Abstract
Background and aims
Crop species differ in phosphorus (P) acquisition in P-limiting environments. However, it is not fully understood how elevated atmospheric CO2 concentrations affects these P acquisition mechanisms and the plant's ability to acquire P from soil. This study aimed to investigate the effect of elevated CO2 on P acquisition in crop species with contrasting P acquisition mechanisms.
Methods
White lupin, faba bean, canola and near-isogenic wheat lines with and without citrate efflux were grown for 70 days in a P-deficient Chromosol soil under ambient (400 ppm) and elevated (800 ppm) CO2. Plant P uptake and P transformation in the rhizosphere were determined.
Results
Elevated CO2 promoted total P uptake in white lupin and canola by 84% and 48%, respectively, and decreased the P uptake in the non-citrate-exuding wheat (by 24%) but not the exuding wheat. In white lupin, elevated CO2 enhanced phosphatase activity and depletion of organic P in the rhizosphere. Elevated CO2 increased P uptake by increasing root length which allowed canola to exploit a greater volume of soil for P. In the rhizosphere of faba bean, NaOH-extractable inorganic P was greater under elevated CO2.
Conclusion
Crops which rely on organic acid exudation and phosphatases appear to be better adapted to acquiring P under elevated CO2.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Atmospheric CO2 concentrations are predicted to rise to 700–800 ppm by the end of the century (IPCC 2013). Elevated atmospheric CO2 has been shown to increase carbon-fixation in many C3 plant species which can lead to increases in above- and below-ground biomass and decreases in nutrient concentration (Ainsworth and Long 2005; Jin et al. 2012; Lam et al. 2012). A key regulator of plant response to elevated CO2 is plant P status. Severe plant P deficiency decreases plant growth, which can minimise elevated-CO2-induced increases in primary productivity. This indicates that soil P status contributes to how primary productivity is affected by elevated CO2 (Jin et al. 2015).
Phosphorus exists in various organic and inorganic soil pools with only a small proportion of total P being available to plants as the free orthophosphate ion. Given the unique behaviour of P compared to other nutrients, some plants species are relatively more efficient than others at obtaining P from non-labile sources particularly in an elevated CO2 environment (Khan et al. 2008; Nuruzzaman et al. 2005).
Although organic P pools represent a significant proportion of total soil P, the mineralisation of organic P is required before plant use. The mineralisation of organic P is carried out by both microorganisms and plants that produce phosphatase enzymes that hydrolyse the phosphate ester linkages in organic P compounds. There is variation in the activity of phosphatases in the rhizospheres of different crop species with white lupin having higher phosphatase activity relative to wheat under P deficiency (Tadano et al. 1993). Furthermore, elevated CO2 has been shown to upregulate phosphatase activity in the rhizosphere, indicating that elevated CO2 may promote the utilisation of organic P by plants (Barrett et al. 1998).
A large proportion of inorganic P is unavailable to the plant and is associated with the soil solid phase which is due to precipitation with Al, Fe and Ca or through specific adsorption with soil colloids. One way in which plants can access precipitated or sorbed forms of P is through the exudation of organic acids such as citrate and malate. The exuded organic acids can lead to enhanced dissolution and desorption of P in the rhizosphere (Barrow et al. 2018; Guppy et al. 2005). White lupin and some wheat lines have a strong organic acid efflux which allows these plants to access these largely unavailable forms of P (Shen et al. 2005). As elevated CO2 enhances belowground C allocation, it is expected that root exudation of organic acids increases (Cotrufo and Gorissen 1997). Haase et al. (2007) found that elevated CO2 significantly increase malate exudation in common bean which could potentially allow the plants to access greater amounts of precipitated and sorbed P in an elevated CO2 environment.
Increased soil exploration through altering root architecture in response to low P availability has been observed in species such as wheat and faba bean (Nuruzzaman et al. 2005). As roots explore a greater volume of soil, the roots intercept and hence assimilate more P from soil (Richardson et al. 2009). Elevated CO2 enhances belowground C allocation which not only increases root exudation but also root length which can aid the plant in exploiting a greater volume of soil for P. Furthermore, greater root length exposes more soil to rhizosphere processes such as release of phosphatase enzymes and organic acids, which may further aid the uptake of P. Unique to white lupin is the ability to produce dense regions of rootlets on lateral roots, termed cluster roots, under P deficiency. Cluster roots not only increase root surface area but are also the site of high exudation of organic acid anions, phosphatase release and rhizosphere acidification (Neumann et al. 2000; Wasaki et al. 2005).
Phosphorus acquisition mechanisms can be divided into two categories, physiological and morphological mechanisms. Physiological adaptations include phosphatase production, organic acid exudation and rhizosphere acidification to promote the dissolution, desorption or mineralisation of non-labile P (Lyu et al. 2016). Morphological adaptations are mechanisms where the plant alters its root architecture to intercept more P (Lambers et al. 2006). This may include the extension of root length, increasing the root-to-shoot ratio or root hair length. Given that crop species can display a range of physiological and morphological adaptations to P deficiency, elevated CO2 may not consistently affect P uptake across all crop species equally. Crop species such as white lupin, deposit phosphatases and organic acids into the rhizosphere and may be more adapted to acquiring P under elevated CO2 due to enhanced exudation of organic acids, phosphatase production and number of cluster roots (Campbell and Sage 2002; Wasaki et al. 2005). Crop species such as wheat have very limited organic acid exudation and therefore may not be able to acquire additional P under elevated CO2.
This study aimed to compare the effects of elevated CO2 on plant acquisition of P from soil and associated mechanisms of crop species differing in P acquisition traits. It was hypothesised that elevated CO2 would benefit crop species which rely on organic acid efflux or phosphatases to acquire P compared to those that do not, through a greater ability to access non-labile P.
Materials and methods
Soil was collected from a permanent grass pasture in Hamilton, Victoria, Australia (37°42′S 142°07′E) at a depth of 0–15 cm. The soil was then air-dried and sieved (<2 mm). It was classified as a Chromosol (Isbell 2016) and had the following properties: pH(CaCl2) 5.0, total C 38 g kg−1, total N 3.4 g kg−1, K2SO4-extractable NO3− 20 mg kg−1, Bray-P 5.1 mg kg−1 and Olsen-P 15 mg kg−1. Based on the Bray-P, the soil would be considered mildly P deficient (Six et al. 2013).
Experimental setup
The experiment consisted of two levels of CO2 and five crop species; each treatment was replicated four times. The two CO2 levels were 400 ppm and 800 ppm for ambient and elevated CO2, respectively. 800 ppm was chosen for the elevated CO2 treatment based on the predicted CO2 concentration by the end of this century (IPCC 2013) and within the concentration range used in the literature (Du et al. 2019). The five crop species used were white lupin (Lupinus albus L. cv. Kiev), faba bean (Vicia faba L. cv. Nura), canola (Brassica rapus L. cv. Hyola 50) and two wheat lines. White lupin is widely regarded for having a strong physiological response for P acquisition and can utilise large amounts of non-labile P; faba bean has a balanced morphological and physiological acquisition trait with some organic acid exudation, but can also increase its root length and root-to-shoot ratio in response to P deficiency, whereas canola is largely unable to utilise non-labile sources of P and relies largely on root architecture to assimilate P but can significantly acidify its rhizosphere in response to P deficiency (Lyu et al. 2016; Nuruzzaman et al. 2005; Vu et al. 2010). The two wheat lines were near-isogenic lines: one with no citrate efflux (Triticum aestivum L. cv. Egret) and another with a constitutive citrate efflux gene (Triticum aestivum L. cv. Egret TaMATE1B) which confers aluminium tolerance and may help to acquire P associated with the soils solid phase (Han et al. 2016). An additional set of four columns without plants were also included. In total 44 columns were used.
The experiment was performed in four CO2-controlled growth chambers (Fitotron SGC 120, Weiss Technik, Loughborough, UK). Plants were grown in PVC columns (10 cm diameter and 45 cm in height) which were lined with low-density polyethylene bags. In each column, 3.2 kg of air-dried soil was loaded. Before soil was added to the columns, basal nutrients were added at the following composition (mg kg−1): K2SO4, 120; CaCl2‧2H2O, 180; MgSO4‧7H2O, 50; Fe-EDTA, 1.3; MnSO4‧H2O 15; ZnSO4‧7H2O, 9; CuSO4‧5H2O, 6; Na2MoO4‧2H2O, 0.4. Six germinated wheat seeds and four of the remaining crop species were sown and the seedlings were thinned to 3 per column for wheat and 2 per column for the remaining crop species after 2 weeks. At sowing, white lupin and faba bean were inoculated with rhizobial strains WU425 and WSM1455, respectively. Nitrogen was applied weekly as urea for non-legumes at a rate of 15 mg N kg−1 soil which was based on existing soil N levels and plant density (Rogers et al. 1993). Columns were wetted to 80% field capacity by watering columns daily with reverse-osmosis water.
The plants were grown under 20 °C days at a photon flux 400 μmol m−2 s−1 at the canopy level for 14 h with 18 °C nights at a constant humidity of 70%. The columns were alternated weekly between two ambient and two elevated growth cabinets with the columns randomised to eliminate differences in environment between cabinets.
Columns were destructively harvested 70 days after sowing. Shoot and roots were harvested by cutting the shoots at ground level. Shoots were washed in 0.1 M HCl. Roots were carefully removed from the soil and lightly shaken, any soil that was adhered to the root was considered as rhizosphere soil and immediately sieved (<2 mm) and stored at 4 °C for fresh analysis. Roots were washed and scanned, root length was determined using WinRhizo Pro 2016 (Regent Instruments, Quebec City, Canada).
Microbial biomass C was determined using the chloroform-fumigation method according to Vance et al. (1987). For microbial biomass C, 8 g of fresh soil was weighed out into glass vials and placed in a vacuum desiccator with alcohol-free chloroform and fumigated for 24 h. Non-fumigated soils were immediately extracted using 0.5 M K2SO4, shaken for 1 h and then filtered through Whatman #42 whereas fumigated samples were fumigated first and then extracted. The concentrations of organic C in extracts were determined using a TOC analyser (GE Sievers InnovOx TOC, CO, USA). Microbial biomass C was estimated as the difference between fumigated and non-fumigated samples over the extraction efficiency (KEC = 0.45) (Joergensen 1996). Microbial P was determined using a liquid fumigation method, in which 2 g fresh rhizosphere soil was extracted in Milli-Q water over 16 h with anion-exchange membranes (Kouno et al. 1995). Fumigated samples received 1 ml of hexanol. Membranes were eluted in 0.1 M HCl/NaCl. Phosphorus concentration of the extracts was measured using malachite green (Motomizu et al. 1983). Here we considered the difference between fumigated and non-fumigated samples as microbial P, no conversion factor was used which would underestimate the true microbial P value (Bilyera et al. 2018). Rhizosphere soil pH was measured in 0.01 M CaCl2 in a 1:5 soil-solution ratio after 1-h shaking.
Phosphatase activity was measured using 4-methylumbelliferyl phosphate (4-MUB-P) according to Marx et al. (2001) and calculated according to German et al. (2011). Briefly, 0.5 g of fresh rhizosphere soil was briefly sonicated in 50 ml of 50 mM sodium acetate buffer (pH 5.2). Whilst being stirred, 100 μl of soil homogenate was pipetted into a black 96-well micro-plate followed by 100 μl of 200 μM 4-MUB-P solution. Each sample had 3 technical replicates. Plates were incubated at 20 °C for 2 h and measured at 0, 0.5, 1 and 2 h after substrate addition to ensure a linear increase in fluorescence. Fluorescence was measured at an excitation wavelength of 360 nm and emission of 420 mm (Varioskan, Thermo Fisher Scientific, Waltham, MA, USA).
Air-dried rhizosphere soil was sequentially extracted using a modified Hedley fractionation method using 0.5 M NaHCO3 and shaking for 16 h, followed by a 0.1 M NaOH extraction for 16 h and 1 M HCl for 16 h, these extractants indicate labile, moderately non-labile and stable P pools, respectively (Guppy et al. 2000). Total P of soil extracts was determined by digesting extracts using ammonium persulphate in an autoclave at 121 °C at 103 kPa for 1 h. Organic P (Po) of extracts was calculated by subtracting the inorganic P (Pi) from the total P of extracts. Phosphorus concentration of extracts was determined using malachite green.
Plant analysis
Shoot and root materials were ground through a 0.5-mm sieve (ZM200, Retsch GmbH, Haan, Germany) and digested in conc. HNO3:HClO4 (4:1). The concentrations of P in shoot and roots were determined using malachite green. Phosphorus acquisition-efficiency (PAE) was calculated by dividing total P uptake by root length.
Statistical analysis
A two-way analysis of variance (ANOVA) was performed to compare the effects of crop species, CO2 concentration and their interactions. Residuals were checked for normality by plotting actual values against the predicted values. Differences between means were tested using Tukey’s honest significant difference (HSD). All statistical analyses were performed in R version 3.6.3 (R Core Team 2020) using the agricolae package version 1.3–2 (de Mendiburu 2020).
Results
Plant growth and P uptake
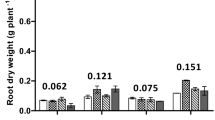
Elevated CO2 had not significantly affected shoot dry weight but increased root weight of faba bean by 29% (Fig. 1a and b, Table 1). There was a significant CO2 × crop species interaction for root length (F4,30 = 10.57, P < 0.001) with elevated CO2 significantly increasing the root length of faba bean and canola by 32% and 115%, respectively (Fig. 1c, Table 1).
Dry weights of shoot (a) and roots (b), root length (c) and root-to-shoot ratio (d) of wheat without (wheat -citrate) and with citrate exudation (wheat +citrate), white lupin, faba bean and canola grown for 70 days under ambient (400 ppm) and elevated (800 ppm) CO2 concentrations. Error bars represent standard error of the mean. Means that do not share a common letter are significantly different from each other (P < 0.05, Tukey HSD)
A significant CO2 × crop species interaction was also observed for shoot P concentration (F4,30 = 3.50, P = 0.018). The shoot P concentration in the wheat line that did not exude citrate decreased by 26% in response to elevated CO2, but this did not occur in the citrate-exuding wheat line. There was also a significant CO2 × crop species interaction on total P uptake (F4,30 = 3.90, P = 0.012) in white lupin and canola as P uptake increased by 84% and 48% under elevated CO2 (Table 2). In contrast, elevated CO2 decreased shoot P uptake of faba bean (data not shown).
A significant CO2 × crop species interaction for PAE (F4,30 = 2.70, P = 0.049) with elevated CO2 increasing PAE in white lupin (by 82%) but not other species (Table 2).
Rhizosphere soil properties
Elevated CO2 and crop species showed no significant effect on the NaHCO3-Pi fraction (Table 1). On average, plants depleted the NaHCO3-Pi pool by 12% compared to the no-plant control (Fig. 2a). The size of the NaHCO3-Po pool was significantly affected by crop species, elevated CO2 and their interaction. Elevated CO2 decreased NaHCO3-Po by 7% in the rhizosphere of non-citrate exuding wheat plants but increased it by 8% in the rhizosphere of citrate-exuding wheat line (Fig. 2b, Table 1). It decreased the NaHCO3-Po pool by 13% in the white lupin rhizosphere. Across all treatments, plant growth depleted the NaOH-Pi pool by 4.3 mg kg−1 compared to the no-plant control. In the rhizosphere of faba bean, NaOH-Pi was 5% higher under elevated CO2 (F4,30 = 3.60, P = 0.019) (Fig. 2c, Table 1). A significant CO2 × crop species interaction on the NaOH-Po pool were observed (F4,30 = 6.81, P < 0.001, Table 1). Organic P was greater under elevated CO2 in the rhizosphere of the non-citrate and citrate-exuding wheat line, increasing by 6.6% and 6.1%, respectively. Oppositely, the NaOH-Po pool was depleted under elevated CO2 by 5.6% in the rhizosphere of white lupin (Fig. 2d). HCl-extractable Pi was not affected by CO2 level, crop species or their interaction (Fig. 2e, Table 1).
Sequential phosphorus fractionation of soil showing NaHCO3- and NaOH-extractable inorganic (Pi) (a, c) and organic P (Po) (b, d) in the rhizosphere of wheat without (wheat -citrate) and with (wheat +citrate) citrate exudation, white lupin, faba bean and canola grown under ambient (400 ppm) and elevated (800 ppm) CO2 concentration for 70 days. Horizontal dashed line represents the no-plant control. Error bars represent standard error of the mean. Means that do not share a common letter are significantly different from each other (P < 0.05, Tukey HSD)
Phosphatase activity was 489 μmol MUB g−1 h−1 in the no-plant control which was similar in the rhizosphere of all crop species except white lupin where phosphatase activity was 899 μmol MUB g−1 h−1 under ambient CO2. Elevated CO2 further enhanced phosphatase activity in the rhizosphere of white lupin by 43% (F4,30 = 4.71, P = 0.005) (Fig. 3b, Table 1).
Extractable organic carbon (a) and phosphatase activity (b) in the rhizosphere of wheat without (wheat -citrate) and with (wheat +citrate) citrate exudation, white lupin, faba bean and canola grown for 70 days under ambient (400 ppm) and elevated (800 ppm) CO2 concentrations. Horizontal line represents the no-plant control. Error bars represent standard error of the mean. Means that do not share a common letter are significantly different from each other (P < 0.05, Tukey HSD)
Extractable organic C in the rhizosphere of white lupin was increased by 34% under elevated CO2 (Fig. 3a, Table 1). Although elevated CO2 did not affect rhizosphere pH, there was an alkalinisation effect compared to the no-plant controls (pH 5.1) with the rhizosphere pH of non-citrate exuding wheat, white lupin and faba bean being 5.22, 5.25 and 5.17, respectively (F4,30 = 8.54, P < 0.001) (Table 3). In addition, resin P, microbial biomass C and microbial biomass P in the rhizosphere soil were not significantly affected by elevated CO2, crop species or their interaction (Table 3).
Discussion
The present study was the first to use crop species of contrasting P acquisition mechanisms, including near-isogenic lines of wheat lines contrasting in citrate exudation to assess the effect of elevated CO2 on P acquisition. This study showed that elevated CO2 (800 ppm) could enhance, not affect and decrease P uptake compared with ambient CO2 when grown in the P-deficient Chromosol, and that these effects were linked to the P acquisition mechanism of the crop species. Crop species that have highly physiological mechanisms such as organic acid exudation and phosphatase production might be upregulated under elevated CO2, and hence improved the utilisation of non-labile P in soil. Furthermore, elevated CO2-induced increases in root length could also prove advantageous for P acquisition. Whilst elevated CO2 did not significantly affect biomass production in wheat and white lupin, it could still affect below-ground processes.
Elevated CO2 increased P uptake in white lupin and this was likely attributed to greater phosphatase activity which depleted the organic P pool. It is evident that elevated CO2 enhanced phosphatase activity in the rhizosphere of white lupin (Fig. 3c). The results are supported by Wasaki et al. (2005) who found a trend of increased phosphatase activity in the rhizosphere of the same species grown under elevated CO2. White lupin is known to produce large amounts of phosphatases in response to P deficiency to mineralise organic P sources as also evident in this experiment (Gilbert et al. 1999). It may upregulate the activity of extracellular phosphatases under elevated CO2 due to an increase in P demand because plant growth is more limited by the deficiency of P than C under elevated CO2 conditions (Rogers et al. 1993). The elevated-CO2-induced increase in phosphatase activity promoted the mineralisation of organic P and hence further enhanced P uptake. It is shown that the concentrations of NaHCO3- and NaOH-extractable organic P in the rhizosphere of white lupin were lower under elevated than ambient CO2 (Fig. 2d). Whilst both soil microbes and plants produced phosphatases, it is likely that the enhanced activity of phosphatases had resulted from changes in root physiology because there are no significant shifts in microbial biomass C and P and hence the P demand of soil microbes was unlikely to change under elevated CO2 (Heuck et al. 2015). Elevated CO2 enhanced the activity of phosphatases, which is a strong physiological P acquisition strategy, led to the depletion of the non-labile organic P pools, suggesting that white lupin grown under elevated CO2 had utilised non-labile P.
Elevated CO2 decreased P uptake in faba bean and non-citrate-exuding wheat but increased it in white lupin and canola. This species variation might be attributed to differences in the root exudation rates of the species and the effects of elevated CO2, which in turn influences access to non-labile P in the rhizosphere. Faba bean exudes organic acids to aid in P acquisition; however, it is not the dominant P acquisition strategy of this species (Lyu et al. 2016; Pearse et al. 2006). In the rhizosphere of faba bean, NaOH-extractable Pi (non-labile P) was greater under elevated CO2, indicating that organic acid exudation might have reduced and hence solubilisation of non-labile inorganic P decreased. This is supported in barley where elevated CO2 decreased dissolved organic C exudation but evidence of this in faba bean is lacking (Calvo et al. 2019). Unlike faba bean, elevated CO2 increased the extractable organic C in the rhizosphere of white lupin which could indicate enhanced exudation of organic acids as organic acids represent a large proportion of white lupin rhizodeposits (Nuruzzaman et al. 2006). It is unclear if increased extractable organic C in the rhizosphere of white lupin would aid in the acquisition of non-labile P due to small effect of CO2 on NaOH-extractable inorganic P. Future research should explore the effect of elevated CO2 on root exudation and its subsequent effect on P acquisition from non-labile P sources. These results outline the possible contrasting effects of elevated CO2 on root exudation and how this affects P uptake.
Citrate appeared to be important for maintaining the P status of wheat under elevated CO2. Elevated CO2 decreased the P concentration in the non-citrate-exuding wheat but did not affect the citrate-exuding wheat line. Elevated CO2 frequently reduces the concentrations of nutrients within above-ground plant tissues, most notably N and P (Huang et al. 2015). The decreased nutrient concentrations have often been attributed to a dilution effect due to growth promotion under elevated CO2 conditions (Lam et al. 2012). In our present study, elevated CO2 did not affect shoot dry weight of either wheat line. As the two wheat lines used in this study are genetically identical except for a constitutive citrate exudation gene which is expressed in the roots, the differences observed in shoot P concentration and total P uptake could be attributed entirely due to citrate exudation (Han et al. 2016). In a previous study, decreased P uptake in a wheat line that did not exudate citrate might be due to microbial immobilisation of P in the earlier stages of plant growth (Jin et al. 2014). The citrate-exuding wheat line was able to sustain P uptake under elevated CO2 through further enhancement of desorption and dissolution reactions of P. This is indicated by the citrate-exuding wheat line depleting NaOH-extractable inorganic P in response to elevated CO2 (Fig. 2c). Given the absence of citrate exudation and decreased shoot P concentration in the wheat line without citrate exudation, the importance of organic acids in P acquisition in an elevated CO2 environment is outlined in these two wheat lines.
Changes in root morphology under elevated CO2 also contributed to the improved P uptake. Canola displayed greater root length and a larger root-to-shoot ratio under elevated CO2, which enabled canola to exploit a larger volume of soil for P. Elevated-CO2-induced effects on canola root architecture would be the dominant driver for the enhancement in P uptake as there are no significant effects of elevated CO2 on the P fractions in the rhizosphere, which is supported by its lack of ability to acquire P from non-isotopically exchangeable P pools (Vu et al. 2010). Whilst increasing root length was not advantageous for P uptake in faba bean, canola root architecture contrasts that of faba bean due to the high proportion of fine roots that significantly increases the surface area of canola roots (Rose et al. 2009). Furthermore, elevated CO2 increases production of fine roots which can further contribute to enhanced canola P uptake under elevated CO2 (Piñeiro et al. 2017). It is likely that fine roots are more effective than coarse roots in acquiring P and its enhancement under elevated CO2 promotes P uptake.
Elevated CO2 did not significantly enhance shoot dry weight in many crop species possibly due to P limitations. A range of previous experiments have shown that shoot dry weight increases with higher atmospheric CO2 concentrations (Ainsworth and Long 2005; Du et al. 2019; Lam et al. 2012). In a number of studies, P-deficient plants have been shown to not respond to elevated levels of CO2 due to severe P limitation (Ellsworth et al. 2017; Jin et al. 2015). Evidence of P limitation can be seen in the shoot P concentration being less than the internal critical concentration of those in other experiments for the same crop species (Reuter and Robinson 1997). However, due to the absence of treatments with added P, we cannot conclusively attribute the lack of CO2 response in some species to P limitation. Other factors such as light intensity and N-form might regulate plant response to elevated CO2 (Pérez-López et al. 2013; Rubio-Asensio and Bloom 2017). Although elevated CO2 might still increase C fixation at the earlier growth stages, P deficiency intensified over time, which induced changes the rhizosphere organic P pools.
Conclusions
This was the first study to use crop species of contrasting P acquisition mechanisms and near-isogenic lines of wheat to assess how P acquisition mechanisms drive P uptake under elevated CO2. Elevated CO2 was shown to not universally increase the uptake of P across the crop species. Crop species that have physiological P acquisition strategies were more adapted to acquiring non-labile P in an elevated CO2 environment due to enhanced phosphatase activity and rhizodeposition of organic C whereas the species with root morphological P strategies were less adapted to acquiring non-labile P under elevated CO2. Future research should explore the effect of elevated CO2 on the exudation of organic acid anions under P deficiency and its subsequent effect on the mobilisation efficiency of non-labile P in the rhizosphere.
References
Ainsworth EA, Long SP (2005) What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol 165:351–372. https://doi.org/10.1111/j.1469-8137.2004.01224.x
Barrett DJ, Richardson AE, Gifford RM (1998) Elevated atmospheric CO2 concentrations increase wheat root phosphatase activity when growth is limited by phosphorus. Aust J Plant Physiol 25:87–93. https://doi.org/10.1071/PP97045
Barrow NJ, Debnath A, Sen A (2018) Mechanisms by which citric acid increases phosphate availability. Plant Soil 423:193–204. https://doi.org/10.1007/s11104-017-3490-8
Bilyera N, Blagodatskaya E, Yevdokimov I, Kuzyakov Y (2018) Towards a conversion factor for soil microbial phosphorus. Eur J Soil Biol 87:1–8. https://doi.org/10.1016/j.ejsobi.2018.03.002
Calvo OC, Franzaring J, Schmid I, Fangmeier A (2019) Root exudation of carbohydrates and cations from barley in response to drought and elevated CO2. Plant Soil 438:127–142. https://doi.org/10.1007/s11104-019-03998-y
Campbell CD, Sage RF (2002) Interactions between atmospheric CO2 concentration and phosphorus nutrition on the formation of proteoid roots in white lupin (Lupinus albus L.). plant. Cell Environ 25:1051–1059. https://doi.org/10.1046/j.1365-3040.2002.00883.x
Cotrufo MF, Gorissen A (1997) Elevated CO2 enhances below-ground C allocation in three perennial grass species at different levels of N availability. New Phytol 137:421–431. https://doi.org/10.1046/j.1469-8137.1997.00839.x
de Mendiburu F (2020) Agricolae: statistical procedures for agricultural research. R package version 1:3–2 https://CRAN.R-project.org/package=agricolae
Du C, Wang X, Zhang M, Jing J, Gao Y (2019) Effects of elevated CO2 on plant C-N-P stoichiometry in terrestrial ecosystems: a meta-analysis. Sci Total Environ 650:697–708. https://doi.org/10.1016/j.scitotenv.2018.09.051
Ellsworth DS, Anderson IC, Crous KY, Cooke J, Drake JE, Gherlenda AN, Gimeno TE, Macdonald CA, Medlyn BE, Powell JR, Tjoelker MG, Reich PB (2017) Elevated CO2 does not increase eucalypt forest productivity on a low-phosphorus soil. Nat Clim Chang 7:279–282. https://doi.org/10.1038/nclimate3235
German DP, Weintraub MN, Grandy AS, Lauber CL, Rinkes ZL, Allison SD (2011) Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies. Soil Biol Biochem 43:1387–1397. https://doi.org/10.1016/j.soilbio.2011.03.017
Gilbert GA, Knight JD, Vance CP, Allan DL (1999) Acid phosphatase activity in phosphorus-deficient white lupin roots. Plant Cell Environ 22:801–810. https://doi.org/10.1046/j.1365-3040.1999.00441.x
Guppy CN, Menzies NW, Moody PW, Compton BL, Blamey FPC (2000) A simplified, sequential, phosphorus fractionation method. Commun Soil Sci Plant Anal 31:1981–1991. https://doi.org/10.1080/00103620009370556
Guppy CN, Menzies NW, Moody PW, Blamey FPC (2005) Competitive sorption reactions between phosphorus and organic matter in soil: a review. Aust J Soil Res 43:189–202. https://doi.org/10.1016/j.soilbio.2011.03.017
Haase S, Neumann G, Kania A, Kuzyakov Y, Römheld V, Kandeler E (2007) Elevation of atmospheric CO2 and N-nutritional status modify nodulation, nodule-carbon supply, and root exudation of Phaseolus vulgaris L. Soil Biol Biochem 39:2208–2221. https://doi.org/10.1016/j.soilbio.2007.03.014
Han C, Zhang P, Ryan PR, Rathjen TM, Yan ZH, Delhaize E (2016) Introgression of genes from bread wheat enhances the aluminium tolerance of durum wheat. Theor Appl Genet 129:729–739. https://doi.org/10.1007/s00122-015-2661-3
Heuck C, Weig A, Spohn M (2015) Soil microbial biomass C:N:P stoichiometry and microbial use of organic phosphorus. Soil Biol Biochem 85:119–129. https://doi.org/10.1016/j.soilbio.2015.02.029
Huang W, Houlton BZ, Marklein AR, Liu J, Zhou G (2015) Plant stoichiometric responses to elevated CO2 vary with nitrogen and phosphorus inputs: evidence from a global-scale meta-analysis. Sci Rep 5:1–8. https://doi.org/10.1038/srep18225
IPCC (2013). Climate change 2013: the physical science basis. Contribution of the working group I to the fifth assessment report of the intergovernmental panel on climate change
Isbell RF (2016) The Australian soil classification. CSIRO Publishing, Collingwood
Jin J, Tang C, Armstrong R, Sale P (2012) Phosphorus supply enhances the response of legumes to elevated CO2 (FACE) in a phosphorus-deficient vertisol. Plant Soil 358:91–104. https://doi.org/10.1007/s11104-012-1270-z
Jin J, Tang C, Robertson A, Franks AE, Armstrong R, Sale P (2014) Increased microbial activity contributes to phosphorus immobilization in the rhizosphere of wheat under elevated CO2. Soil Biol Biochem 75:292–299. https://doi.org/10.1016/j.soilbio.2014.04.019
Jin J, Tang C, Sale P (2015) The impact of elevated carbon dioxide on the phosphorus nutrition of plants: a review. Ann Bot 116:987–999. https://doi.org/10.1093/aob/mcv088
Joergensen RG (1996) The fumigation-extraction method to estimate soil microbial biomass: calibration of the kEC value. Soil Biol Biochem 28:25–31. https://doi.org/10.1016/0038-0717(95)00102-6
Khan FN, Lukac M, Turner G, Godbold DL (2008) Elevated atmospheric CO2 changes phosphorus fractions in soils under a short rotation poplar plantation (EuroFACE). Soil Biol Biochem 40:1716–1723. https://doi.org/10.1016/j.soilbio.2008.02.008
Kouno K, Tuchiya Y, Ando T (1995) Measurement of soil microbial biomass phosphorus by an anion exchange membrane method. Soil Biol Biochem 27:1353–1357. https://doi.org/10.1016/0038-0717(95)00057-L
Lam SK, Chen D, Norton R, Armstrong R, Mosier AR (2012) Nitrogen dynamics in grain crop and legume pasture systems under elevated atmospheric carbon dioxide concentration: a meta-analysis. Glob Chang Biol 18:2853–2859. https://doi.org/10.1111/j.1365-2486.2012.02758.x
Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas EJ (2006) Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Ann Bot 98:693–713. https://doi.org/10.1093/aob/mcl114
Lyu Y, Tang H, Li H, Zhang F, Rengel Z, Whalley WR, Shen J (2016) Major crop species show differential balance between root morphological and physiological responses to variable phosphorus supply. Front Plant Sci 7:1–15. https://doi.org/10.3389/fpls.2016.01939
Marx MC, Wood M, Jarvis SC (2001) A microplate fluorimetric assay for the study of enzyme diversity in soils. Soil Biol Biochem 33:1633–1640. https://doi.org/10.1016/S0038-0717(01)00079-7
Motomizu S, Wakimoto T, Tôei K (1983) Spectrophotometric determination of phosphate in river waters with molybdate and malachite green. Analyst 108:361–367. https://doi.org/10.1039/an9830800361
Neumann G, Massonneau A, Langlade N, Dinkelaker B, Hengeler C, Römheld V, Martinoia E (2000) Physiological aspects of cluster root function and development in phosphorus-deficient white lupin (Lupinus albus L.). Ann Bot 85:909–919. https://doi.org/10.1006/anbo.2000.1135
Nuruzzaman M, Lambers H, Bolland MDA, Veneklaas EJ (2005) Phosphorus benefits of different legume crops to subsequent wheat grown in different soils of Western Australia. Plant Soil 271:175–187. https://doi.org/10.1007/s11104-004-2386-6
Nuruzzaman M, Lambers H, Bolland MDA, Veneklaas EJ (2006) Distribution of carboxylates and acid phosphatase and depletion of different phosphorus fractions in the rhizosphere of a cereal and three grain legumes. Plant Soil 281:109–120. https://doi.org/10.1007/s11104-005-3936-2
Pearse SJ, Veneklaas EJ, Cawthray GR, Bolland MDA, Lambers H (2006). Carboxylate release of wheat, canola and 11 grain legume species as affected by phosphorus status plant soil 288:127-139 https://doi.org/10.1007/s11104-006-9099-y
Pérez-López U, Miranda-Apodaca J, Muñoz-Rueda A, Mena-Petite A (2013) Lettuce production and antioxidant capacity are differentially modified by salt stress and light intensity under ambient and elevated CO2. J Plant Physiol 170:1517–1525. https://doi.org/10.1016/j.jplph.2013.06.004
Piñeiro J, Ochoa-Hueso R, Delgado-Baquerizo M, Dobrick S, Reich PB, Pendall E, Power SA (2017) Effects of elevated CO2 on fine root biomass are reduced by aridity but enhanced by soil nitrogen: a global assessment. Sci Rep 7:1–9. https://doi.org/10.1038/s41598-017-15728-4
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Reuter D, Robinson JB (1997) Plant analysis: an interpretation manual. CSIRO Publishing, Melbourne, Melbourne
Richardson AE, Hocking PJ, Simpson RJ (2009) George TS. Plant mechanisms to optimise access to soil phosphorus Crop Pasture Sci 60:124–143. https://doi.org/10.1071/Cp07125
Rogers GS, Payne L, Milham P, Conroy J (1993). Nitrogen and phosphorus requirements of cotton and wheat under changing atmospheric CO2 concentrations plant soil 155:231-234 https://doi.org/10.1007/bf00025026
Rose TJ, Rengel Z, Ma Q, Bowden JW (2009) Crop species differ in root plasticity response to localised P supply. J Plant Nutr Soil Sci 172:360–368. https://doi.org/10.1002/jpln.200800031
Rubio-Asensio JS, Bloom AJ (2017) Inorganic nitrogen form: a major player in wheat and Arabidopsis responses to elevated CO2. J Exp Bot 68:2611–2625. https://doi.org/10.1093/jxb/erw465
Shen J, Li H, Neumann G, Zhang F (2005) Nutrient uptake, cluster root formation and exudation of protons and citrate in Lupinus albus as affected by localized supply of phosphorus in a split-root system. Plant Sci 168:837–845. https://doi.org/10.1016/j.plantsci.2004.10.017
Six L, Smolders E, Merckx R (2013) The performance of DGT versus conventional soil phosphorus tests in tropical soils—maize and rice responses to P application. Plant Soil 366:49–66. https://doi.org/10.1007/s11104-012-1375-4
Tadano T, Ozawa K, Sakai H, Osaki M, Matsui H (1993) Secretion of acid phosphatase by the roots of crop plants under phosphorus-deficient conditions and some properties of the enzyme secreted by lupin roots. Plant Soil 155:95–98. https://doi.org/10.1007/bf00024992
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707. https://doi.org/10.1016/0038-0717(87)90052-6
Vu DT, Armstrong RD, Sale PWG, Tang C (2010) Phosphorus availability for three crop species as a function of soil type and fertilizer history. Plant Soil 337:497–510. https://doi.org/10.1007/s11104-007-9516-x
Wasaki J, Rothe A, Kania A, Neumann G, Römheld V, Shinano T, Osaki M, Kandeler E (2005) Root exudation, phosphorus acquisition, and microbial diversity in the rhizosphere of white lupine as affected by phosphorus supply and atmospheric carbon dioxide concentration. J Environ Qual 34:2157–2166. https://doi.org/10.2134/jeq2004.0423
Acknowledgements
We would like to thank Mr. Mark Richards from New South Wales Department of Primary Industry for providing the white lupin and chickpea seeds and Professor Manny Delhaize from CSIRO for providing the wheat seeds. JBO was supported by an Australian Government Research Training Program Scholarship
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Honghua He.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
O’Sullivan, J.B., Jin, J. & Tang, C. Elevated CO2 promotes the acquisition of phosphorus in crop species differing in physiological phosphorus-acquiring mechanisms. Plant Soil 455, 397–408 (2020). https://doi.org/10.1007/s11104-020-04698-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-020-04698-8