Abstract
Plant growth promoting rhizobacteria influence host functional and adaptive traits via complex mechanisms that are just started to be clarified. Azospirillum brasilense acts as a probiotic bacterium, but detailed information about its molecular mechanisms of phytostimulation is scarce. Three interaction systems were established to analyze the impact of A. brasilense Sp245 on the phenotype of Arabidopsis seedlings, and underlying molecular responses were assessed under the following growth conditions: (1) direct contact of roots with the bacterium, (2) chemical communication via diffusible compounds produced by the bacterium, (3) signaling via volatiles. A. brasilense Sp245 improved shoot and root biomass and lateral root production in the three interaction systems assayed. Cell division, quiescent center, and differentiation protein reporters pCYCB1;1::GUS, WOX5::GFP, and pAtEXP7::GUS had a variable expression in roots depending of the nature of interaction. pCYCB1;1::GUS and WOX5::GFP increased with volatile compounds, whereas pAtEXP7::GUS expression was enhanced towards the root tip in plants with direct contact with the bacterium. The auxin reporter DR5::GUS was highly expressed with diffusible and volatile compounds, and accordingly, auxin signaling mutants pin3, slr1, arf7arf19, and tir1afb2afb3 showed differential phytostimulant responses when compared with the wild type. By contrast, ethylene signaling was not determinant to mediate root changes in response to the different interactions, as observed using the ethylene-related mutants etr1, ein2, and ein3. Our data highlight the diverse effects by which A. brasilense Sp245 improves plant growth and root architectural traits and define a critical role of auxin but not ethylene in mediating root response to bacterization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The plant microbiome supports growth, development, and adaptation, and many bacterial species are integral to host functioning via production of phytohormones, nitrogen fixation, solubilization of phosphate, and induction of defense (Lugtenberg and Kamilova 2009; Vacheron et al. 2013). A very few bacterial species that promote plant growth have been characterized in detail, some release auxin and other phytohormones, diffusible compounds, or produce volatile organic compounds (VOCs), which target basic physiological programs such as photosynthesis or cell division and/or elongation (Ryu et al. 2003, 2004; Kanchiswamy et al. 2015). In particular, the molecular mechanisms underlying the effects of bacterial diffusible compounds and VOCs in plants are largely unknown, and the signaling networks orchestrating these responses are not easy to dissect in crops or horticultural species. This makes model plants such as Arabidopsis very valuable in order to decipher the plant-rhizobacteria molecular dialogue (Ryu et al. 2003; Zhang et al. 2007; Pérez-Flores et al. 2017; Tahir et al. 2017). The availability of Arabidopsis mutants and transgenic lines altered in proteins with key roles in different signaling pathways and in different morphological processes permits to explore the molecular biology and biochemistry of the plant responses to rhizobacteria. Thus, the auxin mutants tir1afb2afb3, pin3, slr1, arf7arf9, impaired in auxin perception, efflux, sensitivity, and transcription, respectively, have been found to be affected in root growth and/or lateral root formation, which are critical traits in rhizosphere biology (Dharmasiri et al. 2005; Blilou et al. 2005; Fukaki et al. 2002; Okushima et al. 2007). As such, the transgenic line DR5::GUS, which harbors a synthetic auxin-responsive promoter fused to the GUS encoding sequence, permits detailed evaluation of auxin-inducible and tissue-specific responsiveness to rhizobacterial metabolites, which can be linked to cellular programs through the cell cycle, quiescent center, and cell differentiation reporter genes CYCB1;1::GUS, WOX5::GFP and AtEXP7::GUS, respectively.
The genus Azospirillum has been typified as a plant growth promoting rhizobacterium (PGPR), which colonizes roots and enhances biomass production in several plant species (Cassan and Diaz-Zorita 2016). The role of auxins and other phytoregulators produced by Azospirillum in improving plant growth has been demonstrated (Cassan et al. 2014; Strzelczyk et al. 1994; Bottini et al. 1989, 2004; Cohen et al. 2008), but whether VOCs, ethylene, quorum-sensing signals, and/or secondary metabolites emitted by this bacterium could also modulate plant growth remains unknown.
Morphological changes induced by Azospirillum include increased lateral root growth, root hair formation, as well as a reduction in the main root length. These changes have been mostly related to the production of auxins (Dobbelaere et al. 1999; Spaepen et al. 2008, 2014). Noteworthy, the expression of ethylene-responsive genes (ETR2, ERF11, ERF15, ERF59 and EBF2) as induced by Azospirillum brasilense Sp245 has been reported, indicating that the signaling coordinated by auxin, ethylene, or both may orchestrate the plant morphological reconfiguration during the interaction (Spaepen et al. 2014). However, developmental, genetic, and molecular evidences to answer this question remain to be gathered.
The configuration of the root system is controlled by cell division, elongation, and differentiation (Petricka et al. 2012). Meristem activity is largely controlled by a group of initial cells and the quiescent center (QC), which instructs the undifferentiated status (Aichinger et al. 2012). Studies on plant growth and root morphology promoted by A. brasilense have been performed under conditions in which the bacteria directly colonize the plant root. However, the contribution of diffusible or volatile bacterial compounds to plant growth and changes in root morphology has not been clearly determined. The aim of this research was to analyze in what way different interaction systems such as Arabidopsis/Azospirillum interaction systems involving either direct contact, communication via diffusible compounds, or bacterial volatile emissions influence molecular and morphological changes in roots and concomitantly improve growth, as well as the role of auxin and ethylene signaling in these responses.
Materials and methods
Arabidopsis growth and bacterial cultivation
The plant materials used in this study are listed in Table 1. The wild-type ecotype of Arabidopsis thaliana was Col-0. Seeds were disinfected with 95% (vol/vol) ethanol for 5 min and 20% (vol/vol) bleach (Cloralex) for 5 min, followed by five washes with sterile distilled water. The seeds, stored at 4 °C for 2 days prior to sowing, were germinated and grown on agar plates containing 0.2× Murashige and Skoog (MS) medium, pH 7 (Murashige and Skoog 1962) with 0.9 g/L MS salt, 6 g/L sucrose, and 10 g/L Agar Plant TC (PhytoTechnology Laboratories, St, Lenexa, KS, USA). Plants were placed vertically in a plant growth chamber (Percival Scientific AR-95L) at 22 °C with a photoperiod of 16 h light (100 μmol/m/s) and 8 h darkness.
Wild-type Azospirillum brasilense Sp245 and the Azospirillum brasilense Sp245 carrying the pJBA21Tc vector which constitutively expresses the gusA gene encoding for the β-glucuronidase activity and used to monitor wheat root interactions in Azospirillum were kindly donated by Dr. Gladys Alexandre (University of Tennessee, USA). Bacteria were grown in LB medium, pH 7 (10 g/L peptone, 5 g/L yeast extract, 5 g/L NaCl, supplemented with 0.30 g/L MgSO4 and 0.277 g/L CaCl2; for solidified medium, 15 g/L agar was added). To prepare the inoculum, the bacteria were treated according to Spaepen et al. (2014). Cultures were grown 20 h (exponential phase) at 28 °C with rotation at 140 rpm. The cultures were then washed twice in 0.9% NaCl by centrifugation (4300×g, 10 min, 4 °C), resuspended in 0.01 M MgSO4, and adjusted by sequential dilutions to a desired final concentration of colony-forming units (CFU) for use as inoculum.
Seedling treatments
Four days after germination, seedlings were aseptically transplanted into Petri dishes containing 0.2× MS medium, pH 7. Different bacterial concentrations (103, 104, 105, and 106 CFU/mL) were analyzed to establish a dose-response effect on plant growth.
The different interactions were performed as follows: for direct contact assay, bacteria were mixed in plant medium (~ 37 °C) and allowed to solidify in Petri dishes; seedlings were immediately transplanted. For diffusible compounds produced by the bacteria, 10 μL of the bacteria, autoclaved bacteria (121 °C at a pressure of 15 psi, 20 min), Escherichia coli XL1 (1.0 OD600), or two different IAA (indole acetic acid) concentrations (0.5 and 1 μM) were placed in drops in 0.5-cm sterilized filter paper discs, 5 cm from the plant root. For volatile compounds treatment, similar experiment to diffusible compounds was performed, but without IAA and in this case using divided Petri dishes.
Root colonization was analyzed to direct contact and diffusible compounds with A. brasilense expressing the gusA gen and by quantifying the CFU per root. Plant growth parameters primary root length, lateral root number and density, shoot and fresh weight, and root hair number and length were analyzed after treatments. To test for diffusibility of molecules in the experimental design, we first tested the effect of IAA on the expression of the DR5::GUS reporter in root meristems.
For all A. brasilense treatments, a bacterial concentration of 103 CFU/mL was used. Petri dishes were sealed with a plastic wrap and incubated vertically into a plant growth chamber.
Analysis of plant growth
For all experiments, plant growth parameters were analyzed 6 days after transplant. The fresh weight per plant was determined with an analytical balance (Ohaus V14130 Voyager). Primary root growth was measured using a ruler. The number of lateral roots was determined using a stereoscopic microscope (Leica MZ6) to count the lateral roots present in the primary root from the tip to the root/stem transition zone. Lateral root density (LRD) was obtained by dividing the number of lateral roots by the length of the primary root and is expressed as LR number per centimeter. Emerged lateral roots was monitored with an Eclipse E200LED MV R microscope (Nikon Corp., Tokyo, Japan) each day post-inoculation in WT seedlings clarified according to Malamy and Benfey (1997). Root hairs were analyzed using a stereoscopic microscope, and the number of root hairs within 1 mm of the root was counted starting from the first radical hair above the root tip. To analyze the primary root meristem, the root was clarified (Malamy and Benfey 1997) and analyzed using a Leica DFC450C microscope (Nomarski Optics). The meristem length was defined as the distance between the quiescent center (QC) and the first cell of the cortex that was elongated. Digital images obtained from the roots were analyzed for meristem length and root hair formation with ImageJ software (https://imagej.nih.gov/ij/).
Cell division was analyzed with the cell cycle marker CYCB1;1::GUS, cell differentiation with the expansin marker AtEXP7::GUS, and meristem organization with the transcription factor WOX5::GFP. Responses to auxin were evaluated using DR5::GUS transgenic seedlings. For positive control in DR5::GUS experiments, seedlings were incubated during 10 h into liquid MS medium with 0.05 μM of indole-3-acetic acid (IAA).
Histochemical and fluorescence analysis
Transgenic lines expressing the uidA reporter gene (GUS: β-glucuronidase) were stained with 0.1% X-Gluc (5-bromo-4-chloro-3-indolyl β-d-glucuronide) dissolved in phosphate buffer (NaH2PO4 and Na2HPO4, 0.1 M, pH 7), 2 mM potassium ferrocyanide, and 2 mM potassium ferricyanide overnight at 37 °C, except for pAtEXP7::GUS, which was stained for 20 min. Plants were cleared and fixed as previously described (Malamy and Benfey 1997). The processed plants were mounted on glass slips and sealed with commercial nail varnish. Subsequently, the slides were observed and photographed using a Leica DFC450C microscope. To determine the fluorescence in the transgenic line WOX5::GFP, plants were treated with a 10-mg/mL propidium iodide solution to visualize the cell contour and subsequently analyzed using an Olympus FV1000 confocal microscope (Olympus Corp., Tokyo, Japan). Propidium iodide fluorescence was visualized using an argon laser at 568 nm for excitation and an emission window of 585–610 nm, whereas GFP fluorescence was visualized with excitation and emission wavelengths of 488 nm and 505–550 nm, respectively. All reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise noted.
Statistical analysis
All experiments were repeated at least three times. The data were analyzed using the STATISTICA program version 8.0.550. Univariate and multivariate analyses followed by a post hoc test (Tukey’s HSD) were used to examine differences between experimental groups. Different letters indicate significant differences (P < 0.05).
Results
Effect of A. brasilense on A. thaliana growth
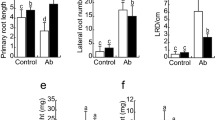
The effect of A. brasilense on the growth of A. thaliana was first analyzed by transferring 4-day-old A. thaliana seedlings to agar solidified MS medium with different densities of bacteria (103, 104, 105, and 106 CFU/mL), and plant growth was determined 6 days later. The rhizobacterium decreased primary root length according to the concentration (Fig. 1a). A conspicuous increase in lateral root number, root fresh weight, and shoot fresh weight at all rhizobacterial inoculum density was observed (Fig. 1b–d). On the basis of these results, the lower concentration of bacteria affecting plant growth (103 CFU/mL; Fig. 1e) was used for all subsequent experiments.
Effect of A. brasilense Sp245 on A. thaliana growth. Seedlings were transplanted to Petri dishes with different bacterial concentrations, and after 6 days, growth parameters were measured. a–d Primary root length, lateral root number, root fresh weight, and shoot fresh weight, respectively. e Representative images of seedlings grown in presence of two different concentrations of the rhizobacterium. n = 30. CFU = colony forming units. The letters on the bars indicate statistically significant differences according to Tukey’s HSD test, P < 0.05
Three Azospirillum/Arabidopsis interaction systems were established as follows: (1) direct contact of roots with the bacterium, (2) chemical communication via diffusible compounds produced by the bacterium, and (3) signaling via volatiles. These interactions were probed to associate each system with their effects on morphological changes and molecular responses in the root of the Arabidopsis seedlings. In the direct contact system, an assay of root colonization was performed and compared with the diffusible compound system. The results showed that root colonization was observed in direct contact with the rhizobacterium and not in the diffusible compound system (Supplementary Fig. 1a, b).
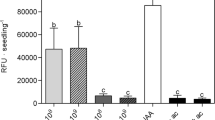
In the diffusible compound system, the effects on root morphology and plant growth of A. brasilense were compared with the autoclaved rhizobacterium and the non-rhizobacterium E. coli XL1 (Supplementary Fig. 2a). Changes in plant growth parameters and root architecture promoted by A. brasilense were abolished by the autoclaved rhizobacterium and slightly stimulated by E. coli (Supplementary Fig. 2b–f). A detailed analysis of root morphology showed that root hair formation was not stimulated by the autoclaved rhizobacterium (Supplementary Fig. 2g–i). The effect of diffusible molecules on plant growth changes was first confirmed by using IAA. This phytohormone stimulated changes in the root morphology (Supplementary Fig. 2j–l) and in the expression of DR5::GUS marker in root meristem (Supplementary Fig. 2m, n).
In the case of volatile compounds production, a similar experiment to diffusible compounds was performed, but using divided Petri dishes. In these plants, volatiles of A. brasilense increased primary root length, lateral root number and density, and shoot and fresh weight. These effects were not observed with autoclaved rhizobacterium or with E. coli (Supplementary Fig. 3a–f). Root hair number and length were stimulated at lower levels than with diffusible compounds (Supplementary Fig. 3g–i).
Overall, the results confirm that the changes promoted by the different interactions established between Azospirillum and Arabidopsis are due to diffusible and volatile bioactive molecules produced by the rhizobacterium or by the combination of these molecules acting during the direct contact.
Effect of different Arabidopsis-Azospirillum interactions on plant biomass and root architecture
Differential effects of A. brasilense on plant growth were observed for the three different interactions with Arabidopsis (Fig. 2a). In all three conditions, A. brasilense increased shoot and root fresh weight, but a higher effect was observed with volatile compounds (Fig. 2b, c), in relation to untreated control. An increase in the primary root length was only appreciated with volatile compounds (Fig. 2d). Although all interactions increased lateral root number and density, a higher effect was seen in direct contact, in contrast with the other two interactions (Fig. 2e, f). A time course analysis of emerged lateral roots indicated that its promotion started by the third day in all interactions and progressively increased until the end of the experiment (Fig. 2g), indicating a similar stimulation in the lateral root formation in the three systems.
Effects of A. brasilense Sp245 on the growth of A. thaliana seedlings under direct contact, diffusible, and volatile compounds production. a Representative images of plants 6 days after transplant in the different experimental conditions. Bar = 1 cm. b–f Shoot fresh weight, root fresh weight, primary root length, lateral root number, and lateral root density (LRD), respectively. g Kinetics of lateral root formation. The bacterial concentration used was 103 CFU per milliliter. n = 30. The letters on the bars and in panel g indicate statistically significant differences according to Tukey’s HSD test, P < 0.05
Analyzing the effect of the interactions specifically on the meristem root, seedlings exposure to the volatiles increased ~ 40% meristem length when compared with controls, whereas roots in direct contact or in the presence of diffusible compounds manifested roughly ~ 35 and ~ 25% reduction in meristem size, respectively (Fig. 3a, b). In addition, the number of root hairs and length was variable, with direct contact and diffusible compounds showing higher increases in these traits, compared with volatile compounds. In addition, the formation of root hairs occurred closer to the root tip in the direct contact interaction, suggesting that the rhizobacteria stimulated cell differentiation near the meristem (Fig. 3c–e).
Effect of A. brasilense Sp245 on the primary root morphology under different experimental conditions. a Representative images of the root meristem of seedlings 6 days after transplant. The dotted lines indicate the root meristem zone. Bar = 100 μm. b Meristem length, considered as the distance between the quiescent center and the first elongated cell from the cortex, where the cell elongation zone begins. c Representative images of root hairs. Bar in a = 1 mm. d Root hair number. The number of root hairs was determined in 1 mm segment of the root, starting with the first root hair formed. e Box plot of root hair length. The bacterial concentration used was 103 CFU per milliliter. n = 30 roots and 300 root hairs. The letters on the bars indicate statistically significant differences according to Tukey’s HSD test, P < 0.05
These data show that although A. brasilense behaves as a plant growth promoting bacterium, morphological changes and biomass production depend of the interaction type established with the plant.
Effect of A. brasilense on meristem activity
To further study the stimulated changes in meristem root, a molecular approach was performed focused on cell division, quiescent center identity, and cell differentiation in the primary root meristem. Using A. thaliana plants expressing cyclin-GUS, we analyzed patterns of mitotic activity in response to the different interactions. Interestingly, 6 days after treatment, the area of reporter expression was reduced in plants in direct contact and with diffusible compounds, whereas with volatile compounds, it exhibited increased expression and intensity, relative to control (Fig. 4a, b). These results show that the rhizobacteria may affect differentially cell division in the meristem depending upon the mechanism of interaction. Using WOX5::GFP transgenic plants to visualize QC structure, low levels of WOX5 expression were observed in direct contact, whereas with diffusible and volatile compounds, transgenic plants showed an increase in the fluorescence intensity (Fig. 4c, d). In relation to cell differentiation, in direct contact with rhizobacteria or in presence of diffusible compounds, roots showed a sharp increase in expansin-GUS expression and closer to the root tip. These increases were higher than that seen for the volatile compounds (Fig. 4e, f). The results show that the rhizobacteria can stimulate plant tissue differentiation in several ways and that the observed changes in root morphology result in the different molecular responses which depend on the kind of interaction.
Effect of A. brasilense Sp245 on cell division, quiescent center, and differentiation in Arabidopsis primary roots. Expression and relative quantification of the cell division marker CYCB1;1::GUS (a, b), quiescent center marker WOX5::GFP (c, d), and cell differentiation marker EXP7::GUS (e, f). n = 30. The bacterial concentration used was 103 CFU per milliliter, and the expression of the markers was analyzed 6 days after transplant. Bars in a, b, c = 100, 50, 200 μm, respectively. The experiments were performed three times with similar results
Role of auxin and ethylene signaling in the phytostimulation by A. brasilense
We further analyzed the role of some phytoregulators potentially involved in the plant-bacterium interaction, namely auxin and ethylene. First, the auxin-signaling pathway was analyzed through the expression of the auxin-inducible marker DR5::GUS in A. thaliana seedlings, 6 days after different interactions (Supplementary Fig. 4). Similar DR5::GUS expression to control was observed in the shoot, independently of the interaction type. However, in direct contact, the expression was strongly increased along the root and in lateral roots, whereas with diffusible and volatile compounds, the expression was localized only to the lateral roots. DR5::GUS expression was higher in root meristems in diffusible and volatile compounds than in direct contact. The exogenous addition of 0.05 μM IAA strongly increased β-glucuronidase expression in shoot and in root tissues.
Subsequent analysis of auxin signaling in plants was performed using the wild-type A. thaliana and tir1afb2afb3, pin3, slr1, and arf7arf19 mutants. Null or decreased root hair formation was seen for all mutants regardless of the kind of interaction established, suggesting that the sensing and signaling of this phytohormone is required for promotion of root hair by the rhizobacterium and its metabolites (Fig. 5a) (Supplementary Table 1).
Effect of A. brasilense Sp245 on root morphology in Arabidopsis mutant plants carrying mutations in genes of the auxin and ethylene signaling pathways. a Representative images of mutants impaired in sensing and signaling of auxins. b Representative images of mutants impaired in sensing and signaling of ethylene. The plants were analyzed 6 days after transplant (see description of the mutants in Table 1). The experiments were performed three times with similar results
Likewise, the role of ethylene in the three different interactions with A. brasilense was evaluated in etr1-1, ein2-1, and ein3-1 Arabidopsis mutants. All mutants showed similar morphology in the primary root to that observed to the wild type, regardless of interaction system (Fig. 5b) (Supplementary Table 2). These data suggest that ethylene signaling has a minor role in modulating root hair development in Arabidopsis during the different interactions with Azospirillum.
Discussion
Most previous analyses of plant growth promotion by A. brasilense have been performed under conditions that involve direct contact between the rhizobacteria and plant roots (Dobbelaere et al. 1999; Spaepen et al. 2008, 2014). Thus, the effect of diffusible and volatile compounds produced by A. brasilense Sp245 on plant growth remained unclear. Here we observed that this rhizobacterium could promote plant growth in three different co-cultivation systems in which direct contact and/or contact with diffusible or volatile compounds are established. Interestingly, the different interaction types had varying effects on plant growth, such that plants exposed to volatile compounds produced by rhizobacteria had a larger increase in biomass and root length, relative to the other two interaction types and axenic seedlings. The phenotypic differences in the three conditions indicate that the rhizobacterium can emit various types of molecules that differentially regulate plant growth, perhaps changing the endogenous levels of phytohormones.
Although sucrose, a sugar that plays a role as a signaling molecule that regulates a variety of genes affecting development in higher plants (Ohto et al. 2001), is a constituent of the medium where the different in vitro interactions such as plant-rhizobacterium were established, the changes in the morphology and growth in Arabidopsis resulted in the interaction with the rhizobacterium because they were not observed in control plants. However, in the addition of sucrose in in vitro plant-rhizobacterium interaction, studies must avoid excluding its known effects on plant development.
The phenotype observed in direct contact was similar to that previously reported in wheat and Arabidopsis plants inoculated with A. brasilense and with the exogenous addition of IAA (Dobbelaere et al. 1999; Dubrovsky et al. 1994; Spaepen et al. 2008, 2014). It has been reported that auxins and other molecules produced by Azospirillum such as cytokinins, gibberellins, acetic acid, and nitric oxide play an important role during this type of interaction (Bashan and de-Bashan 2010). A. brasilense also stimulates biochemical and physiological changes in Arabidopsis such as the increase in chlorophyll, carotenoid, anthocyanin, and abscisic acid (ABA) productions, as well as a retarded water loss. Overall, these changes improved plant seed yield and plant survival (Cohen et al. 2015).
Besides phytohormones, rhizobacteria can produce other biochemical compounds with ability to impact plant growth, such as volatile organic compounds or ethylene, which may have an effect on plant growth and in the formation of lateral roots and root hairs (Niu et al. 2011; Kai and Piechulla 2009; Ryu et al. 2003; Masle 2000). We found that volatile compounds produced by A. brasilense Sp245 increased shoot and root fresh weight as well as primary root length, but the chemical nature of the volatiles emitted by this rhizobacterium remains to be determined. Similar effects on the root system architecture of volatiles produced by rhizobacteria were observed by Delaplace et al. (2015) in the model grass Brachypodium distachyon (L.) P. Beauv., although the authors did not identified the responsible compounds of these changes.
The analysis of cell division in the root tip using the reporter CyCB1,1::GUS showed that the expression of this cell division marker was higher for plants exposed to volatile compounds, relative to those in the direct contact or in the vicinity of the inoculum. This expression correlated with increased root length. Previous reports evidenced that rhizobacteria of the genus Pseudomonas and Bacillus induce changes in cell division and differentiation, which alter root architecture in the host plant (López-Bucio et al. 2007; Zamioudis et al. 2013). Thus, the differences in root growth under different interaction types could be due to the changes in cell division stimulated by the rhizobacterium. Accordingly, WOX5::GFP expression was reduced in the direct contact group, whereas in the diffusible and volatile compound groups, it was slightly increased relative to WT plants. The WOX5 transcription factor is expressed only in the QC and controls the identity of stem cells that give rise to all meristem cell types (Sarkar et al. 2007; Aichinger et al. 2012). WOX5 loss-of-function causes terminal differentiation in distal stem cells and abates cell division in the meristem, indicating the importance of these cells in maintaining meristem organization and function (Sarkar et al. 2007; Forzani et al. 2014). The high degree of root differentiation stimulated by direct contact of roots to A. brasilense Sp245 likely resulted from the decreased expression of this marker. The changes in root differentiation were supported by AtEXP7::GUS expression levels, which were higher in the direct contact group but lower in the volatile compound group.
We also tested the response of the auxin-inducible marker DR5::GUS to examine effects of rhizobacteria on auxin signaling pathways. This marker was highly expressed along the root in direct contact with bacteria, whereas with diffusible and volatile compounds, the high expression of this marker was observed only in leaf edges and lateral roots as in the control condition. Hormonal redistribution was previously shown to alter cell division and differentiation patterns (Blilou et al. 2005; Dello Ioio et al. 2007, 2008). These results suggest that the auxin redistribution, mediated by the type of interaction, contributes to the root architectural reconfiguration in Arabidopsis.
Arabidopsis mutants affected in the machinery of sensing and response to auxin and ethylene showed that auxin appears to be required to root hair formation and elongation in roots exposed to A. brasilense, but not ethylene. These results were similar with a previous report that showed a contribution of auxin to the elongation of root hairs in Arabidopsis induced by Pseudomonas, but disagree with the role of ethylene (Zamioudis et al. 2013).
Thus, the effect of bacterial contact or secondary metabolites on plant growth seems to be due to a mixture of effects caused by either elicitors, diffusible compounds, or volatiles.
Together, our results showed that the effects of A. brasilense Sp245 on Arabidopsis are complex and involve elements in the auxin and in minor level ethylene pathways, which synergistically control different processes such as cell division and differentiation, underpinning their complimentary roles in plant growth and development during a probiotic interaction.
References
Aichinger E, Kornet N, Friedrich T, Laux T (2012) Plant stem cell niches. Ann Rev Plant Biol 63:615–636. https://doi.org/10.1146/annurev-arplant-042811-105555
Bashan Y, de-Bashan LE (2010) How the plant growth-promoting bacterium Azospirillum promotes plant growth - a critical assessment. Adv Agro 108:77–136. https://doi.org/10.1016/S0065-2113(10)08002-8
Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, Heidstra R, Aida M, Palme K, Scheres B (2005) The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433:39–44. https://doi.org/10.1038/nature03184
Bottini R, Fulchieri M, Pearce D, Pharis RP (1989) Identification of gibberellins a(1), a(3), and iso-a(3) in cultures of Azospirillum lipoferum. Plant Physiol 90:45–47. https://doi.org/10.1104/pp.90.1.45
Bottini R, Cassán F, Piccoli P (2004) Gibberellin production by bacteria and its involvement in plant growth promotion and yield increase. Appl Microbiol Biotechnol 65:497–503. https://doi.org/10.1007/s00253-004-1696-1
Cassan F, Diaz-Zorita M (2016) Azospirillum sp. in current agriculture: from the laboratory to the field. Soil Biol Biochem 103:117–130. https://doi.org/10.1016/j.soilbio.2016.08.020
Cassan F, Vanderleyden J, Spaepen S (2014) Physiological and agronomical aspects of phytohormone production by model plant-growth-promoting rhizobacteria (PGPR) belonging to the genus Azospirillum. J Plant Growth Reg 33:440–459. https://doi.org/10.1007/s00344-013-9362-4
Chao Q, Rothenberg M, Solano R, Roman G, Terzaghi W, Ecker JR (1997) Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE3 and related proteins. Cell 89:1133–1144. https://doi.org/10.1016/s0092-8674(00)80300-1
Cho HT, Cosgrove DJ (2002) Regulation of root hair initiation and expansin gene expression in Arabidopsis. Plant Cell 14:3237–3253. https://doi.org/10.1105/tpc.006437
Cohen AC, Bottini R, Piccoli PN (2008) Azospirillum brasilense Sp245 produces ABA in chemically-defined culture medium and increases ABA content in Arabidopsis plants. Plant Growth Reg 54:97–103. https://doi.org/10.1007/s10725-007-9232-9
Cohen AC, Bottini R, Pontin M, Berli FJ, Moreno D, Boccanlandro H, Travaglia CN, Piccoli PN (2015) Azospirillum brasilense ameliorates the response of Arabidopsis thaliana to drought mainly via enhancement of ABA levels. Physiol Plant 153:79–90
Colón-Carmona A, You R, Haimovitch-Gal T, Doerner P (1999) Spatio-temporal analysis of mitotic activity with a labile cyclin-GUS fusion protein. Plant J 20:503–508. https://doi.org/10.1046/j.1365-313x.1999.00620.x
Delaplace P, Delory BM, Baudson C, Mendaluk-Saunier de Cazenave M, Spaepen S, Varin S, Brostaux Y, du Jardin P (2015) Influence of rhizobacterial volatiles on the root system architecture and the production and allocation of biomass in the model grass Brachypodium distachyon (L.) P. Beauv. BMC Plant Biol 15:195. https://doi.org/10.1186/s12870-015-0585-3
Dello Ioio R, Linhares FS, Scacchi E, Casamitjana-Martinez E, Heidstra R, Costantino P, Sabatini S (2007) Cytokinins determine Arabidopsis root-meristem size by controlling cell differentiation. Curr Biol 17:678–682. https://doi.org/10.1016/j.cub.2007.02.047
Dello Ioio R, Nakamura K, Moubayindin L, Perilli S, Taniguchi M, Morita MT, Aoyama T, Costantino P, Sabatini S (2008) A genetic framework for genetic control of cell division and differentiation in the root meristem. Science 322:1380–1384. https://doi.org/10.1126/science.1164147
Dharmasiri N, Dharmasiri S, Weijers D, Lechner E, Yamada M, Hobbie L, Ehrismann JS, Jürgens G, Estelle M (2005) Plant development is regulated by a family of auxin receptor F box proteins. Dev Cell 9:109–119. https://doi.org/10.1016/j.devcel.2005.05.014
Dobbelaere S, Croonenborghs A, Thys A, Broek AV, Vanderleyden J (1999) Phytostimulatory effect of Azospirillum brasilense wild type and mutant strains altered in IAA production on wheat. Plant Soil 212:155–164. https://doi.org/10.1023/A:1004658000815
Dubrovsky JG, Puente ME, Bashan Y (1994) Arabidopsis thaliana as a model system for the study of the effect of inoculation by Azospirillum brasilense Sp-245 on root hair growth. Soil Biol Biochem 26:1657–1664. https://doi.org/10.1016/0038-0717(94)90318-2
Forzani C, Aichinger E, Sornay E, Willemsen V, Laux T, Dewitte W, Murray JAH (2014) WOX5 suppresses CYCLIN D activity to establish quiescence at the center of the root stem cell niche. Curr Biol 24:1939–1944. https://doi.org/10.1016/j.cub.2014.07.019
Friml J, Wisniewska J, Benková E, Mendgen K, Palme K (2002) Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415:806–809. https://doi.org/10.1038/415806a
Fukaki H, Tameda S, Masuda H, Tasaka M (2002) Lateral root formation is blocked by a gain-of-function mutation in the SOLITARY-ROOT/IAA14 gene of Arabidopsis. Plant J 29:153–168. https://doi.org/10.1046/j.0960-7412.2001.01201.x
Guzmán P, Ecker JR (1990) Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell 2:513–523. https://doi.org/10.1105/tpc.2.6.513
Hua J, Meyerowitz EM (1998) Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell 94:261–271. https://doi.org/10.1016/s0092-8674(00)81425-7
Kai M, Piechulla B (2009) Plant growth promotion due to rhizobacterial volatiles - an effect of CO2? FEBS Lett 583:3473–3477. https://doi.org/10.1016/j.febslet.2009.09.053
Kanchiswamy CN, Malnoy M, Maffei ME (2015) Chemical diversity of microbial volatiles and their potential for plant growth and productivity. Front Plant Sci 6:151. https://doi.org/10.3389/fpls.2015.00151
López-Bucio J, Campos-Cuevas JC, Hernández-Calderón E, Velásquez-Becerra C, Farías-Rodríguez R, Macías-Rodríguez LI, Valencia-Cantero E (2007) Bacillus megaterium rhizobacteria promote growth and alter root-system architecture through an auxin- and ethylene-independent signaling mechanism in Arabidopsis thaliana. Mol Plant-Microbe Inter 20:207–217. https://doi.org/10.1094/MPMI-20-2-0207
Lugtenberg B, Kamilova F (2009) Plant-growth-promoting rhizobacteria. Ann rev Microbiol 63:541–556. https://doi.org/10.1146/annurev.micro.62.081307.162918
Malamy JE, Benfey PN (1997) Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124:33–44
Masle J (2000) The effects of elevated CO2 concentrations on cell division rates, growth patterns, and blade anatomy in young wheat plants are modulated by factors related to leaf position, vernalization, and genotype. Plant Physiol 122:1399–1415. https://doi.org/10.1104/pp.122.4.1399
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Niu Y, Jin C, Jin G, Zhou Q, Lin X, Tang C, Zhang Y (2011) Auxin modulates the enhanced development of root hairs in Arabidopsis thaliana (L.) Heynh. Under elevated CO2. Plant Cell Environ 34:1304–1317. https://doi.org/10.1111/j.1365-3040.2011.02330
Ohto M, Onai K, Furukawa Y, Aoki E, Araki T, Nakamura K (2001) Effects of sugar on vegetative development and floral transition in Arabidopsis. Plant Physiol 127:252–261. https://doi.org/10.1104/pp.127.1.252
Okushima Y, Overvoorde PJ, Arima K, Alonso JM, Chan A, Chang C, Ecker JR, Hughes B, Lui A, Nguyen D, Onodera C, Quach H, Smith A, Yu G, Theologis A (2005) Functional genomic analysis of the AUXIN RESPONSE FACTOR gene family members in Arabidopsis thaliana: unique and overlapping functions of ARF7 and ARF19. Plant Cell 17:444–463. https://doi.org/10.1105/tpc.104.028316
Okushima Y, Fukaki H, Onoda M, Theologis A, Tasaka M (2007) ARF7 and ARF19 regulate lateral root formation via direct activation of LBD / ASL genes in Arabidopsis. Plant Cell 19:118–130. https://doi.org/10.1105/tpc.106.047761
Pérez-Flores P, Valencia-Cantero E, Altamirano-Hernández J, Pelagio-Flores R, López-Bucio J, García-Juárez P, Macías-Rodríguez L (2017) Bacillus methylotrophicus M4-96 isolated from maize (Zea mays) rhizoplane increases growth and auxin content in Arabidopsis thaliana via emission of volatiles. Protoplasma 254:2201–2213. https://doi.org/10.1007/s00709-017-1109-9
Petricka JJ, Winter CM, Benfey PN (2012) Control of Arabidopsis root development. Ann Rev Plant Biol 63:563–590. https://doi.org/10.1146/annurev-arplant-042811-105501
Ryu CM, Farag MA, Hu CH, Reddy MS, Wei HX, Paré PW, Kloepper JW (2003) Bacterial volatiles promote growth in Arabidopsis. Proc Natl Acad Sci U S A 100:4927–4932. https://doi.org/10.1073/pnas.0730845100
Ryu CM, Farag MA, Hu CH, Reddy MS, Kloepper JW, Paré PW (2004) Bacterial volatiles induced resistance in Arabidopsis. Plant Physiol 134:1017–1026. https://doi.org/10.1104/pp.103.026583
Sarkar AK, Luijten M, Miyashima S, Lenhard M, Hashimoto T, Nakajima K, Scheres B, Heidstra R, Laux T (2007) Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature 446:811–814. https://doi.org/10.1038/nature05703
Spaepen S, Dobbelaere S, Croonenborghs A, Vanderleyden J (2008) Effects of Azospirillum brasilense indole-3-acetic acid production on inoculated wheat plants. Plant Soil 312:15–23. https://doi.org/10.1007/s11104-008-9560-1
Spaepen S, Bossuyt S, Engelen K, Marchal K, Vanderleyden J (2014) Phenotypical and molecular responses of Arabidopsis thaliana roots as a result of inoculation with the auxin-producing bacterium Azospirillum brasilense. New Phytol 201:850–861. https://doi.org/10.1111/nph.12590
Strzelczyk E, Kampert M, Li CY (1994) Cytokinin-like substances and ethylene production by Azospirillum in media with different carbon sources. Microbiol Res 149:55–60
Tahir HAS, Gu Q, Wu H, Raza W, Hanif A, Wu L, Colman MV, Gao X (2017) Plant growth promotion by volatile organic compounds produced by Bacillus subtilis SYST2. Front Microbiol 8:171. https://doi.org/10.3389/fmicb.2017.00171
Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ (1997) Aux/lAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9:1963–1971. https://doi.org/10.1105/tpc.9.11.1963
Vacheron J, Desbrosses G, Bouffaud ML, Touraine B, Moënne-Loccoz Y, Muller D, Legendre L, Wisniewski F, Prigent-Combaret C (2013) Plant growth-promoting rhizobacteria and root system functioning. Front Plant Sci 4:356. https://doi.org/10.3389/fpls.2013.00356
Zamioudis C, Mastranesti P, Dhonukshe P, Blilou I, Pieterse CMJ (2013) Unraveling root developmental programs initiated by beneficial Pseudomonas spp. Bacteria. Plant Physiol 162:304–318. https://doi.org/10.1104/pp.112.212597
Zhang H, Kim MS, Krishnamachari V, Payton P, Sun Y, Grimson M, Farag MA, Ryu CM, Allen R, Melo IS, Paré PW (2007) Rhizobacterial volatile emissions regulate auxin homeostasis and cell expansion in Arabidopsis. Planta 226:839–851. https://doi.org/10.1007/s00425-007-0530-2
Acknowledgments
This study was supported by the Coordinación de la Investigación Científica, Universidad Michoacana de San Nicolás de Hidalgo, México. We thank the Consejo Nacional de Ciencia y Tecnología (CONACYT) for the postgraduate scholarship to MMG (276783). We also sincerely thank León Francisco Ruiz-Herrera for technical assistance for confocal microscopy.
Author information
Authors and Affiliations
Contributions
MMG and EGP designed the experiments. MMG and SBO conducted the experiments. JLB and ECM provided materials, reagents, and technical support. MMG, EGP, and JLB analyzed the data and wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Ulrike Mathesius
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Figure 1.
Characterization of the direct contact Azospirillum-Arabidopsis interaction. (a) Root images exposed to direct contact or diffusible compounds of the rhizobacterium. Bar = 200 μm. (b) Colonization analysis in roots. CFU = Colony Forming Units; C = Control (untreated roots); DC = Direct Contact; Dif = Diffusible compounds. n = 30. (PNG 347 kb)
Supplementary Figure 2.
Characterization of the interaction Azospirillum-Arabidopsis through the production of diffusible compounds. (a) Representative images of seedlings 6 d after transplant to medium containing A. brasilense (Ab), Autoclaved Ab (A-Ab), or E.coli. Bar = 1 cm. (b-f) Primary root length, lateral root number, lateral root density, shoot fresh weight and root fresh weight, respectively. (g) Representative images of root hair formation. Bar = 1 mm. (h,i) Root hair number and box-plot of root hair length, respectively. (j) Representative images of root hair formation after diffusible IAA treatment. Bar = 1 mm. (k,l) Root hair number and box-plot of root hair length, respectively. (m,n) Expression of the auxin response marker DR5::GUS. Bar = 100 μm. The rhizobacterium was used at 103 CFU/mL, and E. coli XL1 at 1.0 OD600. The letters on the bars indicate statistically significant differences according to Tukey's HSD test, P <0.05. (PNG kb)
Supplementary Figure 3.
Characterization of the interaction Azospirillum-Arabidopsis through the production of volatile compounds. (a) Representative images of seedlings 6 d after transplant to divided Petri dishes with medium containing A. brasilense (Ab), Autoclaved Ab (A-Ab), or E.coli. Bar = 1 cm. (b-f) Primary root length, lateral root number, lateral root density, shoot fresh weight and root fresh weight, respectively. (g) Representative images of root hair formation. Bar = 1 mm. (h,i) Root hair number and box-plot of root hair length, respectively. The rhizobacterium was used at 103 CFU/mL, and E. coli XL1 at 1.0 OD600. The letters on the bars indicate statistically significant differences according to Tukey's HSD test, P <0.05. (PNG 1.78 mb)
Supplementary Figure 4.
Effect of A. brasilense Sp245 on expression of the auxin response marker DR5::GUS. Representative images of the shoot, root and primary root meristem of seedlings 6 days after transplant with 103 CFU/mL of the rhizobacterium. As a positive control, plants were treated for 10 h with 0.05 μM of IAA in liquid MS medium. Bars in shoot and root = 1 mm; bar in root meristem = 100 μm. n = 30. The experiments were performed least three times with similar results. (PNG 1219 kb)
Supplementary Table 1
(PNG 236 kb)
Supplementary Table 2
(PNG 208 kb)
Rights and permissions
About this article
Cite this article
Méndez-Gómez, M., Barrera-Ortiz, S., Castro-Mercado, E. et al. The nature of the interaction Azospirillum-Arabidopsis determine the molecular and morphological changes in root and plant growth promotion. Protoplasma 258, 179–189 (2021). https://doi.org/10.1007/s00709-020-01552-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-020-01552-7