Abstract
The effects of lipopolysaccharides (LPS) from Azospirillum brasilense Sp245, a plant growth-promoting rhizobacteria, and Pseudomonas aeruginosa PAO1, a pathogenic bacterium, on plant growth and peroxidase (POD) activity were assessed on wheat seedlings. A. brasilense LPS (100 µg/mL) increased total length, and total fresh weight in wheat seedlings 4 days after treatment. P. aeruginosa LPS did not show effect on plant growth. A. brasilense LPS increased root hairs length similar to whole cells, while P. aeruginosa LPS increased root hairs density and slightly root hairs length. Both LPS increased POD activity and hydrogen peroxide (H2O2) content in root; however, the LPS from the pathogenic bacterium generated higher increments. The peroxidase inhibitor salicylhydroxamic acid (SHAM) inhibited plant growth, which was not recovered by the addition of LPS neither A. brasilense nor P. aeruginosa. POD activity stimulated by LPS was calcium-dependent as confirmed by the addition of the calcium channel blocker LaCl3. The results suggest that plant cells sense differentially LPS from beneficial or pathogenic bacteria and that calcium is needed to respond to the presence of both LPS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lipopolysaccharides (LPS) are major components of the outer membrane (OM) of Gram-negative bacteria that contribute to membrane integrity and stability. LPS also contribute to cell defense against external stress factors by providing a permeability barrier against many different classes of molecules including antibiotics and metals (Silipo and Molinaro 2017; Molinaro et al. 2009).
LPS are involved in adhesion and colonization in host-bacterium interactions. In addition, LPS are potent elicitors of innate immune responses during pathogenesis of Gram-negative infections in both plant and animal hosts (Takeuchi and Akira 2010; Ranf 2016; Ranf et al. 2016). All LPS share three common structural components: a lipophilic moiety termed lipid A, a core oligosaccharide, and an O-specific polysaccharide (also known as O-chain or O-antigen), a hydrophilic glycan. The core oligosaccharide contains a carbohydrate that is specific to LPS: 3-deoxy-d-manno-oct-2-ulosonic acid (Kdo), as well as several heptose moieties. The O-specific polysaccharide is joined to the Kdo domain and is oriented outwards, whereas lipid A is embedded in the OM to anchor LPS to the membrane (Whitfield and Trent 2014).
LPS from plant pathogens trigger the metabolomics reconfiguration of primary and secondary metabolites synthetized from various metabolic pathways (Mareya et al. 2020). Furthermore, the intact LPS structure is required for its complete biological activity, as the lipid A and the polysaccharide (O-antigen-core) chain reprogramming cellular activities at low levels than intact LPS (Madala et al. 2012). Although, the lipid A alone is able to reprogram the cellular metabolism related with immunity and defense (Madala et al. 2011).
Gram-negative bacteria that contain LPS include the plant growth-promoting rhizobacteria (PGPR) Azospirillum brasilense and the opportunistic pathogen Pseudomonas aeruginosa. Azospirillum is the most studied PGPR and is a widely used model of bacterial interactions with many plant species (Cassán et al. 2020; Pereg et al. 2016; Steenhoudt and Vanderleyden 2000). A. brasilense LPS are highly active in interactions with plant roots (Fedonenko et al. 2001; Matora et al. 1995) and in induction of plant responses (Evseeva et al. 2011). Furthermore, A. brasilense Sp245 LPS can promote wheat plant growth in vitro (Vallejo-Ochoa et al. 2018) and under greenhouse conditions (Chavez-Herrera et al. 2018).
Pseudomonas is a bacterial genus that has a wide distribution and these bacteria inhabit various ecological niches. As a phytopathogenic bacteria, Pseudomonas colonize surfaces of plant leaves to form dense bacterial populations that do not cause disease due to an inability to penetrate the leaf epidermis directly, but rather enter into plant tissue through natural surface openings such as stomata or wounds (Gimenez-Ibanez and Rathjen 2010; Starkey and Rahme 2009).
Pseudomonas aeruginosa is an opportunistic pathogen of plants and is ubiquitously distributed in soil and aquatic habitats (Sitaraman 2015). The P. aeruginosa strain PAO1 is the most commonly used strain for research on this opportunistic pathogen, and has become the reference strain to study the genetics, physiology and metabolism of this gamma proteobacterium (Chahtane et al. 2018; Klockgether et al. 2010). P. aeruginosa can also infect plant roots and thus is also used to study the plant defense responses against bacterial pathogens (Walker et al. 2004).
Although LPS have been isolated from some Pseudomonas strains, for example from P. aeruginosa PAC1 (Chester and Meadow 1975), their effects on plant growth and some biochemical responses such as peroxidase activity are unknown.
Peroxidases (PODs, E.C. 1.11.1.7) are hemoproteins expressed by a range of organisms. These enzymes catalyze the oxidation of numerous substrates using hydrogen peroxide and are involved in a variety of biological processes (Kalsoom et al. 2015). Class III PODs are specific to plants and are key proteins that control plant differentiation and development (Renard et al. 2020; de Carvalho Oliveira et al. 2019; Yan et al. 2019; Francoz et al. 2015). They belong to large, multigenic families with 73 members reported for A. thaliana, 181 for Eucalyptus grandis, 138 for Oryza sativa and 143 for Brachypodium distachyon (Fawal et al. 2013).
Different PODs have antagonistic activities that contribute to the loosening and stiffening of the cell wall that occurs during plant growth and cell expansion. To stiffen the cell wall, PODs oxidize aromatic compounds in the cell wall such monolignols and cinnamic acids in the presence of H2O2 to promote covalent bond formation. To loosen the cell wall, PODs generate radical oxygen species (ROS) such as hydroxyl radical (OH·) that break covalent bonds in cell wall polymers (Schopfer 2001). PODs can also control cell elongation through their auxin oxidase activity (Cosio et al. 2009). Plant PODs contain two calcium ions (Ca2+) that are critical for maintaining structural stability around the heme moiety and also for preserving thermal stability (Maranon and Huystee 1994; Shiro et al. 1986).
POD activity is induced by A. brasilense in inoculated Handroanthus impetiginosus shoots (Larraburu et al. 2016) and by Pseudomonas in wheat plants (Minaeva et al. 2018). However, less is known about how LPS of these bacteria affect POD activity. In this work, we compared the effect of LPS from A. brasilense Sp245 and P. aeruginosa PAO1 on early growth and root peroxidase activity in wheat plants.
Materials and Methods
Materials and Growth Conditions
The strains A. brasilense Sp245 (Baldani et al. 1986) and P. aeruginosa PAO1 (Klockgether et al. 2010) were used in this study. Both strains were routinely grown on solid LB medium (10 g/L tryptone, 5 g/L yeast extract, 5 g/L NaCl, 0.186 g/L MgSO4, 0.277 g/L CaCl2, 15 g/L agar). To prepare inocula, cultures were grown on liquid LB medium for 16 h (exponential phase) at 27 °C with shaking at 100 rpm. The cultures were then washed twice in 0.9% NaCl by centrifugation (4300×g, 10 min, 4 °C), resuspended in sterile water, and adjusted to a final concentration of 106 colony-forming units (CFU)/mL.
Triticum aestivum seeds, cv Nana F2007, were kindly provided by Dr. Mario González-Chavira (Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias—Celaya, Guanajuato, México). For germination, seed were washed with 1% sodium dodecyl sulfate (SDS) for 3 min. The seeds were then surface sterilized with 1% sodium hypochlorite for 5 min and rinsed four times with sterile distilled water. The seed were place in a petri dish containing a wet sterile filter paper and incubated in the dark at 28 ℃ for 3 days.
Inoculation and Treatments
After germination, seedlings were transferred to sterilized test tubes (15 cm long and 2 cm wide) and only the roots were immersed in 10 mL liquid Murashige and Skoog (MS) medium (pH 5.7). Inoculation with the bacteria was performed by adding 106 CFU/mL in MS medium. For LPS treatment, LPS was added to the liquid medium at two different concentrations (10 or 100 µg/mL). Four days after the LPS treatments, plant growth was assayed in terms total fresh weight as well as the length of leaves, roots, and total plant length. Images of the root were recorded with a stereomicroscope (Leica MZ6).
Salicylhydroxamic acid (SHAM; 100 µM), lanthanum chloride (LaCl3; 0.5 mM), and calcium chloride (CaCl2; 100 µM) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Chemicals were added to MS medium at the indicated concentrations 30 min before the addition of LPS.
Extraction of Bacterial LPS
LPS extraction was performed as previously described (Renukadevi et al. 2012). Cell wall fractions were isolated from 50 mL of a culture grown for 16 h in liquid LB medium (27 °C). Cells were first pelleted by centrifugation at 4000×g, for 10 min and resuspended in 5 mL sterile distilled water with 5 mL phenol equilibrated with 10 mM Tris–HCl, pH 8 and incubated at 65 °C for 20 min with shaking. The samples were then incubated at 4 °C for 24 h. The crude LPS were then purified by dialysis for 3 days at 5 °C against deionized water. The LPS were concentrated by alcohol precipitation wherein sodium acetate was added to a final concentration of 0.15 M followed by dropwise addition of ice cold 96% ethanol to yield a final ethanol proportion of 1:4. The mixture was incubated for 24 h at − 20 °C. The pellet was then collected by centrifugation at 4000×g for 20 min and suspended in distilled water. LPS were stored at 4 °C until use.
SDS-PAGE and LPS Silver Staining
Preparations were developed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) (Laemmli 1970). LPS preparations were separated on an SDS-PAGE gel with 4% and 12.5% acrylamide in the stacking and separating gels, respectively. Electrophoresis was performed at 12 mA through the stacking gel and 25 mA in the separating gel.
The LPS silver staining procedure was modified from Fomsgaard et al. (1990). Gels were fixed overnight in 100 mL 40% (vol/vol) ethanol in 5% (vol/vol) acetic acid. The gels were then subjected to a 5 min oxidation in 100 mL 0.7% periodic acid, 40% (vol/vol) ethanol, 5% (vol/vol) acetic acid. After oxidation, the gels were washed four times for 1 h each with 100 mL distilled water before incubation for 10 min in freshly prepared silver staining solution consisting of 18.66 mL 0.1 N sodium hydroxide, 1.33 mL 29.4% ammonium hydroxide and 3.33 mL 20% (wt/vol) silver nitrate, with distilled water added to yield a final volume of 100 mL. After staining, the gels were washed three times for 15 min each with 100 mL distilled water. The gels were then developed for 10 to 20 min by incubation in 100 mL freshly prepared developer solution consisting of 5 mg citric acid and 0.05 mL 37% formaldehyde, in 100 mL distilled water, at 37 °C. After visualizing the LPS bands, the gel was transferred to a stop bath (100 mL 0.35% [vol/vol] acetic acid in distilled water) and incubated for 1 h. Finally, the gel was washed with 100 mL distilled water and stored in sealed plastic bags with a small amount of water to prevent dehydration. Gel images were recorded with a digital camera (C-3030 Zoom, Camedia, Olympus, Tokyo, Japan).
Total POD Assay
Soluble POD assays were performed according to Svalheim and Robertsen (1990). Roots (~ 100 mg) were ground with a mortar and pestle in liquid nitrogen and the resulting powder was resuspended in 10 mM sodium phosphate, pH 6.0 and homogenized using a vortex. The suspension was assayed for POD activity by the formation of tetraguaiacol measured as an increase in absorbance at 470 nm in a spectrophotometer (Beckman Instruments, Fullerton, CA). Each reaction mixture (1 mL) consisted of 10 μL root extract and 990 μL guaiacol solution containing 0.25% guaiacol (v/v) in 10 mM sodium phosphate buffer pH 6.0 and 0.125% H2O2 (v/v). The reaction was monitored for 1 min. The protein content of the extracts was determined (Bradford 1976) using the Bio-Rad Protein Assay dye reagent (Bio-Rad, Hercules, CA). Bovine serum albumin was used as the standard.
For in situ detection of POD, wheat roots were transferred to 20 mL of medium containing 0.1 M Tris–acetate (pH 5), 0.1 mM 2,6-dichlorophenolindophenol (2,6-DCPI), 0.9 mM H2O2, at pH 5. After a 30 min incubation at room temperature, a colorimetric reaction occurred in the tissue (Córdoba-Pedregosa et al. 2003). The root was immediately photographed using a stereomicroscope.
H2O2 Analysis
The H2O2 content was assayed according to a modified procedure described by García-Pineda et al. (2010). Root tissue (100 mg) was powdered in liquid nitrogen and homogenized in 1.5 mL of deionized water. The samples were centrifuged at 4000×g, for 5 min. H2O2 was measured by incubating 500 µL of the supernatant with an equal volume of a reagent mixture containing 0.05% guaiacol (440 μL/L) and horseradish peroxidase (350 μL/L; 2,500 U/mL) dissolved in 25 mM sodium phosphate buffer (pH 7.0). The mixture was incubated for 15 min at room temperature in the dark and then the absorbance was measured immediately at 450 nm. Commercial H2O2 was used to generate a standard curve.
H2O2 localization in the root was assayed according to the procedure described by Thordal-Christensen et al. (1997). Roots were placed in 1 mg/mL 3,3′-diaminobenzidine (DAB), pH 3.8 and incubated in the dark for 2 h before transfer to 96% ethanol and analysis with a stereomicroscope.
Data Analysis
Experiments were repeated at least three times and data are expressed as mean ± standard error (SE). Statistical analyses were done with one-way analysis of variance (ANOVA) followed by Duncan’s multiple range tests for independent samples. In all cases, the confidence coefficient was set at p < 0.05.
Results
Effect of LPS on Root Morphology and Plant Growth
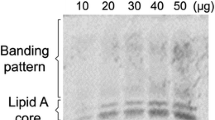
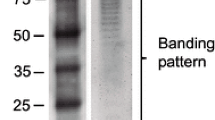
LPS from both A. brasilense and P. aeruginosa were extracted and verified by gel electrophoresis (Fig. 1a). The band pattern obtained was different to LPS from the two bacteria, suggesting structural differences of the molecules. Seedlings 3 days after germination were incubated with LPS at 10 or 100 µg/mL for 4 days and the effect on plant growth and root morphology was then compared. LPS from A. brasilense at 100 µg/mL increased the total length and total fresh weight of the seedlings. In contrast, LPS from P. aeruginosa at either concentration had no effect on these growth parameters (Fig. 1b). The effect of LPS treatment (100 µg/mL) and the bacteria (106 CFU/mL) on root morphology, specifically on the formation of hair roots, was visually compared. Both types of bacteria induced an increase in hair root length compared to uninoculated seedlings. Interestingly, A. brasilense LPS increased hair root length by a similar amount as that induced by incubation with whole cells, whereas root hairs of plants treated with P. aeruginosa LPS were shorter and more dense compared to that seen for A. brasilense LPS (Fig. 1c).
Effect of LPS on plant growth and root morphology in wheat seedlings. a Analysis of LPS on SDS-PAGE. b Analysis of different concentrations of LPS on plant growth. c Hair root analysis after LPS (100 µg/mL) treatment. Barr = 1 mm. Data are mean ± SE of three independent experiments (n = 10). Letters above bars indicate significant differences according to a Duncan test (p < 0.05)
The effect of both types of bacteria and both types of LPS on leaf length and root length was next compared. For A. brasilense, exposure to whole cells resulted in a marked decrease in root length, whereas root length increased following treatment with A. brasilense LPS. Meanwhile, no change in root length was observed with either whole P. aeruginosa cells or with only P. aeruginosa LPS (Fig. 2).
POD Activity and H2O2 Production in Roots in Response to Different LPS
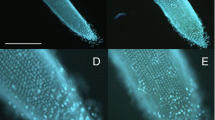
POD activity was analyzed in root seedlings after treatment with 100 µg/mL LPS from either A. brasilense or P. aeruginosa. The enzyme activity of PODs analyzed 4 days after treatment increased following treatment with both types of LPS relative to untreated control seedlings, although a larger increase was seen for LPS from P. aeruginosa compared to that from A. brasilense. An in situ analysis using 2,6-DCPI showed that the POD content increased in root tips but the increase was more notable for seedlings treated with P. aeruginosa LPS compared to those exposed to A. brasilense LPS, suggesting that the biochemical response varied according to LPS type. In addition, the different LPS had varying potential to increase the POD content, which correlated with the levels of total POD activity (Fig. 3a).
Effect of LPS (100 µg/mL) on POD activity and H2O2 production in wheat root. a Wheat seedlings were treated with different LPS and after 4 days POD activity and H2O2 content were analyzed in roots. b In situ detection of POD and H2O2 in roots. 2,6-DCPI = 2,6-dichlorophenolindophenol; DAB = 3,3′-diaminobenzidine. Barr = 1 mm. Data are mean ± SE of three independent experiments (n = 10). Letters above bars indicate significant differences according to a Duncan test (p < 0.05)
H2O2 production in roots as a biochemical response to LPS treatment was also analyzed. Treatment with both types of LPS notably increased the accumulation of H2O2 relative to untreated seedlings, but the accumulation was higher for plants treated with LPS from P. aeruginosa compared to that from A. brasilense. An in situ analysis of the location and content of H2O2 in root using DAB showed a similar pattern to that observed for POD enzymes, wherein P. aeruginosa LPS more effectively stimulated H2O2 accumulation (Fig. 3b).
The role of POD in responses stimulated by LPS was next analyzed using the POD inhibitor SHAM. Seedlings treated with 100 µM SHAM followed by 100 µg/mL LPS showed a decrease in plant growth. This effect was most clearly manifested in root length and weight (Fig. 4a). The same effect of LPS was observed for POD activity in roots, suggesting that this enzyme could mediate the biological activity of LPS (Fig. 4b).
Effect of SHAM on plant growth and POD activity. Wheat seedlings were treated with SHAM (100 µM), incubated by half hour and then LPS (100 µg/mL) were added. Plant growth (a) or POD activity (b) were analyzed 4 days after treatment. Data are mean ± SE of three independent experiments (n = 10). Letters above bars indicate significant differences according to a Duncan test (p < 0.05)
Effect of Calcium on Plant Responses Stimulated by LPS
The role of calcium on responses stimulated by LPS treatment was analyzed with LaCl3, a calcium channel blocker, as well as by the addition of CaCl2. Increases in total length and total fresh weight of the seedlings and POD activity in the root were inhibited with the addition of the channel blocker and were not recovered following LPS treatment. In contrast, these parameters were increased in a similar manner to that seen for stimulation with LPS when calcium was added, but the increases were not additive with those seen in presence of LPS (Table 1). These results suggested that LPS requires calcium mobilization to stimulate the observed cellular responses.
Discussion
There is limited information concerning whether plants exhibit different responses to LPS from beneficial or pathogenic bacteria. In the present study, we compared several morphological and biochemical responses of wheat seedlings to LPS isolated from one type of beneficial rhizobacteria, A. brasilense, and one opportunistic plant pathogen, P. aeruginosa. LPS from A. brasilense dose-dependently increased plant growth and root hair length in a manner similar to that seen for incubation with whole cells, indicating that these molecules play a role in stimulating changes in root morphology that could drive plant growth. Meanwhile, neither concentration of LPS from P. aeruginosa exerted a stimulatory effect on plant growth or root hair length.
Results of previous reports indicated that phytohormones, such as auxins, gibberellins and cytokinins produced by PGPR are required for root morphogenesis (Ambreetha et al. 2018; Zaidi et al. 2015). However, in this study we showed that LPS alone derived from the PGPR A. brasilense could induce changes in plant growth and root morphology. The results suggest that A. brasilense can alter root morphology via structural molecules such as LPS, in addition to phytohormones production. The results also showed that plant roots react differently to LPS derived from a pathogenic microorganism in that LPS from P. aeruginosa did not affect plant growth.
In contrast to a decrease in root length observed upon incubation of seedlings with A. brasilense cells, treatment with A. brasilense LPS was associated with increased root length. Although the reason for this contrasting result is unclear, it does suggest that phytohormones produced by rhizobacteria mediate decreases in root length, as was previously reported (Cassan et al. 2014). Phytohormones produced by rhizobacteria could mask the potential effects of LPS during the interaction between plant and bacteria, and it is possible that LPS must directly contact root tissue to exert a stimulatory effect.
POD activity is stimulated by both bacterial plant pathogens (Farahani and Taghavi 2016; Jang et al. 2004) and beneficial rhizobacteria (Lavania et al. 2006), but less is known about how LPS from these bacteria affect POD activity. Here we compared the effect of LPS on POD activity and found that while both types of LPS increased POD activity, LPS from the pathogenic bacteria were associated with larger increases in POD activity, suggesting that plants may be able to distinguish between LPS from PGPR and bacterial pathogens.
PGPR LPS provide beneficial effects for plant growth as was reported by Evseeva et al. (2018). These authors demonstrated that LPS from A. brasilense Sp245 specifically stimulated the regenerative capacity of wheat culture tissues and increased cell division in the wheat root meristem (Evseeva et al. 2011). In contrast, LPS from plant pathogens carry pathogen-/microbe-associated molecular patterns (PAMP/MAMP) that are recognized by the immune system and activate defense responses (Kutschera and Ranf 2019). Whether the same molecular mechanism is involved in sensing and transducing stimulatory effects by different LPS requires more investigation.
Based on studies of recognition of LPS by mammal cells, plants likely carry cellular receptors for LPS (Kagan 2017). However, whether plant cells can sense LPS from beneficial and pathogenic bacteria using the same receptor is unknown, as is whether plant cells have different receptors and different molecular components that respond to LPS from different bacteria. Information regarding the presence of receptors to LPS in plants is emerging, and will likely address this question. Although there are no reported receptor candidates that could sense LPS from PGPR, the lectin S-domain receptor kinase AtLORE was identified that could sense LPS from Pseudomonas spp. and Xanthomonas spp., a pathogenic bacteria. This putative receptor appears to specifically sense some LPS, but not LPS from Escherichia coli, Salmonella enterica or Burkholderia spp. and the receptor may even be specific to the Brassicaceae plant family (Ranf et al. 2015). Another interesting possibility by which plant cells distinguish different types of LPS is that plant receptors may perhaps recognize different structural components among different LPS. We have studies underway to address this question.
References
Ambreetha S, Chinnadurai C, Marimuthu P, Balachandar D (2018) Plant-associated Bacillus modulates the expression of auxin-responsive genes of rice and modifies the root architecture. Rhizosphere 5:57–66
Azevedo de Carvalho Oliveira R, Silveira de Andrade A, Oliveira Imparato D, Silva de Lima G, Machado de Almeida RV, Matos Santos Lima JP, de Bittencourt Pasquali MA, Siqueira Dalmolin RJ (2019) Analysis of Arabidopsis thaliana redox gene network indicates evolutionary expansion of class III peroxidase in plants. Sci Rep 9:15741
Baldani VL, de B. Alvarez MA, Baldani JI, Döbereiner J (1986) Establishment of inoculated Azospirillum spp. in the rhizosphere and in roots of field grown wheat and sorghum. Plant Soil 90:35–46
Bradford M (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Ann Biochem 72:48–254
Cassan F, Vanderleyden J, Spaepen S (2014) Physiological and agronomical aspects of phytohormone production by model plant-growth-promoting rhizobacteria (PGPR) belonging to the genus Azospirillum. J Plant Growth Regul 33:440–459
Cassán F, Coniglio A, López G, Molina R, Nievas S, Noir Le, de Carlan C, Donadio F, Torres D, Rosas S, Olivera Pedrosa F, de Souza E, Díaz Zorita M, de-Bashan L, Mora V (2020) Biol Fertil Soils 56:461–479
Chahtane H, Nogueira Fuller T, Allard P-M, Marcourt L, Ferreira Queiroz E, Shanmugabalaji V, Falquet J, Wolfender J-L, Lopez-Molina L (2018) The plant pathogen Pseudomonas aeruginosa triggers a DELLA-dependent seed germination arrest in Arabidopsis. eLife 7:e37082
Chavez-Herrera E, Hernandez-Esquivel AA, Castro-Mercado E, Garcia-Pineda E (2018) Effect of Azospirillum brasilense Sp245 lipopolysaccharides on wheat plant development. J Plant Growth Reg 37:859–866
Chester IR, Meadow PM (1975) Heterogeneity of the lipopolysaccharide from Pseudomonas aeruginosa. Eur J Biochem 58:273–282
Cosio C, Vuillemin L, De Meyer M, Kevers C, Penel C, Dunand C (2009) An anionic class III peroxidase from zucchini may regulate hypocotyl elongation through its auxin oxidase activity. Planta 229:823–836
Córdoba-Pedregosa MC, Córdoba F, Villalba JM, González-Reyes JA (2003) Zonal changes in ascorbate and hydrogen peroxide contents, peroxidase, and ascorbate-related enzyme activities in onion roots. Plant Physiol 131:697–706
Evseeva NV, Matora LYu, Burygin GL, Dmitrienko VV, Shchyogolev SYu (2011) Effect of Azospirillum brasilense Sp245 lipopolysaccharide on the functional activity of wheat root meristematic cells. Plant Soil 346:181–188
Evseeva NV, Tkachenko O, Burygin GL, Matora LYu, Lobachev YV, Shchyogolev SYu (2018) Effect of bacterial lipopolysaccharides on morphogenetic activity in wheat somatic calluses. World J Microbiol Biotechnol 34:3
Farahani AS, Taghavi M (2016) Changes of antioxidant enzymes of mung bean [Vigna radiata (L.) R. Wilczek] in response to host and non-host bacterial pathogens. J Plant Protect Res 56:95–99
Fawal N, Li Q, Savelli B, Brette M, Passaia G, Fabre M, Mathé C, Dunand C (2013) PeroxiBase: a database for large-scale evolutionary analysis of peroxidases. Nucleic Acids Res 41:D441–D444
Fedonenko YP, Egorenkova IV, Konnova SA, Ignatov VV (2001) Involvement of the lipopolysaccharides of Azospirilla in the interaction with wheat seedling roots. Microbiology 70:329–334
Fomsgaard A, Freudenberg MA, Galanos C (1990) Modification of the silver staining technique to detect lipopolysaccharide in polyacrylamide gels. J Clin Microbiol 28:2627–2631
Francoz E, Ranocha P, Nguyen-Kim H, Jamet E, Burlat V, Dunand C (2015) Roles of cell wall peroxidases in plant development. Phytochemistry 112:15–21
García-Pineda E, Benezer-Benezer M, Gutiérrez-Segundo A, Rangel-Sánchez G, Arreola-Cortés A, Castro-Mercado E (2010) Regulation of defence responses in avocado roots infected with Phytophthora cinnamomi (Rands). Plant Soil 331:45–56
Gimenez-Ibanez S, Rathjen JP (2010) The case for the defense: plants versus Pseudomonas syringae. Microbes Infect 12:428–437
Jang IC, Park SY, Kim KY, Kwon SY, Kim JG, Kwak SS (2004) Differential expression of 10 sweet potato peroxidase genes in response to bacterial pathogen, Pectobacterium chrysanthemi. Plant Physiol Biochem 42:451–455
Kagan JC (2017) Lipopolysaccharide detection across the kingdoms of life. Trends Immunol 38:696–704
Kalsoom U, Bhatti HN, Asgher M (2015) Characterization of plant peroxidases and their potential for degradation of dyes: a review. Appl Biochem Biotechnol 176:1529–1550
Klockgether J, Munder A, Neugebauer J, Davenport CF, Stanke F, Larbig KD, Heeb S, Schöck U, Pohl TM, Wiehlmann L, Tümmler B (2010) Genome diversity of Pseudomonas aeruginosa PAO1 laboratory strains. J Bacteriol 192:1113–1121
Kutschera A, Ranf S (2019) The multifaceted functions of lipopolysaccharide in plant-bacteria interactions. Biochimie 159:93–98
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Larraburu EE, Yarte ME, Llorente BE (2016) Azospirillum brasilense inoculation, auxin induction and culture medium composition modify the profile of antioxidant enzymes during in vitro rhizogenesis of pink lapacho. Plant Cell Tiss Organ Cult 127:381–392
Lavania M, Chauhan PS, Chauhan SVS, Singh HB, Nautiyal CS (2006) Induction of plant defense enzymes and phenolics by treatment with plant growth–promoting rhizobacteria Serratia marcescens NBRI1213. Curr Microbiol 52:363–368
Madala NE, Leone MR, Molinaro A, Dubery IA (2011) Deciphering the structural and biological properties of the lipid A moiety of lipopolysaccharides from Burkholderia cepacia strain ASP B 2D, in Arabidopsis thaliana. Glycobiology 21:184–194
Madala NE, Molinaro A, Dubery IA (2012) Distinct carbohydrate and lipid-based molecular patterns within lipopolysaccharides from Burkholderia cepacia contribute to defense-associated differential gene expression in Arabidopsis thaliana. Innate Immun 18:140–154
Maranon MJR, Van Huystee RB (1994) Plant peroxidases: interaction between their prosthetic groups. Phytochemistry 37:1217–1225
Mareya CR, Tugizimana F, Di Lorenzo F, Silipo A, Piater LA, Molinaro A, Dubery AA (2020) Adaptive defence-related changes in the metabolome of Sorghum bicolor cells in response to lipopolysaccharides of the pathogen Burkholderia andropogonis. Sci Rep 10:7626
Matora L, Solovova G, Serebrennikova O, Selivanov N, Shchyogolev S (1995) Immunological properties of Azospirillum cell surface: the structure of carbohydrate antigens and evaluation of their involvement in bacteria-plant contact interactions. In: Fendrik I, del Gallo M, Vanderleyden J, de Zamaroczy M (eds) Azospirillum VI and related microorganisms. NATO ASI series (series G: ecological sciences), vol 37. Springer, Berlin
Minaeva OM, Akimova EE, Tereshchenko NN, Zyubanova TI, Apenysheva MV, Kravets AV (2018) Effect of Pseudomonas bacteria on peroxidase activity in wheat plants when infected with Bipolaris sorokiniana. Russ J Plant Physiol 65:717–725
Molinaro A, Newman MA, Lanzetta R, Parrilli M (2009) The structures of lipopolysaccharides from plant-associated gram-negative bacteria. Eur J Org Chem 34:5887–5896
Pereg L, de-Bashan LE, Bashan Y (2016) Assessment of affinity and specificity of Azospirillumfor plants. Plant Soil 399:389–414
Ranf S (2016) Immune sensing of lipopolysaccharide in plants and nimals: same but different. PLoS Pathog 12(6):e1005596
Ranf S, Gisch N, Schaffer M, Illig T, Westphal L, Knirel YA, Sánchez-Carballo PM, Zähringer U, Hückelhoven R, Lee J, Scheel D (2015) A lectin S-domain receptor kinase mediates lipopolysaccharide sensing in Arabidopsis thaliana. Nat Immunol 16:426–433
Ranf S, Scheel D, Lee J (2016) Challenges in the identification of microbe-associated molecular patterns in plant and animal innate immunity: a case study with bacterial lipopolysaccharide. Mol Plant Pathol 17:1165–1169
Renard J, Martínez-Almonacid I, Sonntag A, Molina I, Moya-Cuevas J, Bissoli G, Muñoz-Bertomeu J, Faus I, Niñoles R, Shigeto J, Tsutsumi Y, Gadea J, Serrano R, Bueso E (2020) PRX2 and PRX25, peroxidases regulated by COG1, are involved in seed longevity in Arabidopsis. Plant Cell Environ 43:315–326
Renukadevi KP, Angayarkanni J, Karunakaran G (2012) Extraction and characterization of lipopolysaccharide from Serratia rubidaea and its cytotoxicity on lung cancer cell line-nci-h69. Acta Tech Corviniensis 2:97–101
Schopfer P (2001) Hydroxyl radical-induced cell-wall loosening in vitro and in vivo: implications for the control of elongation growth. Plant J 28:679–688
Shiro Y, Kurono M, Morishima I (1986) Presence of endogenous calcium ion and its functional and structural regulation in horseradish peroxidase. J Biol Chem 261:9382–9390
Silipo A, Molinaro A (2017) Lipid A structure. In: Knirel YA, Valpano MA (eds) Bacterial lipopolysaccharides. Springer, Cham
Sitaraman R (2015) Pseudomonas spp. as models for plant-microbe interactions. Front Plant Sci 6:787
Starkey M, Rahme LG (2009) Modeling Pseudomonas aeruginosa pathogenesis in plant hosts. Nat Protoc 4:117–124
Steenhoudt O, Vanderleyden J (2000) Azospirillum, a free-living nitrogen-fixing bacterium closely associated with grasses: genetic, biochemical and ecological aspects. FEMS Microbiol Rev 24:487–506
Svalheim O, Robertsen B (1990) Induction of peroxidase in cucumber hypocotyls by wounding and fungal infection. Physiol Plant 78:261–267
Takeuchi O, Akira S (2010) Pattern recognition receptors and inflammation. Cell 140:805–820
Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley—powdery mildew interaction. Plant J 11:1187–1194
Vallejo-Ochoa J, López-Marmolejo M, Hernández-Esquivel AA, Méndez-Gómez M, Nicolasa Suárez-Soria L, Castro-Mercado E, García-Pineda E (2018) Early plant growth and biochemical responses induced by Azospirillum brasilense Sp245 lipopolysaccharides in wheat (Triticum aestivum L.) seedlings are attenuated by procyanidin B2. Protoplasma 255:685–694
Walker TS, Bais HP, Deziel E, Schweizer HP, Rahme LG, Fall R, Vivanco JM (2004) Pseudomonas aeruginosa-plant root interactions. Pathogenicity, biofilm formation, and root exudation. Plant Physiol 134:320–331
Whitfield C, Trent MS (2014) Biosynthesis and export of bacterial lipopolysaccharides. Annu Rev Biochem 83:99–128
Yan J, Su P, Li W, Xiao G, Zhao Y, Ma X, Wang H, Nevo E, Kong L (2019) Genome-wide and evolutionary analysis of the class III peroxidase gene family in wheat and Aegilops tauschii reveals that some members are involved in stress responses. BMC Genom 20:666
Zaidi A, Ahmad E, Khan MS, Saif S, Rizvi A (2015) Role of plant growth promoting rhizobacteria in sustainable production of vegetables: current perspective. Sci Hortic 193:231–239
Funding
This study was supported by funds from the Coordinación de la Investigación Científica, Universidad Michoacana de San Nicolás de Hidalgo, and with a Grant to AAHE (No. 606506) from CONACYT, México.
Author information
Authors and Affiliations
Contributions
AAHE conducted the experiments. ECM contributed with technical assistance to experimental setup. AAHE and EGP discussed the results. EGP was the author of project planning and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding authors state that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hernández-Esquivel, A.A., Castro-Mercado, E. & García-Pineda, E. Comparative Effects of Azospirillum brasilense Sp245 and Pseudomonas aeruginosa PAO1 Lipopolysaccharides on Wheat Seedling Growth and Peroxidase Activity. J Plant Growth Regul 40, 1903–1911 (2021). https://doi.org/10.1007/s00344-020-10241-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-020-10241-x