Abstract
Soil aggregate stability is considered feasible and important indicator for understanding complex interactions between soils’ physico-chemical and biological properties and soil structure. The present study was therefore, conducted to find out the land-use change induced alteration in soil organic carbon (C) pool in response to changed restored engineering. The present study was conducted to reveal the distribution of water stable aggregates, aggregate stability, aggregate associated C of macro-and micro-aggregates, C preservation capacity of aggregate, and the labile and non-labile C fractions of variable oxidizability due to land-use change from the uncultivated soils to under rice-wheat, seed sugarcane, ratoon sugarcane and permanent grasslands in north-western India. These results showed that water stable aggregates, macro-and micro-aggregates, C preservation capacity, aggregate ratio and total organic carbon (TOC) stocks were significantly (p < 0.05) higher in permanent grassland and uncultivated soils. Ratoon sugarcane soils had ~ 10.3% higher TOC pool than the seed sugarcane. A significant decrease in TOC pool by ~ 11.3–11.9% occurred in soils under seed sugarcane cultivation, compared to others. Soils under seed sugarcane had ~ 11.5% lower C stocks, compared with the rice–wheat soils. As compared with the uncultivated soils, highest C loss of 3.3–3.7 Mg C ha−1 occurred in soils under seed sugarcane, followed by almost equal in rice-wheat (1.9–2.0 Mg C ha−1) and ratoon sugarcane (1.9–2.1 Mg C ha−1). The greatest C loss in soils under seed sugarcane was ascribed to increased tillage intensity. More intensified tillage under seed sugarcane cultivation resulted in decreased proportion of macro-aggregates (> 0.25 mm) and greater stabilization of organic C in relatively recalcitrant C pool as compared to those under ratoon sugarcane. Active C (Fract. 1 + Fract. 2) pool in surface soil layer under ratoon sugarcane was significantly higher by ~ 25.1–64.9%, compared with others. Conversely, the passive C pool (Fract. 3 + Fract. 4) was significantly lower in soils under seed sugarcane, while the highest in grassland. The proportion of macro-aggregates in soils under different land-use systems exhibited a linear significant relationship with the TOC pool (R2=0.964*; p < 0.05). Soils under seed sugarcane have significantly lower C preservation capacity of macro-aggregates by ~ 42.5%, compared with the ratoon sugarcane. Rice–wheat ecosystem had significantly higher C preservation capacity of macro-aggregates (> 0.25 mm) by ~ 0.70 g C kg−1 soil (~ 80.5%) than the seed sugarcane. The sensitivity index showed significantly higher sensitivity of TOC pool for soils under seed sugarcane (by ~ 8.6–21.8%), followed by ratoon sugarcane (~ 10.3–13.6%) and rice–wheat (~ 7.6–11.8%), while the lowest for grassland ecosystems (~ 0.2–0.5%) following the land-use change from uncultivated lands. Among the three cropland ecosystems, C preservation capacity of macro-aggregates was significantly higher than the sugarcane-based ecosystems. Considering uncultivated lands as reference, the soils under ratoon sugarcane had significantly higher C management index (CMI) than the other compared land-use systems. The highest values of the CMI in soils under ratoon sugarcane indicate C rehabilitation, while the lower values for seed sugarcane indicate C degradation. We put forward general management suggestions for different land-use and focus on better measures for the management of rice-wheat and seed sugarcane to reduce C losses by increasing aggregate stability of soils under different land-use systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The atmospheric concentration of carbon dioxide (CO2) has progressively increased from ~ 280 ppmv in the pre-industrial period to ~ 408 ppmv today, and is expected to rise by ~ 0.5% year−1 (~ 3.3 Pg C year−1) (IPCC 2001; Yi et al. 2006). The cultivated lands are considered a major source of CO2, accounting for ~ 1/5th of total CO2 emissions via soil and root respiration. Soils act both as carbon (C) sink and source and thereby contribute towards global C budgets (Singh and Benbi 2020a). As per estimates, cropland ecosystems have lost up to 2/3rd of their antecedent total organic carbon (TOC) pool due to land-use change, resulting in a cumulative loss of ~ 30–40 Mg C ha−1 (Lal 2004; Singh et al. 2021a). The deterioration in quality and quantity of soil organic matter induced by the adoption of improper cropping systems following land-use change has engrossed worldwide attention, and prompted severe concerns about agricultural production systems and their long-term sustainability (Lal 2004; Singh et al. 2021a; Mandal et al. 2022; Bhatt et al. 2022; Avtar-Singh et al. 2022). Soil organic matter quality under different agricultural croplands varied significantly due to change in crop production and soil management practices, which eventually influence above- and below-ground C input (Singh et al. 2020; Singh and Benbi 2022; Avtar-Singh et al. 2022) and its quality (Singh and Benbi 2018a; Sharma et al. 2020a, b), soil microbial biomass, enzymatic activity (Sharma et al. 2022a, b; Singh et al. 2023), and the rate of C stabilization (Sainju et al. 2007; Singh and Benbi 2020a, b, 2023; Sharma et al. 2020a, b). The cropping systems have significant influence on below-ground C cycling due to changed crop cover, plant-mediated C input, C quality, and substrate availability for micro-organisms (Doran and Zeiss 2000; Sharma et al. 2022c). Land conversion from the uncultivated to cropland ecosystems leads to biodiversity loss and soil organic matter depletion (Beheshti et al. 2012), besides a change in soil physico-chemical and biological properties (Gregorich et al. 1994; Mandal et al. 2022; Sharma et al. 2022c).
Soil management strategies used in various agricultural production systems are thought to significantly influence soils’ biological activities which affect soil quality (Benbi et al. 2015; Bhatt et al. 2022; Singh et al. 2023). Because soil is a living and dynamic resource, biologically mediated processes are considered essential for its sustainable ecological functioning (Benbi et al. 2012). A change in land-use has a direct impact on soil nutrient supply and distribution, as well as on biological changes occurring in soil rhizosphere (Li et al. 2009; Singh and Benbi 2018b). Consequently, a change in land-use and associated management could have a favorable and/or detrimental impact on the microbial ecology of soil rhizosphere (Li et al. 2009; Mandal et al. 2022). The plant species differ greatly in their rooting behavior, more particularly with respect to root architecture and geometry which produce variable quantity of root exudates and secretions in the rhizosphere and serve as a substrate for soil micro-organisms (Benbi et al. 2016; Singh and Benbi 2018a). It is generally understood that crops have variable nutritional demands, and therefore, crop species differ greatly in terms of the quantity and quality of litter produced (Notaro et al. 2014; Singh et al. 2021b), which affect the diversity and composition of micro-organisms (Adak and Sachan 2009). The changes in land-use system cause wide variations in above- and below-ground ecosystems, resulting in soil C depletion and biodiversity loss (Doran and Zeiss 2000), and thereby present variable impact on soil organic matter pool, quality, and enzyme activity (Li et al. 2009; Sharma et al. 2022a, b; Singh and Benbi 2021). It has been well established that research on the effect of land-use change on TOC dynamics is insufficient without understanding their likely impact on soil C pools and the biological activity (Singh and Benbi 2018b; Sharma et al. 2022b). Soil micro-organisms are considered important players in accelerating soil enzymatic activity, and are therefore critically important for ecosystems’ sustainability (Singh and Benbi 2018a). Soil microbiological characteristics are thought to be very sensitive indicator of soil ecological stress (Chan et al. 2001). For that, diversification of land-use systems has been a multi-disciplinary approach that includes an intimate connection among soil, water, climate, livestock, vegetation, and socio-economic elements in developing the most profitable, environmentally friendly, and ecologically sustainable policies (Sharma et al. 2022b). These aspects entail a complicated interaction between soil management regimes, soil biological components, and environmental quality; all of which are important for agriculture and the environment (Sharma et al. 2022b; Mandal et al. 2022). However, less is known about the impact of land-use change from uncultivated soils to intensively cultivated soils in terrestrial ecosystems (Li et al. 2009; Benbi et al. 2015).
In north-western India, rice-wheat has been the most predominant cereal-based cropping system (Bhatt et al. 2019). In this region, sugarcane has been the most important commercial crop cultivated widely under diverse crop production and soil management strategies, which typically result in differential C input and stability under the effect of varying moisture regimes (Benbi et al. 2015; Singh and Benbi 2020a, b). These diverse cropping systems (rice-wheat vis-à-vis sugarcane) are cultivated side-by-side in the entire north-western India, and are established under contrasting moisture regimes, e.g., rice-wheat under alternatively aerobic (during wheat growing season) and anaerobic (during rice growing season), and sugarcane exclusively under aerobic environment, and may therefore have differential impacts on quality of soil organic matter (Singh and Benbi 2021; Avtar-Singh et al. 2023). In rice soils, soil organic matter becomes more lignified (Benbi et al. 2012), and therefore, C accumulation occurs more in recalcitrant pools (Sharma et al. 2020b; Singh and Benbi 2021). Nonetheless, sugarcane is established both as freshly sown (seed sugarcane) and ratoon crop (crop which sprouts from stubble), and has differential impacts on plant-mediated C input in response to contrasting soil and crop production management practices followed in both establishment methods. The intensified tillage under rice-wheat cropping system is considered important and responsible for breakdown of the stable aggregates as macro- and micro-aggregates (Singh and Benbi 2021; Sharma et al. 2022a), and eventual loss of encapsulated C (Six et al. 2000). On the other hand, reduced tillage intensity under ratoon crop as compared to the seed sugarcane with more intense tillage exerts differential impact on stability of water stable aggregates. A change in organic C concentration significantly influences soil physical properties by increasing soil aggregate stability, lowering the soil bulk density, and enhancing strong physico-chemical bonds among soil particles (Blanco-Canqui and Benjamin 2013; Singh and Benbi 2016). Soil aggregates play an important role in the bio-geochemical cycling and help improve biodiversity, nutrient cycling, and water availability (Vourlitis et al. 2015; Piedallu et al. 2016). The organic C protected in aggregate fractions is closely associated with soil C pool (Yu et al. 2015), and is presumed to be key for C preservation (Six et al. 2004; Kleber et al. 2015), and inherent C storage (Zhang et al. 2022).
The studies on C dynamics in soils under sugarcane ecosystems showed a significant change in formation and disruption of water stable aggregates in response to tillage intensity (Choudhury et al. 2014), but the studies investigated response on TOC pools and C preservation capacity of macro- and micro-aggregates remains scarce (Benbi et al. 2015; Singh and Benbi 2022). Therefore, investigations on change in land-use necessitate appropriate assessment of soil C dynamics in relation to a natural ecosystem to develop a long-term management strategy (Lal 2004). The present study therefore investigated the effect of different land-use systems, viz. rice-wheat, seed sugarcane, ratoon sugarcane, and permanent grassland vis-à-vis uncultivated lands on change in TOC pool and C preservation capacity of micro- and macro-aggregates. We hypothesized that tillage intensity and moisture regimes under which these cropland ecosystems are established would lead to differential stabilization of soil organic matter in different C pools of varying oxidizability, and encapsulation of C within water stable aggregates of different sizes. More specifically, we aimed at studying C dynamics among different C fractions of varying lability/oxidizability and management induced changes in C preservation capacity of macro- and micro-aggregates in soils under different cropland ecosystems, relative to the uncultivated lands. The quantification of TOC pool would help estimate loss of C due to land-use change and to frame policies for rehabilitation of degraded land-use systems with robust soil management interventions to build organic matter in soils.
2 Material and Methods
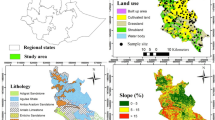
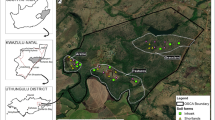
2.1 Description of the Study Area
The study was conducted in the Kapurthala district of Punjab extends between longitudes of 31°22′N and latitude of 75°23′ E, and lies between the Beas River and the “Kali-Bein” River, locally known as floodplains or Bet area. The total area of the district is ~ 1633 square km of which ~ 909 square km is in Tehsil Kapurthala, ~ 304 square km in Tehsil Phagwara, and ~ 451 square km area in Tehsil Sultanpur Lodhi. The economy of the district has been predominantly agricultural; wheat, rice, sugarcane, potato, and maize are the major crops in the district. The climate of the study region is typically sub-tropical steppe, semi-arid, with discrete rainy and dry seasons (Raj-Kumar et al. 2008). Mean monthly minimum temperature varied between 0.8 and 1.0 °C during mid-December to mid-January and monthly maximum temperatures of 41.8° and 47.0 °C during May–June (https://weatherspark.com/y/108387/Average-Weather-in-Kap%C5%ABrthala-India-Year-Round). The long-term data (1981–2010) revealed average highest temperature of 29.9 °C, average lowest temperature of 16.0 °C, average rainfall of ~ 637 mm, and the average relative humidity as ~ 70% in the district (https://en.wikipedia.org/wiki /Kapurthala). Major proportion (~ 80–85%) of total average annual rainfall is received in monsoon season extending between July and September. The original slope and form of the eolian sheets has been highly modified by the agricultural activities of the farmers in the study region.
2.2 Crop Production and Soil Management Practices
Out of total of 163,000 ha area, ~ 82.2% has been under cultivation, 2000 ha (~ 1.3%) under forest, and remaining ~ 16.5% under the non-agricultural use. Cropping intensity of the study region is ~ 205% with rice, sugarcane, maize, and potato as major field crops, while kinnow, orange, lemon, mango, pear, peach, and plum are the main horticultural crops. Conventionally, rice is established after puddling (wet-tillage) which involves mechanically stirring and mixing the surface soil layer with stagnant water converts soil into a muddy paste (Singh and Benbi 2016; Singh et al. 2018). About 80% of the total rice area in the study region has been under puddled transplanted rice, while the remaining ~ 20% is under direct seeded rice (DSR) (Bhatt and Singh 2022). Rice seedlings are manually transplanted after 30–35 days (depending upon variety) and the crop is harvested in October. Rice productivity in the region varied between 6.5 and 7.5 Mg ha−1 (Bhatt and Singh 2022). Wheat crop is established in the last week of October to end of November with seed-cum-fertilizer drill. Wheat crop is irrigated based on physical inspection of the crop and rainfall. Usually, depending upon weather and soil texture, 3–5 irrigations are applied by the farmers to wheat. The crop is harvested in first fortnight of April at physiological maturity. For sugarcane cultivation, early and mid-late sugarcane cultivars (CoPb 95, CoPb 96, CoPb 92, CoPb 93, CoPb 94, and Co 238) recommended for cultivation in the state are preferred by the farmers. Farmers perform cross sub-soiling at an interval of 1.0 m once every 3–4 years for breaking any underlying hard layer for proper growth of root growth. Sugarcane is planted in two different times during the years, viz. September–October (for autumn) and during February–March (for spring) in 75 cm widely spaced rows by maintaining 20–25 cm deep trenches using seed rate of 7.5–8.8 Mg ha−1. Depending on the received rainfall, 15–18 irrigations are provided to the cane crop.
2.3 Collection of Soil Samples and Analyses
The surface (0–15 cm) and sub-surface (15–30 cm) soil samples were collected in the months of March–April 2021 after wheat harvesting with post hole auger (inner diameter = 7.2 cm). A total of 57 locations were selected to represent the entire study region, which includes 17 samples from rice–wheat, 16 from seed sugarcane, 14 from ratoon sugarcane, and 5 each from permanent grasslands and uncultivated lands. At each location, four pseudo-replicates were created by collecting separate soil samples from at least 0.4 ha plot size. Large roots, litter, and stones from collected samples were meticulously removed. Soil samples were spread out for 48 h in the shade to dry. Intact soil cores of 7.5 cm height were used for the estimation of soil bulk density (Blake and Hartage 1986). Soil samples were analyzed for pH (1:2; soil:water) and electrical conductivity (E.C.1:2, dS m−1) (Jackson 1967). Soil’s textural class was determined by using hydrometer method.
2.4 Total Organic C and Its Fractions of Varying Oxidizability
The TOC was determined by reacting with 1N K2Cr2O7 solution at 150 °C for 60 min (Snyder and Trofymow 1984). Total organic C was apportioned into four fractions of variable lability under a gradient of oxidizing conditions using H2SO4-aqueous solution ratios. These ratios of 0.5:1, 1:1, and 2:1 corresponding to 12N, 18N, and 24N H2SO4, respectively (Chan et al. 2001), and represent Fract. 1, Fract. 2, and Fract. 3, respectively (Eqs. 1, 2, and 3). The recalcitrant C pool (Fract. 4) was estimated as a difference in TOC pool and 24N H2SO4 (Eq. 4).
The active C pool was estimated as sum of Fract. 1 + Fract. 2, while the passive C pool was computed as a sum of Fract. 3 + Fract. 4 (Eqs. 5 and 6).
2.4.1 Total Organic Carbon Stocks
The TOC stock in surface (0–15 cm) soils layer under different land-use systems was estimated by multiplying their respective TOC concentration (%) with soil bulk density (\({\mathrm{B}}_{\mathrm{D}};\) Mg cm−3) and depth (m) of soil sampling (Eq. 7).
2.4.2 Carbon Management Index (CMI)
The CMI was determined using mathematical procedure described by Blair et al. (1995). The CMI was estimated as a product of C pool index (CPI) and lability index (LI) using Eq. 8.
The CPI and LI were estimated using Eqs. 9 and 10, considering uncultivated lands as reference soil.
2.5 Distribution of Aggregates and C Preservation Capacity
The proportion of macro-aggregates (> 0.25 mm) and micro-aggregates (< 0.25 mm) was estimated using empirical relationships established for the regional soils (Singh and Benbi 2021). The relationships for the prediction of macro- and micro-aggregates in soils were based on soils’ fine fraction (i.e., silt + clay content) (see Eqs. 11 and 12).
The estimation of water stable aggregates was based on sum of macro-aggregates and micro-aggregates (Eq. 13).
The aggregate ratio was estimated as a ratio of proportion of macro-and micro-aggregates (Eq. 14).
Aggregate (macro- and micro-aggregate) associated C was estimated using Eqs. 15 and 16.
The estimation of C preservation capacity of macro-aggregates was based on empirical relationship established for the regional soils by Singh and Benbi (2021) based on relative proportion of macro-aggregates using Eq. 17.
2.6 Soil Enzyme Activity Analysis
For soil enzymes activity analysis, macro- and micro-aggregates (after removing crop residues) were stored at 4 °C until used for assaying of dehydrogenase (DHA) and alkaline phosphatase (Alk-P) activities. The dehydrogenase activity was estimated by reducing 2, 3, 5-triphenylterazolium chloride (Casida et al. 1964). The Alk-P activity was assayed on the basis of p-nitrophenol (pNP) release after cleavage of enzyme-specific synthetic substrates (Tabatabai and Bremner 1969).
2.7 Sensitivity Index
The sensitivity index (or the percent change in TOC pool) was determined as a ratio of difference in TOC pool in different cropland ecosystems (viz. rice-wheat, seed sugarcane, ratoon sugarcane, and grassland) and a reference soil (i.e., uncultivated land) to that of the reference soil (Eq. 18) (Singh and Benbi 2018a).
2.8 Statistical Analysis
Data were statistically analyzed using analysis of variance (ANOVA) technique in randomized block design (RBD) with unequal number of samples using SPSS software for Windows 21.0 (SPSS Inc., Chicago, USA). Soil sampling sites were treated as replicates (random effects) and land-use systems as treatments (fixed effects). Means for treatment effects were separated based on Duncan’s Multiple Range Test (DMRT) post hoc test at p < 0.05.
3 Results
3.1 Basic Soil Properties
Soil pH was significantly (p < 0.05) lower in rice-wheat, and the highest in soils under ratoon sugarcane (Table 1). Conversely, the E.C. was significantly lower in surface (0–15 cm) and sub-surface (15–30 cm) soils under ratoon sugarcane, compared with the other land-use systems. These results revealed significantly highest E.C. of the surface layer of rice-wheat soils, which was higher by ~ 10.7–34.8% than the sugarcane soils. The sand proportion varied between 60.1 and 73.8% in surface layer and 62.7 and 75.0% in sub-surface, and was significantly higher for soils under seed sugarcane. The clay content varied between 10.0 and 13.2% in surface layer and between 9.4 and 13.5% in sub-surface soil layer.
3.2 Total Organic C Pool and Its Fractions of Varying Oxidizability
TOC pool in the surface soil layer was significantly lower under seed sugarcane, while the highest in uncultivated soils (Table 2). As compared with the seed sugarcane, soils under ratoon sugarcane had ~ 10.3% higher TOC pool. Sugarcane cultivation resulted in a significant decrease in TOC pool by ~ 11.9 and 11.3%, respectively, in surface and sub-surface soils layers. The TOC pool in soils under rice–wheat and ratoon sugarcane did not differ significantly. The Fract. 1 and Fract. 2 were significantly lower in soils under permanent grasslands, while the highest in ratoon sugarcane. However, Fract. 3 was significantly lower under sugarcane, and highest in permanent grassland. Fract. 1 comprised ~ 5.8–10.3% of TOC in the surface, while between 4.7 and 9.4% in the sub-surface soil layer (Table 2). Likewise, Fract. 2 comprised between 7.9 and 14.7% of TOC in surface, and between 6.8 and 13.1% in the sub-surface soil layer. As a proportion of TOC pool, Fract. 1 and Fract. 2 were significantly lower under permanent grasslands, while the higher in ratoon sugarcane. However, Fract. 3 comprised the largest fraction of TOC pool, ~ 19.6–34.4% of TOC in surface and ~ 17.4–30.9% in sub-surface soil layer. The rice–wheat soils had ~ 38.5 and 26.7% higher Fract. 3 in surface and sub-surface soil layers, respectively, compared with seed sugarcane. The ratoon sugarcane had ~ 24.8 and 20.1% higher Fract. 3 in surface and sub-surface soil layers, respectively, over seed sugarcane. Grasslands had ~ 15.8% higher Fract. 3 in surface, and ~ 8.4% in sub-surface layer, compared to uncultivated lands. Fract. 4 was the largest fraction, comprised ~ 51.9 and 58.1% of TOC pool in surface, and between ~ 57.6 and 61.2% in sub-surface soil layer (Table 2).
3.3 Soil Bulk Density and TOC Stocks
Soil bulk density was significantly lower for soils under rice-wheat and seed sugarcane, while higher for other land-use systems, which themselves did not differ significantly (Table 2). Soils under seed sugarcane had ~ 11.5% lower C stocks (data pooled for soil depths), compared with the rice-wheat soils. However, C stocks in soils under rice-wheat and ratoon sugarcane did not differ significantly. As compared with seed sugarcane, ratoon sugarcane had ~ 11.9 and 13.3% higher C stocks in the surface and sub-surface soil layers, respectively. These results revealed significant loss of C stocks following land-use change from uncultivated land. The highest C loss (of 3.3–3.7 Mg C ha−1) occurred under seed sugarcane, followed by rice-wheat (1.9–2.0 Mg C ha−1) and ratoon sugarcane (1.9–2.1 Mg C ha−1) (Fig. 1). The minimum C loss up to 0.3 Mg C ha−1 occurred in soils under grassland ecosystem, compared with uncultivated soils. The C loss under rice-wheat and ratoon sugarcane ecosystems following land-use change from uncultivated soils was statistically at par.
Loss in total soil organic C (TOC) stocks after land-use change in surface (0–15 cm) and sub-surface (15–30 cm) soil layers under different land-use systems in north-western India. Bars indicate standard error from mean. Mean values followed by different letter are significantly different at p < 0.05 by Duncan’s Multiple Range Test (DMRT)
3.4 Soil Enzymatic Activity
The enzymatic activity of dehydrogenase, alkaline phosphatase, and asparaginase was significantly higher in soils under grasslands (Table 2). The uncultivated lands had similar enzymatic activity to that of the soils under ratoon sugarcane. Seed sugarcane soils had significantly lower enzymatic activity, compared with rice-wheat soils. Ratoon sugarcane help enhanced soil enzymatic activity to a significant extent, as compared to the seed sugarcane. Regardless of the land-use system, enzymatic activity was higher in the surface layer, compared with that in the sub-surface soil layer.
3.5 Soil Organic Matter Quality and C Management Index
The active C pool (Fract. 1 + Fract. 2) was significantly lower in grasslands, compared with other investigated land-use systems (Table 3). The active C pool in surface soil layer under ratoon sugarcane was significantly higher by ~ 25.1–64.9%, compared with others. In the sub-surface soil layer, active C pool was significantly lower in grasslands and highest in ratoon sugarcane. The passive C pool (Fract. 3 + Fract. 4) was significantly lower in soils under seed sugarcane, highest in grasslands. The lability index was significantly lower for rice-wheat soils, compared with soils under sugarcane cultivation. The comparison between rice-wheat and grasslands/uncultivated lands revealed non-significant difference in lability index among themselves. The CPI was significantly lower under seed sugarcane, highest in grasslands, while rice-wheat/ratoon sugarcane in between. The CMI was significantly higher under ratoon sugarcane by ~ 26.9 and 22.9%, respectively, in surface and sub-surface layers, compared with seed sugarcane. These results reaffirm significantly higher concentration of passive C pool in soils under ratoon sugarcane, compared with the soils under seed sugarcane. The sensitivity index estimated for TOC pool showed significantly higher C sensitivity under seed sugarcane (8.6–21.8%), followed by ratoon sugarcane (10.3–13.6%), rice-wheat (7.6–11.8%), while the lowest for grassland ecosystems (0.2–0.5%) following land-use change from uncultivated lands (Fig. 2). The compassion of rice-wheat and ratoon sugarcane showed significantly higher sensitivity index for soils under ratoon sugarcane.
3.6 Distribution of Water Stable Aggregates, Aggregate Associated C
The total water stable aggregates varied between 70.0 and 74.4% in surface layer, while between 69.7 and 73.5% in sub-surface soil layer under different land-use systems (Table 4). Results revealed significantly lower proportion of total water stable aggregates under seed sugarcane, compared with other investigated land-use systems. Rice-wheat system had significantly higher proportion of water stable aggregates, compared with sugarcane ecosystems. Although the grasslands and uncultivated lands had highest proportion of total water stable aggregates, but these two land-use systems themselves did not differ significantly. Of the total water stable aggregates, macro-aggregates (> 0.25 mm) had the largest proportion (38.3–46.8% in surface and 37.3–45.7% in sub-surface layer), while the proportion of micro-aggregates (< 0.25 mm) varied between 27.5 and 31.7% in surface and between 27.7 and 32.4% in sub-surface layer. The proportion of macro-aggregates was significantly higher in undisturbed ecosystems (grasslands and uncultivated lands), followed by rice-wheat system, while the lowest under seed sugarcane. The soils under ratoon sugarcane had ~ 9.7 and 8.6% higher macro-aggregates, respectively, in surface and sub-surface layer than seed sugarcane. Conversely, the proportion of micro-aggregates was significantly lower in soils under undisturbed ecosystems (grasslands and uncultivated lands), while the highest in ratoon sugarcane (Table 4). Aggregate ratio was significantly lower for soils under seed sugarcane, and highest for uncultivated lands. The rice-wheat soils had ~ 30.6 and 32.2% higher aggregate ratio, compared with seed sugarcane. The proportion of macro-aggregates under different land-use systems exhibited a linear significant relationship with TOC pool (Fig. 3). The relationship between the two variables (R2 = 0.964*; p < 0.05) could best be described by linear function (macro-aggregates (%) = 4.72 (TOC, g kg−1) + 48.6.
Aggregate associated C differs significantly under different land-use systems (Table 4). The macro-aggregates associated C varied between 1.68 and 3.22 g kg−1 aggregates in surface layer, while between 1.55 and 2.97 g kg−1 aggregates in surface soil layer under different land-use systems. Regardless of soil depth, the macro-aggregates associated C (MacA-C) was significantly higher for soils under undisturbed ecosystems (grasslands and uncultivated lands), while the soils under seed sugarcane had the lowest MacA-C. As compared with seed sugarcane, ratoon sugarcane system had higher C concentration by 0.56 g C kg−1 aggregates (~ 33.3%) and 0.43 0.56 g C kg−1 aggregates (27.7%) in surface and sub-surface soil layers, respectively. The compassion of cropland ecosystems, viz. rice-wheat vis-à-vis seed sugarcane, revealed significantly higher MacA-C by ~ 60.7 and 61.9%, respectively, for surface and sub-surface soil layers. The micro-aggregate associated C (MicA-C) exhibited a similar trend for surface and sub-surface soils under different land-use systems. As compared with MacA-C, MicA-C was lower by ~ 3.3–3.9 times for surface, and 3.3–3.7 times for sub-surface soil layer under different land-use systems.
3.7 Carbon Preservation Capacity
The C preservation capacity of macro-aggregates (> 0.25 mm) varied between 0.87 and 1.94 g kg−1 soils for surface layer and between 0.52 and 0.54 g kg−1 soil for sub-surface layer under different land-use systems (Table 4). A significantly lower C preservation capacity of macro-aggregates for surface soil layer under seed sugarcane (by ~ 0.37 g kg−1; 42.5%) was observed, compared with the ratoon sugarcane. Rice–wheat ecosystem had significantly higher C preservation capacity of macro-aggregates by ~ 0.70 g kg−1 soil (80.5%) than the seed sugarcane. The surface layer of undisturbed ecosystems (grasslands/uncultivated land) had significantly higher C preservation capacity of macro-aggregates, compared with the cropland ecosystems.
3.8 Relationship Between Different C Fractions and Soil Properties
Passive C pool showed highly significant linear positive relationship (Pearson’s correlation coefficient, “r”) with macro-aggregate associated C (r = 0.807**), silt + clay (r = 0.816**), Fract. 3 (r = 0.969**), Fract. 4 (r = 0.939**), and silt (r = 0.760**), but negative relationship with sand (r = − 0.760*) (Table 5). Macro-aggregate associated C exhibited a linear positive relationship with TOC pool (r = 0.712*), Fract. 3 (r = 0.874**), silt (r = 0.974**), and clay (r = 0.714*). The TOC pool showed a linear increase with increase in silt and clay, but decrease with increase in sand content in soil. Fract. 3 and Fract. 4 exhibited a negative linear significant relationship with sand (r = − 0.885** and − 0.640*, respectively). Single linkage correlation between different C fractions and soil mineral matrix showed close association between Fract. 3, Fract. 4, and TOC pool in soils under land-use systems (Fig. 4). The finer fraction (silt + clay) showed close linkage with passive C pool which is closely associated with MacA-C.
4 Discussion
Total organic C has been the largest C pool in terrestrial ecosystems, the size of which depends upon the quality and quantity of plant-mediated C inputs and losses from soils as respiration, and the mean residence time of C pool (Li et al. 2009; Singh and Benbi 2018a, 2021). These results may be explained through several factors, viz. (i) high input of organic matter to the rice–wheat system relative to sugarcane soil from the above- and below-ground plant biomass, (ii) relatively low C mineralization rate in grasslands and soils under sugarcane ratoon, (iii) the larger proportion of macro-aggregates in the grasslands than in the other land-use systems, and (iv) the preferential stabilization of soil organic matter in macro-aggregates as opposed to smaller size classes (Howlett et al. 2011; Mosquera-Losada et al. 2015). A significant change in TOC pool takes several years after a land-use change (Gregorich et al. 1994; Sharma et al. 2020b). Land-use systems vary widely for soil organic matter stabilization (Singh and Benbi 2018b, 2021), depending upon crop production and soil management practices (Benbi et al. 2015; Sharma et al. 2021; Singh and Benbi 2021). The contrasting soil moisture regimes under which different cropping systems are established (Olk et al. 1996), tillage intensity (Roger-Estrade et al. 2010; Sharma et al. 2022a,b), nutrient management practices (Singh and Benbi 2018a; Sharma et al. 2020a), type of vegetation, and their root geometry and architecture (Mandal et al. 2022; Singh and Benbi 2022) greatly influence plant-mediated C input and are considered responsible for differential stabilization of soil organic matter (Ghosh et al. 2012; Singh and Benbi 2021). In submerged soils, there occurs a large accumulation of lignin-derived substances (Ye and Wen 1991). The rice-wheat soils are characterized by alteration in soil moisture regime from aerobic during wheat growing season and anaerobic environment during rice, which lead to incorporation of phenolic moieties into young soil organic matter fractions (Olk et al. 1996), and impart significant recalcitrance to soil organic matter (Benbi et al. 2016; Singh and Benbi 2021). The higher TOC pool in soils under rice-wheat cropping as compared to sugarcane-based ecosystem was ascribed to prevailing submerged conditions during rice cultivation, which lead to greater accumulation of C in recalcitrant pool. The TOC storage in the grassland and ratoon sugarcane would have probably been higher than in the rice-wheat cropping if intensive agriculture had been carried out, because intensive agriculture usually afford considerable soil degradation and C depletion (Plaza-Bonilla et al. 2015). Among the sugarcane-based ecosystem (seed and ratoon crop), higher organic C pool in soils under ratoon sugarcane was ascribed to reduced tillage intensity, compared with that under every year’s freshly planted seed sugarcane crop. Intense tillage causes disruption of soil structure and aggregates, thereby exposing the encapsulated organic matter to oxidative micro-organisms (Six et al. 2000; Benbi et al. 2016). Tillage accentuates break-up of macro-aggregates (> 0.25 mm) and leads to the formation of micro-aggregates (< 0.25 mm) (Tisdall and Oades 1982; Six et al. 2000; Benbi et al. 2016). Tillage causes loss of aggregate associated organic C which remains protected from mineralization due to less physical and microbial degradation (Somasundaram et al. 2009; Sharma et al. 2022b).
Land-use change from relatively undisturbed ecosystems (e.g., uncultivated/grasslands in the present study) to those under intense tillage is considered important to discern changes in C pool and its fractions due to altered soil organic matter quality (Li et al. 2009; Mandal et al. 2008; Beheshti et al. 2012). These results revealed significantly lower TOC stocks in soils under seed sugarcane, compared with rice-wheat and ratoon sugarcane. As compared with sugarcane-based ecosystem, rice-wheat cropping maintained relatively higher C stocks, which could be ascribed to higher plant-mediated C input into the soil (Singh and Benbi 2022). In a rice-wheat cropping, total C input through root + shoot biomass and rhizodeposition in different nutrient management treatments varied between 1458 and 3298 kg C ha−1 year−1 (Singh and Benbi 2022). Nonetheless, soils under rice-wheat cropping remain flooded for 3–4 months during rice period which retard the rate of C oxidation and decrease the rate of soil organic matter decomposition by soil microbes (Sharma et al. 2020a). In general, a high proportion of soil macro-aggregates were associated with a low soil BD because macro-aggregates are plant residues that retain an identifiable cell structure (Kogel-Knabner et al. 2008). Therefore, the BD tends to decrease as soil organic matter levels increase (Sakin 2012). Nonetheless, the higher percentage of macro-aggregates under grassland and ratoon sugarcane compared to that of the other land-use systems could also be explained by the downward displacement of the finest particles by the roots, which increase the formation of bio-pores in the soil surface layers, thereby favoring the transport of small particles towards deeper soil layers (Ferreiro-Domínguez et al. 2016).
Soil organic C can regulate the properties of soil and improve soil stability. Organic C accumulation and transformation can directly or indirectly affect biochemical processes, absorption, and release of water and nutrients (Štursová and Baldrian 2011), and C content in soil is closely associated with soil quality and agricultural productivity (Shi et al. 2014). Due to differences in organic substrate availability in soils, the concentration of C pools differed dramatically in cultivated and uncultivated soils under different land uses. The active C pool (Fract. 1 + Fract. 2) constitutes the labile C pool, and is composed of organic C that decomposes quickly in plant materials by microbes (Singh and Benbi 2018a). These labile C components have poor stability which makes them highly oxidizable and causes fast mineralization in the soil (Mandal et al. 2008). Conversely, the stable C pool (Fract. 3 + Fract. 4) has been the most resistant fraction to microbial destruction (Singh and Benbi 2018a). The recalcitrant C (Fract. 4) comprised the greatest proportion in both the disturbed vis-à-vis undisturbed ecosystems under different land uses (Benbi et al. 2012, 2015). Diversity in plant species, soil moisture, and root biomass and intensity of soil disturbance and organic C inputs are considered important contributors of TOC quantity and quality, where such variation shapes the soil structure (Tirgarsoltani et al. 2014). Blair et al. (1995) proposed CMI an index of organic matter degradation and/or rehabilitation. It has been hypothesized as integrated measure of C stability which describes the soil organic matter quality and creates the groundwork for increased production in different land-use systems (Blair et al. 1995; Tirol-Padre and Ladha 2004). The CMI is a comprehensive measure of C quantity and quality (Blair et al. 1995), which is considered highly sensitive indicator to discern changes in response to improvement in soil organic matter quality (Tirol-Padre and Ladha 2004), and accumulation of lignin-derived substances (Olk et al. 1996; Singh and Benbi 2018a). Considering the uncultivated soils as baseline reference, CMI was higher for soils under ratoon sugarcane followed by permanent grasslands and rice-wheat system. It could be ascribed not only to increased soil organic matter as a result of increased annual C addition through above- and below-ground biomass (Singh and Benbi 2020a), but also because of formation of a stable C pool with less decomposition under anaerobic conditions (Tirol-Padre and Ladha 2004). Additionally, CPI was highest in grasslands followed by rice-wheat soils and ratoon sugarcane and the lowest under seed sugarcane. The changes in C lability and build-up of lignin-derived compounds in rice soils suggest improved soil organic matter quality (Tirol-Padre and Ladha 2004; Singh and Benbi 2021). Rice-wheat systems produce less oxidative environment and better physical protection for soil organic matter (Singh and Benbi 2020a, b; Blair et al. 1995).
Land-use change greatly influences the C sequestration rates (Deng et al. 2014). Grassland had the highest C storage, TOC sequestration rate, and sequestration potential, followed by ratoon sugarcane, while rice-wheat and seed sugarcane had the lowest. It could be been ascribed to the fact that (1) due to high ecological functions of grassland ecosystem, the absorption of C is increased (Lange et al. 2015) while (2) the formation of water stable aggregates which protects TOC pool was greater in grassland ecosystems (Six et al. 2004; Singh and Benbi 2018b).
Land-use and associated management practices have profound effects on the characteristics of soil aggregates (Six et al. 2004; Benbi et al. 2016; Zhao et al. 2017). Aggregate stability can promptly respond to land-use change (Liu and Han 2020). Organic matter is the core of the formation of 100–200 μm agglomerates; the surfaces of which can adsorb soil cosmids and cement soil micro-aggregates into large agglomerates (Jastrow 1996). Plant residue mediated C returned to grasslands and ratoon sugarcane can increase the stability of soil aggregates by supplementing them with fresh organic matter, increasing the proportion of humus and the aggregate structure of soil and increasing the activity of soil micro-organisms (Blanco-Canqui and Lal 2009). Returning residue can also reduce the impact of slap and leaching of rain on the soil, reduce the energy of rain falling to the ground (Fang et al. 2018), and reduce damage to the soil aggregate structure in grassland and ratoon sugarcane crop (Blanco and Lal 2008).
The proportion of macro-aggregates was higher in undisturbed ecosystems, compared with the disturbed cropland ecosystems. Ploughing brings the returning straw in close contact with the soil, which results in faster straw decomposition and faster accumulation of soil organic C. But, if the intensity of tillage is too large, it will destroy the original soil structure and increase the effects of drying-rewetting on the soil, intensifying the destruction of large C-rich aggregates in the soil, affecting the formation and stability of large aggregates. Intensified tillage causes the breakage of aggregates and thereby reduces the physical protection of organic C within the aggregates, and forming many small aggregates containing organic C and free organic matter. However, the small aggregates have a limited ability to retain soil organic C (Benbi et al. 2016), and the stability of free organic matter is poor, which accelerates the mineralization of soil organic matter and increases the loss of soil organic C (Six et al. 2004; Yang et al. 2003; Mikha and Rice 2004). However, no tillage, reduced tillage, and other conservation tillage methods cause less disturbance to the soil, reduce extent of destruction of soil aggregates, slow the turnover of macro-aggregates, keep soil aggregates separated between areas of biological accumulation and mineralization, reduce the C mineralization rate in soil aggregates, prolong the storage period of organic C in the aggregates, slow the circulation rate in soil, and thereby increase the soil organic C content (Dalal and Chan 2001; Barto et al. 2010).
These results revealed that C preservation capacity and aggregate ratio was highest for uncultivated and grasslands. It might be due to the breakage of macro-aggregates in micro-aggregates to oxidizing the hidden C by microbes as CO2 in soils under cropland ecosystems (e.g., rice-wheat/sugarcane). Pan et al. (2007) reported that the accumulation of soil organic C increases as proportion of macro-aggregates (> 0.25 mm) increases, while decreases with increase in proportion of micro-aggregates (< 0.25 mm). In rice-wheat cropping system and sugarcane-based cropland ecosystems, there is not enough time between the formation and disruption of macro-aggregates to form significant numbers of new micro-aggregates within macro-aggregates. Therefore, less new crop C gets incorporated into micro-aggregates in rice-wheat cropping and seed sugarcane, compared with the ratoon sugarcane and grasslands. The increased TOC content in aggregates under grasslands was ascribed to the higher organic C input originating from biomass as plant litter and root exudates (Wang et al. 2014). Grassy vegetation cover in grasslands also contributes towards the formation of macro-aggregates by increasing aggregating agents such as root exudates, hyphae, and polysaccharides (Six et al. 2004; Wang et al. 2018). At the same time, enhanced soil aggregate stability under grass cover could reduce soil aggregate breakdown (Singh and Benbi 2018b). Therefore, there would be less C loss under enhanced soil aggregate stability during erosive rainfall (Shi et al. 2017).
Accumulation of C pool in soils has great influence on soil enzymatic activity responsible for cycling of C, N, and P. Soil enzymatic activity and functioning is essential not only to soil health and fertility (Brady and Weil 2016), but also to C sequestration and global C cycling (Chen et al. 2020). Soil enzymes are biological machines, which play a major role in the bio-geochemical processes and are linked to “plant-soil enzymes-soil nutrients” of the soil system (Lino et al. 2015; Naylor et al. 2020). Soil micro-organisms can regulate microbial nutrient use efficiency by secretion of extracellular enzymes (Mosca et al. 2007) to catalyze the enzymatic depolymerization of complex C compounds to produce soluble, low molecular weight compounds which can be readily assimilated in a soil-plant system (Sinsabaugh 2010; Chen et al. 2017). As a key regulator of litter decomposition, soil enzyme activities may have a significant influence on C fractions. The hydrolytic and oxidative enzymes may make different contributions to the formation of C fractions (Li et al. 2016). The organic C affects the microbial biomass and activity by providing habitat (i.e., stable aggregates) and substrate, while in turn microbial processes determine C turnover. At the same time, vegetation restoration protects enzyme substrates by improving soil structure, and promotes the contact of soil enzymes resulting in increased soil enzymatic activity (Grigera et al. 2006).
The correlation analysis revealed that the majority of C pools were significantly inter-related. As a result, the labile and stable C pools were sensitive indicators of TOC change (Singh and Benbi 2018a). Because of their sensitivity towards land-use change, environmental conditions, and consistent reaction to TOC change, these C pools are frequently employed as effective markers for soil quality assessment (Melero et al. 2009). Labile C fractions are a source of energy and have an impact on different soil properties and C stocks. As a result, a substantial link between labile C fractions was predicted. Passive C significantly affected the TOC and recalcitrant C fractions. Besides, a positive effect relationship between C concentration and the silt + clay fraction was also observed. Several studies carried out under different climatic conditions have also shown that grasslands and forest ecosystems contribute to increasing the C associated with both soil micro-aggregates and silt + clay fraction (Singh and Benbi 2021). The significant correlation between TOC and silt suggests an increasing effect of protection mechanisms for TOC in soil with increasing fine fraction content (Six et al. 2000, 2002; Galantini et al. 2004; Singh and Benbi 2021). The C associated with the micro-aggregates and the silt + clay fraction represents very stable (recalcitrant) C that remains in the soil over long periods, something necessary to help mitigate the effects of climate change. Similarly, Bronick and Lal (2005) reported that TOC was positively related to soil C in macro-aggregates. Bashir et al. (2016) also found that TOC in macro-aggregates and micro-aggregates was significantly increased compared with that in other fractions under organic manure application.
5 Conclusion
Land-use change significantly impacts the C storage within an ecosystem because of large influence on aggregate stability. We compared the land-use change from uncultivated lands to grasslands, rice-wheat, and sugarcane ecosystems to study their effects on total organic C and its fractions of variable oxidizability due to change in soil management and crop production regimes. The response of land-use change was assessed via change in total organic C pools, aggregate proportion, and their C preservation capacity in soils under different land-use systems in north-western India. The intensively cultivated ecosystems, e.g., rice-wheat and seed sugarcane, have significantly higher depletion of organic C in aggregate size fractions, decreased aggregate stability, and soil enzymatic activities. A relatively lower C storage in the soils under rice-wheat and seed sugarcane was ascribed to intensified tillage. A linear significant relationship between soil mineral matrix (silt + clay) with total organic C (r = 0.721*), less labile C (Fract. 3; r = 0.884**), and recalcitrant C (Fract. 4; r = 0.638*) for soils under land-use systems indicates overwhelming significance of soils’ fine fraction in enhancing C sequestration in soils. These results revealed higher C preservation capacity of macro-aggregates for soils ratoon sugarcane, as compared with other cropland ecosystems, e.g., rice-wheat/seed sugarcane. Nonetheless, ratoon sugarcane had significantly higher C management index than the other compared land-use systems, indicating greater C rehabilitation due to reduced tillage intensity. Conversely, the lower values of C management index for soils under seed sugarcane indicate C degradation in response to more intensified tillage. Therefore, the adoption of permanent grassland and ratoon sugarcane soils promotes greater incorporation of C in the soils, resulting in better soil structuring which consequently contributes to C preservation capacity, lower soil density, higher biological processes, and C stabilization.
References
Adak T, Sachan R (2009) Effect of co-inoculation of Sinorhizobiummeliloti and Bacillus megaterium on yield and nutrient uptake of fenugreek (Trigonellafoenum-graecum L.) in Mollisol soil. J Med Aromatic Plant Sci 31:124–130. https://doi.org/10.5281/zenodo.812628
Avtar-Singh, Singh P, Mahajan M (2023) Agronomic and biochemical quality attributes and economic indices of sugarcane (Saccharum officinarum L.) cultivation in saline vis-à-vis non-saline soils of south-western Punjab, India. Indian J Agric Sci 93: 106–109. https://doi.org/10.56093/ijas.v93i1.130246
Avtar-Singh, Singh P, Dhillon GPS, Sharma S, Gill RIS (2022) Differential impacts of soil salinity and water logging on Eucalyptus growth and carbon sequestration under mulched vs. unmulched soils in south-western Punjab. India Plant Soil. https://doi.org/10.1007/s11104-022-05700-1
Barto EK, Friese CF, Cipollini D (2010) Arbuscular mycorrhizal fungi protect a native plant from allelopathic effects of an invader. J Chem Ecol 36:351–360. https://doi.org/10.1007/s10886-010-9768-4
Bashir K, Ali S, Ijaz SS, Ahmad I (2016) Effect of organic amendments on distribution, stability and carbon concentration of soil aggregates. Pak J Agri Sci 53:955–961. https://doi.org/10.21162/PAKJAS/16.4205
Beheshti A, Raiesi F, Golchin A (2012) Soil properties, C fractions and their dynamics in land-use conversion from native forests to croplands in northern Iran. Agric Ecosys Environ 148:121–133. https://doi.org/10.1016/j.agee.2011.12.001
Benbi DK, Brar K, Toor AS, Singh P, Singh H (2012) Soil carbon pools under poplar-based agroforestry, rice-wheat, and maize-wheat cropping systems in semi-arid India. Nutrient Cycl Agroecosys 92:107–118. https://doi.org/10.1007/s10705-011-9475-8
Benbi DK, Brar K, Toor AS, Singh P (2015) Total and labile pools of soil organic carbon in cultivated and undisturbed soils in northern India. Geoderma 237–238:149–158. https://doi.org/10.1016/j.geoderma.2014.09.002
Benbi DK, Singh P, Toor AS, Verma G (2016) Manure and fertilizer application effects on aggregate and mineral-associated organic carbon in a loamy soil under rice-wheat system. Commun Soil Sci Plant Anal 47:1828–1844. https://doi.org/10.1080/00103624.2016.1208757
Bhatt R, Singh P (2022) Farmer’s field evaluation of direct seeded rice vis-à-vis puddled transplanted rice in Kapurthala Punjab. Indian J Ext Edu 58:42–46. https://doi.org/10.48165/IJEE.2022.58208
Bhatt R, Hussain A, Singh P (2019) Scientific interventions to improve land and water productivity for climate-smart agriculture in South-Asia. In: Mirza H (ed) Agronomic Crops, 2, Management Practices. Springer, pp 449–458
Bhatt R, Singh P, Kaur G (2022) Soil management vis-à-vis carbon sequestration in relation to land use cover/change in terrestrial ecosystem-a review. In: Hanuzzaman M, Ahammed GJ and Nahar K (eds.) Managing plant production under changing environment. Springer Nature, Singapore. https://doi.org/10.1007/978-981-16-5059-8_3
Blair GJ, Lefroy RDB, Lisle L (1995) Soil carbon fractions, based on their degree of oxidation, and the development of a carbon management index for agricultural systems. Austra J Agric Res 46:1459–1466. https://doi.org/10.1071/AR9951459
Blake GR, Hartage KH (1986) Bulk density In Klute A (Ed.) Methods of soil analysis (part-I). Agron No. 9. Am Soc Agron Madison, USA
Blanco C, Lal R (2008) Principles of soil conservation and management. Springer, Dordrecht. https://doi.org/10.1007/978-1-4020-8709-7
Blanco-Canqui H, Lal R (2009) Crop residue management and soil carbon dynamics. In: Lal R and Folett R F (Eds.), Soil carbon sequestration and the greenhouse effect. SSSA Spec. Publ. 57, Madison, WI, pp 291–310.
Blanco-Canqui H, Benjamin JG (2013) Impacts of soil organic carbon on soil physical behavior. In: Logsdon S, Berli M, Horn R (eds) Quantifying and modeling soil structure dynamics: advances in agricultural systems modeling, vol 3. Soil Science Society of America Inc., Madison, WI, pp 11–40
Brady NC, Weil RR (2016) Nature and properties of soils. 15th Edition ISBN: 978–0133254488
Bronick CJ, Lal R (2005) Soil structure and management: a review. Geoderma 124:3–22. https://doi.org/10.1016/j.geoderma.2004.03.005
Casida LA, Klein DA, Santoro T (1964) Soil dehydrogenase activity. Soil Sci 98:371–376
Chan KY, Bowman A, Oates A (2001) Oxidizible organic car- bon fractions and soil quality changes in oxicpaleus-talf under different pasture leys. Soil Sci 166:61–67. https://pubag.nal.usda.gov/catalog/1999819. Accessed 30 Dec 2015
Chen J, Luo Y, Li J, Zhou X, Cao J, Wang R (2017) Costimulation of soil glycosidase activity and soil respiration by nitrogen addition. Global Change Biol 23:1328–1337. https://doi.org/10.1111/gcb.13402
Chen J, Elsgaard L, van Groenigen KJ, Olesen JE, Liang Z, Jiang Y (2020) Soil carbon loss with warming: new evidence from carbon-degrading enzymes. Global Change Biol 26:1944–1952. https://doi.org/10.1111/gcb.14986
Choudhury SG, Srivastava S, Singh R, Chaudhari SK, Sharma DK, Singh SK, Sarkar D (2014) Tillage and residue management effects on soil aggregation, organic carbon dynamics and yield attribute in rice-wheat cropping system under reclaimed sodic soil. Soil till Res 136:76–83. https://doi.org/10.1016/j.still.2013.10.001
Dalal RC, Chan KY (2001) Soil organic matter in rainfed cropping systems of the Australian cereal belt. Aust J Soil Res 39(3):435–464. https://doi.org/10.1071/SR99042
Deng L, Liu GB, Shangguan ZP (2014) Land-use conversion and changing soil carbon stocks in China’s ‘Grain-for-Green’ program: a synthesis. Global Change Biol 20:3544–3556. https://doi.org/10.1111/gcb.12508
Doran JW, Zeiss MR (2000) Soil health and sustainability: managing the biotic component of soil quality. Appl Soil Ecol 15(1):3–11. https://doi.org/10.1016/S0929-1393(00)00067-6
Fang Y, Nazaries L, Singh BK, Singh BP (2018) Microbial mechanisms of carbon priming effects revealed during the interaction of crop residue and nutrient inputs in contrasting soils. Global Change Biol 24:2775–2790. https://doi.org/10.1111/gcb.14154
Ferreiro-Domínguez N, Rigueiro-Rodríguez A, Rial-Lovera KE, Romero-Franco R, Mosquera-Losada MR (2016) Effect of grazing on carbon sequestration and tree growth that is developed in a silvopastoral system under wild cherry (Prunus avium L.). Catena 142:11–20. https://doi.org/10.1016/j.catena.2016.02.002
Galantini JA, Senesi N, Brunetti G, Rosell R (2004) Influence of texture on organic matter distribution and quality and nitrogen and sulphur status in semiarid Pampean grassland soils of Argentina. Geoderma 123:143–152. https://doi.org/10.1016/j.geoderma.2004.02.008
Ghosh S, Wilson B, Ghoshal S, Senapati N, Mandal B (2012) Organic amendments influence soil quality and carbon sequestration in the Indo-Gangetic Plains of India. Agri Ecosys Environ 156:134–141. https://doi.org/10.1016/j.agee.2012.05.009
Gregorich EG, Carter MR, Angers DA, Monreal CM, Ellert BH (1994) Towards a minimum data set to assess soil organic matter quality in agricultural soils. Canadian J Soil Sci 74:367–385. https://doi.org/10.4141/cjss94-051
Grigera MS, Drijber RA, Eskridge KM, Wienhold WJ (2006) Soil microbial biomass relationships with organic matter fractions in a Nebraska corn field mapped using apparent electrical conductivity. Soil Sci Soc Am J 70:1480–1488
Howlett DS, Moreno G, Mosquera MR, Losada PK, Nair R, Nair VD (2011) Soil carbon storage as influenced by tree cover in the Dehesa cork oak silvo pasture of central-western Spain. J Environ Monitor 13:1897–1904. https://doi.org/10.1039/C1EM10059A
IPCC (2001) Intergovernmental panel on climate change. Climate Change. Cambridge, UK: The Scientific Basis. Cambridge University Press
Jackson ML (1967) Soil chemical analysis. Prentice Hall International Inc, London
Jastrow JD (1996) Soil aggregate formation and the accrual of particulate and mineral associated organic matter. Soil Bio Biochem 28:665–676. https://doi.org/10.1016/0038-0717(95)00159-X
Kleber M, Eusterhues K, Keiluweit M, Mikutta C, Mikutta R, Nico PS (2015) Mineral–organic associations: formation, properties, and relevance in soil environments. Adv Agron 130:1–140. https://doi.org/10.1016/bs.agron.2014.10.005
Kogel-Knabner I, Ekschmitt IK, Flessa H, Guggenberger G, Matzner E, Marschner B, Lützow MV (2008) An integrative approach of organic matter stabilization in temperate soils: linking chemistry, physics and biology. J Plant Nutri Soil Sci 171:5–13. https://doi.org/10.1002/jpln.200700215
Kumar R, Singh B, Kaur P, Beri V (2008) Planning for precision farming in different agro-ecological sub-regions of Punjab-role of natural resources in agricultural research, planning, development, and transfer of technology. Department of Soils, Punjab Agricultural University, Ludhiana-141001, India, p. 72+13 maps
Lal R (2004) Soil carbon sequestration to mitigate climate change. Geoderma 123:1–22. https://doi.org/10.1016/j.geoderma.2004.01.032
Lange M, Eisenhauer N, Sierra CA, Bessler H, Engels C, Griffiths R, Mellado-Vázquez PG, Malik AA, Roy J, Scheu S, Steinbeiss S, Thomson BS, Trumbore SE, Gleixner G (2015) Plant diversity increases soil microbial activity and soil carbon storage. Nature Commun 6:6707. https://doi.org/10.1038/ncomms7707
Li XG, Li YK, Li FM, Ma Q, Zhang PL, Yin P (2009) Changes in soil organic carbon, nutrients and aggregation after conversion of native desert soil into irrigated arable land. Soil till Res 104:263–269. https://doi.org/10.1016/j.still.2009.03.002
Li H, Ilyina T, Müller WA, Sienz F (2016) Decadal predictions of the north Atlantic CO2 uptake. Nature Commun 7:1–7
Lino IAN, Santos VM, Escobar IEC, Araujo SDKA, ASF, Maia LC, (2015) Soil enzymatic activity in Eucalyptus Grandis plantations of different Ages. Land Degrad Dev 27:77–82. https://doi.org/10.1002/ldr.2454
Liu M, Han GL (2020) Assessing soil degradation under land-use change: Insight from soil erosion and soil aggregate stability in a small karst catchment in southwest China. Peer J 8:19
Mandal B, Majumder B, Adhya TK, Bandyopadhyay PK, Gangopadhyay A, Sarkar D, Kundu MC, Chaudhary SC, Hazra GC, Kundu S, Samantray SC, Misra AK (2008) Potential of double-cropped rice ecology to conserve organic carbon under subtropical climate. Global Change Biol 14:1–13. https://doi.org/10.1111/j.1365-2486.2008.01627.x
Mandal A, Toor AS, Dhaliwal SS, Singh P, Sharma VK, Gupta RK, Naresh RK, Kumar Y, Pramanick B, Nanda G, Gaber A, Alkhedaide A, Soliman MM, Hossain A (2022) Long-term field and horticultural crops intensification in semiarid regions influence the soil physico-biochemical properties and nutrients status. Agron 12:1010. https://doi.org/10.3390/agronomy12051010
Melero S, López-Garrido R, Murillo JM, Moreno F (2009) Conservation tillage: short- and long-term effects on soil carbon fractions and enzymatic activities under Mediterranean conditions. Soil Till Res 104:292–298. https://doi.org/10.1016/j.still.2009.04.001
Mikha MM, Rice CW (2004) Tillage and manure effects on soil and aggregate associated carbon and nitrogen. Soil Sci Soc Am J 68:809–816. https://doi.org/10.2136/sssaj2004.8090
Mosca E, Montecchio L, Scattolin L, Garbaye J (2007) Enzymatic activities of three ectomycorrhizal types of Quercus robur L. in relation to tree decline and thinning. Soil Biol Biochem 39:2897–2904
Mosquera-Losada MR, Santiago-Freijanes JJ, Rois-Díaz M, Moreno G, den Herder M, Aldrey-Vázquez JA, Ferreiro-Domínguez N, Pantera A, Pisanelli A, Rigueiro-Rodríguez A (2015) Agroforestry in Europe: a land management policy tool to combat climate change. Land Use Policy 78:603–613. https://doi.org/10.1016/j.landusepol.2018.06.052
Naylor D, Sadler N, Bhattacharjee A, Graham EB, Anderton CR, McClure R, Lipton M, Hofmockel KS, Jansson JK (2020) Soil microbiomes under climate change and implications for carbon cycling. Annual Rev Environ Resour 45:29–59
Notaro KA, de Medeiros EV, Duda GP, Silva AO, Moura PM (2014) Agroforestry systems, nutrients in litter and microbial activity in soils cultivated with coffee at high altitude. Scientia Agricola 71:87–95. https://doi.org/10.1590/s0103-90162014000200001
Olk DC, Cassman KG, Randall EW, Kinchesh P, Sanger LJ, Anderson JM (1996) Changes in chemical properties of organic matter with intensified rice-cropping in tropical lowland soil. European J Soil Sci 47:293–303. https://doi.org/10.1111/j.1365-2389.1996.tb01403.x
Pan GX, Zhou P, Li LQ, Zhang XH (2007) Core issues and research progresses of soil science of carbon sequestration. Acta Pedol Sin 44:328–337
Piedallu C, Gégout JC, Lebourgeois F, Seynave I (2016) Soil aeration, water deficit, nitrogen availability, acidity and temperature all contribute to shaping tree species distribution in temperate forests. J Veg Sci 27:387–399
Plaza-Bonilla D, Arrúe JL, Cantero-Martínez C, Fanlo R, Iglesias A, Álvaro-Fuentes J (2015) Carbon management in dryland agricultural systems: a review. Agron Sustain Develop 35:1319–1334. https://doi.org/10.1007/s13593-015-0326-x
Roger-Estrade J, Anger C, Bertrand M, Richard G (2010) Tillage and soil ecology: partners for sustainable agriculture. Soil till Res 111:33–40. https://doi.org/10.1016/j.still.2010.08.010
Sainju UM, Lenssen A, Caesar-Thonthat T, Waddell J (2007) Dryland plant biomass and soil carbon and nitrogen fractions on transient land as influenced by tillage and crop rotation. Soil till Res 93:452–461. https://doi.org/10.1016/j.still.2006.06.003
Sakin E (2012) Organic carbon, organic matter and bulk density relationships in arid-semi arid soils in Southeast Anatolia region. African J Biotech 11(6):1373–1377. https://doi.org/10.5897/AJB11.2297
Sharma S, Singh P, Kumar S (2020) Responses of soil carbon pools, enzymatic activity and crop yields to nitrogen and straw incorporation in a rice-wheat cropping system in north-western India. Front Sust Food Sys 4:532704. https://doi.org/10.3389/fsufs.2020a.532704
Sharma S, Singh P, Sodhi GPS (2020) Soil organic carbon and biological indicators of uncultivated vis-à-vis intensively cultivated soils under rice–wheat and cotton–wheat cropping systems in South-western Punjab. Carbon Manage 11:681–695. https://doi.org/10.1080/17583004.2020.1840891
Sharma S, Singh P, Choudhary OP, Neemisha (2021) Nitrogen and rice straw incorporation impact nitrogen use efficiency, soil nitrogen pools and enzyme activity in rice-wheat system in north-western India. Field Crops Res 266:108131. https://doi.org/10.1016/j.fcr.2021.108131
Sharma S, Singh P, Angmo P, Satpute S (2022) Total and labile pools of organic carbon in relation to soil biological properties under contrasting land-use systems in a dry mountainous region. Carbon Manage 13:352–371. https://doi.org/10.1080/17583004.2022.2089236
Sharma S, Singh P, Chauhan S, Choudhary OP (2022) Landscape position and slope aspects impacts on soil organic carbon pool and biological indicators of a fragile ecosystem in high altitude cold arid region. J Soil Sci Plant Nutr 22:6235. https://doi.org/10.1007/s42729-022-00831-x
Sharma S, Vashisht BB, Singh P, Singh Y (2022) Changes in soil aggregate-associated carbon, enzymatic activity and biological pools under conservation agriculture-based practice in rice-wheat system. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-021-02144-y
Shi Z, Thomey ML, Mowll W, Litvak M, Brunsell NA, Collins SL, Pockman WT, Smith MD, Knapp AK, Luo Y (2014) Differential effects of extreme drought on production and respiration: synthesis and modeling analysis. Biogeosci 11:621–633. https://doi.org/10.5194/bg-11-621-2014
Shi H, Fan J, Zhao D (2017) Predicting household PM 2.5-reduction behavior in Chinese urban areas: an integrative model of theory of planned behavior and norm activation theory. J Cleaner Produc 145:64–73. https://doi.org/10.1016/j.jclepro.2016.12.169
Singh P, Benbi DK (2016) Effect of inorganic fertilizers and farm yard manure on physical properties of soil under rice-wheat cropping. Agri Res J 53:328–333
Singh P, Benbi DK (2018) Nutrient management effects on organic carbon pools in a sandy loam soil under rice-wheat cropping. Arch Agron Soil Sci 64:1879–1891. https://doi.org/10.1080/03650340.2018.1465564
Singh P, Benbi DK (2018) Soil organic carbon pool changes in relation to slope position and land-use in Indian lower Himalayas. CATENA 166:171–180. https://doi.org/10.1016/j.catena.2018.04.006
Singh P, Benbi DK (2020) Modeling soil organic carbon with DNDC and Roth C models in different wheat-based cropping systems in north-western India. Communi Soil Sci Plant Anal 51:1184–1203. https://doi.org/10.1080/00103624.2020.1751850
Singh P, Benbi DK (2020) Nutrient management impacts on net ecosystem carbon budget and energy flow nexus in intensively cultivated cropland ecosystems of north-western India. Paddy Water Environ 18:697–715. https://doi.org/10.1007/s10333-020-00812-9
Singh P, Benbi DK (2021) Physical and chemical stabilization of soil organic matter in cropland ecosystems under rice-wheat, maize-wheat and cotton-wheat cropping systems in north-western India. Carbon Manage 12:603–21. https://doi.org/10.1080/17583004.2021.1992505
Singh P, Benbi DK (2022) Nutrient management effects on carbon input through root and shoot biomass in a rice-wheat system. Agri Res J 59:135–145
Singh P, Benbi DK (2023) Organic carbon in soils’ fine fraction: thresholds in saturation capacity and its relationship with carbon stabilization. Tropical Ecol. https://doi.org/10.1007/s42965-022-00288-0
Singh G, Singh P, Sodhi GPS (2018) Status of crop management practices for rice and basmati cultivation in south-western Punjab. J Commun Mobilization Sustain Develop 13:457–462
Singh P, Singh G, Sodhi GPS (2020) Energy and carbon foot-prints of wheat establishment following different rice residue management strategies vis-à-vis conventional tillage coupled with rice residue burning in north-western India. Energy 200:117554. https://doi.org/10.1016/j.energy.2020.117554
Singh P, Benbi DK, Verma G (2021) Nutrient management impacts on nutrient use efficiency and energy, carbon, and net ecosystem economic budget of rice-wheat cropping system in north-western India. J Soil Sci Plant Nutri 21:559–577. https://doi.org/10.1007/s42729-020-00383-y
Singh P, Sharma S, Nisar S, Choudhary OP (2023) Structural stability and organic matter stabilization in soils: differential impacts of soil salinity and sodicity. J Soil Sci Plant Nutr. https://doi.org/10.1007/s42729-023-01136-3
Singh P, Bhatt R, Kaur G (2021b) Phosphorus availability in soils and use efficiency for food and environmental sustainability. R. Bhatt et al. (eds.), Input use efficiency for food and environmental security. https://doi.org/10.1007/978-981-16-5199-1_12
Sinsabaugh RL (2010) Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol Biochem. 42:391–404
Six J, Paustian K, Elliott ET, Combrink C (2000) Soil structure and organic matter: I. Distribution of aggregate-size classes and aggregate-associated carbon. Soil Sci Soc Am J 64:681–689. https://doi.org/10.2136/sssaj2000.642681x
Six J, Conant RT, Paul EA, Paustian K (2002) Stabilization mechanisms of soil organic matter: implications for C-sequestration of soil. Plant Soil 241:155–176
Six J, Bossuyt H, Degryze S, Denef K (2004) A history of research on the link between (micro)aggregates, soil biota, and soil organic matter dynamics. Soil and Till Res 79:7–31. https://doi.org/10.1016/j.still.2004.03.008
Snyder JD, Trofymow JA (1984) A rapid accurate wet oxidation diffusion procedure for determining organic and inorganic carbon in pot and soil samples. Communi Soil Sci Plant Anal 15:587–597. https://doi.org/10.1080/00103628409367499
Somasundaram J, Singh RK, Parandiyal AK, Prasad SN (2009) Micronutrient status of soils under different land-use systems in Chambal ravines. J Indian Soc Soil Sci 57:307–312
Štursová M, Baldrian P (2011) Effects of soil properties and management on the activity of soil organic matter transforming enzymes and the quantification of soil-bound and free activity. Plant Soil 338:99–110. https://doi.org/10.1007/s11104-010-0296-3
Tabatabai MA, Bremner JM (1969) Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Bio Biochem 1:301–307. https://doi.org/10.1016/0038-0717(69)90012-1
Tirgarsoltani MT, Gorji M, Mohammadi MH, Millan H (2014) Evaluation of models for description of wet aggregate size distribution from soils of different land uses. Soil Sci Plant Nutr 60:123–133. https://doi.org/10.1080/00380768.2013.878642
Tirol-Padre A, Ladha JK (2004) Assessing the reliability of permanganate-oxidizable carbon as index of soil labile carbon. Soil Sci Soc Amer J 98:969–978. https://doi.org/10.2136/sssaj2004.9690
Tisdall JM, Oades JM (1982) Organic matter and water-stable aggregates in soils. European J Soil Sci 33:141–163. https://doi.org/10.1111/j.1365-2389.1982.tb01755.x
Vourlitis GL, Francisco DAL, Pinto OB, Zappia A, Dalmagro HJ, De Arruda PHZ (2015) Variations in aboveground vegetation structure along a nutrient availability gradient in the Brazilian Pantanal. Plant Soil 389:307–321. https://doi.org/10.1007/s11104-014-2364-6
Wang JG, Li ZX, Cai CF, Ma RM (2014) Particle size and shape variation of Ultisol aggregates affected by abrasion under different transport distances in overland flow. Catena 123:153–162. https://doi.org/10.1016/j.catena.2014.07.020
Wang J, Han X, Yide L, Mingxian L, Zhang Z, Tushou L, Dexiang C (2018) Effects of topographic heterogeneity on community structure and diversity of woody plants in Jianfengling tropical montane rainforest. Scientia Silvae Sinicae 54:1–11
Yang H, Griffiths PR, Tate JD (2003) Comparison of partial least squares regression and multi-layer neural networks for quantification of nonlinear systems and application to gas phase fourier transform infrared spectra. Anal Chim Acta 489:125–136. https://doi.org/10.1016/s0003-2670(03)00726-8
Ye W, Wen QX (1991) Characteristics of humic substances in paddy soils. Pedosphere 1:229–239
Yi S, Arain AM, Woo MK (2006) Modifications of a land surface scheme for improved simulation of ground freeze-thaw in northern environments. Geophysics Res Lett 33:L13501. https://doi.org/10.1029/2006GL026340
Yu H, Gong R, Zhou Y, Cha T, Nie L, Lv Z (2015) Characteristic of soil aggregate stability and soil organic carbon under four typical artificial plantations in Beijing Badaling Mountain area. J Soil Water Conser 29:162–166. https://doi.org/10.21203/rs.3.rs-845068/v1
Zhang P, Wang Y, Xu L, Sun H, Li R, Zhou J (2022) Factors controlling the spatial variability of soil aggregates and associated organic carbon across a semi-humid watershed. Sci Total Environ 809:151155. https://doi.org/10.1016/j.scitotenv.2021.151155
Zhao XG, Wu L, Li A (2017) Research on the efficiency of carbon trading market in China. Renew Sustain Energy Rev 79:1–8. https://doi.org/10.1016/j.rser.2017.05.034
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bhatt, R., Singh, P. & Sharma, S. Changes in Soil Organic Pool and Carbon Preservation Capacity of Macro- and Micro-aggregates in Response to Land-Use Change in North-Western India. J Soil Sci Plant Nutr 23, 2849–2867 (2023). https://doi.org/10.1007/s42729-023-01239-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-023-01239-x