Abstract
Surfactants toxicity has induced a worldwide alert followed by various regulations. There are still concerns about the biodegradability and ecofriendliness of surfactants. Reviews on surfactants are available, but a concise manuscript covering surfactant types, primary and secondary toxicity of surfactants, evaluating the level of surfactant pollution worldwide, is needed. We review here the safety of surfactants in the aquatic system, in terrestrial ecosystems and for humans. We discuss strategies to solve surfactant contamination. Remediation methods include ozonation, UV radiation and catalyst-coupled auto-oxidation. We focus on the biodegradation of the anionic detergents sodium dodecyl sulfate and linear alkyl benzene sulfonate. Finally, the relevance and role of biosurfactants as alternatives to synthetic detergents are also described.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Traced back from the ancestral Babylonian ash-oil soap formula to the currently available soaps, cleansers and detergents, surfactants appear in various forms. Of these, detergents indeed have become indispensable elements of man’s life all along his steps aiming cleanliness and tidiness. Apart from serving as cleansing agents, surfactants find many industrial applications as additives in paints, as textile softeners, as antistatic agents, in metal processing and in oil drilling operations. Some surfactants have antimicrobial properties which provide the basis for their utility as biocides (Ginkel 1989).
Industries worldwide discharge a wide range of surfactants, or surface-active agents, to their wastewater treatment facilities. Once used, surfactants enter the water bodies, where they can cause problems if they persist long, leading to the accumulation of potentially toxic or otherwise harmful substances (Deschenes et al. 1996) and cause serious environmental problems (Abd-Allah 1995). Surfactants are ubiquitous and in untreated effluents, certain classes of surfactants can be present in sufficient concentrations to constitute toxicity problems to aquatic organisms (Ankley and Burkhard 1992), even between 0.4 and 40 mg/L (Abel 1974).
Extensive research on the surfactant toxicity does exist (Lewis 1991; Schweigert et al. 2000; Chaturvedi and Kumar 2010a); however, assessment of contemporary pollution profile of surfactants is relevant. The toxicity and biodegradation of the most commonly used anionic surfactants sodium dodecyl sulfate (SDS) and linear alkyl benzene sulfonate (LAS) are detailed in this review, with special reference to its biodegradability and safe disposal. Information available on the metabolic pathway and molecular mechanism of surfactant degradation, methods and alternatives to combat the problem of surfactant contamination are also discussed. The relevance and utility of biosurfactants as an alternative to current synthetic detergents are also reviewed. This article is an abridged version of the chapter by Rebello et al. (2013) published in the book series Environmental Chemistry for a Sustainable World.
Chemistry of surfactants
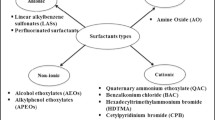
Surfactants are chemicals capable of reducing surface tension of liquids or interfaces of liquids, endowed by its hydrophobic tail and hydrophilic head. Surfactants do occur as simple monomers, but sometimes exist as more complex polymers. Based on the charge of the hydrophilic group of surfactants, they are classified as anionic (negatively charged), cationic (positively charged), nonionic (without any charge) and ampholytic/zwitter ionic (both charges). The anionic surfactant SDS is synthesized by sulfonation of petrochemical or oleo chemical-based lauryl alcohols, whereas LAS is exclusively synthesized from petrochemical by-products. SDS is linear molecule with an alkyl tail of 12 carbon atoms, attached to a sulfate group giving the molecule the amphiphilic properties required of a surfactant. LAS have a hydrophobic alkyl chain and a hydrophilic head with a benzene ring and a sulfonate group. It is not a single compound, but ideally a mixture of 20 compounds, of closely related homologs and isomers.
Surfactant pollution: worldwide and the Indian Scenario
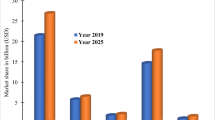
The per capita detergent consumption in India is around 2.7 kg/year, whereas in places like Philippines and Malaysia, it is 3.7 kg, and in USA, it is around 10 kg. The high consumption rates of detergents also develop a high detergent concentration in our water bodies (Gonzalez et al. 2012) as depicted in Fig. 1. The accumulation of surfactants in river sediments (Rico-Rico et al. 2009), marine water and sediments (Petrovic et al. 2002), infiltrated ground water (Field et al. 1992) and sewage effluents with concentrations up to 1,090 μg/L (Holt et al. 1989) has been observed. LAS was found in treated sludge at high concentrations of up to 30,200 mg/kg dry weight (Berna et al. 1989) and in surface waters at concentrations of up to 416 μg/L (Fox et al. 2000), which is quite above its predicted no effect concentration of 250 μg/L (van de Plassche et al. 1999). In various developing countries, the usage of phosphate-based detergents continues at alarmingly high rates (Khurana 2002), leading to excessive growth of algae.
Most of the ponds in Varanasi city have become eutrophic and are highly contaminated especially with high amount of detergents (Chaturvedi and Kumar 2010b). Detergent pollution resulting from textile industry of Tirupur of Tamil Nadu also contributes to environmental pollution (De Neve 2009). Surfactants were detected in water samples collected from surface and ground (2–62 μg/L), bore wells and open wells (22–427 μg/L) in Tirupati of Andhra Pradesh, and open municipal drainage waste waters (50–720 μg/L) (Kanchi et al. 2012).
Safety concerns on surfactants
The cycle of surfactant toxicity starts from its very synthesis, disposal and subsequent exposure to the environment. Surfactant synthesis critically affects the environment aggravating the problems related to global warming, climate change, ozone layer depletion and greenhouse gas emission which cannot be totally avoided. Both petrochemical and oleo chemical-based surfactant production result in atmospheric emission (NOx, CO2, SO2, hydrocarbons), waterborne wastes and solid wastes capable of causing eutrophication and acidification of rivers and lakes (Stalmans et al. 1995).
Toxicity on microbial world
The impacts of surfactants on the microbial world vary with each species and extend of pollution. Bacterial degradation of surfactants is well established (which will be discussed in biodegradation of surfactants). However, surfactants are also found to have deleterious effects on various other bacteria such as phosphate solubilizing Acinetobacter junii (Ivankovic et al. 2009), autotrophic ammonia oxidizing Nitrosomonas and Nitrosospira strains (Brandt et al. 2001) and bioluminescent Vibrio fischeri (Lima et al. 2011). Surfactant-based membrane lysis, DNA damage and starvation are found as counterparts even in bacteria capable of utilizing SDS as sole carbon source (Klebensberger et al. 2006). Effect of LAS on inherent populations of bacteria, fungi and actinomycetes in soil gave statistically significant results for bacteria and fungi (Asok and Jisha 2012b).
Surfactants primarily affect the growth, motility and photosynthetic ability of algae, the extent of toxicity dependant on the surfactants (type, concentration) and algal type (Lewis 1990). SDS even at 0.1 mg/mL causes inhibition of asexual and sexual reproduction of Closterium ehrenbergii resulting in no zygospore formation or defective/abnormal spores (Matsui and Park 2000). The photosynthetic ability of algae decreased exponentially with increasing surfactant concentration and decrease in algal biomass (Maksimov and Parshikova 2006), with cationic surfactants causing the most potent inhibition.
Toxicity on soil and plants
Even at low concentrations, surfactants seem to alter soil physics, soil chemistry and soil biology significantly, whereby sorption processes play a dominant role (Kuhnt 1993). Surfactants primarily affect the roots of plants suppressing or killing the roots, with comparatively less inhibition in the shoots of wheat seedlings (Rinallo et al. 1988). Use of detergent-contaminated water for cultivation reduces the photosynthetic rate and chlorophyll content in bean plants (Jovanic et al. 2010). The continuous application of the anionic surfactant LAS to the soil increased the acid and alkaline phosphatase activity and arylsulfatase activity, whereas the soil dehydrogenase activity was decreased on continuous LAS exposure (Sanchez-Peinado et al. 2009).
Toxicity to aquatic system
Surfactant toxicity has been reported since late 1960s, during which it was observed that the exposure of fish Ictalurus natalis to detergent levels (0.5 ppm) even much lower than its sublethal concentrations caused disruption of chemoreceptors (Bardach et al. 1965). Chronic and sublethal toxicities of anionic and nonionic surfactants to aquatic animals occur at concentrations usually greater than 0.1 mg/L (Lewis 1991). Adverse biological effects on aquatic organisms occur especially when surfactants occur at relatively high concentrations (Romanelli et al. 2004).
The aftermath of surfactant exposure can be clearly visualized in various organs like gills (Mallatt 1985), liver, kidney (Rosety-Rodríguez et al. 2002), spleen and intestines (Ribelles et al. 1995) of fishes. Exposure to high surfactant concentration results in gill epithelial disruption causing subsequent asphyxiation or osmoregulatory failure (Mallatt 1985), while exposure to sublethal concentrations of surfactants causes gill epithelial hyperplasia, oxidative stress and mucus layer damage of fishes, which predispose them to microbial attack (Susmi et al. 2010). The detrimental effects of SDS on crustaceans are found to be less pronounced, yet reports on detrimental effects of this surfactant on filter feeding habits of bivalves (Ostroumov 2003) and mussel suspension feeding (Ostroumov and Widdows 2006) do exist.
Effect on higher vertebrates
In humans, anionic surfactants target mainly the stratum corneum of membrane bilayer of sensitive skin resulting in dermatitis (Marrakchi and Maibach 2006) and aphthous ulcers (Chahine et al. 1997). The American Cancer Society denied that SDS is carcinogenic and points out that the substance, while undoubtedly a skin irritant, is a strong detergent intended to remove oil and soil, but there is no link between use of this product and cancer risk (Doyle 2010). Thus, it is likely that the toxicity of these anionic surfactants is relatively low in human and wild animals as the molecular weight increases, probably due to lower adsorption in the intestine. An acute toxic effect by anionic surfactants is therefore not to be likely but a chronic effect can, however, be more possible since a regular dosage of a human is about 5 mg/person from drinking waters, detergents, toothpaste and food.
Several cytotoxicity tests reveal that nonionics have the least toxicity in the order as cationic > anionic > amphoteric > nonionic (Grant et al. 1992). Some nonionic surfactants are found to anaesthetize the eye ball, and thus, combinations of nonionic surfactants with anionics would make many shampoos gentle to eye (Conry 1980).
Secondary toxicity of surfactants
Generally, the presence of surfactants helps in the degradation of polycyclic hydrocarbons, but the degradation of PAH was inhibited by SDS because this surfactant was preferred as a growth substrate (Tiehm 1994). This suggests that the presence of this detergent in the water bodies would indirectly lead to bioaccumulation of other hydrocarbons. Reports are also available that adding SDS and Pseudomonas aeruginosa UG2 biosurfactants inhibit polycyclic aromatic hydrocarbon biodegradation in a weathered creosote-contaminated soil (Deschenes et al. 1996) .The presence of surfactants may be important for the fate of pesticides at effluent-irrigated sites because they may increase the apparent solubility of hydrophobic pesticides (Vigon and Rubin 1989).
Tackling surfactant pollution
Surfactant toxicity has aroused worldwide attempts to reduce after effects of these silent toxicants. The strict regulations on usage of phosphate free surfactants, remediation of waste water before disposal and promotion of green surfactants are manifestations of these attempts. The usage of liquid detergents than powder forms results in less surfactants toxicity according to a case study at Ludhiana (Goel and Kaur 2012). Interesting suggestions for hands on preparation of laundry and liquid detergents for hard and soft water could also add to our attempts to reduce surfactant toxicity (Khurana 2002). The use of nontoxic-biodegradable natural soaps and soapnuts is yet another promising approach (Ghai 2011).
Remediation before disposal
The influence and relevance of surfactants in mans life are too immense, that totally avoiding them from our day-to-day life seems to be impossible and unpractical. Better management of surfactant use and disposal has become the need of the hour, both at industrial and domestic level. Strict regulations in the effective remediation of surfactants before disposal should be done. This section describes the various methods of surfactant remediation and various steps that could reduce surfactant pollution at domestic level.
Physical and chemical methods
Various physical, chemical and biological methods of surfactant detoxification are reported. Physical treatment of surfactants by ozonation and advanced oxidation using various combinations of ozone, hydrogen peroxide, ultraviolet light irradiation and iron salts were found effective in degrading recalcitrant surfactants, including LAS, alkylphenol ethoxylates and quaternary ammonium surfactants (Ikehata and El-Din 2004). Various other techniques like electrocoagulation (Yuksel et al. 2009), nanofiltration (Korzenowski et al. 2012), sonochemical degradation, foam fractionation and wet air oxidation are also used.
Oxidation-based methodologies Detoxification of micropollutants such as surfactants, pesticides, herbicides and microtoxins from drinking water mainly relies on high oxidizing capacity of ozone (Beltran et al. 2000). LAS ozonation in surface waters intended for human consumption demonstrated that combinatorial use of O3 and powdered activated carbon approach is the most efficacious than traditional O3- or H2O2-based oxidation systems, considerably increasing the LAS removal rate and also reducing the concentration of dissolved organic carbon (Rivera-Utrilla et al. 2006).
Photocatalytic degradation The photocatalytic degradation of surfactants in water is done by solar fenton-like oxidation reaction (Bhatkhande et al. 2002). The photodegradation of SDS and LAS in reactors in presence of TiO2 catalyst and UV light is also well studied (Hidaka 1998). According to this study, the surfactant competitively binds to TiO2 and on exposure to UV light, radical attack on the aromatic ring and alkyl chain brings about degradation.
Foam fractionation Foam fractionation is a chemical process in which hydrophobic molecules are preferentially separated from a liquid solution using rising columns of foam. It has been shown to be an effective method of removing anionic or cationic surfactants from effluent streams. Cationic surfactants were easily removed from water by foam fractionation than the anionic surfactants studied (Tharapiwattananon et al. 1996).
Sonochemical degradation The utility of sonochemical reactors for anionic surfactant degradation from waste waters is well studied the rate of degradation proportionally increasing with sonication time, but decreasing with surfactant increase (Dehghani et al. 2010). The potential of using ultrasonic irradiation for the removal of LAS revealed that it increases with the frequency of radiation, and the addition of NaCl or H2O2 to this system had adverse effect on LAS conversion, while addition of Fe2+ either alone or in conjunction with H2O2 (fenton reagent) had a positive effect on degradation of LAS (Manousaki et al. 2004). Usage of 20 kHz ultrasound at 40 °C, pH at 2.5 throughout and addition of extra amounts of zero valent iron and H2O2 during the degradation resulted in 93 % reduction in LAS (Naldoni et al. 2011).
Electrochemical degradation Electrocoagulation can be addressed as a method of wastewater treatment when electric current goes through an electrolysis cell supplied with soluble electrodes (Sequeira 1994). This technique is also effective in the treatment of the strongly acidic effluents arising from electrokinetic surfactant-aided soil flushing of polluted soils using aluminum electrodes (anodes and cathodes) (Lopez-Vizcaino et al. 2012).
Biological methods
Microbial biodegradation provides a safer, environmentally benign and cost-effective alternative to physicochemical methods for surfactant remediation (Oya and Hisano 2010). Biodegradation of surfactants is most often performed by diverse soil or aquatic microorganisms leading to generation of water and carbon dioxide gas (Schleheck et al. 2000). Surfactant degradation is predominantly carried out by various species of Pseudomonas, yet many other bacterial species are also reported to participate in surfactant remediation as listed in Table 1.
Biodegradation of anionic surfactants was initiated in early 1960s (Payne and Feisal 1963), with the isolation of two unknown bacterial soil isolates capable of degrading short- or long-chained organic acids and alcohols of SDS and three of five phenyl placement isomers of dodecyl benzene sulfonate. But isomers with phenyl placement at carbon 4 or 5 were toxic and killed the bacteria. The primary SDS splitting enzyme was reported soon (Hsu 1963) and identified as a primary alcohol sulfatase. Anionic surfactant degradation is initiated by alkylsulfatases which convert them to corresponding alcohol by removing the sulfate/sulfonate moiety. Growth of the bacteria on SDS as the sole carbon source induced glyoxylate bypass enzymes, isocitrate lyase and malate synthetase, in addition to alkylsulfatases (Williams and Payne 1964).
Metabolism of SDS by the detergent-degrading bacterium Pseudomonas C12B using a 14C radiotracer which showed that 70 % of the radiolabel was released as 14CO2 at completion, where as the remaining isotope was incorporated cells (Thomas and White 1989). As depicted in Fig. 2, SDS was degraded yielding with the sequential production from [1−14C] SDS of 1-dodecanol, dodecanal and dodecanoic acid. Biodegradation of LAS begins at the terminus of the alkyl chain with an omega-oxidation and is followed by successive cleavage of C2 fragments (β-oxidation) (Huddleston and Allred 1963; Swisher 1963). These intermediates are further biodegraded by oxidative removal of the aromatic ring and cleavage of the sulfonate group (Setzkorn and Huddleston 1965; Swisher 1967). Core research aiming optimized surfactant degradation has been carried out in the case of anionic surfactants SDS and LAS (Abboud et al. 2007; Asok and Jisha 2012a). SDS is found be highly degradable both in aerobic and anaerobic conditions. LAS biodegradation in turn is found to be inhibited by anaerobic conditions (Mungray and Kumar 2009).
Analysis of alkyl sulfatase in parent and cured strains of Pseudomonas confirmed that both enzymes are encoded by the chromosome. The nucleotide sequence of two chromosomally located genes sdsA and sdsB, coding for alkylsulfatase and its transcriptional regulator, respectively, were identified to play significant role in SDS degradation (Davison et al. 1992). Evidence to the transcriptional regulation of sdsA gene by sdsB protein was further proved (Jovcic et al. 2010). The ability of Pseudomonas C12 B to utilize alkyl benzene sulfonate also appears to be coded by the chromosome (Kostal et al. 1998). A novel alkylsulfatase gene, sdsAP, was cloned from a newly isolated bacterium Pseudomonas sp. S9 and expressed in heterologous host of E. coli (Long et al. 2011). Plasmid-encoded character often plays significant role in bacterial adaptation to xenobiotics in the environment (Kado and Liu 1981). Reports on plasmid-encoded surfactant degradation also do support this (Yeldho et al. 2011).
Sewage treatment plants
A sewage treatment plant represents the practical manifestations of biological surfactants degradation. The presence of surfactants (alcohol sulfates) in industrial effluents inhibited the anaerobic digestion of even readily biodegradable compounds like starch and other carbohydrates (Feitkenhauer and Meyer 2002), substantiating the need for remediation. The incorporation of surfactant degrading bacterial cultures in household and industrial sewage could be a cost-effective method of anionic surfactants elimination reducing the BOD, COD and methylene blue active levels (Hosseini et al. 2007) in the water bodies. The presence of properly functioning sewage treatment plants in several places has resulted in low surfactant concentrations in the environment (van de Plassche et al. 1999). The use of fluidized bed reactors enabled the anaerobic degradation of LAS by microbial consortia (Bacteroidetes, Firmicutes, Actinobacteria and Proteobacteria) in different support material giving 99 ± 2 % removal rates (de Oliveira et al. 2010).
Green surfactants
The idea of going green has launched the use of renewable materials for surfactant synthesis resulting in so-called green surfactants. This new class of biodegradable and biocompatible products is a response to the increasing consumer demand for products that are both “greener,” milder and more efficient (Benvegnu and Sassi 2010). The use of renewable resources for surfactant synthesis rather than petrochemicals would reduce the liberated CO2 levels by 37 % in EU (Patel et al. 1999). Green surfactants are defined as biobased amphiphilic molecules obtained from nature or synthesized from renewable raw materials. Various renewable raw materials particularly triglycerides, carbohydrate sources and organic acids (produced by fermentation) serve as starting materials in surfactant synthesis, of which, triglycerides/sterols contribute to the hydrophobic part while sugars/amino acids contribute to the hydrophilic part of green surfactants. Green surfactants can be synthesized from renewable raw materials either by chemical modification or utilizing the biosynthetic machinery of biotic community (plants, microbes, yeast, etc.) yielding biosurfactants.
Chemically derived green surfactants Triglycerides, regardless of their source, utilize a variety of standard oleochemical transformations—hydrogenation, hydrolysis, trans-esterification as well as certain specific modifications to yield various surfactants and surfactant precursors including fatty acid methyl ester, methyl ester sulfonate, fatty alcohols, fatty amines, fatty acid anhydrides, fatty chlorides, fatty acids, fatty acid carboxylates and alkylpolyglucosides. (Foley et al. 2012). In recent years, due to the large increase in petroleum cost, there has been a re-emergence of interest in large-volume production of fermentation chemicals. Biotechnology is providing new, low-cost and highly efficient fermentation processes for the production of chemicals from biomass resources. Moreover, with a wide range of microorganisms available and many more recently discovered, fermentation of sugars represents an important route for the production of new bioproducts. On account of the performance and the high quality, regarding the light color and the good odor, alkylpolyglucoside is particularly appropriate to cosmetic lotions and creams (Weuthen et al. 1995). Alkylpolyglucosides are found to be superior to various carbohydrate based surfactants and are extensively produced on account of its good performance, mildness and completely renewable-based nature. Methyl ester sulfonate offers an environmentally friendly and viable alternative to the currently used workhorse surfactant LAS due to its high biodegradability (Ghazali 2002).
As noted earlier, surfactants could be synthesized either from petrochemicals or natural oleochemicals, but the use of green surfactants does not always bring a solution to ecotoxicity as the surfactants chemically remains the same irrespective of the mode of its synthesis. For example, while surfactants like linear sulfate and secondary alkyl sulfonates are purely petrochemical-based surfactants like alcohol sulfates, alcohol ether sulfates and alkyl ethoxylates are partly fossil fuel based or partly oleo chemical based.
Biosurfactants as alternate to synthetic surfactants Biosurfactants are biological compounds with high surface-active properties (Georgia and Poe 1931), produced by microorganisms, plants, animals and humans (Christofi and Ivshina 2002). They are produced on microbial cells surfaces or excreted extracellularly and contain both hydrophilic and hydrophobic moieties. They have several advantages over the chemical surfactants, such as lower toxicity higher biodegradability (Zajic et al. 1977), better environmental compatibility (Georgiou et al. 1992), higher foaming ability (Razafindralambo et al. 1996), high selectivity and specific activity at extreme temperatures, pH and salinity (Velikonja and Kosaric 1993) and the ability to be synthesized from renewable feed stock (Desai and Banat 1997). In general, biosurfactants are more effective and efficient, and their CMC is about 10–40 times lower than that of chemical surfactants, i.e., less surfactant is necessary to get a maximum decrease in surface tension (Desai and Banat 1997), and biosurfactants also have higher EC50 than synthetic surfactants (Poremba et al. 1991). Biosurfactants constitute an interesting alternative to the commercial chemical surfactants with potential use in several industries (Vaz et al. 2012).
Biosurfactants are of different types including glycolipids, lipopeptides, phospholipids, surface-active antibiotics, fatty acids/neutral lipids, polymeric surfactants and particulate (Muthusamy et al. 2008). These compounds are produced during the growth of microorganisms on water-soluble and water immiscible substrates. Diverse ranges of prokaryotic and eukaryotic microorganisms are capable of producing surfactants (Lang 2002). Bacterial surfactant—producing organisms—includes P. aeruginosa (mono- and di-rhamnolipid biosurfactants), Corynebacterium, Nocardia and Rhodococcus spp. (phospholipids, trehalose dimycolates/dicorynomycolates, glycolipids etc.), Bacillus subtilis (surfactin), Bacillus licheniformis (lipopeptide similar to surfactin), Arthrobacter paraffineus (trehalose and sucrose lipids) and others. Fungi involved in surfactant production include the yeasts Torulopsis spp. (sophorolipids) and Candida spp. (liposan, phospholipids) (Christofi and Ivshina 2002). The potential application of biosurfactants in Table 2 depicts the various fields in which the use of synthetic detergents can be replaced by biosurfactants.
Some practical approaches have been adopted to make biosurfactant production process economically attractive including use of cheaper raw materials, optimized and efficient bioprocesses and overproducing mutant and recombinant strains for obtaining maximum productivity (Muthusamy et al. 2008). Agro-industrial wastes such as olive oil mill effluent (Mercade et al. 1993), soap stock (Shabtai 1990; Benincasa et al. 2002); molasses (Patel and Desai 1997), starch-rich wastes (Nitschke and Pastore 2004) and vegetable oils (Makkar et al. 2011) are used for surfactant production. Optimized biosurfactant production by an integrated rational whole-cell biocatalyst and bioprocess design methodology, termed systems biotechnology, has also been described (Muller and Hausmann 2011). A novel method of SDS-based rhamnolipid synthesis is also been introduced yielding a high substrate to product conversion ratio (Rebello et al. 2013). However, current biosurfactant research has advanced far ahead providing significant opportunities to replace chemical surfactants with sustainable biologically produced alternatives in bulk commercial products. The growing demand for ecofriendly, truly bio-based surfactants, along with developments biosurfactant production has commercialized their production as shown in Table 3.
Conclusions
Tracing back from the ancient ashes, to modern petrochemical or nonrenewable raw material based surfactants, the surfactant industry is constantly evolving and expanding to a highly competitive sector yielding a myriad of brands to meet the various demands of mankind. Their consumption is increasing day by day with no limits and restrictions, equally contributed by domestic purposes and industry. Such accumulation of these silent toxicants to the ecosystem could lead to drastic environmental problems including global warming, terrestrial and aquatic toxicity of the ecosystem and its inhabitants. Total banning of surfactants is impossible in such a modernized lifestyle needing surfactants in our food, cosmetics, cleansers, etc.
Surfactants are quite often regarded harmless, on basis of its biodegradability and speculated low concentrations in the environment. But statistical analysis of surfactant concentrations worldwide reveals the fact that these pollutants are found in concentrations higher than their predicted no effect concentrations. Thus, regarding surfactants as nonpollutants is a mistake. Visible manifestations of surfactant toxicity are available in the case of microbes, plants and animals.
The problem of surfactants toxicity should be thus addressed with cautiousness in every nation. Wise and limited usage of surfactants right from household level to large scale industries thus could reduce the intensity of surfactant pollution. The use of various physical, chemical and bioremediation strategies could help to reduce the toxicity of surfactants before their disposal into the environment. In such a scenario, going green by choosing the right surfactants, especially phosphate free and ecofriendly ones, gains relevance. Ultimately the utilization of biosurfactants could lower the extent of synthetic surfactants prevalence in environment and its associated toxicity.
References
Abboud MM, Khleifat KM, Batarseh M, Tarawneh KA, Al-Mustafa A, Al-Madadhah M (2007) Different optimization conditions required for enhancing the biodegradation of linear alkylbenzosulfonate and sodium dodecyl sulfate surfactants by novel consortium of Acinetobacter calcoaceticus and Pantoea agglomerans. Enzym Microb Technol 41:432–439. doi:10.1016/j.enzmictec.03.011
Abd-Allah AMA (1995) Determination of long chain alkylbenzenes in sediment samples from Alexandria Coast, Egypt. Toxicol Environ Chem 47:83–88. doi:10.1080/02772249509358130
Abel PD (1974) Toxicity of synthetic detergents to fish and aquatic invertebrates. J Fish Biol 6:279–298. doi:10.1111/j.1095-8649.1974.tb04545.x
Ambily PS, Jisha MS (2011) Characterization of alkyl sulfatase required for the biodegradation of sodium dodecyl sulphate (SDS). Eur J Exp Biol 1:41–49
Ambily PS, Jisha MS (2012) Biodegradation of anionic surfactant, sodium dodecyl sulfate by Pseudomonas aeruginosa MTCC 10311. J Environ Biol 33(4):717–720
Ankley GT, Burkhard LP (1992) Identification of surfactants as toxicants in a primary effluent. Environ Toxicol Chem 11:1235–1248
Asok AK (2011) Bioremediation of the anionic surfactant linear alkylbenzene sulphonate (las) by Pseudomonas sp. isolated from soil. PhD thesis. Mahatma Gandhi University, Kottayam, India 192
Asok AK, Jisha MS (2012a) Biodegradation of the anionic surfactant linear alkylbenzene sulfonate (LAS) by autochthonous Pseudomonas sp. Water Air Soil Pollut 223(8):5039–5048. doi:10.1007/s11270-012-1256-8
Asok AK, Jisha MS (2012b) Assessment of soil microbial toxicity on acute exposure of the anionic surfactant linear alkylbenzene sulfonate. J Environ Sci Technol 5:354–363. doi:10.3923/jest.2012.354.363
Bardach JE, Fujiya M, Holl A (1965) Detergents: effects on the chemical senses of the fish Ictalurus natalis (le Sueur). Science 148:1605–1607
Beltran FJ, Garcia-Araya JF, Alvarez PM (2000) Sodium dodecylbenzene sulfonate removal from water and wastewater. Kinetics of decomposition by ozonation. Ind Eng Chem Res 39:2214–2220. doi:10.1021/ie990721a
Benincasa M, Contiero J, Manresa MA, Moraes IO (2002) Rhamnolipid production by Pseudomonas aeruginosa LBI growing on soapstock as the sole carbon source. J Food Eng 54:283–288. doi:10.1016/S0260-8774(01)00214-X
Benvegnu T, Sassi JF (2010) Oligomannuronates from seaweeds as renewable sources for the development of green surfactants. Carbohydr Sustain Dev I:143–164
Berna JL, Ferrer J, Moreno A, Prats D, Ruiz Bevia F (1989) The fate of LAS in the environment. Tenside Surfactants Deterg 26:101–107
Bhatia M, Singh HD (1996) Biodegradation of commercial linear alkyl benzenes by Nocardia amarae. J Biosci 21:487–496
Bhatkhande DS, Pangarkar VG, Beenackers AACM (2002) Photocatalytic degradation for environmental applications—a review. J Chem Technol Biotechnol 77:102–116. doi:10.1002/jctb.532
Bird JA, Cain RB (1972) Metabolism of linear alkylbenzenesulfonates by a Vibrio sp. Biochem J 127(2):46
Brandt KK, Hesselso M, Roslev P, Henriksen K, So J (2001) Toxic Effects of linear alkylbenzene sulfonate on metabolic activity, growth rate, and microcolony formation of Nitrosomonas and Nitrosospira strains. Appl Environ Microbiol 67:2489–2498
Chahine L, Sempson N, Wagoner C (1997) The effect of sodium lauryl sulfate on recurrent aphthous ulcers: a clinical study. Compend Contin Educ Dent 18(12):1238–1240
Chaturvedi V, Kumar A (2010a) Bacterial utilization of sodium dodecyl sulfate. Int J Appl Biol Pharmaceut Tech 3:1126–1131
Chaturvedi V, Kumar A (2010b) Isolation of sodium dodecyl sulfate degrading strains from a detergent polluted pond situated in Varanasi city, India. J Cell Mol Biol 2:103–111
Chaturvedi V, Kumar A (2011) Diversity of culturable sodium dodecyl sulfate (SDS) degrading bacteria isolated from detergent contaminated ponds situated in Varanasi city, India. Int Biodeterior Biodegrad 65:961–971
Chen J, Song X, Zhang H, Qu Y, Miao J (2006) Sophorolipid produced from the new yeast strain Wickerhamiella domercqiae induces apoptosis in H7402 human liver cancer cells. Appl Microbiol Biotechnol 72:52–59. doi:10.1007/s00253-005-0243-z
Christofi N, Ivshina IB (2002) Microbial surfactants and their use in field studies of soil remediation. J Appl Microbiol 93:915–929. doi:10.1046/j.1365-2672.01774
Conry T (1980) Consumer’s guide to cosmetics. Ancor Press/Doubleday, Garden City, p 74
Das K, Mukherjee AK (2007) Differential utilization of pyrene as the sole source of carbon by Bacillus subtilis and Pseudomonas aeruginosa strains: role of biosurfactants in enhancing bioavailability. J Appl Microbiol 102:195–203
Davison J, Fo Brunel, Phanopoulos A, Prozzi D, Terpstra P (1992) Cloning and sequencing of Pseudomonas genes determining sodium dodecyl sulfate biodegradation. Gene 114:19–24
De Neve G (2009) Power, inequality and corporate social responsibility: the politics of ethical compliance in the South Indian garment industry. Econ Polit Wkly 44:63–71
De Oliveira LL, Costa RB, Okada DY, Vich DV, Duarte IC, Silva EL, Varesche MB (2010) Anaerobic degradation of linear alkylbenzene sulfonate (LAS) in fluidized bed reactor by microbial consortia in different support materials. Bioresour Technol 101:5112–5122. doi:10.1016/j.biortech.2010.01.141
Dehghani MH, Najafpoor AA, Azam K (2010) Using sonochemical reactor for degradation of LAS from effluent of wastewater treatment plant. Desalination 250:82–86
Desai JD, Banat IM (1997) Microbial production of surfactants and their commercial potential. Microbiol Mol Biol Rev 61:47–64
Deschenes L, Lafrance P, Villeneuve JP, Samson R (1996) Adding sodium dodecyl sulfate and Pseudomonas aeruginosa UG2 biosurfactants inhibits polycyclic aromatic hydrocarbon biodegradation in a weathered creosote-contaminated soil. Appl Microbiol Biotechnol 46:638–646
Doyle C (2010) Powerful choices podcast: dispelling cancer myths. www.cancer.org
Eniola KT (2012) Effect of nitrogen supplementation on aerobic degradation of LAS by consortia of bacteria. J Xenobiot 2(e5):24–27
Farzaneh H, Fereidon M, Noor A, Naser G (2010) Biodegradation of dodecylbenzene sulfonate sodium by Stenotrophomonas maltophilia biofilm. Afr J Biotechnol 9:55–62
Feitkenhauer H, Meyer U (2002) Anaerobic digestion of alcohol sulfate (anionic surfactant) rich wastewater batch experiments. Part II: influence of the hydrophobic chain length. Bioresour Technol 82:123–129
Field JA, Leenheer JA, Thorn KA, Barber LLB, Rostad C, Macalady DL, Daniel SR (1992) Identification of persistent anionic surfactant-derived chemicals in sewage effluent and groundwater. J Contam Hydrol 9:55–78
Foley P, Beach ES, Zimmerman JB (2012) Derivation and synthesis of renewable surfactants. Chem Soc Rev 41:1499–1518
Fox K, Holt M, Daniel M, Buckland H, Guymer I (2000) Removal of linear alkylbenzene sulfonate from a small Yorkshire stream: contribution to great-er project 7. Sci Total Environ 251:265–275
Georgia FR, Poe CF (1931) Study of bacterial fluorescence in various media: I. Inorganic substances necessary for bacterial fluorescence. J Bacteriol 22:349
Georgiou G, Lin SC, Sharma MM (1992) Surface-active compounds from microorganisms. Nat Biotechnol 10:60–65
Ghai VU (2011) Say no to chemical detergents. http://EzineArticles.com/?expert=Vineet_U_Ghai
Ghazali R (2002) The effect of disalt on the biodegradability of methyl ester sulfonates (MES). J Oil Palm Res 14:45–50
Ginkel CG (1989) Complete degradation of xenobiotic surfactants by consortia of aerobic microorganisms. Biodegradation 7:151–164
Goel G, Kaur S (2012) A study on chemical contamination of water due to household laundry detergents. J Hum Ecol 38:65–69
Gonzalez S, Lopez-Roldan R, Cortina JL (2012) Presence and biological effects of emerging contaminants in Llobregat River basin: a review. Environ Pollut 161:83–92. doi:10.1016/j.envpol.2011.10.002
Grant RL, Yao C, Gabaldon D, Acosta D (1992) Evaluation of surfactant cytotoxicity potential by primary cultures of ocular tissues: I. Characterization of rabbit corneal epithelial cells and initial injury and delayed toxicity studies. Toxicology 76:153–176
Hidaka H (1998) Photodegradation of surfactants with TiO2 semiconductor for the environmental wastewater treatment. J Chem Sci 110:215–228
Holt MS, Matthus E, Waters J (1989) The concentrations and fate of linear alkylbenzene sulfonate in sludge amended soils. Water Res 23:749–759
Hosseini F, Malekzadeh F, Amirmozafari N, Ghaemi N (2007) Biodegradation of anionic surfactants by isolated bacteria from activated sludge. Int J Environ Sci Technol 4:127–132
Hsu YC (1963) Detergent (sodium lauryl sulfate)-splitting enzyme from bacteria. Nature 200:1091–1092. doi:10.1038/2001091b0
Huddleston RL, Allred RC (1963) Microbial oxidation of sulfonated alkylbenzenes. Dev Ind Microbiol 4:24–38
Ikehata K, El-Din MG (2004) Degradation of recalcitrant surfactants in wastewater by ozonation and advanced oxidation processes: a review. Ozone Sci Eng 26:327–343. doi:10.1080/01919510490482160
Ishigami Y, Suzuki S (1997) Development of biochemicals-functionalization of biosurfactants and natural dyes. Prog Org Coat 31:51–61
Ivankovic T, Hrenovic J, Gudelj I (2009) Toxicity of commercial surfactants to phosphate-accumulating bacterium. Acta Chim Slov 56:1003–1009
Jovanic BR, Bojovic S, Panic B, Radenkovic B, Despotovic M (2010) The effect of detergent as polluting agent on the photosynthetic activity and chlorophyll content in bean leaves. Health 2:395–399
Jovcic B, Venturi V, Davison J, Topisirovic L, Kojic M (2010) Regulation of the sdsA alkyl sulfatase of Pseudomonas sp. ATCC 19151 and its involvement in degradation of anionic surfactants. J Appl Microbiol 109:1076–1083
Kado CI, Liu ST (1981) Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol 145:1365–1373
Kanchi S, Niranjan T, Babu Naidu K, Naidu Venkatasubba N (2012) Monitoring the status of anionic surfactants in various water systems in urban and rural areas of Tirupati, Andhra Pradesh, South India. Int J Res Chem Environ 2:144–156
Khurana R (2002) Detergents: counting the cost of cleanliness. Toxic Link Fact Sheet 16:1–4
Klebensberger J, Rui O, Fritz E, Schink B, Philipp B (2006) Cell aggregation of Pseudomonas aeruginosa strain PAO1 as an energy-dependent stress response during growth with sodium dodecyl sulfate. Arch Microbiol 185:417–427
Korzenowski C, Martins MBO, AaM Bernardes, Ferreira JZ, Duarte ECNF, De Pinho MN (2012) Removal of anionic surfactants by nanofiltration. Desalin Water Treat 44:269–275
Kostal J, Suchanek M, Klierova H, Demnerova K, Kralova B, McBeth DL (1998) Pseudomonas C12B, an SDS degrading strain, harbours a plasmid coding for degradation of medium chain length n-alkanes. Int Biodeterior Biodegrad 42:221–228
Kuhnt G (1993) Behavior and fate of surfactants in soil. Environ Toxicol Chem 12:1813–1820
Kumar CG, Mamidyala SK (2011) Extracellular synthesis of silver nanoparticles using culture supernatant of Pseudomonas aeruginosa. Colloids Surf B Biointerfaces 84(2):462–466. doi:10.1016/j.colsurfb.2011.01.042
Lang S (2002) Biological amphiphiles (microbial biosurfactants). Curr Opin Colloid Interface Sci 7:12–20
Lewis MA (1990) Chronic toxicities of surfactants and detergent builders to algae: a review and risk assessment. Ecotoxicol Environ Safe 20:123–140
Lewis MA (1991) Chronic and sublethal toxicities of surfactants to aquatic animals: a review and risk assessment. Water Res 25:101–113
Lima TM, Procopio LC, Brandao FD, Leao BA, Totola MR, Borges AC (2011) Evaluation of bacterial surfactant toxicity towards petroleum degrading microorganisms. Bioresour Technol 102:2957–2964
Long M, Ruan L, Li F, Yu Z, Xu X (2011) Heterologous expression and characterization of a recombinant thermostable alkylsulfatase (sdsAP). Extremophiles 15:293–301
Lopez-Vizcaino R, Saez C, Canzares P, Rodrigo MA (2012) Electrocoagulation of the effluents from surfactant-aided soil-remediation processes. Sep Purif Technol 98:88–93
Makkar RS, Cameotra SS, Banat IM (2011) Advances in utilization of renewable substrates for biosurfactant production. AMB Express 1:1–19
Maksimov VN, Parshikova TV (2006) Influence of surfactants on the photosynthetic activity of algae. Hydrobiol J 42:67–76
Mallatt J (1985) Fish gill structural changes induced by toxicants and other irritants: a statistical review. Can J Fish Aquat Sci 42:630–648
Manousaki E, Psillakis E, Kalogerakis N, Mantzavinos D (2004) Degradation of sodium dodecylbenzene sulfonate in water by ultrasonic irradiation. Water Res 38:3751–3759
Marrakchi S, Maibach HI (2006) Sodium lauryl sulfate-induced irritation in the human face: regional and age-related differences. Skin Pharmacol Physiol 19:177–180
Matsui S, Park H (2000) Morphological effects and ecotoxicity of nonionic and anionic surfactants to Closterium ehrenbergii using AGZI (algal growth and zygospore inhibition) test. Environ Eng Res 5(2):63–69
Mercade ME, Manresa MA, Robert M, Espuny MJ, De Andres C, Guinea J (1993) Olive oil mill effluent (OOME). New substrate for biosurfactant production. Bioresour Technol 43:1–6
Mireles JR, Toguchi A, Harshey RM (2001) Salmonella enterica serovar typhimurium swarming mutants with altered biofilm-forming abilities: surfactin inhibits biofilm formation. J Bacteriol 183:5848–5854
Mizumoto S, Hirai M, Shoda M (2007) Enhanced iturin A production by Bacillus subtilis and its effect on suppression of the plant pathogen Rhizoctonia solani. Appl Microbiol Biotechnol 75:1267–1274
Mukherjee AK (2007) Potential application of cyclic lipopeptide biosurfactants produced by Bacillus subtilis strains in laundry detergent formulations. Lett Appl Microbiol 45:330–335
Muller MM, Hausmann R (2011) Regulatory and metabolic network of rhamnolipid biosynthesis: traditional and advanced engineering towards biotechnological production. Appl Microbiol Biotechnol 91(2):251–264. doi:10.1007/s00253-011-3368-2
Mungray AK, Kumar P (2009) Fate of linear alkylbenzene sulfonates in the environment: a review. Int Biodeterior Biodegrad 63:981–987
Muthusamy K, Gopalakrishnan S, Ravi TK, Sivachidambaram P (2008) Biosurfactants: properties, commercial production and application. Curr Sci 94:736–747
Naldoni A, Schiboula A, Bianchi C, Bremner D (2011) Mineralisation of surfactants using ultrasound and the advanced fenton process. Water Air Soil Pollut 215:487–495
Nguyen TT, Sabatini DA (2009) Formulating alcohol-free microemulsions using rhamnolipid biosurfactant and rhamnolipid mixtures. J Surfactants Deterg 12:109–115
Nitschke M, Pastore GM (2004) Biosurfactant production by Bacillus subtilis using cassava-processing effluent. Appl Biochem Biotechnol 112:163–172
Noordman WH, Janssen DB (2002) Rhamnolipid stimulates uptake of hydrophobic compounds by Pseudomonas aeruginosa. Appl Environ Microbiol 68:4502–4508
Ostroumov SA (2003) Studying effects of some surfactants and detergents on filter-feeding bivalves. Hydrobiologia 500:341–344
Ostroumov SA, Widdows J (2006) Inhibition of mussel suspension feeding by surfactants of three classes. Hydrobiologia 556:381–386
Oya M, Hisano N (2010) Decreases in surface activities and aquatic toxicities of linear alkylbenzene sulfonate and alcohol ethoxylates during biodegradation. J Oleo Sci 59:31–39
Patel RM, Desai AJ (1997) Biosurfactant production by Pseudomonas aeruginosa GS3 from molasses. Lett Appl Microbiol 25:91–94
Patel MK, Theiss A, Worrell E (1999) Surfactant production and use in Germany: resource requirements and CO2 emissions. Resour Conserv Recycl 25:61–78
Payne WJ, Feisal VE (1963) Bacterial utilization of dodecyl sulfate and dodecyl benzene sulfonate. Appl Microbiol 11:339–344
Perez T, Sarrazin L, Rebouillon P, Vacelet J (2002) First evidences of surfactant biodegradation by marine sponges (Porifera): an experimental study with a linear alkylbenzene sulfonate. Hydrobiologia 489:225–233
Petrovic M, Fernandez-Alba AR, Borrull F, Marce RM, Mazo EG, Barcelo D (2002) Occurrence and distribution of nonionic surfactants, their degradation products, and linear alkylbenzene sulfonates in coastal waters and sediments in Spain. Environ Toxicol Chem 21:37–46
Poremba K, Gunkel W, Lang S, Wagner F (1991) Toxicity testing of synthetic and biogenic surfactants on marine microorganisms. Environ Toxicol Water Qual 6:157–163
Ramarathnam R, Bo S, Chen Y, Fernando WGD, Xuewen G, De Kievit T (2007) Molecular and biochemical detection of fengycin- and bacillomycin D-producing Bacillus spp., antagonistic to fungal pathogens of canola and wheat. Can J Microbiol 53:901–911
Razafindralambo H, Paquot M, Baniel A, Popineau Y, Hbid C, Jacques P, Thonart P (1996) Foaming properties of surfactin, a lipopeptide biosurfactant from Bacillus subtilis. J Am Oil Chem Soc 73:149–151. doi:10.1007/BF02523463
Rebello S, Asok AK, Joseph SV, Joseph BV, Jose L, Mundayoor S, Jisha MS (2013a) Bioconversion of sodium dodecyl sulphate to rhamnolipid by Pseudomonas aeruginosa: a novel and cost-effective production strategy. Appl Biochem Biotechnol 169(2):418–430. doi:10.1007/s12010-012-9988-x
Rebello S, Asok AK, Mundayoor S, Jisha MS (2013b) Surfactants toxicity and remediation. Pollutant diseases, remediation and recycling in. Environ Chem Sustain World 4:277–320
Ribelles A, Carrasco MC, Rosety M, Aldana M (1995) A histochemical study of the biological effects of sodium dodecyl sulfate on the intestine of the gilthead seabream, Sparus aurata L. Ecotoxicol Environ Safe 32:131–138
Rico-Rico A, Temara A, Behrends T, Hermens JLM (2009) Effect of sediment properties on the sorption of C12-2-LAS in marine and estuarine sediments. Environ Pollut 157:377–383
Rinallo C, Bennici A, Cenni E (1988) Effects of two surfactants on Triticum durum Desf. plantlets. Environ Exp Bot 28:367–374
Rivera-Utrilla J, Mendez-Diaz J, Sanchez-Polo M, Ferro-Garcia MA, Bautista-Toledo I (2006) Removal of the surfactant sodium dodecylbenzene sulfonate from water by simultaneous use of ozone and powdered activated carbon: comparison with systems based on O3 and O3/H2O2. Water Res 40:1717–1725
Rodrigues L, Banat IM, Teixeira J, Oliveira R (2006) Biosurfactants: potential applications in medicine. J Antimicrob Chemother 57:609–618
Romanelli MF, Moraes MCF, Villavicencio ALCH, Borrely SI (2004) Evaluation of toxicity reduction of sodium dodecyl sulfate submitted to electron beam radiation. Radiat Phys Chem 71:411–413
Rosety-Rodríguez M, Ordonez FJ, Roldan S, Rosety JM, Rosety M, Ribelles A, Carrasco C, Rosety I (2002) Acute effects of sodium dodecyl sulfate on the survival and on morpho-histochemical characteristics of the trunk kidney of juvenile turbot Scophthalmus maximus L. Eur J Histochem 46:179–184
Sanchez-Peinado MM, Rodelas B, Martinez-Toledo MV, Gonzalez-Lopez J, Pozo C (2009) Response of soil enzymes to linear alkylbenzene sulfonate (LAS) addition in soil microcosms. Soil Biol Biochem 41:69–76
Schleheck D, Dong W, Denger K, Heinzle E, Cook AM (2000) An α-proteobacterium converts linear alkylbenzenesulfonate surfactants into sulfophenyl carboxylates and linear alkyldiphenyletherdisulfonate surfactants into sulfodiphenyl-ethercarboxylates. Appl Environ Microbiol 66:1911–1916
Schleheck D, Knepper TP, Fischer K, Cook AM (2004) Mineralization of individual congeners of linear alkylbenzene sulfonate by defined pairs of heterotrophic bacteria. Appl Environ Microbiol 70:4053–4063
Schweigert MK, Mackenzie DP, Sarlo K (2000) Occupational asthma and allergy associated with the use of enzymes in the detergent industry a review of the epidemiology, toxicology and methods of prevention. Clin Exp Allergy 30:1511–1518
Sequeira CAC (1994) Environmental oriented electrochemistry. Studies in environmental science 59. Elsevier, Amsterdam
Setzkorn EA, Huddleston RL (1965) Ultraviolet spectroscopic analysis for following the biodegradation of hydrotropes. J Am Oil Chem Soc 42:1081–1084
Shabtai Y (1990) Production of exopolysaccharides by Acinetobacter strains in a controlled fed-batch fermentation process using soap stock oil (SSO) as carbon source. Int J Biol Macromol 12:145–152
Shah V, Doncel GF, Seyoum T, Eaton KM, Zalenskaya I, Hagver R, Azim A, Gross R (2005) Sophorolipids, microbial glycolipids with anti-human immunodeficiency virus and sperm-immobilizing activities. Antimicrob Agents Chemother 49:4093–4100. doi:10.1128/AAC.49.10.4093-4100.2005
Shukor MY, Husin WSW, Rahman MFA, Shamaan NA, Syed MA (2009) Isolation and characterization of an SDS-degrading Klebsiella oxytoca. J Environ Biol 30:129–134
Singh KL, Kumar A (1998) Short communication: Bacillus cereus capable of degrading SDS shows growth with a variety of detergents. World J Microbiol Biotechnol 14:777–779
Singh S, Patel P, Jaiswal S, Prabhune AA, Ramana CV, Prasad BLV (2009) A direct method for the preparation of glycolipid-metal nanoparticle conjugates: sophorolipids as reducing and capping agents for the synthesis of water re-dispersible silver nanoparticles and their antibacterial activity. New J Chem 33:646–652
Stalmans M, Berenbold H, Berna JL, Cavalli L, Dillarstone A, Franke M et al. (1995) European life-cycle inventory for detergent surfactants production. Tenside Surfactants Deterg 32(2):84–109
Standard PG, Ahearn DG (1970) Effects of alkylbenzene sulfonates on yeasts. Appl Microbiol 20:646
Stipcevic T, Piljac A, Piljac G (2006) Enhanced healing of full-thickness burn wounds using di-rhamnolipid. Burns 32:24–34
Susmi TS, Rebello S, Jisha MS, Sherief PM (2010) Toxic effects of sodium dodecyl sulfate on grass carp Ctenopharyngodon idella. Fish Technol 47(2):157–162
Swisher RD (1963) Biodegradation of ABS in relation to chemical structure. J (Water Pollut Control Fed) 35:877–892
Swisher RD (1967) Biodegradation of LAS benzene rings in activated sludge. J Am Oil Chem Soc 44:717–724
Tharapiwattananon N, Scamehorn JF, Osuwan S, Harwell JH, Haller KJ (1996) Surfactant recovery from water using foam fractionation. Sep Sci Technol 31:1233–1258
Thomas OR, White GF (1989) Metabolic pathway for the biodegradation of sodium dodecyl sulfate by Pseudomonas sp. C12B. Biotechnol Appl Biochem 11:318–327
Tiehm A (1994) Degradation of polycyclic aromatic hydrocarbons in the presence of synthetic surfactants. Appl Environ Microbiol 60:258–263
USEPA (2008) Zonix. www.epa.gov/pesticides/biopesticides/ingredients/product/prod_110029.htm
Vakil H, Sethi S, Fu S, Stanek A, Wallner S, Gross R (2010) Sophorolipids decrease pulmonary inflammation in a mouse asthma model. Mod Pathol 23:392A
Van Bogaert INA, Saerens K, De Muynck C, Develter D, Soetaert W, Vandamme EJ (2007) Microbial production and application of sophorolipids. Appl Microbiol Biotechnol 76:23–34
Van de Plassche EJ, de Bruijn JHM, Stephenson RR, Marshall SJ, Feijtel TCJ, Belanger SE (1999) Predicted no effect concentrations and risk characterization of four surfactants: linear alkyl benzene sulfonate, alcohol ethoxylates, alcohol ethoxylated sulfates, and soap. Environ Toxicol Chem 18:2653–2663
Vaz DA, Gudina EJ, Alameda EJ, Teixeira JA, Rodrigues LR (2012) Performance of a biosurfactant produced by a Bacillus subtilis strain isolated from crude oil samples as compared to commercial chemical surfactants. Colloids Surf B 89:167–174
Velikonja J, Kosaric N (1993) Biosurfactants in food applications. In: Kosaric N (ed) Biosurfactants—production, properties and applications. New York: Marcel Dekker, pp 419–446
Vigon BW, Rubin AJ (1989) Practical considerations in the surfactant-aided mobilization of contaminants in aquifers. J Water Pollut Control Fed 61(7):1233–1240
Weuthen M, Kawa R, Hill K, Ansmann A (1995) Long chain alkyl polyglycosides—a new generation of emulsifiers. Lipid/Fett 97:209–211
Williams J, Payne WJ (1964) Enzymes induced in a bacterium by growth on sodium dodecyl sulfate. Appl Microbiol 12:360–362
Xie Y, Ye R, Liu H (2007) Microstructure studies on biosurfactant-rhamnolipid/n-butanol/water/n-heptane microemulsion system. Colloids Surf A 292:189–195
Yadav JS, Lawrence DL, Nuck BA, Federle TW, Reddy CA (2001) Biotransformation of linear alkylbenzene sulfonate (LAS) by Phanerochaete chrysosporium: oxidation of alkyl side-chain. Biodegradation 12:443–453
Yeldho D, Rebello S, Jisha MS (2011) Plasmid-mediated biodegradation of the anionic surfactant sodium dodecyl sulfate, by Pseudomonas aeruginosa S7. Bull Environ Contam Toxicol 86:110–113
York JD, Firoozabadi A (2008) Comparing effectiveness of rhamnolipid biosurfactant with a quaternary ammonium salt surfactant for hydrate anti-agglomeration. J Phys Chem B 112(3):845–851. doi:10.1021/jp077271h
Yuksel E, Sengil IA, Ozacar M (2009) The removal of sodium dodecyl sulfate in synthetic wastewater by peroxi-electrocoagulation method. Chem Eng J 152:347–353
Zajic JE, Guignard H, Gerson DF (1977) Properties and biodegradation of a bioemulsifier from Corynebacterium hydrocarboclastus. Biotechnol Bioeng 19:1303–1320
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rebello, S., Asok, A.K., Mundayoor, S. et al. Surfactants: toxicity, remediation and green surfactants. Environ Chem Lett 12, 275–287 (2014). https://doi.org/10.1007/s10311-014-0466-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-014-0466-2