Abstract
The increasing atmospheric [CO2] would alter soil–plant nutrient dynamics depending on crop species, soil type, and climate. Insights on the impacts of the predicted level of elevated [CO2] (e[CO2]) on the soil–plant-environment system are, therefore, important for strategic nutrient management for future environments. The impacts of e[CO2] environment on soil phosphorus (P) bioavailability and soil–plant P dynamics in chickpea are uncertain in tropical alkaline Vertisols. An open-top chamber–based experiment with e[CO2] (570 ± 30 ppmv) and ambient [CO2] treatments aimed to investigate the impacts of e[CO2] on soil–plant P dynamics, physiology, and yield of chickpea in a moderately alkaline Vertisol of subtropical central India. Experimental findings revealed that the e[CO2] treatment increased Olsen P at flowering stage (+ 13%, p < 0.05), water-soluble carbon (11–14%), and KMnO4-C (5–14%) at both branching and flowering stages (p < 0.05). Results demonstrated that the increased mobilization of dissolved non-reactive P (NaHCO3-Po, NaOH-Po) (from branching to flowering) and competitive sorption with higher soluble carbon possibly contributed to the higher available P (Olsen P) under the e[CO2] environment. The e[CO2] treatment had a significant impact on photosynthetic rate (+ 5.3%), stomatal conductance (− 16.5%), and leaf chlorophyll content (+ 5.1%) over the ambient (p < 0.05) but did not alter leaf nitrate reductase activity. The e[CO2] treatment increased plant biomass (+ 25%) and productivity (+ 11.6%), P uptake (+ 16.6%), and physiological P use efficiency (+ 7.1%) (p < 0.05). Thus, it can be concluded that e[CO2] (~ 570 ppmv) could enhance P availability in alkaline Vertisols of subtropical regions favoring P nutrition, physiological activity, and yield of chickpea.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The atmospheric carbon dioxide (CO2) concentration ([CO2]) has increased over time, which is predicted to reach 750 ppmv by the end of the twenty-first century (IPCC 2014). The rising trend of atmospheric [CO2] is a global sustainability concern for terrestrial and oceanic ecosystems (Lougheed et al. 2020; Wang et al. 2021). The potential impacts of elevated [CO2] (e[CO2]) on crops have been studied in different agro-regions, and meta-analysis reports explicate that the effects are variable with crop species and environments (Taub et al. 2008; Wang et al. 2012; McLachlan et al. 2020). An e[CO2] condition can alter/affect plant physiological functions and yield at variable scales depending on crop species (Wang et al. 2012; Jena et al. 2018), soil type (De Graaff et al. 2006), climate (Feng et al. 2008), and management practices (Hazra et al. 2019). Therefore, assessing the impacts of e[CO2] on food crops under different agro-climatic and edaphic conditions is important.
Chickpea (Cicer arietinum L.) is a major cool season legume crop, cultivated in 13.7 M ha globally, with average productivity of 1.03 t ha−1 (FAOSTAT 2020). Southeast Asia contributes ~ 80% of global chickpea production (ICRISAT 2015), and the crop is predominantly grown in Vertisols of central India (subtropical climate) (Kumar et al. 2021). Chickpea is highly sensitive to environmental variables (Jha et al., 2021). Several studies reported the impacts of e[CO2] on grain legumes like black gram (Sathish et al., 2014), pigeon pea (Saha et al. 2011), lentil (Nasser et al. 2008), cowpea (Dey et al. 2017), and chickpea (Lamichaney et al., 2021) in tropical environments primarily focusing on crop growth, physiology, and yield as the response parameters. However, studies on the impact of e[CO2] environment on soil P bioavailability and crop nutrition are scanty, particularly in Vertisols of subtropical India. Given the increased significance of optimal P nutrition for sustaining chickpea productivity in tropical soils, particular attention is warranted to understand the impact e[CO2] on soil P bioavailability and P nutrition in chickpea-grown P-deficient Indian Vertisols.
Mostly, e[CO2] condition favors biomass accumulation in leguminous C3 crops and thus can increase the demand for plant nutrients (Jin et al. 2012). An imbalance or non-synchrony in the plant nutrient demand and supply could affect crop yield under e[CO2] conditions (Satapathy et al. 2015). Suboptimal P nutrition due to the inefficiencies in the tropical soil system (high P sorption and low bioavailability) has yield-limiting impacts on grain legumes (Hazra et al. 2018). Quantifying the changes in soil biological processes and plant-mediated enzymes, soil P pools, soil labile carbon (C) pool, and plant rhizospheric properties would provide valuable insights on P cycling as altered with e[CO2] environments (Jin et al. 2013, 2014). Furthermore, investigating the changes in organic and inorganic forms of P at different crop growth stages under contrasted [CO2] environments may advance our understanding of soil processes influencing P availability (Jin et al. 2017). Presently, P acquisition and intra-plant P use efficiency in relation to changes in physiological functions in chickpea crop under e[CO2] environments are not well understood in tropical agro-regions (particularly central India—a major chickpea growing belt) and therefore a topic of research interest.
Thus, an open-top chamber–based study was conducted to investigate the effect of e[CO2] on temporal soil P dynamics during crop season, soil–plant P relations, physiology, growth, and yield of chickpea in a moderately alkaline Vertisol of subtropical climate (central India). The major objectives of this study were to (i) determine the impact of e[CO2] on temporal dynamics of P pools, labile C pool, and biochemical properties in chickpea growth stages in alkaline Vertisol; (ii) to quantify the changes in growth, physiological functions of chickpea with e[CO2] condition over ambient (iii) to assess intra-plant P distribution, P use efficiency, and soil–plant P relations in chickpea as altered with e[CO2] environment.
2 Materials and Methods
2.1 Site and Soil Characteristics
The experiment was conducted in the open-top poly-carbonated chambers (OTC) located at the experimental station of ICAR–Indian Institute of Soil Science, Bhopal (23°15′ N, 77°25′ E, and 427 m above mean sea level). The climate of the study site is a subtropical sub-humid with dry winters. During the crop growth period, the average maximum temperature, minimum temperature, relative humidity, average pan evaporation, and rainfall were 26.9 °C, 11.5 °C, 65.6%, 2.8 mm, and 2.2 mm, respectively (Supplementary Fig. 1). For the study, we used deep Vertisol (> 50% clay), which is classified as Isohyperthermic Typic Haplustert (World reference Base soil Classification). The experimental soil had 6.7 g kg−1 soil organic C (Walkley and Black 1934), 113.4 mg kg−1 available nitrogen (KMnO4–N) (Subbaiah and Asija 1956), 5.64 mg kg−1 available P (Olsen P) (Olsen 1954), and 68.8 mg kg−1 available potassium (NH4OAc-K) (Jackson 1973). The experimental soil was non-saline [electrical conductivity 0.27 dS m−1 (1:2.5 soil suspension ratio)] and moderately alkaline in reaction (pH 7.96) (Jackson 1973).
2.2 Treatment Description
The open-top chamber (OTC) experiment was conducted during the winter season of 2018–2019. Two different CO2 concentration treatments, i.e., ambient [CO2] (without an external supply of CO2) and elevated [CO2] (e[CO2]), were evaluated on chickpea crops. The average CO2 concentration during the crop-growing season was recorded 370 ± 10 ppmv in the ambient e[CO2] treatment and 570 ± 30 ppmv in the e[CO2] treatment, respectively. There were six replications for each CO2 treatment, and inside each OTC, chickpea plants were grown in pots (six pots in each OTC). The OTCs were made up of polycarbonate sheets, and their dimension was 4 m × 4 m × 4 m. The CO2 chamber located adjacent to the OTCs was used to supply CO2 gas (99.5% purity) at a pressure of 2.0 kg cm–2 through a manifold system. In the elevated CO2 chambers, CO2 was released through nozzles connected to polyvinyl tubes. The desired gas concentration was monitored through an automated control facility connected to data loggers and SCADA software.
2.3 Crop Management
For the experiment, soils were collected from an undisturbed field and uniformly mixed and processed. The pots (top diameter 15 cm, bottom diameter 15 cm, depth 50 cm) were filled with 10 kg soils. A basal dose of fertilizers rate of 20–40–50 kg ha−1 (N: P2O5:K2O) was calculated for the pot soil weight basis and mixed thoroughly at the time of pot filling. The sources of fertilizer N, P, and K were urea [CO(NH)2, 46% N], single superphosphate (CaH6O8P2+2, 16% P2O5), and muriate of potash (KCl, 60% K2O). Then, the pots were watered and allowed to settle before sowing. Five seeds of desi chickpea (cv. RVG–202) were sown in each pot on 22 November 2018. The seeds were treated with Rhizobium culture (> 107 bacteria g−1 inert material) at 20 g kg seeds−1. After the emergence of the seedlings, three healthy seedlings were retained in each pot. The pots were watered at regular intervals, and to avoid overwatering, a predetermined volume of water was added to each pot using a handheld sprinkler to maintain 80% of field capacity. During the crop season, both the pot and soil were not disturbed. As there was no incidence of disease and insect pests during the crop season, no plant protection chemicals were applied.
2.4 Crop Growth and Yield Estimations
Periodical growth observations and plant dry weights were recorded at branching (~ 55 days after sowing), flowering (~ 75 days after sowing), and maturity stages by the destructive plant sampling method. The plant samples were oven-dried at 65 ± 2 °C for 72 h to estimate dry biomass and expressed as g plant−1. At maturity, grains and remaining plant parts were harvested separately. Both grain and stover at maturity were expressed on oven-dry weight basis. The harvest index, defined as the ratio of grain yield to aboveground biomass at harvest, was calculated and expressed as a percentage.
2.5 Crop Physiological Observations
At the pre-flowering stage, plant physiological parameters like stomatal conductance, transpiration rate, photosynthetic rate, and leaf temperature were recorded using a LiCor 6400 XT photosynthesis analysis system (LiCor Corporate, Nebraska, USA). Leaf chlorophyll a, chlorophyll b, and nitrate reductase activity at the branching and flowering stages were analyzed. Chlorophyll content in the leaves was analyzed as per Hiscox and Israelstam (1979). In brief, the chlorophyll content of leaf samples was extracted with dimethyl sulfoxide (DMSO). After incubating the leaf sample at 50 °C for 2 ½ h, the supernatant was decanted, and leaf tissues were discarded. The optical density was read at λ = 663 and 645 nm using 80% acetone as a blank by a spectrophotometer (Model UV–1900i UV–Vis Spectrophotometer, Shimadzu). Then, chlorophyll a (Eq. 1) and chlorophyll b contents (Eq. 2) in leaf tissue was calculated according to Arnon (1949) formula and expressed as μg g−1 fresh leaf tissue.
The nitrate reductase activity of leaf tissue was estimated colorimetrically following the method of Cazetta and Villela (2004). In brief, 0.1 gm leaf sample was taken in a test tube followed by 3 ml of each phosphate buffer (pH 7.6), and 0.2 M KNO3 solution and propanol were added. Then, the test tubes or samples were incubated at 30 °C in dark conditions. Then, the test tubes were kept in a hot water bath for 20–25 min to end the reaction. After that, 1 ml of aliquot was taken in a fresh test tube, and 1 ml each of sulfanilamide (prepared in 1% HC) and 1 ml 0.025% N-(1-naphthyl)-ethylenediamine dihydrochloride (NEDD) was added and kept for 20 min for allowing pink color to develop. After that, the readings were taken at 540 nm. The nitrate reductase activity was determined by multiplying the reading value 406.8 and expressed as nanomole NO2– g−1 fresh weight h−1.
2.6 Soil Sampling and Soil Analysis
Soil samples were collected, mixed, air-dried, and passed through a 2-mm sieve. The procedure of Hedley et al. (1982) was followed to estimate P fractions. The method of Murphy and Riley (1962) was used for determining inorganic P fractions (Pi) irrespective of extractants. The ammonium persulfate digestion method (EPA, 1971) was selected for the determination of total P(Pt). Organic P (Po) was determined by subtracting the value of Pt from Pi. Water-soluble and permanganate oxidizable C were determined following the methods of McGill et al. (1986) and Blair et al. (1995), respectively. Acid and alkaline phosphatases were determined following the method described by Tabatabai and Bremner (1969), using p-nitrophenyl phosphate as a substrate. β-Glucosidase was determined by using p-nitrophenyl-β-d-glucopyranoside as substrate as per the method described by Eivazi and Tabatabai (1988).
2.7 Plant P Uptake and P Use Efficiency Calculation
At the maturity, whole plant samples were collected, and the grains, leaves, and stems were separated and oven-dried at 65 ± 2 °C. The plant samples (grains, leaves, and stems) were ground, passed through a 0.5-mm sieve, and kept in paper packets for laboratory nutrient analysis. Total P content in plant samples was determined using the sulfuric-nitric-perchloric acid digestion method (Jackson 1973). Phosphorus accumulated in different plant parts (leaf, stem, and grain) was calculated by multiplying phosphorus concentration in the different plant parts with their corresponding biomass dry weight and then added to estimate the total P uptake (or total aboveground P accumulation). Physiological P use efficiency (Eq. 3), internal P use efficiency (Eq. 4), and P harvest index (Eq. 5) were determined using the following formula:
2.8 Statistical Analysis
Comparison of elevated and ambient [CO2] treatments was made following the Student t-test procedure of two independent variables (Gomez and Gomez 1984). The two treatments were compared at 95% level of significance. The percent and ratio data were subjected to logit transformation before statistical analysis.
3 Results
3.1 Treatment-Induced Changes in the Microenvironment
Results showed that the e[CO2] treatment marginally altered the OTC environment. The e[CO2] treatment slightly increased air temperature (inside the OTC) in the month December (+ 0.49 °C), January (+ 0.42 °C), February (+ 0.90 °C), and March (+ 0.70 °C) (Fig. 1). The morning and evening soil temperatures were comparable in both the ambient [CO2] and e[CO2] treatments (Fig. 2).
3.2 Crop Physiology, Growth, and Yield
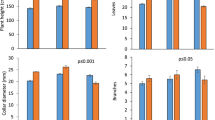
The e[CO2] treatment had a significant impact on growth and physiological attributes of chickpea. Higher chlorophyll a and chlorophyll b were recorded under the e[CO2] treatment over the ambient [CO2] treatment at both branching and flowering stages (p < 0.05) (Fig. 3a,b,d,e). The [chlorophyll a/chlorophyll b] ratio was 5.17:1 in the ambient [CO2] treatment at the branching stage, which was reduced to 4.68:1 in the e[CO2] treatment. The ratio value was lower at the flowering stage (3.7:1) compared to the branching stage (4.9:1) (p < 0.05). The e[CO2] treatment did not alter nitrate reductase activity (Fig. 3c,f). The e[CO2] treatment increased photosynthesis rate (+ 5%, p < 0.05) (Fig. 4a) and reduced stomatal conductance (− 16%, p < 0.05) (Fig. 4b), where the transpiration rate and canopy temperature depression remain unchanged with the [CO2] treatments (p > 0.05) (Fig. 4c,d).
Effect of elevated and ambient [CO2] treatments on leaf chlorophyll content (mg g–1 fresh leaf) and nitrate reductase activity (nanomole NO2–1 g–1 fresh leaf h–1) at pre-flowering (a–c) and pod development (d–f) stages. Error bar represents ± standard error of mean. The different lowercase letters within the treatments are significantly different at p < 0.05. Amb. [CO2] = ambient [CO2] (370 ppmv); Elev. [CO2] = elevated [CO2] (570 ppmv)
Effect of elevated and ambient [CO2] treatments on photosynthetic rate (a), stomatal conductance (b), transpiration rate (c), and canopy temperature depression (d) at pre-flowering stage of chickpea. Error bar represents ± standard error of mean. The different lowercase letters correspond to the treatment are significantly different at p < 0.05. Amb. [CO2] = ambient [CO2] (370 ppmv); Elev. [CO2] = elevated [CO2] (570 ppmv)
The e[CO2] treatment increased plant height over the ambient [CO2] treatment (p < 0.05) (Table 1). Plant biomass was higher in the e[CO2] treatment at all the growth stages. The incremental changes in crop growth rate with e[CO2] treatment during sowing to branching, branching to pre-flowering, and pre-flowering to maturity were 20%, 24%, and 26%, respectively (p < 0.05). The e[CO2] treatment increased grain and biological yield by 10% and 19%, respectively, over the ambient (p < 0.05) but reduced harvest index (p < 0.05).
3.3 Soil-Available P and P Pools
The e[CO2] treatment increased Olsen P (13%, p < 0.05) at the flowering stage (Fig. 5b), but the effect was non-significant at the branching stage (Fig. 5a). The effect of elevated [CO2] treatment on NaHCO3-Pi, NaHCO3-Po, NaOH-Pi, and NaOH-Po pools was similar to the ambient in all the growth stages, i.e., branching, flowering, and maturity stages (Table 2). The temporal dynamics of P fractions were prominent in the study. For instance, a reduction in NaHCO3-Po was observed after the flowering stage, where the values of NaOH-Po followed the order maturity > branching > flowering (p < 0.05). The dissolved non-reactive P pool (NaHCO3-Po + NaOH-Po) was reduced from 62.4 to 45.6 mg kg−1 (27%, p < 0.05) during branching to flowering stage in ambient condition, while the corresponding reduction in the e[CO2] treatment was 61.2 to 38.7 mg kg−1 (37%, p < 0.01). From the branching to the flowering stage, the incremental change in NaHCO3-Pi was higher in the e[CO2] treatment (54%) over the ambient [CO2] treatment (26%).
Effect of elevated and ambient [CO2] treatments on Olsen P at branching (a) and flowering stages (b). Error bar represents ± standard error of mean. The different lowercase letters correspond to the treatment are significantly different at p < 0.05. Amb. [CO2] = ambient [CO2] (370 ppmv); Elev. [CO2] = elevated [CO2] (570 ppmv)
3.4 Soil Enzymes and Carbon
The e[CO2] treatment did not alter acid phosphatase and alkaline phosphatase activities (p > 0.05) (Fig. 6a,b,d,e). Notably, the activity scale of alkaline phosphatase was relatively higher than acid phosphatase in the soil. Likewise, the effect of e[CO2] treatment on β-glucosidase activity was also non-significant (Fig. 6c,f). The content of KMnO4-C was higher in the e[CO2] treatment at both the branching stage (+ 5%) and flowering stage (+ 14%) over the ambient [CO2] treatment (p < 0.05) (Table 2). The positive effect of e[CO2] treatment on water-soluble C was prominent at the branching stage (+ 11%) and flowering stage (+ 14%) (p < 0.05). In the study, the depletion of KMnO4-C was observed during the branching to the flowering stage, higher in the ambient (22%) over the e[CO2] treatment (15%).
Effect of elevated and ambient [CO2] treatments on acid phosphatase (µg p-nitrophenol g–1 soil h–1), alkaline phosphatase (µg p-nitrophenol g–1 soil h–1), ß-glucosidase (µg p-nitrophenol g–1 h–1), at pre-flowering (a–c) and pod development (d–f) stages of chickpea. Error bar represents ± standard error of mean. The different lowercase letters correspond to the treatment are significantly different at p < 0.05. Amb. [CO2] = ambient [CO2] (370 ppmv); Elev. [CO2] = elevated [CO2] (570 ppmv)
3.5 Phosphorus Uptake and P Input Use Efficiency
Grains and stover P accumulations (or uptake) were significantly higher in e[CO2] treatment, which was recorded 9% (p < 0.05) and 25% (p < 0.01) higher over the ambient [CO2] treatment (Table 3). Subsequently, the total P accumulation was 17% higher in the e[CO2] treatment over the ambient [CO2] treatment (p < 0.01). The e[CO2] treatment increased physiological P use efficiency by 7% (p < 0.05), but did not change internal P use efficiency and P harvest index over the ambient [CO2] treatment.
4 Discussion
The present study indicated a noticeable alteration in soil processes influencing P availability under the e[CO2] environment. Our results demonstrate that e[CO2] environment could mobilize P in chickpea grown alkaline Vertisol. In contrast, the short-term exposure to e[CO2] environment failed to have a significant impact on NaHCO3-Pi, NaOH-Pi, and dissolved non-reactive P fractions (NaHCO3-Po, NaOH-Po) when compared with ambient [CO2] environment. Previous reports also concluded that short-term exposure to e[CO2] environment may not be sufficient to trigger major changes in the soil–plant system that could alter the soil inorganic P pools (Wasaki et al., 2005; Jin et al., 2013). According to Jin et al. (2012) and Jin et al. (2013), e[CO2] built up NaOH-extractable organic P in soils with legume cropping, which is not certain in the alkaline Vertisol of subtropical region. The possible reason could be the enhanced rate of organic to inorganic P conversion to meet the elevated demand of P by chickpea plants under e[CO2] environment. Our results demonstrate that the ambient and e[CO2] environments have a differential impact on the relative changes of P fractions over the crop growth stages (temporal dynamics). For instance, the higher rate of depletion of dissolved non-reactive P fractions (NaHCO3-Po, NaOH-Po) from branching to flowering stage (p < 0.05) could be a reason for the incremental changes in Olsen P (19%) and NaHCO3-Pi (54%) under e[CO2] environment. The significant depletion of KMnO4-C from branching to flowering stages further verifies a higher mineralization rate in the tropical soil irrespective of the [CO2] treatments. The increased Olsen P at flowering with the e[CO2] environment might be attributed to the increase in the labile C compounds (particularly water-soluble C). The low-molecular-weight soluble C compounds can increase P availability by competitive sorption in the soil matrix (Guppy et al., 2005). Chickpea exudates a large amount of low-molecular-weight organic acids to mobilize P in the rhizosphere (Veneklaas et al. 2003). This may be further interpreted that the increased photosynthate accumulation under the e[CO2] environment might have higher rhizodeposition of water-soluble C compounds. According to Kant et al. (2007), wheat under e[CO2] (600 ppmv) produces 49% more dissolved organic C than ambient environment. On the same line, Das et al. (2011) conceptualized that subsided microbial activity in the ambient conditions failed to utilize optimum native water-soluble carbohydrate C in the soil while a part of total carbohydrate C transforms into a water-soluble form.
A build-up of mineralizable organic compounds triggers phosphatase enzymes’ activities; however, in the study, the increase in water-soluble C and KMnO4-C with e[CO2] treatment did not stimulate phosphatase enzymes activities. Plants also release phosphatase to mineralize organic P to soluble Pi, and this is often viewed as an adaptation strategy under P-deficient conditions (Gomez and Carpena 2014). Therefore, this is possible that lower P availability in ambient conditions might have increased phosphatase released by chickpea plants leading to a non-significant treatment difference. Other workers have also reported the inconspicuous impact of e[CO2] on phosphatase in different grain legumes (Haase et al. 2008; Jin et al. 2013).
Our results demonstrate that e[CO2] environment has a favorable influence on crop growth and physiology of chickpea under subtropical climate. An increased rate of photosynthesis coupled with reduced oxidative reaction of Rubisco under e[CO2] environment leads to top up the biomass production in C3 plants (Long et al. 2006). Our results also verify that the increased photosynthetic rate under the e[CO2] environment leads to higher biomass accumulation in the leguminous crop. The results of crop growth rate suggest that the increase in photosynthates accumulation with e[CO2] treatment is consistent throughout the crop stages, even at the later growth stage (pre-flowering to maturity). The reduced stomatal conductance under e[CO2] condition would directly impact crop water use efficiency, possibly higher for the rainfed/dryland crop like chickpea. In the study, the transpiration rate was not affected by e[CO2] treatment despite significantly reducing stomatal conductance under e[CO2] conditions. This is possible because of the non-deficit soil moisture condition and, thereby, retention of leaf water flux (Mishra and Agrawal 2015). Considering this, the effect of e[CO2] on chickpea under water-limited environments would be much more interesting, particularly concerning water use efficiency and photosynthates assimilations.
Studies have reasoned that carbohydrate build-up and leaf N concentration diminution impeding normal nitrate reductase activity vis-à-vis nitrate assimilation under e[CO2] condition (Reich et al. 2006). However, our result indicates that the e[CO2] environment does not necessarily affect nitrate reductase activity in the chickpea plant. Several studies have reasoned that the legumes will be at an advantage over non-legumes, as their growth and N fixation increase under e[CO2] conditions (Ross et al. 2004). Hence, an increased biological N fixation under e[CO2] condition might have maintained the nitrate reductase activity in chickpea leaves. The chlorophyll a/b ratio ([C55H72O5N4Mg]/[C55H70N4O6Mg]) indicates an N-limiting condition in the later stages of chickpea growth, which is possible because of nodule senescence and subsequent dilution of tissue N with increased growth. As mentioned, the non-significant difference in the chlorophyll a/b ratio indicates that N is not a limiting factor, and that is why facilitated the photosynthates assimilation. Free-air CO2 studies showed a higher photosynthetic rate over ambient because of the higher chlorophyll content (Dey et al. 2017, 2019).
The higher uptake of P in the e[CO2] treatment was undoubtedly attributed to the increased crop growth (higher biomass accumulation) and plant productivity sustained by the increased availability of labile Pi (Olsen P). The incremental change in P accumulation with e[CO2] was much higher in the stover part than the grain. This implies that the intra-plant distribution of P was altered, leading to a reduced P use efficiency (internal P use efficiency). This is particularly important that the acquired P by the plants is effectively utilized for grain development to improve P use efficiency, particularly concerning the depleting global P reserve, increased cost of fertilizers, and fertilizer P pollutions. However, The physiological P use efficiency, which demonstrates the utilization of acquired P for biomass production, is significantly increased under the e[CO2] condition, suggesting that the scale of change in photosynthates accumulation was more prominent over the magnitude of increase in P uptake by the chickpea plants. Hence, the study suggests that in tropical environments, the effects of e[CO2] are primarily favorable for chickpea growth and plant nutrition. Thus, there is no valid sustainability concern with increasing atmospheric [CO2] in the major chickpea growing belts of central India (subtropical sub-humid climate). Nevertheless, future studies are warranted in different soil conditions, climates, and other legumes to verify the results.
5 Conclusions
The study concluded that the elevated CO2 environment (~ 570 ppmv) could enhance P availability in tropical Vertisol and improve P nutrition of chickpea. Our results indicated that the increased rate of mineralization of organic P and the competitive sorption with the increased water-soluble carbon compound(s) due to higher plant P demand could be the possible reasons for the increased availability of P under elevated CO2 conditions. Results further suggested that elevated CO2 at 570 ppmv has no adverse impact on chickpea crop growth and physiology, instead favored higher biomass accumulation attributed to increased photosynthetic rate and leaf chlorophyll content. The higher physiological P use efficiency with the elevated CO2 treatment indicated the greater incremental change of biomass over P acquisition under elevated CO2 conditions.
References
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta Vulgaris Plant Physio 24:1. https://doi.org/10.1104/pp.24.1.1
Blair GJ, Lefroy RD, Lisle L (1995) Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural systems. Aust J Agric Res 46:1459–1466. https://doi.org/10.1071/AR9951459
Cazetta JO, Villela LCV (2004) Nitrate reductase activity in leaves and stems of tanner grass (Brachiaria radicans Napper). Sci Agric 61:640–648. https://doi.org/10.1590/S0103-90162004000600012
Das S, Bhattacharyya P, Adhya TK (2011) Interaction effects of elevated CO2 and temperature on microbial biomass and enzyme activities in tropical rice soils. Environ Monit Assess 182:555–569. https://doi.org/10.1007/s10661-011-1897-x
De Graaff MA, Van Groenigen KJ, Six J, Hungate B, van Kessel C (2006) Interactions between plant growth and soil nutrient cycling under elevated CO2: a meta–analysis. Glob Change Biol 12:2077–2091. https://doi.org/10.1111/j.1365-2486.2006.01240.x
Dey SK, Chakrabarti B, Prasanna R, Pratap D, Singh SD, Purakayastha TJ, Pathak H (2017) Elevated carbon dioxide level along with phosphorus application and cyanobacterial inoculation enhances nitrogen fixation and uptake in cowpea crop. Arch Agron Soil Sci 63:1927–1937. https://doi.org/10.1080/03650340.2017.1315105
Dey SK, Chakrabarti B, Purakayastha TJ, Prasanna R, Mittal R, Singh SD, Pathak H (2019) Interplay of phosphorus doses, cyanobacterial inoculation, and elevated carbon dioxide on yield and phosphorus dynamics in cowpea. Environ Monit Assess 191:1–11. https://doi.org/10.1007/s10661-019-7378-3
Eivazi F, Tabatabai MA (1988) Glucosidases and galactosidases in soils. Soil Biol Biochem 20:601–606. https://doi.org/10.1016/0038-0717(88)90141-1
EPA–Environmental Protection Agency, (1971) Methods for chemical analysis of waters and wastes. Environmental Protection Agency, Cincinnati, Ohio
FAOSTAT database collections (2020) Food and Agriculture Organization of the United Nations (FAO). Rome. http://www.fao.org/faostat/en/#home.
Feng Z, Kobayashi K, Ainsworth EA (2008) Impact of elevated ozone concentration on growth, physiology, and yield of wheat (Triticum aestivum L.): a meta–analysis. Glob Change Biol 14:2696–2708. https://doi.org/10.1111/j.1365-2486.2008.01673.x
Gomez DA, Carpena RO (2014) Effect of 1–naphthaleneacetic acid on organic acid exudation by the roots of white lupin plants grown under phosphorus–deficient conditions. J Plant Physiol 171:1354–1361. https://doi.org/10.1016/j.jplph.2014.05.008
Gomez KA, Gomez AA (1984) Statistical procedures for agricultural research. John Wiley & Sons
Guppy CN, Menzies NW, Moody PW, Blamey FPC (2005) Competitive sorption reactions between phosphorus and organic matter in soil: a review. Soil Res 43:189–202. https://doi.org/10.1071/SR04049
Haase S, Rothe A, Kania A, Wasaki J, Römheld V, Engels C, Kandeler E, Neumann G (2008) Responses to iron limitation in Hordeum vulgare L. as affected by the atmospheric CO2 concentration. J Environ Qual 37:1254–1262. https://doi.org/10.2134/jeq2006.0136
Hazra KK, Singh SS, Nath CP, Borase DN, Kumar N, Parihar AK, Swain DK (2018) Adaptation mechanisms of winter pulses through rhizospheric modification in mild–alkaline soil. Nat Acad Sci Lett 41:193–196. https://doi.org/10.1007/s40009-018-0648-8
Hazra S, Swain DK, Bhadoria PBS (2019) Wheat grown under elevated CO2 was more responsive to nitrogen fertilizer in Eastern India. Eur J Agron 105:1–12. https://doi.org/10.1016/j.eja.2019.02.001
Hedley MJ, Stewart JWB, Chauhan B (1982) Changes in inorganic and organic soil phosphorus fractions induced by cultivation practices and by laboratory incubations 1. Soil Sci Soc Am J 46:970–976. https://doi.org/10.2136/sssaj1982.03615995004600050017x
Hiscox JD, Israelstam GF (1979) A method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot 57:1332–1334. https://doi.org/10.1139/b79-163
ICRISAT 2015 http://www.icrisat.org/what–we–do/crops/ChickPea/Chickpea.htm (Accessed on: 02.07.2021)
IPCC (2014) Summary for policymakers. Climate change, mitigation of climate change. Contribution of Working Group III to the fifth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge, pp 1–31
Jackson ML (1973) Soil chemical analysis. Prentice-Hall of India Pvt. Ltd., New Delhi
Jena UR, Swain DK, Hazra KK, Maiti MK (2018) Effect of elevated [CO2] on yield, intra-plant nutrient dynamics, and grain quality of rice cultivars in eastern India. J Sci Food Agric 98:5841–5852. https://doi.org/10.1002/jsfa.9135
Jha UC, Nayyar H, Palakurthi R, Jha R, Valluri V, Bajaj P, Chitikineni A, Singh NP, Varshney RK, Thudi M (2021) Major QTLs and potential candidate genes for heat stress tolerance identified in chickpea (Cicer arietinum L.). Front Plant Sci 12:655103. https://doi.org/10.3389/fpls.2021.655103
Jin J, Armstrong R, Tang C (2017) Long-term impact of elevated CO2 on phosphorus fractions varies in three contrasting cropping soils. Plant Soil 419:257–267. https://doi.org/10.1007/s11104-017-3344-4
Jin J, Tang C, Armstrong R, Butterly C, Sale P (2013) Elevated CO2 temporally enhances phosphorus immobilization in the rhizosphere of wheat and chickpea. Plant Soil 368:315–328. https://doi.org/10.1007/s11104-012-1516-9
Jin J, Tang C, Armstrong R, Sale P (2012) Phosphorus supply enhances the response of legumes to elevated CO2 (FACE) in a phosphorus–deficient vertisol. Plant Soil 358:91–104. https://doi.org/10.1007/s11104-012-1270-z
Jin J, Tang C, Robertson A, Franks AE, Armstrong R, Sale P (2014) Increased microbial activity contributes to phosphorus immobilization in the rhizosphere of wheat under elevated CO2. Soil Biol Biochem 75:292–299. https://doi.org/10.1016/j.soilbio.2014.04.019
Kant PC, Bhadraray S, Purakayastha TJ, Jain V, Pal M, Datta SC (2007) Active carbon–pools in rhizosphere of wheat (Triticum aestivum L.) grown under elevated atmospheric carbon dioxide concentration in a Typic Haplustept in sub–tropical India. Environ Pollut 147:273–281. https://doi.org/10.1016/j.envpol.2006.07.018
Kumar T, Hamwieh A, Swain N, Sarker A (2021) Identification and morphological characterization of promising kabuli chickpea genotypes for short–season environment in central India. J Genet 100:1–8. https://doi.org/10.1007/s12041-021-01280-8
Lamichaney A, Tewari K, Basu PS, Katiyar PK, Singh NP (2021) Effect of elevated carbon–dioxide on plant growth, physiology, yield and seed quality of chickpea (Cicer arietinum L.) in Indo-Gangetic plains. Physiol Mol Biol Plants 27:251–263. https://doi.org/10.1007/s12298-021-00928-0
Long SP, Ainsworth EA, Leakey AD, Nösberger J, Ort DR (2006) Food for thought: lower–than–expected crop yield stimulation with rising CO2 concentrations. Science 312:1918–1921. https://doi.org/10.1126/science.1114722
Lougheed VL, Tweedie CE, Andresen CG, Armendariz AM, Escarzaga SM, Tarin G (2020) Patterns and drivers of carbon dioxide concentrations in aquatic ecosystems of the Arctic coastal tundra. Global Biogeochem Cycles 34:e2020GB006552. https://doi.org/10.1029/2020GB006552
McGill WB, Cannon KR, Robertson JA, Cook FD (1986) Dynamics of soil microbial biomass and water–soluble organic C in Breton L after 50 years of cropping to two rotations. Can J Soil Sci 66:1–19. https://doi.org/10.4141/cjss86-001
McLachlan B, Van Kooten GC, Zheng Z (2020) Crop yields, carbon dioxide, and temperature: a meta–analysis. Department of Economics, University of Victoria Victoria, Canada, REPA Resource Economics & Policy Analysis Research Group
Mishra AK, Agrawal SB (2015) Biochemical and physiological characteristics of tropical mung bean (Vigna radiata L.) cultivars against chronic ozone stress: an insight to cultivar-specific response. Protoplasma 252:797–811. https://doi.org/10.1007/s00709-014-0717-x
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36. https://doi.org/10.1016/S0003-2670(00)88444-5
Nasser RR, Fuller MP, Jellings AJ (2008) Effect of elevated CO2 and nitrogen levels on lentil growth and nodulation. Agron Sustain Dev 28:175–180. https://doi.org/10.1051/agro:2007056
Olsen SR (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate (No. 939). US Department of Agriculture Washington DC, USA.
Reich PB, Hobbie SE, Lee T, Ellsworth DS, West JB, Tilman D, Knops JM, Naeem S, Trost J (2006) Nitrogen limitation constrains sustainability of ecosystem response to CO2. Nature 440:922–925. https://doi.org/10.1038/nature04486
Ross DJ, Newton PC, Tate KR (2004) Elevated [CO2] effects on herbage production and soil carbon and nitrogen pools and mineralization in a species-rich, grazed pasture on a seasonally dry sand. Plant Soil 260:183–196
Saha S, Chakraborty D, Pal M, Nagarajan S (2011) Impact of elevated CO2 on utilization of soil moisture and associated soil biophysical parameters in pigeon pea (Cajanus cajan L.). Agric Ecosys Environ 142:213–221. https://doi.org/10.1016/j.agee.2011.05.008
Satapathy SS, Swain DK, Pasupalak S, Bhadoria PBS (2015) Effect of elevated [CO2] and nutrient management on wet and dry season rice production in subtropical India. Crop J 3:468–480. https://doi.org/10.1016/j.cj.2015.08.002
Sathish P, Vijay Kumar G, Jyothi Lakshmi N, Vanaja M, Yadav SK, Vagheera P (2014) Impact of CO2 enhancement on photosynthesis and protein profile–response studies with a CO2 responsive black gram genotype. Int J Appl Biol Pharm 5:441–450
Subbaiah VV, Asija GK (1956) A rapid procedure for utilization of available nitrogen in soil. Curr Sci 26:258–260. https://doi.org/10.1016/0038-0717(88)90014-4
Tabatabai MA, Bremner JM (1969) Use of p–nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol Biochem 1:301–307. https://doi.org/10.1016/0038-0717(69)90012-1
Taub DR, Miller B, Allen H (2008) Effects of elevated CO2 on the protein concentration of food crops: a meta–analysis. Global Change Biol 14:565–575. https://doi.org/10.1111/j.1365-2486.2007.01511.x
Veneklaas EJ, Stevens J, Cawthray GR, Turner S, Grigg AM, Lambers H (2003) Chickpea and white lupin rhizosphere carboxylates vary with soil properties and enhance phosphorus uptake. Plant Soil 248:187–197. https://doi.org/10.1023/A:1022367312851
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci 37:29–38. https://doi.org/10.1097/00010694-193401000-00003
Wang C, Sun Y, Chen HY, Ruan H (2021) Effects of elevated CO2 on the C: N stoichiometry of plants, soils, and microorganisms in terrestrial ecosystems. CATENA 201:105219. https://doi.org/10.1016/j.catena.2021.105219
Wang D, Heckathorn SA, Wang X, Philpott SM (2012) A meta–analysis of plant physiological and growth responses to temperature and elevated CO2. Oecologia 169:1–13. https://doi.org/10.1007/s00442-011-2172-0
Wasaki J, Rothe A, Kania A, Neumann G, Römheld V, Shinano T, Osaki M, Kandeler E (2005) Root exudation, phosphorus acquisition, and microbial diversity in the rhizosphere of white lupine as affected by phosphorus supply and atmospheric carbon dioxide concentration. J Environ Qual 34:2157–2166. https://doi.org/10.2134/jeq2004.0423
Acknowledgements
The first author thankfully acknowledges the research support provided by the ICAR–Indian Institute of Soil Science, Bhopal, during his professional training program to carry out this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dutta, A., Lenka, N.K., Praharaj, C.S. et al. Impact of Elevated CO2 on Soil–Plant Phosphorus Dynamics, Growth, and Yield of Chickpea (Cicer arietinum L.) in an Alkaline Vertisol of Central India. J Soil Sci Plant Nutr 22, 1904–1914 (2022). https://doi.org/10.1007/s42729-022-00781-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-022-00781-4