Abstract
Background
Increasing zinc (Zn) concentration of rice seed has potential benefits for human nutrition and health. Enhanced levels of Zn in grain also contributes greatly to crop production through better germination and seedling vigor of rice plants grown on soils with limited Zn supply.
Aims and methods
This study evaluated the effect of soil and/or foliar Zn fertilizer application on grain yield and grain Zn concentration of rice grown in 17 field trials conducted in 2008 to 2010 in China, India, Lao PDR, Thailand and Turkey on soils ranging in pH from 4.8 to 8.8 and DTPA- extractable Zn from 0.5 to 6.5 mg kg−1.
Results
Zinc fertilization had little effect on rice grain yield with the exception of increases of up to 10 % in some locations in China and India. As an average of all trials, Zn application increased grain yield by about 5 %. Grain Zn concentrations were, however, more effectively increased by Zn fertilization, especially with foliar Zn applications. On average, Zn concentration in brown rice (whole caryopsis with husk removed) was increased by 25 % and 32 % by foliar and foliar + soil Zn applications, respectively, and only 2.4 % by soil Zn application. The Zn concentration of un-husked rice (whole grain with husk), which was increased by 66 % by foliar Zn, showed a close association with the Zn in brown and white rice, indicating a possible penetration of Zn from the husk into the inner layers of the rice endosperm. Increase in grain Zn concentration by foliar Zn spray was significantly affected by the timing of the foliar application. More distinct increases in grain Zn by foliar Zn application were achieved when Zn was applied after flowering time, e.g., at early milk plus dough stages.
Conclusions
Foliar Zn spray offers a practical and useful means for an effective biofortification of rice grain with Zn. This practice consistently and significantly contributed to increases in grain Zn of rice irrespective of cultivars, environmental conditions and management practices in 5 different countries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Zinc (Zn) deficiency is one of the most critical global health problem, affecting nearly one-third of world population (Welch and Graham 2004; Hotz and Brown 2004). Low dietary Zn intake is considered to be the major reason for widespread occurrence of Zn deficiency in human populations, especially in developing countries. In South and Southeast Asia, over half a billion people are estimated to be at risk from inadequate Zn intake (Hotz and Brown 2004; Gibson et al. 2007), and the well-known high incidence and severity of childhood infectious diseases in those regions are commonly associated with Zn deficiency (Black et al. 2008). Rice, the main staple food of Asia, is inherently very low in Zn and its high consumption relative to other foods contributes to high incidence of Zn deficiency in human populations in Asia (Gibson et al. 2007; Stein et al. 2007).
Today, increasing grain Zn concentration of rice represents an important challenge to be met by using agricultural tools such as breeding and fertilization. Zinc concentrations of both polished and unpolished rice are inherently too low to meet human demands for Zn (Lee et al. 2009; Bouis and Welch 2010). The Zn concentration of rice germplasm at the International Rice Research Institute, which included advanced breeding lines, modern and traditional varieties and grown at Los Baños, Philippines averaged only 25.4 mg Zn kg−1 for brown rice, compared with an average of 35.0 mg Zn kg−1 for wheat germplasm of the International Maize and Wheat Improvement Center grown at El Batan in Mexico (Graham et al. 1999). In a screening study including about 1,000 genotypes, it has been found that there is a four-fold range of rice grain Zn concentration among the rice genotypes (e.g., 13.5–58.4 mg Zn kg−1) (Graham et al. 1999). This impressive genotypic variation has led to a suggestion that such substantial genetic potential for Zn concentration in rice should be exploited through plant breeding (Welch and Graham 2004; Bouis and Welch 2010). Raising the Zn concentration of rice appears to be one of the most cost effective means to overcome this malnutrition problem among low income rice eaters. In addition, sowing seeds containing high Zn concentration has a potential to benefit crop growth and yield by improving germination and seedling vigor, especially in Zn deficient soils (Cakmak 2008; Rengel and Graham 1995; Yilmaz et al. 1998). For example, in the US Zn coated rice seed (with 1.0–4.7 g Zn kg−1) germinated better and more rapidly into seedlings with longer roots and better shoot growth than untreated seed (Slaton et al. 2001).
In recent years, a considerable progress has been made on the impact of foliar Zn fertilization on biofortification of cereal grains with Zn, particularly in wheat (Cakmak 2008; Peck et al. 2008; Wissuwa et al. 2008; Zhang et al. 2010). In wheat, foliar Zn spray, especially at later growth stages (e.g., early milk stage and dough stage), was very effective in increasing Zn concentration of both whole grain and also in the endosperm fraction, while soil Zn applications remained less effective (Cakmak et al. 2010). Similarly in rice grown in Philippines, soil application of Zn had very little increasing effect on Zn concentrations of brown rice while foliar Zn application caused greater increases (Wissuwa et al. 2008). To our knowledge, there is very little published data on the effectiveness of foliar Zn applications on Zn concentrations of un-husked or brown or white rice. Regarding the role of timing of foliar Zn spray in increasing grain Zn there is also no published data for rice. Very recently, Boonchauy et al. showed that applying Zn to foliar after flowering time caused more increases in grain Zn when compared to the applications realized before flowering in rice plants grown under field conditions (PLSO9297paper is under review). Spraying Zn at heading stage to rice improved Zn concentrations of white rice in a single trial in China (Fang et al. 2008).
When same rice varieties were grown on different soils, up to 90 % difference was observed in their grain Zn concentration (Graham et al. 1999). Wissuwa et al. (2008) also showed a significant variation in grain Zn concentration (e.g., from 8 to 47 mg kg−1) for a given rice genotype when grown on different soil types. These results indicate that environmental conditions have significant impact on grain Zn concentrations. It would be interesting to study how soil and/or foliar Zn applications affect grain Zn accumulation under different soil conditions. In this study, field experiments have been conducted on rice grown in different locations of China, India, Lao PDR, Thailand and Turkey to study the effect of individual or combined applications of soil and foliar Zn treatments on grain Zn concentration and grain yield over 2 years. In one experiment, the effect of timing of foliar Zn spray on grain Zn accumulation has been also examined. To our knowledge, this is the first study demonstrating the impact of soil and/or foliar Zn applications on Zn concentrations in un-husked, brown and white rice grown in different countries with diverse soil conditions.
Materials and methods
Rice (Oryza sativa L.) plants were grown in five different countries, namely, China, India, Lao PDR, Thailand and Turkey over 2 years (Fig. 1). The names of the locations and rice cultivars used in the trials are presented in Table 1. The rice cultivars used in the trials were commonly grown by farmers, except IR68144 which was included in one trial in Thailand (Table 1). Experimental design was randomized complete block design with 4 to 6 replicates. Plants were gown under 4 different Zn applications as follows: i) nil (no Zn treatment), ii) soil Zn application, iii) foliar Zn application and iv) soil + foliar combined Zn application. The soil Zn treatment consisted of 50 kg ZnSO4.7H2O ha−1 applied to the soil and incorporated into top 15–20 cm soil before transplanting. In the case of the foliar Zn treatment, a 0.5 % (w/v) aqueous solution of ZnSO4.7H2O was sprayed on the plants until the solution started to run-off from the leaves. The foliar Zn was applied twice, first one at panicle initiation and the second 1 week after flowering. The soil and foliar treatments were combined to give the soil + foliar Zn treatment. Generally 500 to 600 l of the 0.5 % (w/v) aqueous solution of ZnSO4.7H2O was applied per ha in the foliar Zn treatment. The soil at different locations had considerable variation for soil pH (ranging from acidic to alkaline, pH 4.8 to 8.8); DPTA extractable Zn (ranging from 0.5 to 6.5 mg Zn kg−1) and other fertility characteristics (Table 2). Different rates of basal NPK fertilizers were also applied to the different trials in those countries based on their locally recommended management practices (Table 3).
Before the first foliar Zn application, samples were taken from the youngest emerged leaf blades for Zn analysis. At maturity, grain yield (14 % moisture) was determined from 6 to 10 m2 internal area of each plot. Zinc concentration of brown rice was determined in samples from all trials. Grain samples from China from 2008 and from Lao PDR, Thailand and Turkey in both 2008 and 2009 were sub-sampled for the Zn analysis in un-husked caryopsis with embryo and pericarp intact. Samples from Turkey collected in 2008 and 2009 and from Lao PDR and Thailand in 2009 were also analyzed for Zn concentration in white rice in which the outer layers of the caryopsis including pericarp, testa, nucella and part of the aleurone layer along with the embryo were removed by laboratory milling machine.
An additional experiment was conducted in Turkey in 2009 to determine the effect of timing of foliar Zn application on Zn concentrations of un-husked rice, brown rice and white rice. The foliar Zn treatments were applied twice at the following growth stages i) stem elongation plus booting stages, ii) booting plus milk stages, and iii) milk plus dough stages, in comparison with nil Zn treatment. At maturity, grain yield and Zn concentration in un-husked rice, brown rice and white rice were determined. This experiment was established in a randomized complete block design with 4 replications and conducted as described above in terms of foliar Zn applications.
Samples of un-husked (whole grain with husk), brown (whole caryopsis with husk removed by hand) and white (outer layers of the caryopsis including pericarp, testa, nucella and part of the aleurone layer along with the embryo were removed, by polishing for 30 s in standard laboratory mill) rice were rinsed with distilled de-ionized (DDI) water, dried with tissue papers, oven dried at 45 °C. The dried samples were then subjected to acid-digestion in a closed-vessel microwave system (MarsExpress; CEM Corp., Matthews, NC, USA), and analyzed for Zn and also P (phosphorous) by inductively coupled plasma optical emission spectrometry (ICP-OES) (Vista-Pro Axial; Varian Pty Ltd, Mulgrave, Australia). Measurement of mineral nutrients was checked by using certified standard reference materials which were obtained from the National Institute of Standards and Technology (Gaithersburg, MD, USA).
Results
At nil Zn treatment, rice grain yield in the different trials conducted in the 5 countries ranged from 3.3 t ha−1 in Lao PDR to 10.1 t ha−1 in China (Table 4). Except for occasional and moderate yield increases of up to 10 % in China and India, the Zn fertilizer treatments had generally little effect on rice grain yield. As average of 17 trials, soil Zn application increased grain yield by about 5 % (Table 4). Soil Zn application had also little effect on grain Zn. By contrast, foliar Zn application consistently increased Zn concentrations in all trials. In case of un-husked rice, foliar Zn application increased Zn concentration by about 66 %, but soil Zn application had only little effect (Table 5). Applying Zn to the soil together with foliar Zn had the same effect on Zn concentration of un-husked rice as foliar Zn treatment alone. The Zn concentrations of brown (Table 6) and white rice (Table 7) were also increased by foliar Zn, but very little by soil Zn application. Average increase in Zn concentration by foliar Zn spray was about 25 % in brown rice, and 10 % in white rice. Combination of soil and foliar Zn applications resulted in further increase in Zn concentrations of brown (about 32 %) and white rice (about 15 %) (Tables 6 and 7). As presented in Fig. 2, Zn concentrations of brown and white rice were increasing linearly with increasing Zn in un-husked rice. Furthermore, the Zn concentration of both brown and white rice also increased linearly with increasing P concentration in the flag leaf and in brown rice (Fig. 3). The regression function for Zn concentration remained strongly significant (P < 0.001) for both brown rice (1) and white rice (2), when P concentration in un-husked rice was included as another independent variable along with the un-husked rice Zn.
Relationship between Zn concentration in brown rice and flag leaf P (upper left) and brown rice P (upper right) concentration; and between Zn concentration in white rice and flag leaf P (lower left) and brown rice P (lower right) concentration (Significance of linear regressions by f-test: **, P < 0.01; ***, P < 0.001)
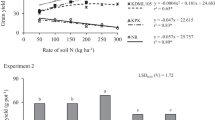
Timing of the foliar Zn application was crucial to its effectiveness in increasing grain Zn concentration (Fig. 4). Foliar Zn applications which had no significant effect on rice grain yield resulted in a marginal effect on rice grain Zn when applied at stem elongation plus booting stage. Major increases in grain Zn concentrations (up to 2-fold in un-husked rice and about 30 % in brown and white rice) were achieved when one or both of the foliar Zn applications were realized when the grain had reached milk stage. Most probably, the large increases in Zn concentrations of un-husked rice might be also related to Zn contamination through foliar spray of Zn.
Rice grain yield (with error bars, left) and Zn concentration (with LSD
P < 0.05, right) in un-husked, brown and white rice as affected by foliar Zn treatments realized at i) stem elongation plus booting stage ( ); ii) at milk plus dough stages (
); ii) at milk plus dough stages ( ) and at booting plus milk stage (
) and at booting plus milk stage ( ). (LSD bars are for comparison of Zn treatments within each category of rice)
). (LSD bars are for comparison of Zn treatments within each category of rice)
Discussion
The level of the DTPA extractable Zn in soil considered to be critical for Zn deficiency for rice is 0.5–0.8 mg Zn kg−1 (Randhawa et al. 1978; Sims and Johnson 1991). The available Zn level in soils of the trials (Table 2) did not appear to be limiting yield, except in China. Despite very high level of DTPA extractable Zn in the Zhejiang location (Table 2), soil and foliar Zn applications caused significant increases in grain yield although the increases were relatively small (3 % to 13 %). The reason for such increases in grain yield by Zn applications despite high DTPA-Zn levels could not be understood, and might be related to higher yield values in the Zhejiang location and thus dilution of Zn in the tissue (Table 4). A possible contribution of S to the observed increases in grain Zn by ZnSO4 application can be excluded because the plants with and without Zn applications had sufficiently high S concentrations in leaves (no data shown). The effectiveness of foliar Zn application in increasing Zn concentration in rice grain was independent of the rice grain yield, which ranged from 3.3 t ha−1 to 11.4 t ha−1. The positive impact of foliar Zn application on grain Zn was consistent over all 17 trials in 2008 to 2010 in 5 different countries with diverse rice cultivars and soil and management conditions. On average, Zn concentration in brown rice was increased by 25 % and 32 % by foliar and foliar + soil Zn applications, respectively, while there was only 2.4 % increase with soil Zn application. In some locations in India and Thailand, foliar Zn application increased brown rice Zn by about 60 % (Table 6). The effectiveness of foliar Zn application on grain Zn varied substantially between years for a given genotype, indicating important role of environmental conditions on seed deposition of foliarly applied Zn. There were also some genotypes which showed very poor response to foliar Zn application such as IR68144 in Thailand. It seems that impact of foliar Zn application on grain Zn can be maximized by selecting genotypes with high ability in leaf absorption and seed deposition of foliarly applied Zn. Field tests in Philippines showed that rice genotypes differ greatly in their response to foliar Zn treatments in terms of increases in grain Zn (Wissuwa et al. 2008).
In addition, when planted on different soils in Chiang Mai (with lower pH, higher DTPA Zn and available P) and Takli (with higher pH, lower DTPA Zn and available P) the variety CNT1 showed large variation in grain Zn concentration (Tables 5 and 6). The lower grain Zn concentration of CNT1 at Takli could not be explained by a dilution effect as it was associated with lower grain yield. Similar findings on variation in grain Zn concentration when the same rice varieties were grown on different soils have been previously reported (Graham et al. 1999; Wissuwa et al. 2008). Stability of a trait over different locations is desirable and an important issue in breeding programs, especially for grain micronutrient concentrations (Pfeiffer and McClafferty 2007). When a highly promising genotype for grain Zn is identified, a special attention should be paid to how the grain Zn concentration of that genotype vary with different soil and environmental conditions.
As shown in wheat (Rengel and Graham 1995; Yilmaz et al. 1998), the higher Zn in un-husked rice should have positive impacts on germination, seedling growth and field establishment, especially on soils where Zn availability is limited. Indeed, increasing Zn supply to the germinating rice seed by coating the seed with Zn before sowing improved germination and crop establishment by improving Zn nutrition of the seedlings (Slaton et al. 2001 and 2005). Thus, increasing grain Zn concentration by Zn fertilization is important not only for human nutrition, but also for the agronomic performance of seeds or seedlings on potentially Zn deficient soil conditions.
The close association between Zn concentration in un-husked rice and the Zn in brown and white rice (Fig. 2) indicated that the foliar applied Zn is probably penetrated into the inner layers of the rice endosperm. Timing of the foliar Zn application seems to be a crucial issue in biofortification of rice grain with Zn. The Zn concentration of un-husked, brown and white rice were all increased much more markedly by the foliar Zn applications made at milk stage while only minimal increases in grain Zn were found when Zn applications were made at tillering and booting stages (Fig. 4). A similar result has been also found very recently in rice grown in Thailand (PLSO9297paper is under review). In a recently published study, a similar result has been shown in wheat plants grown under field conditions in Turkey. Higher increase in grain Zn concentration by foliar application of Zn in the late compared with the early growth stages was also shown in wheat plants (Cakmak et al. 2010).
The significance of the multiple regression functions for Zn concentration in both brown and white rice with P as well as Zn concentration in un-husked rice (P < 0.001) and the linear regressions between Zn concentration in brown and white rice and P concentration in flag leaf and in brown rice (Fig. 3) suggested that the increase in brown and white rice Zn by foliar Zn application may be more than simple physical accretion of the applied Zn on to rice grain. In wheat, it has been shown that grain Zn concentration was increased by application of high rate of N fertilizer, and wheat grain Zn was positively associated with grain protein (Kutman et al. 2011a, b). In rice, while the pattern of protein bodies in the endosperm, the embryo and bran fraction of different rice varieties closely resembled their N concentration, Fe and Zn were only detected in those protein bodies with phytate inclusions (Prom-u-thai et al. 2008). The close associations between the seed concentrations of Zn, Fe, P and N were also found in different cereal germplasms such as wheat, spelt wheat and wild wheat (Gomez-Becerra et al. 2010a, b; Morgounov et al. 2006; Zhao et al. 2009). Lott and Spitzer (1980) showed that in wheat seeds Zn and Fe are mainly associated with both phytate and protein. These results together with results presented here indicate that higher levels of P and N in seeds might be an important sink for the seed transportation of Fe and Zn derived from foliarly applied fertilizers or from the stem and leaf tissues. In recent publication, by using novel analytical methods, Persson et al. (2009) demonstrated that in barley seed Fe and Zn have, however, different speciation and bindings. Based on the results reported for rice, Fe appears to be associated mainly with phytate while Zn is speciated with peptides (Persson et al. 2009). The relationships between P nutrition and seed accumulation of Zn and Fe needs to be further studied.
In conclusion, foliar Zn application to rice during grain formation represents an effective agronomic practice to contribute to the daily Zn intake of rice eaters. Foliar sprayed Zn significantly increased Zn concentrations of both white and brown rice as well as in un-husked rice, and this positive impact of foliar Zn application occurred consistently over a wide range of environments and local management practices, including different rice varieties. It needs to be further explored how wide the window for further increases in grain Zn is. Probably, by modifying the rates, form and timing of foliarly applied Zn, further increases in grain Zn can be achieved resulting in higher nutritional impacts. Related experiments are in progress.
References
Black RE, Lindsay HA, Bhutta ZA, Caulfield LE, De Onnis M, Ezzati M, Mathers C, Rivera J (2008) Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 371:243–260
Bouis HE, Welch RM (2010) Biofortification- a sustainable agricultural strategy for reducing micronutrient malnutrition in the global South. Crop Sci 50:20–32
Cakmak I (2008) Enrichment of cereal grains with zinc: agronomic or genetic biofortification? Plant Soil 302:1–17
Cakmak I, Kalayci M, Kaya Y, Torun AA, Aydin N, Wang Y, Arisoy Z, Erdem H, Gokmen O, Ozturk L, Horst WJ (2010) Biofortification and localization of zinc in wheat grain. J Agric Food Chem 58:9092–9102
Fang Y, Wang L, Xin Z, Zhao L, An X, Hu Q (2008) Effect of foliar application of zinc, selenium, and iron fertilizers on nutrients concentration and yield of rice grain in China. J Agric Food Chem 56:2079–2084
Gibson RS, Manger MS, Krittaphol W, Pongcharoen T, Gowachirapant S, Bailey KB, Winichagoon P (2007) Does zinc deficiency play a role in stunting among primary school children in NE Thailand? Br J Nutr 97:167–175
Gomez-Becerra HF, Abugalieva A, Morgounov A, Abdullayev K, Bekenova L, Yessimbekova M, Sereda G, Shpigun S, Tsygankov V, Zelenskiy Y, Pena RJ, Cakmak I (2010a) Phenotypic correlations, G x E interactions and broad sense heritability analysis of grain and flour quality characteristics in high latitude spring bread wheats from Kazakhstan and Siberia. Euphytica 171:23–38
Gomez-Becerra HF, Yazici A, Ozturk L, Budak H, Peleg Z, Morgounov A, Fahima T, Saranga Y, Cakmak I (2010b) Genetic variation and environmental stability of grain mineral nutrient concentrations in Triticum dicoccoides under five environments. Euphytica 171:39–52
Graham RD, Senadhira D, Beebe S, Iglesias C, Monasterio I (1999) Breeding for micronutrient density in edible portions of staple food crops: conventional approaches. Field Crop Res 60:57–80
Hotz C, Brown KH (2004) Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr Bull 25:94–204
Kutman UB, Yildiz B, Cakmak I (2011a) Improved nitrogen status enhances zinc and iron concentrations both in the whole grain and the endosperm fraction of wheat. J Cereal Sci 53:118–125
Kutman UB, Yildiz B, Cakmak I (2011b) Effect of nitrogen on uptake, remobilization, and partitioning of zinc and iron throughout the development of durum wheat. Plant Soil 342:149–164
Lee S, Jeon US, Lee SJ, Kim YK, Persson DP, Husted S, Schjoerring JK, Kakei Y, Masuda H, Nishizawa NK, An G (2009) Iron fortification of rice through activation of the nicotianamine synthase gene. Proc Natl Acad Sci U S A 106:22014–22019
Lott JNA, Spitzer E (1980) X-ray analysis studies of elements stored in protein body globoid crystals of Triticum grains. Plant Physiol 66:494–499
Morgounov A, Gomez-Becerra HF, Abugalieva A, Dzhunusova D, Yessimbekova M, Muminjanov H, Zelenskiy Y, Ozturk L, Cakmak I (2006) Iron and zinc grain density in common wheat grown in Central Asia. Euphytica 155:193–203
Peck AW, McDonald GK, Graham RD (2008) Zinc nutrition influences the protein composition of flour in bread wheat (Triticum aestivum L.). J Cereal Sci 47:266–274
Persson DP, Hansen TH, Laursen KH, Schjoerring JK, Husted S (2009) Simultaneous iron, zinc, sulfur and phosphorus speciation analysis of barley grain tissues using SEC-ICP-MS and IP-ICP-MS. Metallomics 1:418–426
Pfeiffer WH, McClafferty B (2007) Biofortification: breeding micronutrient-dense crops. In: Kang MS, Priyadarshan PM (eds) Breeding major food staples. Blackwell Science, New York, pp 61–91
Prom-u-thai C, Huang L, Rerkasem B, Thomson G, Kuo J, Saunders M, Dell B (2008) The distribution of protein bodies and phytate rich inclusions in grain tissues in relation to iron density in low and high Fe rice genotypes. Cereal Chem 85:257–265
Randhawa NS, Sinha MK, Takkar PN (1978) Micronutrients. In: Soils and rice, International Rice Research Institute, pp. 581–603
Rengel Z, Graham RD (1995) Importance of seed zinc content for wheat growth on zinc deficient soil. I. vegetative growth. Plant Soil 173:259–266
Sims JT, Johnson GV (1991) Micronutrient soil tests. In: Mortvedt JJ, Cox FR, Shuman LM, Welch RM (eds) Micronutrients in agriculture: second edition. Soil Science Society of America Book Series. Soil Science Society of America, Inc, Madison, pp 427–476
Slaton NA, Wilson CE Jr, Ntamatungiro S, Norman RJ, Boothe DL (2001) Evaluation of zinc seed treatments for rice. Agron J 93:157–163
Slaton NA, Norman RJ, Wilson CE Jr (2005) Effect of zinc source and application time on zinc uptake and grain yield of flood-irrigated rice. Agron J 97:272–278
Stein AJ, Nestel P, Meenakshi JV, Waim M, Sachdev HPS, Bhutta ZA (2007) Plant breeding to control zinc deficiency in India: how cost-effective is biofortification? Publ Health Nutr 10:492–501
Welch RM, Graham RD (2004) Breeding crops for enhanced micronutrient content. Plant Soil 245:205–214
Wissuwa M, Ismail AM, Graham RD (2008) Rice grain zinc concentrations as affected by genotype, native soil-zinc availability, and zinc fertilization. Plant Soil 306:37–48
Yilmaz A, Ekiz H, Torun B, Gültekin I, Karanlik S, Bagci SA, Cakmak I (1998) Effect of different zinc application methods on grain yield and zinc concentration in wheat grown on zinc-deficient calcareous soils in Central Anatolia. J Plant Nutr 20:461–471
Zhang YQ, Shi RL, Karim MR, Zhang FS, Zou CQ (2010) Iron and zinc concentrations in grain and flour of winter wheat as affected by foliar application. J Agric Food Chem 58:12268–12274
Zhao FJ, Su YH, Dunham SJ, Rakszegi M, Bedo Z, McGrath SP, Shewry PR (2009) Variation in mineral micronutrient concentrations in grain of wheat lines of diverse origin. J Cereal Sci 49:290–295
Acknowledgments
This study was financially supported by the HarvestPlus Program (www.harvestplus.org) and the sponsors of the HarvestPlus Global Zinc Fertilizer Project-I. Phase (www.harvestzinc.org) including Mosaic Company, K + S Kali GmbH, International Zinc Association, Omex Agrifluids, International Fertilizer Industry Association and International Plant Nutrition Institute.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Hans Lambers.
Rights and permissions
About this article
Cite this article
Phattarakul, N., Rerkasem, B., Li, L.J. et al. Biofortification of rice grain with zinc through zinc fertilization in different countries. Plant Soil 361, 131–141 (2012). https://doi.org/10.1007/s11104-012-1211-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-012-1211-x