production
The indirect method of GUS activity in the DR5::GUS Arabidopsis reporter line was used to confirm that the ipdC − mutant obtained through insertional mutagenesis was impaired in IAA production and, therefore, unable to modify endogenous plant IAA levels. The maximum GUS activity was detected under exogenous addition of IAA (Fig. 1). Seedlings inoculated with A. argentinense Az39 also showed high fluorescence levels compared to the negative controls, while those inoculated with the ipdC − mutant (at the same titers) did not, corroborating the results of the direct IAA quantification. Besides, inoculation with 108 and 109 CFU·mL−1 of A. argentinense Az39 or ipdC − led to similar responses in terms of GUS activity.
Expression of DR5::GUS in A. thaliana Col-0 seedlings inoculated with A. argentinense Az39 or ipdC − . Seedlings were exposed for 17 h to 108 and 109 CFU·mL−1 of each strain. To obtain a positive control, seedlings were exposed for the same time to 5 µM IAA dissolved in 0.1% acetic acid (ac). Seedlings exposed to Murashige Skoog (MS) medium with or without 0.1% (w/v) ac were used as negative controls
3.4 Selection of Working Variables Based on Preliminary Experiments
Although A. argentinense Az39 showed no visible growth in MS at 24 °C, colonies became detectable after 7 days of incubation at 37 °C, demonstrating that Azospirillum cells stay viable in the culture medium used for Arabidopsis growth (Fig. S1 a, b). As expected, growth in LB was much greater, especially at 37 °C (Fig. S1c, d). The bacterial titers associated with significant changes in the PR length and LR number in A. thaliana Col-0 seedlings were 107 CFU·mL−1 (data not shown) and 108 CFU·mL−1 (Fig. S2); however, the most significant changes occurred at the latter, for which 108 CFU·mL−1 was the bacterial titer selected for further assays.
Regarding IAA concentration and its effects on root morphology (Fig. S3), we detected that 0.10 µg·mL−1 (equivalent to 0.57 µM IAA) induces a root phenotype similar to that obtained with the inoculation of 108 CFU·mL−1 of A. argentinense Az39. These changes included a reduction in the PR length and a significant increase in LR and RH numbers. IAA concentrations higher than 1.0 µg·mL−1 also resulted in a significant PR reduction, but this was accompanied by reductions in LR development. For this reason, 0.10 µg·mL−1 of IAA was the concentration selected for further experiments.
3.5 Azospirillum argentinense Az39 and ipdC − Alter Root Architecture in Both A. thaliana Genotypes
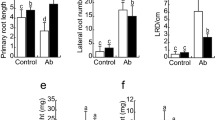
Arabidopsis Col-0 plants inoculated with Az39 showed a significant reduction in the primary root (PR) length (Fig. 2a, b) and an increase in the number of LR (Fig. 2c) and LRD (Fig. 2d). Observations with magnifying glasses also revealed an increased number and length of RH (Fig. 2e). In these seedlings, LR proliferation occurred mainly in the middle and apical root zones (Fig. 2e). Arabidopsis Col-0 seedlings inoculated with the mutant strain ipdC − also showed a significant decrease in the PR length and a significant increase in LR emergence compared to non-inoculated seedlings, but lower than that caused by Az39 (Fig. 2a, b, c). Unlike Az39, the IAA-deficient mutant induced LR development only in the middle zone (Fig. 2e). Additionally, we detected that LR of the seedlings inoculated with the ipdC − mutant tended to be longer than those inoculated with Az39. These results show that, despite the impairment for IAA production of the ipdC − mutant, both A. argentinense strains could change A. thaliana root morphology, leading to a shorter PR and increased root density compared to the uninoculated control (Table S3), due to the formation of more LR, with more abundant and large RH, suggesting that the production of IAA by the bacterium is not a strict requirement to change Arabidopsis root architecture.
Root architecture changes in A. thaliana inoculated with A. argentinense Az39 or ipdC − . Upper panel: Col-0; lower panel: tir1.1 mutant line. a, f plant phenotype; b, g primary root (PR) length; c, h lateral roots (LR) number; d, i lateral root density (LRD); e, j stereoscopic magnifying glass images showing basal, middle, and apical zones of the primary root. The letters on the bars indicate statistically significant differences according to Tukey’s HSD test, p < 0.05
The TIR1 protein is a member of a protein family implicated in ubiquitin-mediated processes and is encoded by the TRANSPORT INHIBITOR RESPONSE 1 (TIR1) gen. Because tir1 mutants are deficient in several auxin-regulated growth processes, including lateral root formation, this Arabidopsis line would further allow testing to what extent bacterial IAA production and/or IAA sensing by the plant are involved in the root architecture changes described above.
Tir1.1 mutant seedlings inoculated with Az39 showed a similar response as that observed in the WT line, with a significant reduction in the PR development (Fig. 2f, g) and a significant increase in the number of LR (Fig. 2h), also resulting in higher LRD (Fig. 2i). Besides, more RH were formed (Fig. 2j, Table S3), as in the wild-type line. However, tir1.1 seedlings inoculated with the ipdC − mutant presented a PR length similar to that of the uninoculated control (Fig. 2f, g). Despite this, the IAA-deficient strain increased LR formation (Fig. 2h) in the middle zone (Fig. 2j) and stimulated RH proliferation in the apical and middle zones (Fig. 2j, Table S3).
Summing up, at different degrees and with different spatial patterns, inoculation treatments induced LR formation and increased the number of RH in both A. thaliana lines, ruling out the strict requirement of bacterial IAA production or IAA sensing by the TIR1 protein to induce these changes. However, the shortening of the PR seems to be a more complex mechanism where the combined effect of IAA level in the roots and IAA sensing by the plant, not necessarily through the TIR1 protein, might be involved. The illustrative images shown in Fig. S4 seem to corroborate this idea, as the exogenous addition of 0.1 µg·mL−1 IAA resulted in the shortening of the PR in both A. thaliana lines.
3.6 Flagellin: a Novel Actor in A. argentinense—Root Interaction?
Because the above-described findings suggested the existence of a mechanism independent of IAA production and sensing behind most morphological changes occurring in Arabidopsis roots after A. argentinense inoculation, we designed a new set of experiments which included the exposure of Arabidopsis Col-0 seedlings to A. argentinense Az39, to non-viable Az39 cells (Az39 Ø), to Escherichia coli DH5α (a flagellated bacterium, not considered PGPR), or to flagellin obtained from A. argentinense Az39.
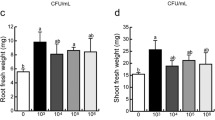
As shown in Fig. 3a, Az39 Ø did not induce relevant macroscopic changes in the A. thaliana root system compared to non-inoculated plants. However, a more detailed analysis using magnifying glasses revealed an increased number of RH in the apical and middle zones (Fig. 3b, Table S3). Moreover, the addition of E. coli DH5α cells also resulted in increased numbers of RH in the apical and middle root zones, with no relevant changes in the PR or RH length. Still, no changes in LR numbers were observed under these treatments. On the other hand, the addition of flagellin from Az39 also led to a similar response, with more number and length of RH than control plants (both in the LR and PR), mainly when used at 1 µM (Fig. 3c), suggesting a dose-dependent effect of this compound. In such conditions, the RH density of Arabidopsis seedlings exposed to flagellin was similar to that observed in plants inoculated with 108 CFU·mL−1 of Az39.
Comparative effects of different treatments on A. thaliana root architecture. Arabidopsis wt seedlings were exposed to A. argentinense Az39, A. argentinense Az39 heat-inactivated (Az39Ø), E. coli DH5ɑ (a, b), and to 200 nM or 1 µM Az39-flagellin (c). Illustrative images obtained by a the naked eye, b a stereoscopic magnifying glass, or c an optical microscope (40 ×). c A segment of a representative lateral root is shown; scale bar: 1 mm at 40 ×
We confirmed by viable cell counting on MMAB agar plates (CFU.ml−1) and optical density (OD595) that A. argentinense Az39 exposed to 57 °C during 45 min (Az39 Ø) was not able to growth and consequently, unable to synthesize IAA. Thus, both the root morphology and root hair proliferation induced by Az39 Ø would be attributed to a mechanism independent of the bacterial capacity to produce IAA (Fig. S6). In this scenario, we assumed that at least part of the changes observed in the root morphology of Arabidopsis seedlings could be attributed to the direct physical contact between bacterial cells (even non-viable) and the roots, where flagellin may be a key signaling molecule, contributing to a more developed root system due to increased root hair formation.
3.7 Gene Expression Patterns in the Roots of Arabidopsis Seedlings Inoculated with Az39 or the ipdC − Mutant Show Temporal Differences
The transcriptome of Arabidopsis roots was assessed 24 h and 7 days after the inoculation with A. argentinense Az39 or A. argentinense ipdC − , and a differential expression genes (DEGs) analysis (FDR < 0.05) was conducted (Fig. 4a, Dataset S1). Plants inoculated with the IAA-deficient mutant differentially expressed a large number of genes (1359 genes) after 24 h, and much fewer (281 genes) after 7 days. Conversely, plants inoculated with Az39 differentially expressed only 2 genes after 24 h, whereas 1260 genes were differentially expressed after 7 days. This finding demonstrates that Az39 affects plant gene expression later than the ipdC − mutant. At the time points of maximum DEGs, most of these genes were upregulated in plants inoculated with ipdC − , whereas in plants inoculated with Az39, they were more balanced.
Differential gene expression analysis of RNA-seq data after inoculation with A. argentinense Az39 or ipdC − . a Number of differentially expressed genes (DEGs; FDR < 0.05) compared to the control at 24 h and 7 days after inoculation. b Top 10 enriched upregulated GO terms from comparisons in (a). c Venn diagram showing common and non-common DEGs. d Top 10 enriched upregulated GO terms from the comparisons in (c), after removing the genes common to all 3 treatments. In b and d, GO terms are colored according to the p-value (Fisher’s exact test), with yellow being the most significant. All p-values > 0.0005 are colored purple. Several GO terms are enriched in more than one sample, as indicated by multiple yellow bars in the row
A Gene Ontology (GO) enrichment analysis revealed that upregulated DEGs in the roots of seedlings exposed to the ipdC − mutant at 24 h after inoculation were enriched for many GO terms related to photosynthesis, while 7 days after inoculation were mostly enriched for cell signaling and defense response terms, particularly jasmonic acid (JA) signaling (Fig. 4b). JA signaling-related DEGs common to both treatments included the JA biosynthesis gene OPC-8:0 COA LIGASE1 (OPCL1; AT1G20510) and the JASMONATE-ZIM-DOMAIN PROTEIN (JAZ) genes JAZ6 (AT1G72450), JAZ7 (AT2G34600), JAZ9 (AT1G70700), and JAZ10 (AT5G13220).
Az39-upregulated DEGs 7 d after inoculation were additionally enriched for abiotic stress responses (response to cold and to water deprivation), which included genes such as the LOW TEMPERATURE-INDUCED (LTI) genes LTI29 (AT1G20450) and LTI30 (AT3G50970).
In a global analysis considering DEGs at 24 h and 7 days after inoculation, we found that many of them were common to the three treatments (control seedlings, inoculated with Az39, inoculated with ipdC −) (971genes) (Fig. 4c). To elucidate differences in gene expression due to developmental stages or bacterial effects, the common DEGs were removed for the subsequent analysis of unique responses. Unique DEGs (i.e., differentially expressed in 1 or 2 of the treatments) were further characterized through a GO term enrichment analysis, which revealed that plants inoculated with ipdC − and Az39 activated unique functional programs (Fig. 4d). Thus, control seedlings had GO terms related to housekeeping functions (e.g., water transport, protein ubiquitination, response to nitrate), while bacterial treatments included some overlapping terms related to defense responses (response to wounding, response to fungus, defense response) as well as unique responses. Specifically, plants inoculated with Az39 were enriched for several abiotic stress responses (response to hypoxia, oxidative stress, and water deprivation), and plants inoculated with ipdC − were enriched for cell signaling responses (plant-type hypersensitive response, jasmonic acid/salicylic acid signaling, protein phosphorylation, regulation of transcription).
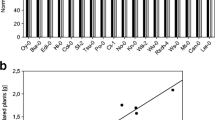
Finally, to better understand how auxin-signaling-related genes were affected by the presence of Az39 or ipdC − cells, we investigated the expression of differentially expressed auxin gene families and genes annotated with auxin-related GO terms (Fig. 5). The chosen auxin gene families were the AUXIN RESPONSE FACTORS (ARF), INDOLE-3-ACETIC ACID (IAA), YUCCA (YUC), and PIN-FORMED (PIN), though no PIN genes were differentially expressed. Overall, most differentially expressed auxin-signaling genes had the highest expression after 7 days compared to 24 h. The highest overall expression was found at 7 days in the Az39 treatment and the lowest at 24 h in the ipdC − treatment. Despite the large number of upregulated DEGs at 24 h in the ipdC − treatment, most auxin-related signaling genes were suppressed in the roots of these plants by that time. On the other hand, plants inoculated with Az39 had a similar DEGs pattern to that of the control after 24 h, but, at day 7, auxin signaling was greatly increased in Az39-inoculated plants and not in control plants.
Differential gene expression (FDR < 0.05) of auxin pathway components. Included are auxin-related gene families (ARF, auxin response factors; IAA, idole-3-acetic acid; YUC, YUCCA) and genes annotated with auxin-related Gene Ontology terms, where applicable. Each gene is colored by a z-score, which is the row-wise scaled expression of each gene. Yellow indicates higher expression, while dark blue indicates lower expression, allowing for sample comparison
4 Discussion
Several reports have shown that the direct effects of plant growth–promoting rhizobacteria, particularly Azospirillum, involve the bacterial production of phytohormones, among which auxins have received great attention (Karimi et al. 2021; Méndez-Gómez et al. 2020), and in many screening works for PGPR selection conducted in the past decades, IAA biosynthetic ability was considered a very important trait.
In this work, changes at the root level induced by A. argentinense Az39 and by a mutant strain deficient for IAA biosynthesis were evaluated in A. thaliana seedlings to elucidate the mechanisms underlying root growth promotion by this PGPR and to understand to what extent bacterial IAA is involved in plant responses.
We found that inoculation of Arabidopsis seedlings with both A. argentinense strains IAA + (Az39) and IAA − (ipdC −) induced a significant reduction in the PR elongation and a significant increase in the number of LR and RH compared to the uninoculated control. Nevertheless, the effects of the IAA + strain were more pronounced.
Based on the results obtained in experiments using A. brasilense Sp245 (now A. baldaniorum Sp245; dos Santos Ferreira et al. 2020), Spaepen et al. (2014) proposed that the inhibition of the PR growth mainly depends on IAA released by the bacterial partner, while the increase in LR would involve other molecules not related to IAA. Recently, Méndez-Gómez et al. (2020) and Carrillo-Flores et al. (2022) showed that the kinase TOR (target of rapamycin) is involved in the development of LR in A. thaliana inoculated with A. brasilense Sp245; previous data suggest that PR growth also depends on TOR through the regulation of cell proliferation and elongation (Deng et al. 2016).
Numerous studies have shown that a similar phenotype can be induced by different PGPR species involving different compounds. For example, Bacillus subtilis GB03 (Zhang et al. 2007), Phyllobacterium brassicacearum STM196 (Contesto et al. 2010), Pseudomonas aeruginosa (Ortíz-Castro et al. 2011), Martelella endophytica YC6887 (Khan et al. 2016), Bacillus amyloliquefaciens UCMB5113 (Asari et al. 2017), and Achromobacter sp. 5B1 (Jiménez-Vázquez et al. 2020) induced changes in the root architecture of A. thaliana by affecting auxin transport, perception, or signaling. However, more than 10 years ago, Shi et al. (2010) reported that plant inoculation with the rhizobacterium Serratia marcescens 90–166 induced a reduction in the PR length and stimulated LR development through both auxin-dependent and auxin-independent pathways, as well as through complex cross-talk between plant hormones including jasmonates, ethylene, and salicylic acid.
Interestingly, we found that genes encoding jasmonic acid synthesis and signaling increased in plants exposed to the IAA + and IAA − strain by day 7 after inoculation. In contrast, genes related to other signaling molecules, such as ethylene or salicylic acid, exhibited differential patterns. These signaling pathways have already been shown to be enriched in the roots of Arabidopsis inoculated with other rhizobacteria species (Stringlis et al. 2018). Recent evidence has demonstrated that plants respond similarly to PGPR as they do to pathogens, with past studies noting an induction of both induced systemic resistance (ISR) and systemic acquired resistance (SAR) dependent on the plant–microbe interaction (van Loon et al 2008; Spaepen et al. 2014). Although hormone responses of SAR (namely salicylic acid) and ISR (primarily jasmonic acid and ethylene) were classically thought to be segregated based upon pathogen lifestyle (Kunkel and Brooks 2002), elaborate cross-talk between these two pathways has been more recently described (Tsuda et al. 2009). In the present study, global RNA sequencing revealed upregulation of genes/GO terms involved in SAR and ISR as a result of both A. argentinese treatments. Enriched DEGs of the shared interaction between Az39 and ipdC − and A. thaliana such as the camalexin biosynthesis gene, PHYTOALEXIN DEFICIENT 3 (PAD3; AT3G26830), and the flagellin responsive kinase BRI1-ASSOCIATED KINASE 1 (BAK1; AT2G13790) have both been noted in other interaction between A. thaliana and A. baldaniorum (formerly A. brasilense) and other PGPR (Spaepen et al. 2014; Pečenková et al. 2017). These reactions stem from the plant sensing PGPR as non-self, due to the presence of microbe-associated molecular patterns (MAMPs) or pathogen-associated molecular patterns (PAMPs) (Zamioudis and Pieterse 2012). As a well-studied MAMP involved in plant defense responses, flagellin (specifically the conserved peptide flg22) has also been shown to induce inhibition of primary root growth as has been noted in plant-PGPR interactions (Gómez et al. 1999; Millet et al. 2010). Induction of the plant defense response by MAMPs or PAMPs results in complex hormonal cross-talk between salicylic acid, jasmonic acid, and ethylene, which may occur even if the detected molecule originates from non-pathogenic organisms (Tsuda et al. 2009). Although the detection of bacterial flagellin is likely responsible for the global changes in gene activity regarding plant immunity, the full repertoire of effectors in PGPRs, and specifically in Az39 and ipdC − have yet to be determined.
Besides, we observed that most genes related to IAA metabolism or transport tended to become more expressed with time, regardless of the strain. Previous reports described gene upregulation in Arabidopsis roots under other rhizobacteria interactions (Desrut et al. 2020; Stringlis et al. 2018; Zhao et al. 2018). Global RNA sequencing results also correspond with the observed phenotype of increased lateral root growth. Shared enriched GO terms of Az39 and ipdC − included response to jasmonic acid and ethylene, while GO terms specific to Az39 included response to auxin and meristematic regulation. Shared upregulated differentially expressed genes included AUXIN RESPONSE FACTOR 11 (ARF11; AT1G19220). Despite ipdC − ’s inability to produce IAA, increased ethylene in the plant, possibly occurring as a result of defense response induction, has been shown to stimulate auxin biosynthesis and transport towards the elongation zone of the root, ultimately inhibiting primary root growth (Růžička et al. 2007). Also shared as a result of treatment with the either A. argentinense strains was the enrichment for GO terms and differentially expressed genes associated with jasmonic acid, whose increased synthesis been reported to inhibit primary root elongation while promoting lateral root development in A. thaliana (Cai et al. 2014; Cheng et al. 2011). Specific only to treatment with Az39 was upregulation for the leucine-rich repeat receptor like kinases, MUSTACHES (MUS; AT1G75640) and MUSTACHES-LIKE (MUL; AT4G36180), which hit within the GO terms pertaining to kinase activity and meristematic regulation, respectively. It has been found that MUS and MUL regulate development of lateral root primordia in A. thaliana, with T-DNA insertional MUS-MUL knockout mutants exhibiting greatly reduced lateral root formation (Xun et al. 2020). Authors Xun et al. (2020) also found MUS transcription to be greatly enhanced as a result of exogenous auxin application. Thus, it is suggested that DEG upregulation as a result of ethylene, jasmonic acid, and auxin, result in the observed phenotype of reduced primary root growth, with enhanced lateral root development after treatment with Az39 and ipdC − strains of A. argentinese. However, further study characterizing the hormone response/synthesis in developing root tissue upon PGPR-plant interaction is required.
It should be noted that Arabidopsis tir1.1 inoculation with Az39 resulted in the same root phenotype as the wild-type Col-0, that is, a shorter PR and a higher number of LR. This finding indicates that IAA bacterial production and IAA plant perception, at least with the involvement of the TIR1 receptor, are not mediating the induction of LR and root hairs in A. argentinense Az39-inoculated seedlings. Nevertheless, we cannot rule out the existence of redundant functions in proteins belonging to the same family (such as AFB2 or AFB3). In this regard, Jiménez-Vázquez et al. (2020) showed that the rhizobacterium Achromobacter sp. 5B1 caused an increase in root branching in tir1-1 and axr3-1 mutants. Both auxin-responsive mutants showed some ability to form lateral roots under controlled conditions, strengthening the idea of an auxin-independent mechanism mediating lateral root maturation in Arabidopsis seedlings exposed to Achromobacter sp. 5B1. However, other auxin-signaling mutants included in that study did not show root growth promotion.
Apart from those involving auxin, other signaling mechanisms or molecules have been suggested to participate in plant growth promotion by rhizospheric microorganisms, particularly in LR and RH formation. For example, cytokinins (López-Bucio et al. 2007), jasmonates, ethylene, salicylic acid (Ribaudo et al. 2006; Shi et al. 2010), and nitric oxide (Creus et al. 2005; Koul et al. 2015) were shown to mediate processes triggered by A. brasilense. Likewise, some bacteria affected plant responses by releasing volatile organic compounds (VOCs); this was corroborated for several Bacillus spp. (Li et al. 2021), B. subtilis GB03, B. amyloliquefaciens IN937a (Ryu et al. 2004), B. megaterium UMCV1 (López-Bucio et al. 2007), B. subtilis SYST2 (Tahir et al. 2017), and Pseudomonas PS01 (Chu et al. 2020). Changes in Arabidopsis root architecture due to the action of VOCs released by phytopathogens such as Penicillium aurantiogriseum have also been documented; root accumulation of auxins and ethylene was detected in this interaction (García-Gómez et al. 2020).
Likewise, the biosynthesis of dimethyl disulfide by Bacillus sp. B55 (Meldau et al. 2013) and phenylacetic acid by Martelella endophytica YC6887 (Khan et al. 2016) was shown to affect plant growth. In these studies, primary root growth arrest seemed to depend on volatile or diffusible bacterial molecules’ sensing, while lateral root development might have been a compensatory mechanism.
Ribaudo et al. (2006) reported that cross-talk between plant ethylene and bacterial auxin is necessary to cause changes in root morphology regarding PR elongation and RH development. These authors suggested that high concentrations of IAA produced by the hyper producer strain A. brasilense FT326 could induce the expression of 1-aminocyclopropane-1-carboxylate synthase (ACS; E.C.4.4.1.14), a key enzyme in ethylene synthesis. Tomato inoculation with A. brasilense FT326 induced LR production and increased RH number and length. On the other hand, Creus et al. (2005) showed that the induction of LR formation in tomato seedlings is mediated by NO produced by A. brasilense Sp245 (now A. baldaniorum Sp245).
So far, there is a good body of evidence that plant-microorganism interactions occur through a highly complex communication network involving a variety of molecules, which alter gene expression in plants, in the associated microbe, or both partners. These mechanisms modulate root morphological changes that result in a more developed root system and better plant use of soil resources (nutrients and water), ultimately leading to improved plant growth and health.
Azospirillum brasilense has a polar flagellum, responsible for bacterial movement in liquid media (swimming), and several lateral flagella, synthesized only when this bacterium grows on solid or semi-solid surfaces (Steenhoudt and Vanderleyden 2000); the key role of A. brasilense Sp7 polar flagellum in cells attachment on wheat roots was documented many years ago (Croes et al. 1993). The polar flagellum of A. brasilense Sp7 consists of flagellin, a glycoprotein of ~ 100 KDa (Moens et al. 1995). In this work, we obtained flagellin from A. argentinense Az39 and tested its action on Arabidopsis seedlings. More root hairs were formed in the presence of Azospirillum flagellin compared to the untreated control, but less if compared with plants inoculated with A. argentinense Az39. Interestingly, this phenotype was dose-dependent.
The positive effect of A. baldaniorum Sp245 flagellin on root meristem mitotic activity was reported in wheat seedlings (Shirokov et al. 2020). Likewise, Elías et al. (2021) communicated recently that flagellin from A. argentinense REC3 can protect strawberry plants against the fungus Macrophomina phaseolina by inducing biochemical, histological, and molecular responses. Here, we report for the first time the ability of Azospirillum argentinense Az39 flagellin to induce RH formation in A. thaliana, thus altering root architecture.
Hernández-Esquivel et al. (2020) proposed that A. brasilense Sp245 can alter root morphology through structural molecules such as lipopolysaccharides (LPS), in addition to phytohormone production. These authors refer that LPS may need to contact the root directly to exert a stimulating effect and that plant cells need to distinguish between different LPS components to allow plant response, as these interactions may be species-specific.
There is scarce information on the effects of microbial LPS perception by plants, although plant receptors for typically pathogen-associated molecules (such as flagellin) have been described (Zipfel et al. 2004). Chávez-Herrera et al. (2018) showed that LPS from A. brasilense affected some aspects of wheat development, but the molecular mechanism involved is still obscure. More recently, Cassán et al. (2020) suggested that certain cellular components of A. argentinense, in direct contact with plant roots, could induce a growth response. In line with these concepts and considering that polar flagellum flagellin from Azospirillum also behaves as a microbe-associated molecular pattern (MAMP) (Elías et al. 2021), it is very likely that flagellin from A. argentinense Az39 could be involved in the signaling processes resulting in root architecture modification, in addition to phytohormone-mediated mechanisms.
5 Conclusions
IAA-dependent and IAA-independent mechanisms seem to mediate root morphological changes in A. thaliana seedlings inoculated with A. argentinense Az39—a well-known PGPR strain frequently used in commercial inoculants in Argentina—, opening new horizons regarding selection criteria when screening Azospirillum strains intended to become biological products for agriculture. Transcriptome analyses may provide valuable clues to shed light on the complex signaling network resulting in root architecture changes under plant–microbe interactions. The physical presence of A. argentinense Az39 cells and the sensing of A. argentinense flagellin by roots may be key points in these less-known, IAA-independent routes of root growth promotion.
Data Availability
All RNA sequencing data have been deposited at the Gene Expression Omnibus (GEO): GSE192383. All other relevant data can be found within the manuscript and its supporting materials.
References
Adu MO, Asare PA, Yawson DO, Ackah FK, Amoah KK, Nyarko MA, Andoh DA (2017) Quantifying variations in rhizosheath and root system phenotypes of landraces and improved varieties of juvenile maize. Rhizosphere 3:29–39. https://doi.org/10.1016/j.rhisph.2016.12.004
Alexeyev MF (1999) The pKNOCK series of broad-host-range mobilizable suicide vectors for gene knockout and targeted DNA insertion into the chromosome of Gram-negative bacteria. Biotechniques 26:824–826. https://doi.org/10.2144/99265bm05
Asari S, Tarkowská D, Rolčík J, Novák O, Palmero DV, Bejai S, Meijer J (2017) Analysis of plant growth-promoting properties of Bacillus amyloliquefaciens UCMB5113 using Arabidopsis thaliana as host plant. Planta 245:15–30. https://doi.org/10.1007/s00425-016-2580-9
Cai XT, Xu P, Zhao PX et al (2014) Arabidopsis ERF109 mediates cross-talk between jasmonic acid and auxin biosynthesis during lateral root formation. Nat Commun 5(1):5833. https://doi.org/10.1038/ncomms6833
Carrillo-Flores E, Arreola-Rivera J, Pazos-Solís DM, Bocanegra-Mondragón M, Fierros-Romero G, Mellado-Rojas ME, Beltrán-Peña E (2022) TOR participation on the root system changes of Arabidopsis during its interaction with Azospirillum. J Appl Biotechnol Bioeng 9:18–23. https://doi.org/10.15406/jabb.2022.09.00280
Cassán F, Coniglio A, López G, Molina R, Nievas S, de Carlan CLN, Donadio F, Torres D, Rosas S, Pedrosa FO, de Souza E, Zorita MD, de-Bashan L, Mora V (2020) Everything you must know about Azospirillum and its impact on agriculture and beyond. Biol Fert Soils 56:461–479. https://doi.org/10.1007/s00374-020-01463-y
Chávez-Herrera E, Hernández-Esquivel AA, Castro-Mercado E, García-Pineda E (2018) Effect of Azospirillum brasilense Sp245 lipopolysaccharides on wheat plant development. J Plant Growth Reg 37:859–866. https://doi.org/10.1007/s00344-018-9782-2
Cheng Z, Sun L, Qi T et al (2011) The bHLH transcription factor MYC3 interacts with the jasmonate ZIM-domain proteins to mediate jasmonate response in Arabidopsis. Mol Plant 4(2):279–288. https://doi.org/10.1093/mp/ssq073
Chu TN, Bui LV, Hoang MTT (2020) Pseudomonas PS01 isolated from maize rhizosphere alters root system architecture and promotes plant growth. Microorganisms 8:471. https://doi.org/10.3390/microorganisms8040471
Coniglio A, Mora V, Puente M, Cassán F (2019) Azospirillum as Biofertilizer for Sustainable Agriculture: Azospirillum brasilense AZ39 as a Model of PGPR and Field Traceability. In: Zúñiga-Dávila, D., González-Andrés, F., Ormeño-Orrillo, E. (eds) Microbial Probiotics for Agricultural Systems. Sustainability in Plant and Crop Protection. Springer, Cham. https://doi.org/10.1007/978-3-030-17597-9_4
Contesto C, Milesi S, Mantelin S, Zancarini A, Desbrosses G, Varoquaux F, Bellini C, Kowalczyk M, Touraine B (2010) The auxin-signaling pathway is required for the lateral root response of Arabidopsis to the rhizobacterium Phyllobacterium brassicacearum. Planta 232:1455–1470. https://doi.org/10.1007/s00425-010-1264-0
Costacurta A, Keijers V, Vanderleyden J (1994) Molecular cloning and sequence analysis of an Azospirillum brasilense indole-3-pyruvate decarboxylase gene. Mol Gen Genet 243:463–472. https://doi.org/10.1007/BF00280477
Creus CM, Graziano M, Casanovas EM, Pereyra MA, Simontacchi M, Puntarulo S, Barassi CA, Lamattina L (2005) Nitric oxide is involved in the Azospirillum brasilense-induced lateral root formation in tomato. Planta 221:297–303. https://doi.org/10.1007/s00425-005-1523-7
Croes C, Moens S, Bastelaere E, Vanderleyden J, Michiels KW (1993) The polar flagellum mediates Azospirillum brasilense adsorption to wheat roots. J Gen Microbiol 139:2261–2269. https://doi.org/10.1099/00221287-139-9-2261
Deng K, Yu L, Zheng X, Zhang K, Wang W, Dong P, Zhang J, Ren M (2016) Target of rapamycin is a key player for auxin signaling transduction in Arabidopsis. Front Plant Sci 7:291. https://doi.org/10.3389/fpls.2016.00291
Desrut A, Moumen B, Thibault F, Le Hir R, Coutos-Thévenot P, Vriet C (2020) Beneficial rhizobacteria Pseudomonas simiae WCS417 induce major transcriptional changes in plant sugar transport. J Exp Bot 71:7301–7315. https://doi.org/10.1093/jxb/eraa396
Di Rienzo JA, Macchiavelli RE, Casanoves F (2012) Modelos lineales mixtos. Aplicaciones en InfoStat. Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. https://www.infostat.com.ar
dos Santos Ferreira N, Hayashi Sant’ Anna F, Massena Reis V, Ambrosini A, Gazolla Volpiano C, Rothballer M, Schwab S, Baura VA, Balsanelli E, Pedrosa FO, Pereira Passaglia LM, Maltempi de Souza E, Hartmann A, Cassán F, Zilli JE (2020) Genome-based reclassification of Azospirillum brasilense Sp245 as the type strain of Azospirillum baldaniorum sp. nov. Int J Syst Evol Microbiol 70:6203–6212. https://doi.org/10.1099/ijsem.0.004517
dos Santos Ferreira N, Coniglio A, Puente M, Hayashi Sant’ Anna F, Maroniche G, García J, Molina R, Nievas S, Gazolla Volpiano C, Ambrosini A, Pereira Passaglia LM, Pedraza RO, Massena Reis V, Zilli JE, Cassán F (2022) Genome-based reclassification of Azospirillum brasilense Az39 as the type strain of Azospirillum argentinense sp. nov. Int J Syst Evol Microbiol 72(10):1099. https://doi.org/10.1099/ijsem.0.005475
Dubrovsky JG, Puente ME, Bashan Y (1994) Arabidopsis thaliana as a model system for the study of the effect of inoculation by Azospirillum brasilense Sp245 on root hair growth. Soil Biol Biochem 26:1657–1664. https://doi.org/10.1016/0038-0717(94)90318-2
Elías JM, Ramírez-Mata A, Albornóz PL, Baca BE, Diaz-Ricci JC, Pedraza RO (2021) The polar flagellin of Azospirillum brasilense REC3 induces a defense response in strawberry plants against the fungus Macrophomina phaseolina. J Plant Growth Regul 41:2992–3008. https://doi.org/10.1007/s00344-021-10490-4
García-Gómez P, Bahaji A, Gámez-Arcas S, Muñoz FJ, Sánchez-López ÁM, Almagro G, Baroja-Fernández E, Ameztoy K, De Diego N, Ugena L, Spíchal L, Doležal K, Hajirezaei MR, Romero LC, García I, Pozueta-Romero J (2020) Volatiles from the fungal phytopathogen Penicillium aurantiogriseum modulate root metabolism and architecture through proteome resetting. Plant Cell Environ 43(10):2551–2570. https://doi.org/10.1111/pce.13817. Epub 2020 Aug 16
Gómez L, Felix G, Boller T (1999) A single locus determines sensitivity to bacterial flagellin in Arabidopsis thaliana. Plant J 18:277–284. https://doi-org.uml.idm.oclc.org/10.1046/j.1365-313X.1999.00451.x
Grover M, Bodhankar S, Sharma A, Sharma P, Singh J, Nain L (2021) PGPR mediated alterations in root traits: way toward sustainable crop production. Front Sust Food Syst 4:618230. https://doi.org/10.3389/fsufs.2020.618230
Gullett J, O’Neal L, Mukherjee T, Alexandre G (2017) Azospirillum brasilense: laboratory maintenance and genetic manipulation. Curr Protoc Microbiol 47:3E.2.1-3E.2.17. https://doi.org/10.1002/cpmc.39
Hernández-Esquivel AA, Castro-Mercado E, García-Pineda E (2020) Comparative effects of Azospirillum brasilense Sp245 and Pseudomonas aeruginosa PAO1 lipopolysaccharides on wheat seedling growth and peroxidase activity. J Plant Growth Regul 40:1903–1911. https://doi.org/10.1007/s00344-020-10241-x
Jiménez-Vázquez KR, García-Cárdenas E, Barrera-Ortiz S, Ortiz-Castro R, Ruiz-Herrera LF, Ramos-Acosta BP, Coria-Arellano JL, Sáenz-Mata J, López-Bucio J (2020) The plant beneficial rhizobacterium Achromobacter sp. 5B1 influences root development through auxin signaling and redistribution. Plant J 103:1639–1654. https://doi.org/10.1111/tpj.14853
Karimi N, Goltapeh EM, Amini J, Mehnaz S (2021) Effect of Azospirillum zeae and seed priming with zinc, manganese and auxin on growth and yield parameters of wheat, under dryland farming. Agric Res 10:44–55. https://doi.org/10.1007/s40003-020-00480-5
Khan A, Hossain MT, Park HC, Yun DJ, Shim SH, Chung YR (2016) Development of root system architecture of Arabidopsis thaliana in response to colonization by Martelella endophytica YC6887 depends on auxin signaling. Plant Soil 405:81–96. https://doi.org/10.1007/s11104-015-2775-z
Koul V, Tripathi C, Adholeya A, Kochar M (2015) Nitric oxide metabolism and indole acetic acid biosynthesis cross-talk in Azospirillum brasilense SM. Res Microbiol 166:174–185. https://doi.org/10.1016/j.resmic.2015.02.003
Kunkel BN, Brooks DM (2002) Cross talk between signaling pathways in pathogen defense. Curr Opin in Plant Biol 5:325–331. https://doi.org/10.1016/S1369-5266(02)00275-3
Li Y, Shao J, Xie Y, Jia L, Fu Y, Xu Z, Zhang N, Feng H, Xun W, Liu Y, Shen Q, Xuan W, Zhang R (2021) Volatile compounds from beneficial rhizobacteria Bacillus spp. promote periodic lateral root development in Arabidopsis. Plant Cell Environ 44:1663–1678. https://doi.org/10.1111/pce.14021
van Loon, LC, Bakker P A, van der Heijdt WH, Wendehenne D, Pugin A (2008) Early responses of tobacco suspension cells to rhizobacterial elicitors of induced systemic resistance. Mol Plant Microbe Interact 21:1609–1621. https://doi-org.uml.idm.oclc.org/10.1094/MPMI-21-12-1609
López-Bucio J, Campos-Cuevas JC, Hernández-Calderón E, Velásquez-Becerra C, Farías-Rodríguez R, Macías-Rodríguez LI, Valencia-Cantero E (2007) Bacillus megaterium rhizobacteria promote growth and alter root-system architecture through an auxin- and ethylene-independent signaling mechanism in Arabidopsis thaliana. Mol Plant-Microbe Interact 20:207–217. https://doi.org/10.1094/MPMI-20-2-0207
Malhotra M, Srivastava S (2008) An ipdC gene knock-out of Azospirillum brasilense strain SM and its implications on indole-3-acetic acid biosynthesis and plant growth promotion. Antonie Van Leeuwenhoek 93:425–433. https://doi.org/10.1007/s10482-007-9207-x
Matsuda F, Miyazawa H, Wakasa K, Miyagawa H (2005) Quantification of indole-3-acetic acid and amino acid conjugates in rice by liquid chromatography-electrospray ionization-tandem mass spectrometry. Biosci Biotechnol Biochem 69:778–783. https://doi.org/10.1271/bbb.69.778
Meldau DG, Meldau S, Hoang LH, Underberg S, Wünsche H, Baldwin IT (2013) Dimethyl disulfide produced by the naturally associated bacterium Bacillus sp. B55 promotes Nicotiana attenuata growth by enhancing sulfur nutrition. Plant Cell 25:2731–2747. https://doi.org/10.1105/tpc.113.114744
Méndez-Gómez M, Castro-Mercado E, Peña-Uribe CA, Reyes-de la Cruz H, López-Bucio J, García-Pineda E (2020) Target of rapamycin signaling plays a role in Arabidopsis growth promotion by Azospirillum brasilense Sp245. Plant Sci 293:110416. https://doi.org/10.1016/j.plantsci.2020.110416
Millet YA, Danna CH, Clay NK, Songnuan W, Simon MD, Werck-Reichhart D, Ausubel, FM (2010) Innate immune responses activated in Arabidopsis roots by microbe-associated molecular patterns. The Plant Cell 22:973–990. https://doi-org.uml.idm.oclc.org/10.1105/tpc.109.069658
Moens S, Michiels K, Vanderleyden J (1995) Glycosylation of the flagellin of the polar flagellum of Azospirillum brasilense, a gram-negative nitrogen-fixing bacterium. Microbiology 141:2651–2657. https://doi.org/10.1099/13500872-141-10-2651
Molina R, Obando D, Torres D, Rivera D, Cassán F (2014) Utilización de un medio de cultivo para la cuantificación y la diferenciación de bacterias presentes en la misma formulación. In: Presentación oral. XV Jornadas Argentinas de Microbiología., Córdoba, Argentina, p. 249
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 13:473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Ortíz-Castro R, Díaz-Pérez C, Martínez-Trujillo M, Del Río RE, Campos-García J, López-Bucio J (2011) Transkingdom signaling based on bacterial cyclodipeptides with auxin activity in plants. PNAS 108:7253–7258. https://doi.org/10.1073/pnas.1006740108
Pečenková T, Janda M, Ortmannová J, Hajná V, Stehlíková Z, Žárský V (2017) Early Arabidopsis root hair growth stimulation by pathogenic strains of Pseudomonas syringae. Ann Bot 120:437–446. https://doi.org/10.1093/aob/mcx073
Pound MP, French AP, Atkinson JA, Wells DM, Bennett MJ, Pridmore T (2003) RootNav: navigating images of complex root architectures. Plant Physiol 162(4):1802–14. https://doi.org/10.1104/pp.113.221531. Epub 2013 Jun 13
Prinsen E, Costacurta A, Michiels K, Vanderleyden J, Van Onckelen H (1993) Azospirillum brasilense indole-3-acetic acid biosynthesis: evidence for a non-tryptophan dependent pathway. Mol Plant Microbe Interact 6:609–615. https://doi.org/10.1094/MPMI-6-609
Puente ML, Gualpa JL, Lopez GA, Molina RM, Carletti SM, Cassán FD (2018) The benefits of foliar inoculation with Azospirillum brasilense in soybean are explained by an auxin signaling model. Symbiosis 76:41–49. https://doi.org/10.1007/s13199-017-0536-x
Ribaudo CM, Krumpholz EM, Cassán FD, Bottini R, Cantore ML, Curá JA (2006) Azospirillum sp. promotes root hair development in tomato plants through a mechanism that involves ethylene. J Plant Growth Regul 25:175–185. https://doi.org/10.1007/s00344-005-0128-5
Robertson SM, Sakariyahu SK, Bolaji A, Belmonte MF, Wilkins O (2022) Growth-limiting drought stress induces time-of-day-dependent transcriptome and physiological responses in hybrid poplar. AoB Plants 14:plac040. https://doi.org/10.1093/aobpla/plac040
Rondina ABL, dos Santos Sanzovo AW, Guimarães GS, Wendling JR, Nogueira MA, Mariangela Hungria M (2020) Changes in root morphological traits in soybean co-inoculated with Bradyrhizobium spp. and Azospirillum brasilense or treated with A. brasilense exudates. Biol Fertil Soils 56:537–549. https://doi.org/10.1007/s00374-020-01453-0
Ruegger M, Dewey E, Gray WM, Hobbie L, Turner J, Estelle M (1998) The TIR1 protein of Arabidopsis functions in auxin response and is related to human SKP2 and yeast Grr1p. Genes Dev 12:198–207. https://doi.org/10.1101/gad.12.2.198
Růžička K, Ljung K, Vanneste S et al (2007) Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell 19:2197–2212. https://doi.org/10.1105/tpc.107.052126
Ryu CM, Farag MA, Hu CH, Reddy MS, Kloepper JW, Paré PW (2004) Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol 134:1017–1026. https://doi.org/10.1104/pp.103.026583
Shi CL, Park HB, Lee JS, Ryu S, Ryu CM (2010) Inhibition of primary roots and stimulation of lateral root development in Arabidopsis thaliana by the rhizobacterium Serratia marcescens 90–166 is through both auxin-dependent and -independent signaling pathways. Mol Cells 29:251–258. https://doi.org/10.1007/s10059-010-0032-0
Shirokov A, Budanova A, Burygin G, Evseeva N, Matora L, Shchyogolev S (2020) Flagellin of polar flagellum from Azospirillum brasilense Sp245: isolation, structure, and biological activity. Int J Biol Macromol 147:1221–1227. https://doi.org/10.1016/j.ijbiomac.2019.10.092
Spaepen S, Bossuyt S, Engelen K, Marchal K, Vanderleyden J (2014) Phenotypical and molecular responses of Arabidopsis thaliana roots as a result of inoculation with the auxin-producing bacterium Azospirillum brasilense. New Phytol 201:850–861. https://doi.org/10.1111/nph.12590
Steenhoudt O, Vanderleyden J (2000) Azospirillum, a free-living nitrogen-fixing bacterium closely associated with grasses: genetic, biochemical and ecological aspects. FEMS Microbiol Rev 24:487–506. https://doi.org/10.1016/S0168-6445(00)00036-X
Stringlis IA, Proietti S, Hickman R, Van Verk MC, Zamioudis C, Pieterse CMJ (2018) Root transcriptional dynamics induced by beneficial rhizobacteria and microbial immune elicitors reveal signatures of adaptation to mutualists. Plant J 93:166–180. https://doi.org/10.1111/tpj.13741
Tahir HAS, Gu Q, Wu H, Raza W, Hanif A, Wu L, Colman MV, Gao X (2017) Plant growth promotion by volatile organic compounds produced by Bacillus subtilis SYST2. Front Microbiol 8:171. https://doi.org/10.3389/fmicb.2017.00171
Torres D, Benavidez I, Donadio F, Mongiardini E, Rosas S, Spaepen S, Vanderleyden J, Pěnčík A, Novák O, Strnad M, Frébortová J, Cassán F (2018) New insights into auxin metabolism in Bradyrhizobium japonicum. Res Microbiol 169:313–323. https://doi.org/10.1016/j.resmic.2018.04.002
Tsuda K, Sato M, Stoddard T, Glazebrook J, Katagiri F (2009) Network properties of robust immunity in plants. PLoS Genet 5:e1000772. https://doi.org/10.1371/journal.pgen.1000772
Vanstockem M, Michiels K, Vanderleyden J, Van Gool AP (1987) Transposon mutagenesis of Azospirillum brasilense and Azospirillum lipoferum: physical analysis of Tn5 and Tn5-Mob insertion mutants. Appl Environ Microbiol 53:410–415. https://doi.org/10.1128/aem.53.2.410-415.1987
Viruega-Góngora VI, Acatitla-Jácome IS, Reyes-Carmona SR, Baca BE, Ramírez-Mata A (2020) Spatio-temporal formation of biofilms and extracellular matrix analysis in Azospirillum brasilense. FEMS Microbiol Lett 367:fnaa037. https://doi.org/10.1093/femsle/fnaa037
Xun Q, Wu Y, Li H et al (2020) Two receptor-like protein kinases, MUSTACHES and MUSTACHES-LIKE, regulate lateral root development in Arabidopsis thaliana. New Phytol 227:1157–1173. https://doi.org/10.1111/nph.16599
Zamioudis C, Pieterse CM (2012) Modulation of host immunity by beneficial microbes. Mol Plant-Microbe Interact 25:139–150. https://doi-org.uml.idm.oclc.org/10.1094/MPMI-06-11-0179
Zhang H, Kim MS, Krishnamachari V, Payton P, Sun Y, Grimson M, Farag MA, Ryu CM, Allen R, Melo IS, Paré PW (2007) Rhizobacterial volatile emissions regulate auxin homeostasis and cell expansion in Arabidopsis. Planta 226:839–851. https://doi.org/10.1007/s00425-007-0530-2
Zhao CZ, Huang J, Gyaneshwar P, Zhao D (2018) Rhizobium sp. IRBG74 alters Arabidopsis root development by affecting auxin signaling. Front Microbiol 8:2556. https://doi.org/10.3389/fmicb.2017.02556
Zipfel C, Robatzek S, Navarro L, Oakeley E, Jones J, Feliz G, Boller T (2004) Bacterial disease resistance in Arabidopsis through flagellin perception. Nature 428:764–767. https://doi.org/10.1038/nature02485
Acknowledgements
We thank Instituto de Investigaciones Agrobiotecnológicas-Consejo Nacional de Investigaciones Científicas y Tecnológicas (INIAB-CONICET) and Universidad Nacional de Río Cuarto (UNRC). We thank Dr. Federico Ariel from Instituto de Agrobiotecnología de Santa Fe (IAL-CONICET) for Col-0 seeds and Prof. Mark Estelle (Department of Cell and Developmental Biology, UC, San Diego, USA) for tir1.1 seeds. We also sincerely thank Dra. Myriam Zawoznik for her valuable comments and suggestions.
Funding
This work was supported by grants from ANPCyT (PICT-2017–0572 to CF; PICT-2018–2271 to MV, PICT-2019–2199 to PR and PICT-2019–0015 to EJM). The work was funded by the project “Plants as a tool for sustainable global development” (CZ.02.1.01/0.0/0.0/16_019/0000827) within the program Research, Development, and Education (OP RDE). This work was supported by ANID–Programa Iniciativa Científica Milenio ICN17_022, NCN2021_010 and Fondo Nacional de Desarrollo Científico y Tecnológico (1200010) to EJM.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mora, V., López, G., Molina, R. et al. Azospirillum argentinense Modifies Arabidopsis Root Architecture Through Auxin-dependent Pathway and Flagellin. J Soil Sci Plant Nutr 23, 4543–4557 (2023). https://doi.org/10.1007/s42729-023-01371-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-023-01371-8