Abstract
Due to a zinc-deficient diet, about 800,000 children die each year worldwide. This aspect is amended by exploiting foliar fertilization, a useful alternative to improve crop yield and nutritional quality of food crops. The aim of this study was then to investigate the leaf uptake and transport of zinc by soybean (Glycine max (L) Merrill). Plant leaves were treated with Zn phosphite and Zn ethylenediamine tetra-acetic acid (EDTA) commercial formulations. X-ray spectroscopy (XRF and XANES) was exploited to trace nutrient movement in the petiolule and scanning electron microscopy (SEM) to evaluate the influence of leaf surface treatments. No radiation damage, in terms of elemental redistribution, was observed during the XRF and XANES measurements. As an alternative to radioisotopes, XRF allowed to detect the movement of Zn from both sources in the plant petiolule. Both fertilizers disintegrated leaf epicuticular wax crystals, yet accumulation of sediments in the vicinity of stomata was noted only for Zn phosphite. Absorption and redistribution of Zn were higher for plants that received Zn phosphite. Zinc supplied as Zn phosphite was transported in a form different from that of the pristine Zn phosphite, whereas Zn supplied as Zn EDTA was transported in its chelated form.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Although being required only as a trace element, zinc is essential for plant and animal (including humans) nutrition. In plants, Zn is involved in several metabolic processes like synthesis of protein and growth hormone, metabolism of carbohydrates, and maintenance of the cell membrane integrity (Broadley et al. 2012). However, the availability of Zn in most cultivated soils is low. A survey relying on approximately 38,000 soil samples from Brazilian agricultural areas revealed that ca. 35% of the soils were Zn deficient (Guilherme et al. 2015). Around the world, ca. 800,000 children under 5 years die annually due to a Zn-deficient diet (Caulfield and Black 2004).
Zinc deficiency in plants can be overcome by fertilization with zinc inorganic compounds (oxides, carbonates, sulfates, chlorides, or nitrates), synthetic chelates (e.g., ethylenediamine tetra-acetic acid—EDTA), natural organic complexes, or inorganic complexes (Montalvo et al. 2016). However, the crop response is usually dependent on the Zn source (Slaton et al. 2005) and on the fertilization method, usually employing soil or foliar application (Cakmak et al. 2010). Once it crosses the cuticle, Zn reaches the foliar vascular system before being transported to the other parts of the plant. To this end, it should penetrate the leaf surface, for example, across the cuticle and/or through the stomatal cavity, and traverse the inner cells via apoplastic or symplastic pathways (Eichert and Fernández 2012; Fernandez and Brown 2013). Nevertheless, the models to explain the permeability of foliar-applied nutrients are still not conclusive (Fernandez et al. 2017).
Foliar applications increased the Zn content in wheat, rice, and soybean grains (Aytaç et al. 2007; Cakmak et al. 2010; Zou et al. 2012; Phattarakul et al. 2012; Singh et al. 2019), demonstrating that foliar fertilization is a useful strategy for grain biofortification and may thereby increase Zn content in the diet of human population. Foliar application of Zn EDTA leads to an increase in Zn concentration and bioavailability in rice grains (Wei et al. 2012). In wheat, efficiency of this application was 1.4–1.7 times more effective relatively to ZnSO4 (Brennan 1996). Zn EDTA is an effective foliar fertilizer also for triticale, increasing the grain yield during drought stress (Kinaci and Gulmezoglu 2007). For chickpea, Zn EDTA increased the Zn concentration in the seeds (Kayan et al. 2015). The combination of soil and foliar fertilization with Zn EDTA led to enhancement of the Zn content in the wheat grain, which was more pronounced then that related to a single application in the soil (Ghasal et al. 2017).
Phosphites are inorganic salts usually obtained by the reaction of cationic compounds and phosphorous acid, H3PO3 (Gozzo and Faoro 2013), which constitute themselves in an alternative to the widely used sulfates and chlorides as sources of foliar fertilizers. Despite the presence of phosphorous, the use of phosphites as the P source is controversial (McDonald et al. 2001). Hence, phosphite salts are traded as fertilizers due to the presence of the cations, such as Zn2+. Besides supplying plant nutrients, phosphite-based compounds present also anti-fungal properties, in which the mechanism of control is not fully clarified (Gozzo and Faoro 2013).
The performance of foliar fertilization may vary according to the physical-chemical properties of the formulation, the plant species, and the environmental conditions. The characteristics of the formulation such as molecular size, solubility, pH, surface tension, and spreading play a relevant role in the efficacy of nutrient uptake by the leaves (Fernandez and Brown 2013). We hypothesize that the uptake velocity as well as the chemical form of Zn during its transportation depends on the Zn source. A clear understanding on the uptake and transport of nutrients by the plants is then fundamental for the development of highly efficient fertilizers. Hence, the aim of this work was therefore to investigate the transport kinetics, the effect on the leaf surface, and the chemical environment of Zn in soybean leaves exposed to Zn EDTA and Zn phosphite. Soybean (Glycine max (L.) Merrill) was chosen as a model plant species due to its economic relevance, and the Zn sources due to their worldwide application by rural producers. Absorption kinetics was monitored in vivo by using X-ray fluorescence spectroscopy (XRF), and X-ray absorption near edge spectroscopy (XANES) uncovered the Zn chemical environment in the treated leaves. Moreover, the leaf structure was characterized by scanning electron microscopy (SEM) after foliar application of Zn.
2 Materials and Methods
2.1 Plant Growth and Foliar Treatments
Soybean plants were cultivated in a growth room at 27 ± 3 °C, with a relative humidity of 80 ± 5% and a photoperiod of 12 h under 6500K LED lamp illumination supplying 250 μmol photons m−2 s−1 (Fig. S1a). When the third trifoliate leaf started expanding, the plants were transferred to the sample holder shown in Fig. S1b.
The treatments consisted of an aqueous solution of commercial Zn phosphite (8.0 wt% Zn and 1.25 g mL−1 density, Agrivalle, BR) and Zn EDTA (15.0 wt% Zn, Alternativa Agrícola, BR) at 23 g L−1 Zn diluted in deionized water. The final pHs of the solutions were 1.5 and 5.9 for Zn phosphite and Zn EDTA. Zn phosphite requires a pH lower than 3.0 to keep the solution stable. Approximately 70 μL of the solutions was spread on half part (from the middle to apex) of a leaflet abaxial surface, by using a brush. This volume was determined as the weight difference before and after the application. Immediately after application, the plants in the sample holder were returned to the growth room where they stayed for 3 days and then analyzed by XRF measurements. The plants were thereafter conditioned inside of a box with a similar environment and transported to the synchrotron facility.
2.2 Redistribution Kinetics
The movement of Zn was monitored in vivo by evaluating the Zn content in the petiolule of the treated leaflet. Measurements were performed in the petiolule, 1 mm far from the leaf edge, before the application and after elapsing 12, 24, 48, and 72 h of it. Aiming at to distinguish possible changes on the Zn concentration in the petiolule due to plant growth, a control plant that did not receive application was analyzed. The experimental setup is shown in the section 1 of the Electronic Supplementary Material.
2.3 Scanning Electron Microscopy
To evaluate the effects of Zn fertilizer deposition on the leaf surface, 20-μL droplets of the above described treatments were applied on the abaxial surface of the middle leaflet of the third youngest leaf. The fertilizers were spread by using a brush, according to ordinary procedures for the kinetics assays. An additional application of water was performed to highlight possible injuries caused by the brush scratch. The leaves were collected after 24 h of foliar Zn application. The sample preparation and analysis conditions are described in the section 2 of the Electronic Supplementary Material.
2.4 Chemical Speciation
The Zn chemical environment during the redistribution process was in vivo evaluated using Zn-K XANES at the XRF beamline of the Brazilian Synchrotron National Laboratory (LNLS). For seeking conciseness, the beamline specifications are presented in the section 3 of the Electronic Supplementary Material.
The leaves were treated following the same above-described procedure. The leaf was covered with a 1-mm Pb foil to avoid Zn fluorescence photons from the leaf surface (Fig. S2), and the measurements were performed approximately 2 mm far from the leaf edge. In order to prevent radiation damage, the measurements were taken in a different spot as described in the section 3 of the Electronic Supplementary Material.
3 Results
3.1 In Vivo Redistribution Kinetics of Zn
Since X-rays may damage fresh or living plant tissues, the impact of irradiation on the analyzed petiolules was firstly investigated. To this end, the content of other detected elements and the intensity of Compton scattering were monitored during the determination of Zn. Compton scattering can be an indicator of tissue dehydration since inelastic scattering decreases with the water content lessening. Using the benchtop equipment, Compton scattering, and the signals of Ca, K, Fe, and Mn underwent only slight modifications for all the plants without any trend (Figs. S4–S6). Thus, it demonstrates that the set instrumental conditions did not cause any artifact and, therefore, the method was adequate for tracing Zn in living tissues.
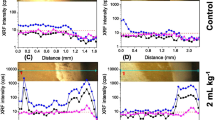
Figure 1 shows the average content of Zn in the petiolules as a function of time based on three measurements. A and B represent the two biological replicates composed of three plants. In order to avoid interferences of the petiolule thickness on the measurements, the Zn content is expressed as the number of Zn-Kɑ photon counts normalized by the rhodium scattering counts. Absorption and redistribution of Zn were higher for plants that received Zn phosphite. Despite the difference of Zn intensity between the two biological replicates, which reflects that different individuals may differently respond, they presented a similar behavior regarding the treatments. For both plants treated with Zn EDTA, the Zn concentration sharply increased after 12 h, then decreased after 24 h, and thereafter remained almost constant for 72 h.
XRF monitoring the Zn concentration in the petiolule of soybean (G. max) as function of time. (a) and (b) represent two biological replicates with three plants each that received Zn phosphite and Zn EDTA treatments. The number of Zn photon counts was normalized by the Rh photon counts aiming to avoid interferences of the petiolule thickness on the measurements. Zinc absorption and redistribution were higher for Zn phosphite treatment compared to Zn EDTA. Error bars represent the standard deviation for three measurements
3.2 Scanning Electron Microscopy Analysis
The control leaf (non-treated sample, Fig. 2a–c) revealed a dense and uniform layer of epicuticular wax crystals (EWC). Figure 2e–f show that the water treatment with a brush was enough to remove mechanically part of the EWC. However, the application of Zn phosphite and Zn EDTA promoted higher EWC removals in comparison with water (Fig. 2g–i and Fig. 2j–l).
SEM micrographs of the abaxial surface of soybean leaves at × 140, × 750, and × 1900 magnification. a, b, c Control sample; d, e, f water treatment; g, h, i Zn phosphite treatment; j, k, l Zn EDTA treatment. The control sample revealed epidermal cells covered by a uniform layer of epicuticular wax crystals. The water treatment with a brush was enough to remove mechanically part of the epicuticular wax crystals (EWC). However, the application of Zn phosphite and Zn EDTA promoted higher removal of EWC than water. The phosphite crystals were observed surrounding the stomata. PC - phosphite crystals, S - stomata of the leaf surface, SC - scalped areas by the brush.
The treatments disintegrated most of EWC from the leaf surface possibly due to a chemical effect caused by the fertilizers. It was also observed that spots of Zn phosphite agglomerated along the leaf surface, mainly around the stomata (Fig. 2 h and i), whereas for Zn EDTA treatment, no evidence of accumulation was detected (Fig. 2l). Additional micrographs recorded for other leaves submitted to the same treatments are presented in Fig. S7 in the Electronic Supplementary Material.
3.3 Chemical Speciation
Figure S8 shows the non-normalized Zn-K XANES spectra recorded for the control plants. Despite the poor signal-to-noise ratio associated to the very low Zn concentration, no photoinduced spectral changes were noted. Similarly, the non-normalized Zn-K XANES spectra for plants treated with Zn phosphite (Fig. S9a) and Zn EDTA (Fig. S9b) did not show consistent spectral changes that could be assigned to radiation damage.
Concerning tissue dehydration, Fig. S10 presents the Compton scattering intensity recorded during the XANES chemical speciation analysis. No significant changes were observed in the scattering intensities. Concomitantly, the intensity of Ca-Kα XRF (Fig. S11) was also recorded, since Ca is one of the major constituents of plant tissues and stress signaler, thus a good radiation damage indicator. The data did not point out any significant signal variation for the Zn phosphite treated plants (Fig. S11a); however, a slight decrease, within error bar range, was noticed during the second scan in point 2 of plant 1, while in plant 2 it slightly increased (Fig. S11b).
Figure S12 shows the stem of a soybean plant (a) prior to and after irradiation (b) by a ca. 30 μm focused beam during 20 min using an Rh anode operating at 45 kV and 900 μA (experimental details provided in section 4 of the Electronic Supplementary Material). It is possible to note the scorching radiation damage caused by the X-ray beam.
Figure 3 a and b present the XANES spectra recorded for Zn phosphite and Zn EDTA reference compound and for petiolules of two different plants whose leaves were treated with Zn phosphite. Analysis of the spectra associated to the standard compounds permits one to infer that Zn from Zn phosphite was transported as a compound different from that applied to the leaf. On the other hand, the spectra recorded for the petiolules of leaves treated with Zn EDTA overlapped that recorded for the pristine material. Figure 3c presents the content of Zn in these plants in terms of XRF counts which represents the Zn concentration in the petiolule. The Zn content in the petiolule was higher for the Zn phosphite treatment, demonstrating the potential for higher absorption of Zn phosphite as compared with Zn EDTA. A similar finding was observed for the XRF approach (Fig. 1).
Zn-K XANES spectra recorded for petiolules of soybean after foliar application of a Zn phosphite and b Zn EDTA plus spectra registered for Zn phosphite and Zn EDTA reference compounds. Overlapping the spectra, one can observe that the Zn from Zn phosphite treatment has been transported as compounds different to that applied to the leaf, whereas the overlay between the spectra presented in b indicates that the Zn chemical environment in the petiolules and Zn EDTA reference compound were mostly the same. c Average XRF intensity from 9.78 to 9.84 KeV in the petiolules of these plants demonstrates higher concentration of Zn for Zn phosphite
4 Discussion
The X-ray beam may modify the spatial distribution and chemical environment of the target element (Scheckel et al. 2004). Changes in the chemical environment during in situ XANES measurements were reported by Smith et al. (2009) during the speciation of arsenic in rice roots and by Scheckel et al. (2004) in the analysis of thallium in Iberis intermedia. Differently from Zn2+, both arsenic and thallium form compounds in multiple oxidation states and are more susceptible to photoreduction or oxidation than Zn2+. The X-ray brilliance, thus the fluence on the sample, varies from one synchrotron beamline to another. This parameter should be then kept in mind during measurements in synchrotron sources. Additionally, recording a XANES spectrum usually subjects a certain point of the sample to longer exposure than that requested for XRF mapping or point analysis (Lombi et al. 2011b). It is important highlighting that scorching symptoms were not observed in the plants submitted to the XRF LNLS beamline. The X-ray flux at this beamline is in the order of 108 photons s−1 mm−2 at 10 keV. Nevertheless, differently from the Rh anode which supplied polychromatic beam (Fig. S12c), the XANES measurements were performed under monochromatic beam, resulting in a much shorter bandwidth. This is especially important, in relation to low energy photons, e.g., < 4 keV, which might modify the biological tissues since their interaction with matter is higher than those around Zn-K edge. Thus, as noted for the time-resolved XRF kinetic measurements, no radiation damage under the conditions used to register the XANES spectra was detected.
The Zn concentration commonly used in field applications is around 1 g Zn L−1 (Cakmak and Kutman 2018). In order to overcome the limits of detection imposed by the in vivo XRF measurements, it was necessary to increase the dose to 23 g Zn L−1. High concentrations of Zn may however negatively influence the uptake and transport of other nutrients such as K, P, Mg, Fe, Cu, and Mo (Santos et al. 2020). In the present study, interference of Zn supply on the concentration of K, Ca, Mn, and Fe in plant petiolule was not observed, which suggests that even in higher concentrations, Zn was not interfering badly the metabolism of other nutrients. Under the set instrumental conditions, the limit of detection for Zn in the petiolule of living plants was estimated as 36 ± 1.3 mg kg−1. The concentration of Zn in the leaves of soybean ranges from 50 to 80 mg kg−1 reaching up to ca. 600 mg kg−1 in plants grown in Zn-contaminated soil (Silva et al. 2014), demonstrating that the experimental design adopted here allowed to quantitatively record the Zn concentration.
The observed agglomeration of Zn phosphite shown by SEM regards the precipitation as the solution dried and the mineral became solid. The inorganic particles of Zn phosphite agglomerated in the stomata may release ions under the influence of air humidity, thus increasing the uptake of Zn via the stomata pathway. A similar behavior was observed by Bala et al. (2019) after applying ZnO nanoparticles in rice. Stomata may take up water and solutes, and even small particles may penetrate leaves through stomata. The mechanisms behind this process are still not fully understood (Avellan et al. 2019; Eichert and Goldbach 2008; Eichert et al. 2008). It is possible that the uptake occurs by diffusion through a liquid water film in the stomata walls, which are formed by increasing the wettability of the stomata pores (Burkhardt 2010; Eichert et al. 2008). Compared with the cuticular pathway, the stomatal pathway is characterized by higher size exclusion limits, which means that it is more accessible for larger molecules (Eichert et al. 2008). On the other hand, since higher stomata density did not lead to higher Zn foliar uptake after the application of ZnSO4, Li et al. (2017, 2018) did not consider stomata the main foliar uptake pathway.
The disintegration of EWC followed the treatments with Zn phosphite and Zn EDTA was also in leaves exposed to abiotic stress, such as hygroscopic aerosols (Burkhardt et al. 2018). The EWC disintegration during the water treatment was caused mechanically by the brush. However, the EWC disintegration caused by Zn phosphite and Zn EDTA can affect more epidermal cells than just the brush use, and it could be the mechanism responsible for the increased uptake of Zn compound through leaf cuticles. Moreover, both Zn phosphite and Zn EDTA are solubilized in a medium with low pH, and leaf cuticles exposed to acidic solutions presented lower fixation of cations such as Zn (Fernandez et al. 2013). One can therefore conclude that the acidic condition of the Zn treatments may be a determinant factor associated Zn entrance trough the leaf.
The epicuticular wax damage caused by Zn EDTA and Zn phosphite raises a concern on possible stress induced by foliar fertilizers. The ability of EWC to recover from stress is dependent on the developmental stage, and, in some cases, it does not recover (Neinhuis et al. 2001). There is a compromise between Zn supplying and cuticle damage, this latter effect may bring undesirable consequences to plant health. From the abiotic stress standing point, the literature pointed out that the EWC has a minor influence in the rate of water movement across the cuticle membrane, in comparison with the intracuticular wax (Goodwin and Jenks 2005). Conversely, the mechanical EWC removal may promote a moderate increase in water permeability (Goodwin and Jenks 2005). In this context, no correlation between quantity of wax cuticular and epidermal water loss was noted for Zea mays (Ristic and Jenks 2002). Regarding biotic stress, it has been demonstrated that adjuvants may alter the epicuticular wax of Vitis vinifera and increase the susceptibility to Botrytis cinerea (Rogiers et al. 2005). The soybean rust, caused by the fungus Phakopsora pachyrhizi, starts the leaf penetration by mechanical and enzymatic disruption of the cuticle (Edwards and Bonde 2011). Hence, one should wonder: Could the EWC damage promoted by Zn EDTA and Zn phosphite increase susceptibility to P. pachyrhizi? The present study highlights that investigations aiming at the development of novel foliar fertilizers should address the impacts of foliar fertilizer applications, the possible leaf stress, and the pathogen susceptibility.
The abaxial surface was chosen as an application site due to its presumed higher capacity to absorb nutrients. Preliminary XRF analysis indicated that Zn is more prone to be absorbed by the abaxial surface of soybean leaves than by the adaxial (data not shown here). Since the trichomes or stomata density seems to not affect Zn absorption (Li et al. 2017, 2018), the higher absorption of the abaxial surface could be related to its thinner cuticle and epidermal cell wall. Compared with the adaxial application, Zn concentration in tomato and citrus leaves was nearly 2-fold higher when Zn nitrate or Zn hydroxide nitrate was applied to the abaxial leaf surface (Du et al. 2015).
One of the reasons why Zn EDTA behaved differently as Zn phosphite might be their different capacities of leaf penetration. In solution, Zn phosphite dissociates, releasing Zn2+(aq) and presenting lower molecular weight than Zn EDTA, at least cuticular favored by smaller molecular size (Fernandez et al. 2017). In addition, acidity might influence the uptake, and the pH values were 2.0 and 6.2 for Zn phosphite and Zn EDTA, respectively. The more effective absorption of Zn2+ from Zn phosphite compared with the Zn EDTA agrees with previous reports. In fact, zinc-amino acid and ZnSO4 increased the Zn concentration in rice grains more consistently when compared with Zn EDTA and Zn citrate (Wei et al. 2012). Another study suggested that Fe EDTA reduced the size of aqueous pores from the leaf epidermis, decreasing the nutrient uptake (Schlegel et al. 2006). In pea leaves treated with Zn EDTA and ZnSO4, Zn was less absorbed as chelate than inorganic salt, whereas its translocation within the plant was higher with Zn EDTA (Ferrandon and Chamel 1988). Zn EDTA was also less mobile than ZnSO4 in wheat leaves (Doolette et al. 2018). Chelated or organically complexed Zn was also less absorbed than inorganic salts, as demonstrated in a study of Peryea (2006) who applied foliar fertilization in apple trees. Since part of the foliar-applied material inevitably falls on the ground, especially for crops with limited leaf area, one advantage that Zn EDTA might have over Zn phosphite is its higher mobility in soil, which leads to increased Zn uptake by roots (Gangloff et al. 2002).
Our study partially agrees with Doolette et al. that treated wheat leaves treated with Zn EDTA at 1000 mg L−1 Zn, their chemical speciation indicated that Zn EDTA was the dominant Zn species near the application point (from 66% to 60%). Their study did not measure the petiolule region; in some sites in the surrounding area of the application point, they found also Zn bonded to phytate, cysteine, and polygalacturonate (Doolette et al. 2018). In the present study, apparently all Zn was transported to the phloem as Zn EDTA. We employed that other four reference compounds were used to identify the chemical form of Zn in the petiolules from the Zn phosphite treatment. Figure S13 shows the XANES spectra recorded for Zn Malate (a), Zn sulfate (b), Zn oxalate (c), and Zn citrate (d), along with the spectra recorded for two plants that received Zn phosphite treatment. The features of the reference compounds spectra did not match with those observed for the samples. Although we the presence of these compounds were not found, one must keep in mind that in linear combination analysis of XAS, a fraction of Zn (less than 5%) would hardly be detectable, since the normalization itself can introduce errors in the order of 10%.
5 Conclusions
The combined absorption and transport rate for Zn phosphite were faster in comparison with Zn EDTA. Since both treatments promoted equivalent dissolution of leaf cuticle, the higher absorption-transport rate for Zn phosphite might be a consequence of the higher diffusion coefficient of the ionic Zn2+ forms from Zn phosphite. However, the accumulation of crystals from Zn phosphite fertilizer in the vicinity of stomata suggests the potential uptake of Zn by the stomata pathway, which could have accelerated the uptake. Of course other factors, such as ionic charge and pH, may also play a role on the absorption and transport velocity.
Inside the petiolule, the Zn supplied as Zn EDTA remained in its pristine form, whereas that from Zn phosphite was transformed and could not be identified. Hence, it is likely that Zn was loaded in the phloem as Zn EDTA; however, the mechanism that would allow a chelate to cross the cell membrane is not clear. Further studies using smaller X-ray beams in the nanometer range shall be performed to trace the chemical species along the Zn pathway. Additionally, it is still not clear in which moment, or tissue, the chelate is break down releasing the Zn2+ ions.
Finally, the present study draws the attention to the deleterious effects that foliar fertilizers may cause on the leaf cuticle. Hence, it would be keen to pursue the development of foliar fertilizers able to accomplish the task of nutrient supply while avoiding damages to leaf cuticle.
Data Availability
The raw data are available in the ESI zip file.
References
Avellan A, Yun J, Zhang YL, Spielman-Sun E, Unrine JM, Thieme J, Li JR, Lombi E, Bland G, Lowry GV (2019) Nanoparticle size and coating chemistry control foliar uptake pathways, translocation, and leaf-to-rhizosphere transport in wheat. ACS Nano 13:5291–5305. https://doi.org/10.1021/acsnano.8b09781
Aytaç S, Çirak C, Ozçelik H (2007) Foliar zinc application on yield and quality characters of soybean. Asian J Chem 19(3):2410–2418
Bala R, Kalia A, Dhaliwal SS (2019) Evaluation of efficacy of ZnO nanoparticles as remedial zinc nanofertilizer for rice. J Soil Sci Plant Nutr 19:379–389. https://doi.org/10.1007/s42729-019-00040-z
Brennan RF (1996) Effectiveness of different sources of manganese foliar sprays in alleviating manganese deficiency of Lupinus angustifolius L grown on manganese deficient soils in western Australia. J Plant Nutr 19:293–304. https://doi.org/10.1080/01904169609365123
Broadley M, Brown P, Cakmak I, Rengel Z, Zhao F (2012) Function of nutrients: micronutrients. In: Marschner P (ed) Marschner’s mineral nutrition of higher plants, 3rd edn. Academic Press, San Diego, pp 191–248
Burkhardt J (2010) Hygroscopic particles on leaves: nutrients or desiccants? Ecol Monogr 80:369–399. https://doi.org/10.1890/09-1988.1
Burkhardt J, Zinsmeister D, Grantz DA, Vidic S, Sutton MA, Hunsche M (2018) Pariyar S (2018) Camouflaged as degraded wax: hygroscopic aerosols contribute to leaf desiccation, tree mortality, and forest decline. Environ Res Lett 13:085001. https://doi.org/10.1088/1748-9326/aad346
Cakmak I, Kutman UB (2018) Agronomic biofortification of cereals with zinc: a review. Eur J Soil Sci 69:172–180. https://doi.org/10.1111/ejss.12437
Cakmak I, Kalayci M, Kaya Y, Torun AA, Aydin N, Wang Y, Arisoy Z, Erdem H, Yazici A, Gokmen O, Ozturk L, Horst WJ (2010) Biofortification and localization of zinc in wheat grain. J Agric Food Chem 58:9092–9102. https://doi.org/10.1021/jf101197h
Caulfield LE, Black RE (2004) Zinc deficiency. In: Ezzati M, Lopez AD, Rodgers AA, Murray CJL (eds) Comparative quantification of health risks: global and regional burden of disease attribution to selected major risk factors. World Health Organization, Geneva, pp 257–279
Doolette CL, Read TL, Li C, Scheckel KG, Donner E, Kopittke PM, Schjoerring JK, Lombi E (2018) Foliar application of zinc sulphate and zinc EDTA to wheat leaves: differences in mobility, distribution, and speciation. J Exp Bot 69:4469–4481. https://doi.org/10.1093/jxb/ery236
Du YM, Kopittke PM, Noller BN, James SA, Harris HH, Xu ZP, Li P, Mulligan DR, Huang LB (2015) In situ analysis of foliar zinc absorption and short-distance movement in fresh and hydrated leaves of tomato and citrus using synchrotron-based X-ray fluorescence microscopy. Ann Bot-London 115:41–53. https://doi.org/10.1093/aob/mcu212
Edwards HH, Bonde MR (2011) Penetration and establishment of Phakopsora pachyrhizi in soybean leaves as observed by transmission electron microscopy. Phytopathology 101(7):894–900. https://doi.org/10.1094/PHYTO-09-10-0248
Eichert T, Fernández V (2012) Uptake and release of elements by leaves and other aerial plant. In: Marschner P (ed) Marschner’s mineral nutrition of higher plants, 3rd edn. Academic Press, San Diego, pp 71–84
Eichert T, Goldbach HE (2008) Equivalent pore radii of hydrophilic foliar uptake routes in stomatous and astomatous leaf surfaces - further evidence for a stomatal pathway. Physiol Plant 132:491–502. https://doi.org/10.1111/j.1399-3054.2007.01023.x
Eichert T, Kurtz A, Steiner U, Goldbach HE (2008) Size exclusion limits and lateral heterogeneity of the stomatal foliar uptake pathway for aqueous solutes and water-suspended nanoparticles. Physiol Plant 134:151–160. https://doi.org/10.1111/j.1399-3054.2008.01135.x
Fernandez V, Brown PH (2013) From plant surface to plant metabolism: the uncertain fate of foliar-applied nutrients. Front Plant Sci 4:289. https://doi.org/10.3389/fpls.2013.00289
Fernandez V, Stiropoulos T, Brown PH (2013) Foliar fertilization: scientific principles and field pratices. International Fertilizer Industry Association. ISBN 979-10-92366-00-6
Fernandez V, Bahamonde HA, Peguero-Pina JJ, Gil-Pelegrin E, Sancho-Knapik D, Gil L, Goldbach HE, Eichert T (2017) Physico-chemical properties of plant cuticles and their functional and ecological significance. J Exp Bot 68:5293–5306. https://doi.org/10.1093/jxb/erx302
Ferrandon M, Chamel AR (1988) Cuticular retention, foliar absorption and translocation of Fe, Mn and Zn supplied in organic and inorganic form. J Plant Nutr 11:247–263. https://doi.org/10.1080/01904168809363800
Gangloff WJ, Westfall DG, Peterson GA, Mortvedt JJ (2002) Relative availability coefficients of organic and inorganic Zn fertilizers. J Plant Nutr 25:259–273. https://doi.org/10.1081/pln-100108834
Ghasal PC, Shivay YS, Pooniya V, Choudhary M, Verma RK (2017) Zinc accounting for different varieties of wheat (Triticum aestivum) under different source and methods of application. Indian J Agric Sci 87:1111–1116
Goodwin SM, Jenks M (2005) Plant cuticle function as a barrier to water loss. In: Jenks M, Hasegawa PM (eds) Plant abiotic stress. Blackwell Publishing, Oxford, pp 14–36
Gozzo F, Faoro F (2013) Systemic acquired resistance (50 years after discovery): moving from the lab to the field. J Agric Food Chem 61:12473–12491. https://doi.org/10.1021/jf404156x
Guilherme LRG, Corguinha APB, Souza GA, Sacco M, Menezes MD (2015) Zinc availability in Brazillian agroecosystems. Proceedings of the 4th International Zinc Symposium: Improving Crop Production and Human Health, São Paulo, Brazil, p 59–49
Kayan N, Gulmezoglu N, Kaya MD (2015) The optimum foliar zinc source and level for improving Zn content in seed of chickpea. Legume Res 38:826–831. https://doi.org/10.18805/lr.v38i6.6731
Kinaci E, Gulmezoglu N (2007) Grain yield and yield components of triticale upon application of different foliar fertilizers. Interciencia 32:624–628
Li C, Wang P, Menzies NW, Lombi E, Kopittke PM (2017) Effects of changes in leaf properties mediated by methyl jasmonate (MeJA) on foliar absorption of Zn, Mn and Fe, Ann. Botany 120(3):405–415. https://doi.org/10.1093/aob/mcx063
Li C, Wang P, Lombi E, Cheng M, Tang C, Howard DL, Menzies NW, Kopittke PM (2018) Absorption of foliar-applied Zn fertilizers by trichomes in soybean and tomato, J experimental Botany69(10). Issue 10(27):2717–2729. https://doi.org/10.1093/jxb/ery085
Lombi E, Scheckel KG, Kempson IM (2011b) In situ analysis of metal(loid)s in plants: state of the art and artefacts. Environ Exp Bot 72:3–17. https://doi.org/10.1016/j.envexpbot.2010.04.005
McDonald AE, Grant BR, Plaxton WC (2001) Phosphite (phosphorous acid): its relevance in the environment and agriculture and influence on plant phosphate starvation response. J Plant Nutr 24:1505–1519
Montalvo D, Degryse F, Silva RC, Baird R, Mclaughlin (2016) Chapter five - agronomic effectiveness of zinc sources as micronutrient fertilizers. Adv Agron 139:215–267. https://doi.org/10.1016/bs.agron.2016.05.004
Neinhuis C, Koch K, Barthlott W (2001) Movement and regeneration of epicuticular waxes through plant cuticles. Planta 213(3):427–434. https://doi.org/10.1007/s004250100530
Peryea FJ (2006) Phytoavailability of zinc in postbloom zinc sprays applied to ‘Golden Delicious’ apple trees. HortTechnology 16:60–65. https://doi.org/10.21273/HORTTECH.16.1.0060
Phattarakul N, Rerkasem B, Li LJ, Wu LH, Zou CQ, Ram H, Sohu VS, Kang BS, Surek H, Kalayci M, Yazici A, Zhang FS, Cakmak I (2012) Biofortification of rice grain with zinc through zinc fertilization in different countries. Plant Soil 361:131–141. https://doi.org/10.1007/s11104-012-1211-x
Ristic Z, Jenks MA (2002) Leaf cuticle and water loss in maize lines differing in dehydration avoidance. J Plant Physiol 159:645–651
Rogiers SY, Whitelaw-Weckert M, Radovanonic-Tesic M, Greer LA, White RG, Steel CC (2005) Effects of spray adjuvants on grape (Vitis vinifera) berry microflora, epicuticular wax and susceptibility to infection by Botrytis cinerea. Aust Plant Pathol 34:221–228. https://doi.org/10.1071/AP05031
Santos LR, Silva BRS, Pedron T, Batista BL, Lobato KS (2020) 24-Epibrassinolide improves root anatomy and antioxidant enzymes in soybean plants subjected to zinc stress. J Soil Sci Plant Nutr 20:105–124. https://doi.org/10.1007/s42729-019-00105-z
Scheckel KG, Lombi E, Rock SA, McLaughlin MJ (2004) In vivo synchrotron study of thallium speciation and compartmentation in lberis intermedia. Environ Sci Technol 38:5095–5100. https://doi.org/10.1021/es049569g
Schlegel TK, Schönherr J, Schreiber L (2006) Rates of foliar penetration of chelated Fe (III): role of light, stomata, species, and leaf age. J Agric Food Chem 54(18):6809–6813
Silva MLDS, Vitti GC, Trevizam AR (2014) Heavy metal toxicity in rice and soybean plants cultivated in contaminated soil. Rev Ceres 61:248–254. https://doi.org/10.1590/S0034-737X2014000200013
Singh P, Shukla AK, Behera SK, Pk T (2019) Zinc application enhances superoxide dismutase and carbonic anhydrase activities in zinc-efficient and zinc-inefficient wheat genotypes. J Soil Sci Plant Nutr 19:477–487. https://doi.org/10.1007/s42729-019-00038-7
Slaton NA, Norman RJ, Wilson CE (2005) Effect of zinc source and application time on zinc uptake and grain yield of flood-irrigated rice. Agron J 97:272–278. https://doi.org/10.2134/agronj2005.0272
Smith E, Kempson I, Juhasz AL, Weber J, Skinner WM, Grafe M (2009) Localization and speciation of arsenic and trace elements in rice tissues. Chemosphere 76:529–535. https://doi.org/10.1016/j.chemosphere.2009.03.010
Wei YY, Shohag MJI, Yang XE (2012) Biofortification and bioavailability of rice grain zinc as affected by different forms of foliar zinc fertilization. Plos One:7. https://doi.org/10.1371/journal.pone.0045428
Zou CQ, Zhang YQ, Rashid A, Ram H, Savasli E, Arisoy RZ, Ortiz-Monasterio I, Simunji S, Wang ZH, Sohu V, Hassan M, Kaya Y, Onder O, Lungu O, Mujahid MY, Joshi AK, Zelenskiy Y, Zhang FS, Cakmak I (2012) Biofortification of wheat with zinc through zinc fertilization in seven countries. Plant Soil 361:119–130. https://doi.org/10.1007/s11104-012-1369-2
Acknowledgments
The authors are grateful to Brazilian Synchrotron Light Source (LNLS) for providing beamtime at XRF beamline (proposals 20180650 & 20180167), to Dr. C.A. Perez for his assistance during beamtime, and to E.W. Kitajima (ESALQ-USP) for providing electron microscopy facilities. We also thank Prof. E.A.G. Zagatto for his invaluable contribution during the writing of the manuscript.
Code Availability
Not applicable.
Funding
This study was funded by São Paulo Research Foundation (FAPESP) under the grants 2015/19121-8, 2015/05942-0, and 2018/13401-7. Partial support by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001 is greatly appreciated.
Author information
Authors and Affiliations
Contributions
M. H. F. Gomes and B. A. Machado carried out plant growth, fertilizer application, and the XRF and XANES measurements. J. P. R. Marques was responsible for obtaining the SEM images. Data interpretation and discussion were carried out by M. H. F. Gomes, J. P. R. Marques, R. Otto, T. Eichert, and H. W. P. Carvalho. M. H. F. Gomes wrote the first manuscript draft which was reviewed and edited by the other authors.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 5956 kb)
Rights and permissions
About this article
Cite this article
Gomes, M.H.F., de Almeida Machado, B., Marques, J.P.R. et al. Foliar Application of Zn Phosphite and Zn EDTA in Soybean (Glycine max (L.) Merrill): In Vivo Investigations of Transport, Chemical Speciation, and Leaf Surface Changes. J Soil Sci Plant Nutr 20, 2731–2739 (2020). https://doi.org/10.1007/s42729-020-00338-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-020-00338-3