Abstract
We explored how two pea varieties, Sarsabz (V1) and Meteor (V2), showed resistance to salt stress by modulating their mechanism under thiamine application. The experiment was performed to evaluate the effect of three levels of foliarly applied thiamine (0, 250, and 500 ppm) on biochemical and morpho-physiological parameters of pea varieties under salt stress (150 mM). Both morpho-physiological and biochemical responses were examined such as growth attributes, chlorophyll contents, total sugars, total proteins, proline content, phenolic content, and antioxidant activities. One hundred fifty millimolar of salinity caused significant reduction in growth and photosynthetic pigments in both pea varieties. The highly significant effect was noted after application of 500 ppm of thiamine in V1 on shoot and root weights, lengths, and photosynthetic pigments as compared to the control. The V2 accumulated the highest Na+, Cl−, and Ca2+ under stressed conditions compared to V1; its homeostasis appeared to be more affected. The results indicated that 500 ppm alleviated the detrimental effects of salinity in both pea varieties by supporting different components of the defense mechanism. Both varieties differed significantly for total soluble sugars, proline, and phenolics. Our results showed that the mitigating effects of 500 ppm of thiamine against salinity proved to be more promising in comparison with other levels. The exogenous supply of thiamine not only protected growth and development but also supported biochemical attributes in terms of detoxification responses for fighting against the stressed conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Semi-arid and arid regions generally contain higher salt contents in the soil across the globe. By the year 2050, approximately 50% loss in agricultural production is estimated as a consequence of the soil salinization of cultivated lands globally (Naheed et al. 2021). Physiological and biochemical attributes of plants are vulnerable to injurious effects caused by the elevated salt contents in the soil (Nabati et al. 2011). For instance, salinity causes a drastic decline in chlorophyll (Chl.) contents (Chl-a, Chl-b, carotenoids) and abnormalities in stomatal functioning leading to reduced photosynthesis and production of reactive oxygen species (ROS) (Abdel Latef 2010; Munns & Tester 2008). Similarly, oxidative stress also occurs as a result of the uptake of salt and the accumulation of Na+ and Cl− ions in different parts of the plants (Hamayun et al. 2010). Besides, plant solutes get concentrated and osmotic potential becomes disrupted by the accumulation of these ions which hampers normal metabolic activities in plants (Brady & Weil 2002).
A diverse range of plant growth regulators control plant growth and development and help them to survive under various environmental stressors. Recent findings revealed that vitamins such as ascorbic acid and thiamine are influential in coping with the lethal effects of biotic and abiotic stresses (Soltani 2017). Thiamine is a growth stimulus and acts as a cofactor in vital plant processes (Colinas & Fitzpatrick 2015). It is found to be involved in energy-generating processes like Calvin and Kreb’s cycles (Du et al. 2011) and also acts as an antioxidant that copes with the oxidative stress in plants (Subki et al. 2018). Antioxidant activities, photosynthetic pigments, and growth were found to be significantly increased with the foliar application of thiamine in different plants (Fallahi et al. 2018). Thiamine also increases total sugars, proteins, and yield attributes in plants (El-Metwally & Sadak 2019).
Pea is one of the extensively grown leguminous crops worldwide (AL-Bayati et al. 2019). Seeds of pea plants are consumed as a vegetable due to their high nutritional quality and plentiful oil contents (Tayyab et al. 2016), and act as an excellent source of vital amino acids and mineral nutrients (Rebello et al. 2014). These plants also play an important environmental role in increasing soil fertility by fixing free nitrogen (El-Hak et al. 2012). Pea crop shows varying sensitivity to salinity at different growth stages. Previous studies have demonstrated that the germination attributes of pea plants like seed vigor, index, and germination rate considerably decline with increasing salinity (Ouerghi et al. 2016). Plant nutrients especially those present in plant leaves are highly affected by salinity (Bharti et al. 2013). Although thiamine has been proven to ameliorate the adverse effects of environmental stresses on plants, its role in the mitigation of salinity stress was unexplored. Therefore, this study was aimed to investigate the crucial role of different levels of foliarly applied thiamine on some morpho-physiological and biochemical traits of pea plants grown under saline soil conditions. The correlation of thiamine and salinity applications with antioxidant defense responses of the pea plants was also studied.
2 Materials and Methods
The experiment was performed to evaluate the effect of foliar application of thiamine on biochemical and morpho-physiological parameters of pea varieties under saline conditions. The experiment was laid out in completely randomized design (CRD) with a two-factor factorial arrangement in the research area of Government College Women University, Faisalabad. Seeds of two pea varieties, namely Sarsabz (V1) and Meteor (V2), were procured from Ayub Agricultural Research Institute, Faisalabad, Pakistan, and subjected to salt stress and three levels of thiamine with the following schedule:
Thiamine was applied as foliarly while salinity was applied to the rooting media. Seeds of pea plants were surface sterilized and ten seeds were sown in each pot filled with soil. Ten seeds were sown in each pot at about 1.5–2 cm deep from the surface of the soil. After 15 days of germination, thinning was done and the 3 plants per pot 10 × 10 × 10 inches (L × W × H) were subjected to 0 mM and 150 mM salt stress and three levels of thiamine once a week. Three consecutive treatments were given. Salinity level was maintained at 150 mM in the soil by measuring electrical conductivity with the help of an EC meter (HI 9835, HANNA, Nusfalau, Romania) and adding the required amount of salt solution. Fifteen days post treatment, the data were recorded for various morphological and physio-biochemical parameters.
2.1 Plant Sampling and Morphological Attributes
Morphological attributes were recorded at the time of plant sampling, i.e. after 15 days of last treatment. Three plants from each replicate were harvested. Shoot and root lengths were measured using the scale while shoot and root fresh weights were measured using an electronic balance. The leaf area was recorded by measuring length and width of one leaf in individual plant from each replicate. These plants were then kept in an oven at 72 °C for 48 h. Completely dried samples were removed from the oven. Root and shoot dry weight of the dried samples was recorded. The samples were saved for further analyses.
2.2 Chlorophyll Contents
Five hundred-milligram fresh plant leaves were extracted in 10 ml of 80% acetone. The extracted material was centrifuged at 4000 rpm for 10 min and the supernatant was transferred to cleaned test tubes. The optical density of each sample was measured at 645 nm, 663 nm, and 480 nm for the determination of Chl-a, Chl-b, and carotenoids, respectively (Arnon 1949).
2.3 Phenolics
Phenolics were assayed using a protocol devised by Julkunen-Tiitto (1985). Five hundred-milligram fresh pea leaves were extracted in 1 ml of 80% acetone and centrifuged for 15 min at 12,000 rpm. Five milliliters of Folin-Ciocalteu reagent was added to the test tube containing 100 µl of supernatant. 2.5 ml of Na2CO3 was immediately added to each of the test tubes and was shaken vigorously. Distilled water was used to maintain the volume up to 5 ml. Then, test tubes were vortexed for a few seconds and kept at room temperature for 15–20 min for reaction stability and then the optical density of the sample was measured at 750 nm.
2.4 Total Soluble Sugars
Total soluble sugars were determined using a method devised by Yemm and Willis (1954) for the sugar determination. The plant samples were completely dried using the oven and 100 mg of this dried plant material was ground with 10 ml of ethanol. The ground plant material was kept on an orbital shaker at 60 °C for 60 min. Three milliliters of anthrone agent was added after removing it from the orbital shaker and the aliquot was heated in a boiling water bath for 10 min. The temperature of the water bath was adjusted to 100 °C. The aliquots were directly transferred to an ice box for the reaction to stop. After removing from the icebox, the samples were kept at room temperature for 10–12 min. The optical density of the samples was determined through a spectrophotometer at 625 nm after running ethanol as the blank.
2.5 Proline
The plant material was ground in 10 ml of 3% sulfo-salicylic acid (each sample) and filtered using filter papers. Dissolving of glacial acetic acid in orthophosphoric acid was facilitated through heating on a hot flame, following the addition of acid ninhydrin. The filtrate was transferred to the test tube and 2 ml acid ninhydrin was added to the test tube containing plant aliquot and heated in a water bath. After removing from the water bath, the samples were shifted to the icebox. The samples were vortexed for a few seconds after adding 4 ml of toluene and then optical density was determined at 570 nm using a spectrophotometer.
2.6 Total Proteins
Protein extraction was done using a method devised by Bradford (1976). Fresh pea leaves were ground in 2 ml of potassium phosphate buffer. Centrifugation was performed at 13,000 rpm for 10 min. Bradford reagent (5 ml) was added in 100 µl of the supernatant obtained from the plant material extraction. The aliquot was vortexed for a few seconds and absorbance was recorded at 595 nm through a spectrophotometer.
2.7 Antioxidants
2.7.1 Peroxidase
Chance and Maehly (1955) assay was followed for the peroxidase analysis. Ice-cold pestle and mortar was used for the grinding of fresh plant material. The grinding was facilitated using 5 ml of potassium phosphate buffer. The pH of the buffer was maintained up to 7.8. The ground plant sample was subjected to centrifugation at 12,000 rpm. The samples were centrifuged for 10 min and 2.7 ml buffer solution was further added to the supernatant. Later, 1 ml guaiacol was added to the sample. The optical density of the sample was read at 470 nm on a spectrophotometer at 0, 30, 60, and 120 s.
2.7.2 Catalase
The supernatant was collected in the same manner as was prepared for peroxidase. However, instead of adding guaiacol, 1 ml of hydrogen peroxide was added to the sample. The optical density of the sample was read at 240 nm on a spectrophotometer at 0, 30, and 60 s.
2.8 Ion Analysis
Ion analysis was performed using a protocol devised by Wolf (1982). One hundred fifty-milligram oven-dried plant material was dipped in 2 ml of concentrated sulfuric acid overnight. The sample was heated on a hot plate at 100 °C for 25–30 min then at 370 °C for 50 min. After cooling the sample at room temperature for 20 min, 2 ml hydrogen peroxidase was added to the flask. The flask containing aliquot was transferred to the hot plate and heated again until the material turns from dark slurry to completely colorless. Volume was maintained up to 50 ml by adding distilled water and saved after filtration through the filter paper. Sample readings were recorded for Na+, K+, and Ca+2 ions using a flame photometer while Cl− ions were determined using a chloride analyzer using the same digestion sample used for the determination of other ions.
2.9 Statistical Data Analysis
The experiment was performed in a completely randomized design (CRD) with two factors and three replications. Graphs were prepared using Origin (v.2021) and statistical program “R (v 4.0.1)” was used for drawing principal component analysis, pairwise t-test, and boxplot comparison (R Development Core Team 2020, OriginLab Corporation 2021). The data was subjected to statistical analysis using Costat version 6.2 (Cohorts Software, 2003, Monterey, CA, USA) for ANOVA (analysis of variance) and Duncan’s multiple range test (DMRT) for mean values comparison (Steel et al. 1997; Kim 2014). Principal component analysis (PCA) was applied for evaluating association among different attributes of plants belonging to two pea varieties.
3 Results
3.1 Morphological Attributes
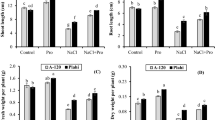
The data analyzed for the shoot and root lengths revealed the significant differences among varieties while the differences at the treatment level were found to be highly significant (Fig. 1a). Maximum reduction was observed in plants exposed to salt stress (T3). Shoot length of both pea varieties decreased up to 15.3% while root length decreased up to 15.3% and 29.4% in V1 and V2 respectively under T3. Besides, root and shoot length was enhanced in plants supplied with 500 ppm of thiamine compared to the control. The application of 500 ppm thiamine aided in the maximum recovery of the plants exposed to the deteriorating effects 150-mM salt stress. The foliar application of 250 ppm thiamine also showed the significant increase in root length both under normal and salt-stressed conditions as shown in T1 and T4, respectively. Both shoot length and root length showed a considerable increase of 15.9% and 22.5% in V1 under T5, whereas root length in V2 increased up to 11.7% under the same treatment. Generally, V1 responded more effectively than V2 for root/shoot length.
Morphological attributes of pea varieties, i.e. shoot and root lengths (a), shoot and root fresh weights (b), shoot and root dry weights (c), number of leaves (d), and leaf area (e) under the effect of various treatments of salt and thiamine. Data are means ± SE (n = 3). Small alphabets above the bars indicate significant differences at P ≤ 0.05 (Duncan’s multiple range test). Similar letters above the bars in each plot are statistically non-significant. T0= 0 ppm thiamine + 0 mM salinity, T1= 250 ppm thiamine + 0 mM salinity, T2 = 500 ppm thiamine + 0 mM salinity, T3 = 0 ppm thiamine + 150 mM salinity, T4 = 250 ppm thiamine + 150 mM salinity, T5 = 500 ppm thiamine + 150 mM salinity
The results for shoot and root fresh weight in both varieties were observed to be highly significant when the data were subjected to statistical analysis at P ≤ 0.001. The reduction in root/shoot fresh and dry weights was more pronounced in V2 (Fig. 1b, c). Both the shoot fresh weight and root fresh weight in V1 experienced 4.4% and 20.9% decrease respectively under T3; however, V2 showed 25% decrease in root fresh weight. The effects of thiamine (500 ppm) were more promising than 250 ppm thiamine and the control. In T5, The ameliorating effects of highest dose of thiamine application in V1 were also observed to be more supporting than its lower dose in salt-stressed conditions. Maximum root (115.9%) and shoot (77.6%) fresh weights were recorded in V2 and V1, respectively, under T2. Under salinity, 56.2% increase was recorded in V1. The data analyzed for root and shoot dry weights disclosed the significant variation among treatments while the difference between varieties was also observed to be highly significant (Fig. 1b, c). Maximum reduction was observed under salt stress for both of these parameters. The highest reduction in shoot (16.1%) and root (32.3%) dry weights was recorded under T3 in V2. Maximum root and shoot dry weights were recorded in T2 where only thiamine was applied; however, minimum values of these attributes were seen only in the sole treatment of salinity (T3) (Fig. 1c). Application of 500 ppm thiamine enhanced the shoot dry weight (37.5%) and root dry weight (42.1%) in V1 under T2.
The statistical analysis for number of leaves per plant revealed a prominent difference among treatments and between varieties (Fig. 1d). Application of salinity (150 mM) caused a clear reduction in both varieties for this parameter. More decrease in number of leaves was observed in V2 (22.2%) as compared to V1 (15.9%) under T3. On the other hand, 500 ppm thiamine proved to be more positive in both varieties under control and salt stress. Minimum number of leaves per plant was recorded in T3, while V1 showed maximum value for this parameter in T2 (Fig. 1d). Both pea varieties experienced 17% increase in number of leaves under T2. Statistically analyzed data for leaf area showed a little variation among all the treatments along with a slight varietal difference. Imposition of salinity (T3) showed a reduction in leaf area for V2 compared to V1. V2 showed a maximum decrease in leaf area (15.3%) as compared to V1 (7.4%) under T3. However, thiamine application enhanced the leaf area in both varieties in both control and salt stress. Application of 500 ppm thiamine enhanced the leaf area in V1 (36.5%) and V2 (38.8%) under T2. The highest value for leaf area was recorded in V1 under T1, while V2 showed minimum leaf area in T3 (Fig. 1e).
3.2 Photosynthetic Pigments
The data regarding chlorophyll a were highly significant both for treatments and varieties. Imposition of 150-mM salinity (T3) considerably (P ≤ 0.01) minimized the chlorophyll a contents in both pea varieties. T3 caused 23.9% and 25% decrease in chlorophyll a in V1 and V2 respectively compared to the control. Chlorophyll a contents in both varieties were enhanced after application of 500 ppm thiamine as shown in Fig. 2. The maximum increase (33%) for this parameter was observed in V1 under T2, whereas maximum reduction (25%) for chlorophyll a was observed in V2 under T3 (Fig. 2a).
Chlorophyll contents of pea varieties, i.e. chlorophyll a contents (a), chlorophyll b contents (b), and carotenoids (c) under the effect of various treatments of salt and thiamine. Data are means ± SE (n = 3). Small alphabets above the bars indicate significant differences at P ≤ 0.05 (Duncan’s multiple range test). Similar letters above the bars in each plot are statistically non-significant. T0 = 0 ppm thiamine + 0 mM salinity, T1 = 250 ppm thiamine + 0 mM salinity, T2 = 500 ppm thiamine + 0 mM salinity, T3 = 0 ppm thiamine + 150 mM salinity, T4 = 250 ppm thiamine + 150 mM salinity, T5 = 500 ppm thiamine + 150 mM salinity, FW, fresh weight
The statistical data for chlorophyll b contents showed significant results (Fig. 2b). T3 (150 mM NaCl) found to be more deleterious for both pea varieties. V1 showed more reduction (38%) in chlorophyll b compared to V2 (30%) with reference to the control. However, the damaging effects of salinity were curtailed in pea plants after supply of 500 ppm thiamine foliarly where it improved the chlorophyll b contents up to 38.3% and 31.4% in V2 and V1, respectively. The data analyzed for carotenoids divulged the significant differences between the treatments and varieties (Fig. 2c). Unlike chlorophyll a and b contents, V2 showed higher concentration of carotenoids compared to V1 in all the treatments. Imposition of salinity (T3) trivialized the carotenoid contents in both pea varieties. Carotenoid contents were reduced to 29.9% and 34%, respectively, in V1 and V2 under T3 compared to the control. The application of 500 ppm thiamine in V2 has been proved to be more positive under T2 and T5. Five hundred parts per million of thiamine enhanced the carotenoid contents in V1 (35.6%) and V2 (45.8%) compared to the control.
3.3 Phenolics
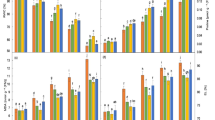
Phenolic contents in pea plants also showed significant effect under salinity and thiamine applications (Fig. 3a). Imposition of 150-mM salinity greatly (P ≤ 0.01) reduced the phenolic contents in both pea varieties. Both pea varieties showed 16.4% decrease in phenolic contents under T3. Thiamine application of 500 ppm impressively (P ≤ 0.001) enhanced the phenolic contents both in control and saline conditions. V1 and V2 showed a respective increase of 10% and 11.9% regarding this parameter in T3. However, maximum increase of 21.9% was recorded in V2 under T2. The highest value for this parameter was recorded in V1 under T2, whereas minimum phenolic contents were found in V2 under T3.
Phenolics (a), proteins (b), proline (c), catalase (d), peroxidase (e), superoxide dismutase (f), and soluble sugars (g) in pea plant varieties under the effect of various treatments of salt and thiamine. *, **, ***, and **** show significance at P ≤ 0.05, ≤ 0.01, ≤ 0.001, and ≤ 0.0001, respectively. T0 = 0 ppm thiamine + 0 mM salinity, T1 = 250 ppm thiamine + 0 mM salinity, T2 = 500 ppm thiamine + 0 mM salinity, T3 = 0 ppm thiamine + 150 mM salinity, T4 = 250 ppm thiamine + 150 mM salinity, T5 = 500 ppm thiamine + 150 mM salinity
3.4 Proteins
Protein contents also varied significantly under the effect of various treatments (Fig. 3b). Pea plants showed a considerable reduction regarding protein contents in both varieties when subjected to 150-mM salt stress (T3). V2 showed lesser reduction (9.3%) compared to V1 (19.4%) with reference to their respective controls under salt stress. Application of 500 ppm thiamine prominently increased the protein contents under both control and stress conditions and this increase was more pronounced in V2 where 500 mM thiamine showed 10% increase under T3.
3.5 Proline and Antioxidants
The statistical analysis for proline contents revealed a noticeable variation between varieties and among different treatments (Fig. 3c). Salt stress (T3) caused a prominent (P ≤ 0.01) increase in proline contents of both pea varieties. A prominent increase of 128.9% in V1 and 67.3% in V2 was observed under T3 in comparison with control. Exogenous application of 500 ppm thiamine also enhanced (P ≤ 0.001) the proline contents compared to 250 ppm thiamine and control as 500 ppm thiamine increased the proline contents in both V1 (38.4%) and V2 (54.5%) under T3.
Statistical analysis of the data recorded for catalase and peroxidase showed considerable variation among treatments along with varietal differences (Fig. 3d, e). Salinity imposition significantly (P ≤ 0.01) decreased the activities of both catalase (CAT) and peroxidase (POD) in pea varieties. V1 showed 4.5% and 49.5% decrease in CAT and POD activities respectively under T3 while V2 experienced 5% and 44% respective decrease. However, thiamine application of 500 ppm thiamine prominently enhanced the activity of both of these antioxidants both in control and saline conditions. Both pea varieties showed more peroxidase activity compared to catalase as V1 experienced 34.1% increase in POD activity while in V2, this increase was 64.2%. In case of catalase, both varieties showed 10% increase under T5. The highest values of CAT and POD were experienced in V2 under T2, whereas minimum activity was recorded in V2 under T3. The same kind of trend was noted for superoxide dismutase (SOD) in both pea varieties in this study (Fig. 3f).
The activity of SOD slightly (P ≤ 0.05) increased when pea plants experienced 150 mM of salinity. Both V1 and V2 experienced 25.2% and 5.6% increase in SOD activity, respectively, under T3. On the other hand, 500 ppm thiamine proved to be more positive compared to 250 ppm thiamine and significantly (P ≤ 0.001) increased the SOD activity under both non-saline and saline conditions. Maximum SOD activity was recorded in V1 (89.2%) compared to V2 (86.6%) under T5. The maximum activity was recorded in V1 under T5; however, V1 showed minimum SOD activity under T0 (Fig. 3f).
3.6 Soluble Sugars
The data examined for soluble sugars divulged the significant variations in varieties and treatments were also observed highly significant (Fig. 3g). Pea plants experienced a considerable reduction (P ≤ 0.01) for soluble sugars but this reduction was more pronounced in V2 where 22.5% decrease in this parameter was recorded as compared to control. Application of 500 ppm thiamine prominently (P ≤ 0.001) enhanced the soluble sugars under both control and stress conditions. Thiamine application enhanced the soluble sugars both in V1 (15%) and V2 (10.6%) under T5. Maximum increase of 23% was recorded in V1 under T2.
3.7 Mineral Ion Quantification
The data examined for the sodium (Na+) and chloride (Cl−) contents were found to be significant among treatments along with considerable difference between varieties (Fig. 4a, d). The application of salinity (T3) prominently (P ≤ 0.001) enhanced the Na+ and Cl− contents in both pea varieties. Salinity imposition enhanced the Na+ contents up to 12% in V1 and 22.3% in V2. On the other hand, T3 increased the Cl− contents in V1 (40.2%) and V2 (65%). Five hundred parts per million thiamine application proved to be helpful in acclimatizing the contents of Na+ and Cl− in pea plants under both control and saline conditions. In V1, Na+ and Cl− contents decreased up to 11.4% and 24.6%, respectively, under T2. The highest amounts of Na+ and Cl− contents were recorded in V2 under T3 compared to T0. On the other hand, V1 showed minimum contents for both of these ions in T2.
Ionic contents of pea plant varieties, i.e. Na+ (a), Ca+2 (b), K+ (c), and Cl− under the effect of various treatments of salt and thiamine. Data are means ± SE (n = 3). Small alphabets above the bars indicate significant differences at P ≤ 0.05 (Duncan’s multiple range test). Similar letters above the bars in each plot are statistically non-significant. T0 = 0 ppm thiamine + 0 mM salinity, T1 = 250 ppm thiamine + 0 mM salinity, T2 = 500 ppm thiamine + 0 mM salinity, T3 = 0 ppm thiamine + 150 mM salinity, T4 = 250 ppm thiamine + 150 mM salinity, T5 = 500 ppm thiamine + 150 mM salinity, DW, dry weight
The results for calcium (Ca2+) and potassium (K+) contents in both varieties were observed to be highly significant (P ≤ 0.01). Salinity imposition (T3) significantly reduced the Ca2+ and K+ contents in pea plants. T3 decreased the Ca2+ and K+ contents up to 44.4% and 8.9% in V1 whereas in V2, this reduction was 56.6% and 14.8%, respectively. The repercussions of 500 ppm thiamine were more positive compared to 250 ppm thiamine both under T2 and T5. The Ca2+ contents showed an increase of 86% and 60% in V1 and V2, respectively. However, in case of K+, only 12% increase was observed under T5. The highest value for both Ca2+ and K+ contents was observed in V1 under T2, whereas minimum value for both of these ions was recorded in V2 under T3 (Fig. 4b, c).
3.8 Principal Component Analysis
PCA-biplot of different attributes in two varieties of pea plants under salt stress and foliar application of thiamine examined the degree of association among the treatments and varieties (Fig. 5a, b). The components of PCA, i.e. Dim1 and Dim2, jointly elaborated almost 70% of variability in data. Dim1 contributed 56.3% of the total variation observed in comparison with Dim2, i.e. 13.2%. Clear variation was observed among all treatments and varieties. The distribution of all the components in the dataset indicates that salinity significantly influenced both varieties in terms of attributes studied. Also, PCA revealed positive and negative correlation among considered characters of Pisum varieties under salinity. We noted that application of salt stress correlated significantly and positively with changes in morphology, biochemistry, ionic partitioning, and antioxidant defense system. A considerable and positive correlation was noted between Na+ and Cl− ions but they appeared to be negatively correlated with plant attributes. Together they caused a significant reduction which is evident from our results (Fig. 5a–c). Similarly, SOD and proline were positively correlated with T5 (Fig. 5a). Na+ and Cl− were positively correlated with T4. Most of the influence of Na+ and Cl− was observed in V2 (Supplementary Table 2a, b). It obviously demonstrated that different variables in control plants did not match significantly with the same variables in salt-stressed Pisum plants.
PCA of Pisum sativum varieties under salt stress and foliar application of thiamine. (a) First plot of the PCA is representing relationship of different treatments on studied attributes. (b) PCA plot is showing relationship of studied attributes and varieties. (c) Corrplot between different studied parameters against different dimensions in PCA. RFWT, root fresh weight; SFWT, shoot fresh weight; RDWT, root dry weight; SDWT, shoot dry weight; RL, root length; SL, shoot length; NOL, number of leaves per plant; LA, leaf area; Na, sodium; K, potassium; Ca, calcium; Cl, chloride; PHEN, phenolics; PROT, proteins; CAT, catalase; POX, peroxidase; SS, soluble sugars; SOD, superoxide dismutase; PROL, proline
4 Discussion
In this study, we validated that exogenous thiamine altered the growth, levels of toxic ions, and organic solute accretion in two pea varieties under salt stress (150 mM) and enhanced antioxidant metabolism as well for salinity tolerance. Herein, using two different varieties of pea, namely Sarsabz (V1) and Meteor (V2), as experimental models, robust proof for role of foliar application of thiamine in mechanism of salinity acclimation inclusive of physio-biochemical regulations is provided (Fig. 5a, b). Presented data evidently establish that application of thiamine played a protective function against impacts of salinity and the response was more distinct in a dose-dependent fashion (500 ppm) against salt stress.
As a common and most obvious response, salt stress abridged the plant growth and yield performance by compromising different attributes (Camalle et al. 2020; Torun 2019). It is recorded in this study that the plants exposed to 150-mM salt stress displayed momentous drop (P ≤ 0.05) in plant attributes while thiamine application improved pea attributes such as fresh and dry weights, shoot length, and root length, leaf area, and photosynthetic pigments in both varieties. The comparative difference between V1 and V2 is indicative of differential tolerance based upon studied attributes. The decline in these traits in salt-stressed pea varieties and comparative improvement after application of thiamine reflects associated physiological, biochemical, and molecular regulatory networks controlling growth of plants (Fig. 1). The decrease in biomass and length of roots and shoots (15.3% and 29.4% in V1 and V2 under T5) can also be justified with the decrease in cell turgor due to salinity, loss of photosynthetic pigments, and production of reactive oxygen species (Bañon et al. 2006; Hasanuzzaman et al. 2013; Sadak 2019; Dawood et al. 2019). On the other hand, as a phyto-regulatory compound, the efficient role of thiamine as an ameliorating agent against various abiotic stresses is of vital importance for the plants (Collin et al. 2008; Sayed & Gadallah 2002; Rady & Mohamed 2015). In this study, maximum root fresh weight (115.9%) and shoot fresh weight (77.6%) were recorded in V2 and V1 respectively under T2. Maximum root and shoot dry weights were recorded in T2 where only thiamine was applied. The corresponding enhancement in growth attributes due to thiamine is representing its stimulatory role in osmoregulation, maintained photosynthetic pigments, initiation of division of meristematic cells, and organ development (Figs. 1 and 2) (El-Metwally & Sadak 2019; Taïbi et al. 2016).
Photosynthesis is known to be the most affected physiological process by salinity in all plants. We recorded a variable response of photosynthetic pigments to salinity depending on variety under high salt and thiamine concentration (Fig. 2). Maximum reduction (25%) for chlorophyll a was observed in V2. The damaging effects of salinity were curtailed in pea plants after foliar supply of 500 ppm thiamine where it improved the chlorophyll b contents up to 38.3% and 31.4% in V2 and V1 respectively. Parallel to this was tissue ionic homeostasis that is crucially needed for proper physiological functioning of the plant cell. The high photosynthetic pigments in thiamine-applied plants were also linked with balance ionic concentration in tissues during the course of experiment. A linked response of photosynthetic pigments and mineral nutrients in both varieties of pea to thiamine highlights connected action-reaction mechanism between stress and thiamine as a protectant. Normally, massive Na+ content in tissue extremely harms cell metabolism (Ahanger & Agarwal 2017; Shabala & Pottosin 2014), and it is highly essential for plants to maintain high K+/Na+ ratio for tolerating salinity (Zörb et al. 2015). Salinity imposition enhanced the Na+ contents up to 12% in V1 and 22.3% in V2. On the other hand, T3 increased the Cl− contents in V1 (40.2%) and V2 (65%). Salt stress results in the leakage of K+ and Ca+2 ions. T3 decreased the Ca2+ and K+ contents up to 44.4% and 8.9% in V1 whereas in V2, this reduction was 56.6% and 14.8% respectively. Thus, under saline conditions, root and shoot analysis of pea varieties presented the decreased concentration of K+ (Shabala 2013) and Ca+2 under the salt stress while the concentration of these ions increased when the plant was exposed to thiamine treatment. The presented decline in plant growth of both varieties is in fact a resistance response due to osmotic adjustments and changes in metabolism. The changes in osmotic adjustment are evident with the help of changes observed in ionic homeostasis. We can also attribute such resistance to accumulation/exclusion of certain ions, i.e. Na+ or Cl− as well as osmotic stress tolerance (Hamayun et al. 2010; Munns & Tester 2008). Here, in salt stress, pea plants with thiamine treatment showed low Na+ and Cl− accretion and augmented K+ (Fig. 4) which maintained a proper K+/Na+ ratio compared to the salt stress only (Allel et al. 2018; Sun et al. 2015). Actually, increased K+ and Ca+ content in thiamine-applied salt-stressed pea plants played a positive role in stabilizing the membrane, osmotic adjustments, and ultimately contributed in controlling stomatal conductance and photosynthetic performance for improved growth under salinity. Our finding is in line with the report of El-Metwally and Sadak (2019) stating depression in plant growth due to the disturbed cell-water relationship, metabolic activities, and production of toxic compounds inside the cell under salt stress (El-Metwally & Sadak 2019; Akhter et al. 2021a).
Reactive oxygen species (ROS), partially reduced forms of oxygen, are used as signaling molecules by plants to regulate abiotic stress responses, defense against pathogens, and systemic signaling etc. (Torun 2019; Qasim et al. 2018, 2021). But, overproduction of ROS is highly destructive for biological processes (Noman et al. 2015; Khalid et al. 2019; Arshad et al. 2016). As a consequence, plant cells have been naturally endowed with effective defense systems, e.g. antioxidants for scavenging and detoxification of ROS. In both V1 and V2, the POX, CAT, and SOD activity exhibited a clearly different response compared to the control after stress application. Both pea varieties showed more peroxidase activity as compared to catalase as V1 experienced 34.1% increase in POD activity while in V2, this increase was 64.2%. In case of catalase, both varieties showed 10% increase under T5. Such variances in POX and CAT activity in stressed plants showed the incidence of oxidative stress (Shalata et al. 2001; Akhter et al. 2021b; Aqeel et al. 2021). Such differential induction of antioxidant enzyme activities in both varieties, according to conditions, i.e. saline and control, indicates obvious differences. This finding is supported by the results of Xiong et al. (Xiong et al. 2006). Similarly, SOD also plays an important and significant role in regulating the physiology of the plants under unwanted environmental conditions. Its overproduction as in this study is also indicative of plant’s effort to cope with the cellular damage (Río et al. 2018). Such differences become highly logical and understandable with application of different doses of thiamine. Exogenous supply of thiamine not only managed activity of these enzymes in plants facing stress but also contributed in accumulation of osmolytes that jointly worked for maintaining plant growth and survival. This proposes that the mitigation of osmotic and oxidative damage by salinity was attained through the combined activity of antioxidant enzymes and organic osmolytes along with total soluble sugars and proteins.
We have opinion that application of thiamine during seedling development and early growth stages protects the plants from damages of ROS and provides additional support by managing soluble proteins and sugars in cells (Goyer 2010; Plaut et al. 2013). V2 showed lesser reduction (9.3%) as compared to V1 (19.4%) with reference to their respective controls under salt stress (Figs. 3 and 5). The observed decline in soluble sugars and proteins in plants under salt stress was comprehensively tackled by thiamine application and even these biochemicals increased with doses of thiamine. From this, we can infer that thiamine induced tolerance against oxidative damages by playing individual and team role (Tunc-Ozdemir et al. 2009). Moreover, the increase in total soluble proteins and sugars indicates involvement of thiamine as functional coenzyme in synthesis of both sugars and proteins. Our results display that the accumulation of proline in controlled treatment plants was almost negligible compared to the stressed ones. The results for proline analysis obtained through current research are in agreement with Abbaspour (2012). In this study, proline accumulation after thiamine supply ameliorated pea varieties facing NaCl stress. Proline accumulation crucially manages the water content in plant cells. Besides, being an excellent osmoregulator and osmoprotector, proline accrues in large amounts in plant cells against salinity (Torun 2019). A prominent increase of 128.9% in V1 and 67.3% in V2 was observed under T3. Five hundred parts per million of thiamine increased the proline contents in both V1 (38.4%) and V2 (54.5%). This thiamine-triggered accumulation of proline is an adaptive response and is supported by different studies conducted on various crop plants under abiotic stresses (Wasti et al. 2012; Shan & Wang 2017; Demiral & Türkan 2005). The production of secondary metabolites during stress condition helps the plant to tolerate the deleterious effects of the stress. The salt stress mainly caused reduction in phenolic compounds (Hussain et al. 2012; Rezazadeh et al. 2012), but the effects have been ameliorated by the application of thiamine. Thiamine application of 500 ppm impressively (P ≤ 0.001) enhanced the phenolic contents both in control and saline conditions. The disturbed enzymatic and photosynthetic activities may cause a reduction in phenolic compounds. The application of thiamine as a phytoregulator improved the tolerance capacities of plants and thus enhanced the production of these secondary metabolites to tolerate the severe impacts of the salt stress. Hence, we affirm that the pea variety V1 proved to be more tolerant to adverse effects of the salt stress owing to its higher photosynthetic pigments and essential ion contents, and its enhanced antioxidative defense mechanism compared to V2.
5 Conclusion
Under salt stress condition, the morpho-physiological attributes of both varieties showed a sharp decline but these deteriorating effects of the salt found to be ameliorated when L3 (500 ppm) of thiamine was applied. The application of thiamine helped the plants in their osmoregulation, ionic compartmentalization, and detoxification of ROS. The effect of thiamine as an ameliorating agent against salt stress has been established; however, more diverse ways of application of thiamine may enhance the positive effects which can be confirmed with further studies. Also, we found that V1 proved to be more tolerant against saline stress and was more responsive to thiamine compared to V2.
References
Abbaspour H (2012) Effect of salt stress on lipid peroxidation, antioxidative enzymes, and proline accumulation in pistachio plants. J Med Plants Res 6(3):526–529
Abdel Latef A (2010) Changes of antioxidative enzymes in salinity tolerance among different wheat cultivars. Cereal Res Commun 38(1):43–55. https://doi.org/10.1556/CRC.38.2010.1.5
Ahanger MA, Agarwal R (2017) Salinity stress induced alterations in antioxidant metabolism and nitrogen assimilation in wheat (Triticum aestivum L) as influenced by potassium supplementation. Plant Physiol Biochem 115:449–460. https://doi.org/10.1016/j.plaphy.2017.04.017
Akhter N, Aqeel M, Hameed M et al (2021a) Foliar architecture and physio-biochemical plasticity determines survival of Typha domingensis pers Ecotypes in nickel and salt affected soil. Environ Pollut 286:117316. https://doi.org/10.1016/j.envpol.2021.117316
Akhter N, Aqeel M, Shahnaz MM et al (2021b) Physiological homeostasis for ecological success of Typha (Typha domingensis Pers) populations in saline soils. Physiol Mol Bio Plants 27(4):687–701. https://doi.org/10.1007/s12298-021-00963-x
AL-Bayati H, Ibraheem F, Allela W, AL-Taey DK (2019) Role of organic and chemical fertilizer on growth and yield of two cultivars of pea (Pisum sativum L). Plant Arch 19(1):1249–1253
Allel D, Ben-Amar A, Abdelly C (2018) Leaf photosynthesis, chlorophyll fluorescence and ion content of barley (Hordeum vulgare) in response to salinity. J Plant Nutr 41(4):497–508. https://doi.org/10.1080/01904167.2017.1385811
Aqeel M, Khalid N, Tufail A et al (2021) Elucidating the distinct interactive impact of cadmium and nickel on growth, photosynthesis, metal-homeostasis, and yield responses of mung bean (Vigna radiata L) varieties. Environ Sci Poll Res 28(21):27376–27390. https://doi.org/10.1007/s11356-021-12579-5
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta Vulgaris Plant Physiol 24(1):1. https://doi.org/10.1104/pp.24.1.1
Arshad M, Ali S, Noman A et al (2016) Phosphorus amendment decreased cadmium (Cd) uptake and ameliorates chlorophyll contents, gas exchange attributes, antioxidants, and mineral nutrients in wheat (Triticum aestivum L) under Cd stress. Arch Agron Soil Sci 62(4):533–546. https://doi.org/10.1080/03650340.2015.1064903
Bañon S, Ochoa J, Franco J, Alarcón J, Sánchez-Blanco MJ (2006) Hardening of oleander seedlings by deficit irrigation and low air humidity. Environ Exp Bot 56(1):36–43. https://doi.org/10.1016/j.envexpbot.2004.12.004
Bharti N, Yadav D, Barnawal D, Maji D, Kalra A (2013) Exiguobacterium oxidotolerans, a halotolerant plant growth promoting rhizobacteria, improves yield and content of secondary metabolites in Bacopa monnieri (L) Pennell under primary and secondary salt stress. World J Microbiol Biotech 29(2):379–387
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Brady N, Weil R (2002) The nature and properties of soils 13th ed Prentice Hall. New Jersey, USA 249. https://doi.org/10.1023/A:1016012810895
Camalle M, Standing D, Jitan M et al (2020) Effect of salinity and nitrogen sources on the leaf quality, biomass, and metabolic responses of two ecotypes of Portulaca oleracea. Agronomy 10(5):656. https://doi.org/10.3390/agronomy10050656
Chance B, Maehly A (1955) [136] Assay of catalases and peroxidases. https://doi.org/10.1016/S0076-6879(55)02300-8
Colinas M, Fitzpatrick TB (2015) Natures balancing act: examining biosynthesis de novo, recycling and processing damaged vitamin B metabolites. Curr Opin Plant Biol 25:98–106. https://doi.org/10.1016/j.pbi.2015.05.001
Collin VC, Eymery F, Genty B, Rey P, Havaux M (2008) Vitamin E is essential for the tolerance of Arabidopsis thaliana to metal-induced oxidative stress. Plant Cell Environ 31(2):244–257. https://doi.org/10.1111/j.1365-3040.2007.01755.x
Dawood M, El-Awadi M, Sadak M, El-Lethy S (2019) Comparison between the physiological role of carrot root extract and β-carotene in inducing Helianthus annuus L drought tolerance. Asian J Biol Sci 12(2):231–241. https://doi.org/10.3923/ajbs.2019.231.241
Demiral T, Türkan I (2005) Comparative lipid peroxidation, antioxidant defense systems and proline content in roots of two rice cultivars differing in salt tolerance. Environ Exp Bot 53(3):247–257. https://doi.org/10.1016/j.envexpbot.2004.03.017
Du Q, Wang H, Xie J (2011) Thiamin (vitamin B1) biosynthesis and regulation: a rich source of antimicrobial drug targets? Int J Biol Sci 7(1):41. https://doi.org/10.7150/ijbs.7.41
El-Hak SG, Ahmed A, Moustafa Y (2012) Effect of foliar application with two antioxidants and humic acid on growth, yield and yield components of peas (Pisum sativum L.). J Hort Sci Ornam Plants 4:318–328. https://doi.org/10.5829/idosi.jhsop.2012.4.3.262
El-Metwally IM, Sadak MS (2019) Physiological role of thiamine and weed control treatments on faba bean and associated weeds grown under salt affected soil. Bull Nat Res Centre 43(1):1–16. https://doi.org/10.1186/s42269-019-0142-6
Fallahi HR, Aminifard MH, Jorkesh A (2018) Effects of thiamine spraying on biochemical and morphological traits of basil plants under greenhouse conditions. J Hort Postharvest Res 1:27–36
Goyer A (2010) Thiamine in plants: aspects of its metabolism and functions. Phytochem 71(14–15):1615–1624. https://doi.org/10.1016/j.phytochem.2010.06.022
Hamayun M, Khan SA, Khan AL et al (2010) Effect of salt stress on growth attributes and endogenous growth hormones of soybean cultivar Hwangkeumkong. Pak J Bot 42(5):3103–3112
Hasanuzzaman M, Nahar K, Fujita M (2013) Plant response to salt stress and role of exogenous protectants to mitigate salt-induced damages. Ecophysiology and responses of plants under salt stress, pp 25–87. Springer. https://doi.org/10.1007/978-1-4614-4747-4_2
Hussain M, Farooq M, Shehzad M, Khan MB, Wahid A, Shabir G (2012) Evaluating the performance of elite sunflower hybrids under saline conditions. Int J Agric Biol 14(1):131–135
Julkunen-Tiitto R (1985) Phenolic constituents in the leaves of northern willows: methods for the analysis of certain phenolics. J Agric Food Chem 33(2):213–217. https://doi.org/10.1021/jf00062a013
Khalid N, Noman A, Aqeel M, Masood A, Tufail A (2019) Phytoremediation potential of Xanthium strumarium for heavy metals contaminated soils at roadsides. Int J Environ Sci Technol 16(4):2091–2100. https://doi.org/10.1007/s13762-018-1825-5
Kim HY (2014) Analysis of variance (ANOVA) comparing means of more than two groups. Restor Dent Endod 39(1):74. https://doi.org/10.5395/rde.2014.39.1.74
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Ann Rev Plant Biol 59651-681 https://doi.org/10.1146/annurev.arplant.59.032607.092911
Nabati J, Kafi M, Nezami A, Moghaddam PR, Masoumi A, Mehrjerdi MZ (2011) Effect of salinity on biomass production and activities of some key enzymatic antioxidants in kochia (Kochia scoparia). Pak J Bot 43(1):539–548
Naheed R, Aslam H, Kanwal H et al (2021) Growth attributes, biochemical modulations, antioxidant enzymatic metabolism and yield in Brassica napus varieties for salinity tolerance. Saudi J Biol Sci 28(10):5469–5479. https://doi.org/10.1016/j.sjbs.2021.08.021
Noman A, Ali S, Naheed F et al. (2015) Foliar application of ascorbate enhances the physiological and biochemical attributes of maize (Zea mays L.) cultivars under drought stress. Arch Agron Soil Sci 61(12):1659–1672. https://doi.org/10.1080/03650340.2015.1028379
OriginLab Corporation (2021) Origin v2021: Scientific Data Analysis and Graphing Software. Northampton, MA 01060, United States, https://www.originlab.com. Assessed 31 Dec 2021.
Ouerghi K, Abdi N, Maazaoui H, Hmissi I, Bouraoui M, Sifi B (2016) Physiological and morphological characteristics of pea (Pisum sativum L.) seeds under salt stress. J New Sci 28.
Plaut Z, Edelstein M, Ben-Hur M (2013) Overcoming salinity barriers to crop production using traditional methods. Crit Rev Plant Sci 32(4):250–291. https://doi.org/10.1080/07352689.2012.752236
Qasim M, Lin Y, Dash CK et al (2018) Temperature-dependent development of Asian citrus psyllid on various hosts, and mortality by two strains of Isaria. Microb Pathogen 119:109–118. https://doi.org/10.1016/j.micpath.2018.04.019
Qasim M, Xiao H, He K et al (2021) Host-pathogen interaction between Asian citrus psyllid and entomopathogenic fungus (Cordyceps fumosorosea) is regulated by modulations in gene expression, enzymatic activity and HLB-bacterial population of the host. Comp Biochem Physiol Part C: Toxicol Pharmacol 248:109112. https://doi.org/10.1016/j.cbpc.2021.109112
R Development Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org. Accessed 05 Jan 2022.
Rady MM, Mohamed GF (2015) Modulation of salt stress effects on the growth, physio-chemical attributes and yields of Phaseolus vulgaris L. plants by the combined application of salicylic acid and Moringa oleifera leaf extract. Sci Hortic 193:105–113. https://doi.org/10.1016/j.scienta.2015.07.003
Rebello S, Asok AK, Mundayoor S, Jisha M (2014) Surfactants: toxicity, remediation and green surfactants. Environ Chem Lett 12(2):275–287. https://doi.org/10.1007/s10311-014-0466-2
Rezazadeh A, Ghasemnezhad A, Barani M, Telmadarrehei T (2012) Effect of salinity on phenolic composition and antioxidant activity of artichoke (Cynara scolymus L) leaves. Res J Med Plant 6(3):245–252. https://doi.org/10.3923/rjmp.2012.245.252
Río LAd, Corpas FJ, López-Huertas E, Palma JM (2018) Plant superoxide dismutases: function under abiotic stress conditions. Antioxidants and antioxidant enzymes in higher plants, pp 1–26. Springer. https://doi.org/10.1007/978-3-319-75088-0_1
Sadak MS (2019) Physiological role of trehalose on enhancing salinity tolerance of wheat plant. Bull Natl Res Cent 43(1):1–10. https://doi.org/10.1186/s42269-019-0098-6
Sayed S, Gadallah M (2002) Effects of shoot and root application of thiamin on salt-stressed sunflower plants. Plant Growth Regul 36(1):71–80. https://doi.org/10.1023/A:1014784831387
Shabala S (2013) Learning from halophytes: physiological basis and strategies to improve abiotic stress tolerance in crops. Ann Bot 112(7):1209–1221. https://doi.org/10.1093/aob/mct205
Shabala S, Pottosin I (2014) Regulation of potassium transport in plants under hostile conditions: implications for abiotic and biotic stress tolerance. Physiol Plant 151(3):257–279. https://doi.org/10.1111/ppl.12165
Shalata A, Mittova V, Volokita M, Guy M, Tal M (2001) Response of the cultivated tomato and its wild salt-tolerant relative Lycopersicon pennellii to salt-dependent oxidative stress: The root antioxidative system. Physiol Plant 112(4):487–494. https://doi.org/10.1034/j.1399-3054.2001.1120405.x
Shan C, Wang Y (2017) Exogenous salicylic acid-induced nitric oxide regulates leaf water condition through root osmoregulation of maize seedlings under drought stress. Brazilian J Bot 40(2):591–597. https://doi.org/10.1007/s40415-016-0355-y
Soltani J (2017) Endophytism in Cupressoideae (Coniferae): a model in endophyte biology and biotechnology. Endophytes: biology and biotechnology, pp 127–143. Springer. https://doi.org/10.1007/978-3-319-66541-2_6
Steel RG, Torrie JH, Dickey DA (1997) Principles and procedures of statistics, McGraw-Hill, New York. USA.
Subki A, Abidin AAZ, Yusof ZNB (2018) The role of thiamine in plants and current perspectives in crop improvement. B Group Vitamins-Current Uses and Perspectives 5(3):33–44. https://doi.org/10.5772/intechopen.79350
Sun Y, Kong X, Li C, Liu Y, Ding Z (2015) Potassium retention under salt stress is associated with natural variation in salinity tolerance among Arabidopsis accessions. PLoS ONE 10(5):e0124032. https://doi.org/10.1371/journal.pone.0124032
Taïbi K, Taïbi F, Abderrahim LA, Ennajah A, Belkhodja M, Mulet JM (2016) Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence systems in Phaseolus vulgaris L. S Afr J Bot 105306-312https://doi.org/10.1016/j.sajb.2016.03.011
Tayyab, Azeem M, Qasim M, Ahmed N, Ahmad R (2016) Salt stress responses of pigeon pea (Cajanus cajan) on growth, yield and some biochemical attributes. Pak J Bot 48(4): 1353–1360. https://pakbs.org/pjbot/PDFs/48(4)/05
Torun H (2019) Time-course analysis of salicylic acid effects on ROS regulation and antioxidant defense in roots of hulled and hulless barley under combined stress of drought, heat and salinity. Physiol Plant 165(2):169–182. https://doi.org/10.1111/ppl.12798
Tunc-Ozdemir M, Miller G, Song L et al (2009) Thiamin confers enhanced tolerance to oxidative stress in Arabidopsis. Plant Physiol 151(1):421–432. https://doi.org/10.1104/pp.109.140046
Wasti S, Mimouni H, Smiti S, Zid E, Ben Ahmed H (2012) Enhanced salt tolerance of tomatoes by exogenous salicylic acid applied through rooting medium. Omics J Integr Biol 16(4):200–207. https://doi.org/10.1089/omi.2011.0071
Wolf B (1982) A comprehensive system of leaf analyses and its use for diagnosing crop nutrient status. Commun Soil Sci Plant Anal 13(12):1035–1059. https://doi.org/10.1080/00103628209367332
Xiong Y-C, Li F-M, Zhang T (2006) Performance of wheat crops with different chromosome ploidy: root-sourced signals, drought tolerance, and yield performance. Planta 224(3):710–718. https://doi.org/10.1007/s00425-006-0252-x
Yemm E, Willis A (1954) The estimation of carbohydrates in plant extracts by anthrone. Biochem J 57(3):508–514. https://doi.org/10.1042/bj0570508
Zörb C, Mühling KH, Kutschera U, Geilfus C-M (2015) Salinity stiffens the epidermal cell walls of salt-stressed maize leaves: is the epidermis growth-restricting? PLoS ONE 10(3):e0118406. https://doi.org/10.1371/journal.pone.0118406
Acknowledgements
The authors are highly thankful to Government College Women University, Faisalabad, Pakistan, for providing experimental station and lab facilities. The authors also extend their appreciation to the deanship of scientific research, King Khalid University, for funding this work through research group program under grant number R.G.P. 2/17/43.
Author information
Authors and Affiliations
Contributions
All authors read and approved the final version of the manuscript. R.N.: conceptualization; formal analysis; resources; project administration; writing—original draft. M.Z.: investigation; formal analysis; resources. M.A.: data curation; methodology; resources; software, visualization; writing—original draft; writing—review and editing. M.F.M.: methodology; formal analysis; writing—review and editing. H.K.: writing—review and editing. N.K.: validation; resources; writing—review and editing. M.H.: funding acquisition, writing—review and editing. S.A. validation; writing—review and editing. A.N.: project administration; writing—review and editing.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Naheed, R., Zahid, M., Aqeel, M. et al. Mediation of Growth and Metabolism of Pisum sativum in Salt Stress Potentially Be Credited to Thiamine. J Soil Sci Plant Nutr 22, 2897–2910 (2022). https://doi.org/10.1007/s42729-022-00854-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-022-00854-4