Abstract
Zinc (Zn) possesses nutritional importance for humans, animals, and plants, making it a crucial element in their dietary requirements. In the current study, the effect of zinc oxide nanoparticles (ZnONPs) solution at four different concentrations (0, 0.5, 1.0 and 5.0 g/L) at 20-day interval on pea plants grown in Zn-deficient soil was assessed for remediation of Zn deficiency and enhanced Zn fortification. Zinc oxide nanoparticles were synthesized by using sol-gel method and characterized by UV-Vis spectroscopy, Fourier transform infrared (FTIR), Scanning Electron Microscopy (SEM), and X-Ray diffraction (XRD) and EDX pattern. The soil samples were analysed for microbial counts, chemical properties, dehydrogenase activity and vegetative characteristics, nutrient profile, and yield parameters according to their respective methods. The change of solution colour to off-white confirmed the synthesis of ZnONPs. ZnONPs were characterized by UV-Vis spectroscopy with a broad peak at 380 nm. The presence of NH/OH, C-H, C-C, C-O, C-N, Cl-C-O functional groups were confirmed by FTIR spectrum. The crystalline structure with hexagonal arrangements was described by the XRD pattern. The EDX pattern of ZnONPs showed the zinc composition as 45.9% and oxygen was 54.05%. The SEM images showed that the size of ZnONPs was of 37 nm. The application of ZnONPs at a concentration of 5.0 g/L significantly improved the growth and yield parameters. However, the highest value for root characteristics was attained with the application of ZnONPs at a concentration of 1.0 g/L. The microbial soil counts and enzyme activities such as viable cell counts, and dehydrogenase activity was highest at 5.0 g/L ZnONPs treatment. The treatment of ZnONPs successfully reverted the symptoms of Zn-deficiency besides the improvement of the Zn content of plant, although the response was concentration dependent. These findings indicate that ZnONPs can be effectively used for remediation and Zn fortification in pea plants cultivated under low soil Zn concentrations. The present study emphasizes the potential of ZnONPs to address micronutrient deficiencies, promote crop growth, and enhance soil health, offering a sustainable and controlled approach to zinc applications in agriculture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In the quest of sustainable agricultural practices, the significance of fertilizers in achieving optimal crop yield and quality cannot be neglected. As global population is increasing day by day, the demand for food production poses pressure on conventional farming methods. Consequently, there is a growing need for innovative and eco-friendly approaches to enhance crop nutrition without compromising natural environment.
Pea plants are well known for their versatile nutritional values and the best example of how the health of the soil and the growth of crops are closely connected.
Peas are rich in protein, starch, fiber, and micronutrients, making them a beneficial addition to a healthy diet with numerous health-promoting advantages. Additionally, peas contain various bioactive compounds such as enzyme inhibitors, lectins, phytic acid, phenolics, and oligosaccharides (Wu et al. 2023). As leguminous plants, they are not only a staple in diets worldwide but also contribute to sustainable farming practices through their ability to fix atmospheric nitrogen. However, like many crops, peas face challenges related to nutrient deficiencies, impacting both yield and nutritional content (Quintieri et al. 2023; Saberi et al. 2023).
Zinc (Zn) deficiency is one of the most worldwide disorders of micronutrient (Zeng et al. 2021) in plants. The proper growth of plants is dependent on Zn element and its importance can be attributed to its major role in various biochemical, metabolic, and physiological processes. These include the necessity for the biogenic machinery of respiratory system, proteins such as cytochrome, structural proteins, nucleotides, proper gene expression, enzymes’ activation, chlorophyll production, maintenance of membrane activity, and enhanced maturation of seeds and stalks (Umair et al., 2020). Additionally, for all major six classes of enzymes, it serves as a cofactor for more than 300 enzymes. Furthermore, in plant zinc plays a central role in detoxification mechanisms, specifically in pathways related to reactive oxygen species (ROS) (Rudani et al. 2018). Therefore, Zn is a vital micronutrient for the optimal growth and development of crop plants and is also essential for their overall health.
Zn concentration in soil has been suggested to improve plant’s growth and yield. Increasing zinc levels up to 60 mg, Zn is beneficial for both agronomy and human health. These strategies include traditional breeding method, fertilizer management, seed priming, and fortification (Rashid et al., 2019). The content of zinc availability changes according to the type of soil. The key factors influencing the Zn availability are high pH carbonate content and low redox potential. Therefore, ZnONPs, a product of an interdisciplinary field of nanotechnology emerging as positive alternative for remediating zinc deficiency in fertilizers.
The ZnONPs application is expected to effectively supply zinc to plants, specific to their small size, easy solubility, diffusible nature, and easy solubility facilitating plant for rapid and complete absorption. In crop plants, it addresses the nutritional needs and deficiencies. Moreover, ZnONPs are taken up more quickly by plants for better growth of plants (Al-Jabri et al., 2022). The reduced hydrophilicity and enhanced dispersibility of ZnONPs in lipophilic substances may improve their ability to penetrate the surface of leaf and release ions across cuticle, surpassing water-soluble ions (Reshma and Meenal 2022). These unique properties of ZnONPs are expected to contribute the higher yield in crops. In the field of agricultural research, the use of nanotechnology, particularly Zinc Oxide Nanoparticles is emerging as a novel approach to address challenges associated with zinc deficiency in soil and enhance crop yield. This study explores innovative approaches for sustainable zinc nutrition and crop yield enhancement in pea plants using ZnONPs. Unlike traditional methods that rely heavily on chemical fertilizers, our research emphasizes sustainable agricultural practices by exploring the unique properties of ZnONPs to improve plant growth and soil health.
Therefore, the present study aimed to analyse the effect of Zinc Oxide Nanoparticles (ZnONPs) as a non-fertilizer on the growth of pea plants. Additionally, it aimed to investigate the impact of varying concentrations of ZnONPs on growth parameters, such as plant height, leaf area, and biomass accumulation, as well as physiological and biochemical responses, including chlorophyll content, photosynthetic efficiency, and antioxidant enzyme activities, under zinc-deficient conditions. The core objective was to develop innovative and sustainable strategies to improve zinc nutrition and enhance crop yield in pea plants, promoting food security and agricultural sustainability.
2 Materials and methods
The experiment of greenhouse pot was conducted at Department of Biological Science, International Islamic University, Islamabad, Pakistan. The initial Zn-deficient soil was collected from National Agricultural Research Center, Islamabad. The initial biological and chemical characteristics of sol are outlined in Table 1. The pea plants were grown in Zn-deficient soil in shallow trays for the period of 3 weeks. Following this, one plant per pot was transplanted in 1 kg of zinc deficient soil and cultured until the onset of typical symptoms associated with Zn deficiency, characterized by leaf browning. After the appearance of symptoms, liquid ZnONPs were introduced in the soil with four different concentrations, i.e., 0, 0.5, 1.0, and 5.0 g/L at 10-day intervals. Each experiment was conducted in triplicates.
2.1 The Chemical Properties of Soil
The assessment of chemical properties for the experimental soil was conducted at 0 and 40 days after treatment (DAT) (Table 1). Soil organic carbon was determined using the rapid titration method (wet digestion) by Sato et al. 2014. Beckmen glass electrode pH meter was used to measure pH and electrical conductivity of soil. Macronutrients like nitrogen (N) were examined by alkaline permanganate method, phosphorus availability was determined through ascorbic acid reduction method (Nisab et al. 2020), and available potassium using a flame photometer with neutral ammonium acetate as an extractant. Micronutrient content in the soil, including Fe, Mn, Zn, and Cu metals, was extracted through DTPA method (Dhaliwal et al. 2021) and quantified using atomic absorption spectroscopy (AAS).
2.2 Synthesis of Zinc Oxide Nanoparticles
ZnONPs were synthesized following the methodology of (Zhou et al. 2023) by using a sol-gel method. 8 g of zinc acetate was mixed with 15 ml of distilled water and stirred for five minutes. 32 g of sodium hydroxide was added into mixture of water and zinc acetate and stirred again for five minutes on a magnetic stirrer. Subsequently, 100 ml of ethanol was titrated drop by drop into the solution of zinc acetate and sodium hydroxide, which resulted in the formation of white precipitates. The solution was centrifuged for 30 min at 300rmp, supernatant was removed, and nanoparticles were collected from the bottom. The nanoparticles were washed three times with distilled water to remove by-products. The nanoparticles were dried at 80 °C overnight resulted in white fine powder and utilized as a nano-fertilizer for the growth of pea plants.
2.3 Characterization of Zinc Oxide Nanoparticles
2.3.1 UV-vis Spectroscopy
UV-Visible Spectrophotometer was used for the characterization of zinc oxide nanoparticles (Perkin Elmer, Lambda 35, Germany). A small amount of zinc oxide nanoparticles was mixed the ethanol solvent for the preparation of sample. The absorbance of ZnONPs was evaluated at wavelength ranges from 300 to 400 nm.
2.3.2 Fourier Transform Infrared Spectroscopy (FTIR)
FTIR spectrometer 1760X (PerkinElmer) with infrared radiation was used for FTIR analysis at room temperature for 1 h. The FTIR was recorded from 400 to 4000 cm− 1 with a resolution of 2 cm− 1. FTIR analysis was used to evaluate the existence of functional groups of synthesized ZnONPs.
2.3.3 X-Ray Diffraction (XRD)
The crystalline structure of zinc oxide nanoparticles was observed using XRD equipped with Cu and Kα radiation source (λ = 1.540562 Å) for 2 h at 30-40kv and 15 mA on 20 scales with a step size of 0.02 degree.
2.3.4 Scanning Electron Microscopy (SEM)
The morphological characteristics and dimensions of ZnONPs were obtained using high vacuum scanning electron microscopy (JEOL JSM 5910 LV) with voltage ranging from 15 to 200 kV and a resolution of 2.4 Å. The ZnONPs sample was affixed to copper adhesive tape for the analysis.
2.3.5 Energy Dispersive Spectroscopy (EDS)
SEM did the elemental analysis with EDS for analysing the metal composition of zin oxide nanoparticles. The exact design of zinc oxide nanoparticles with other elements like impurities was also confirmed by EDS.
2.4 Soil Microbial Properties
The soil microbial properties were analysed according to the methodology of Bala et al. 2019. The quantification of soil microorganisms’ viability involved the analysis of total aerobic bacteria on Nutrient agar, differential counting of pseudomonads on King’s B agar, assessment of actinobacteria on Actinomycetes agar, and enumeration of rhizobia on CR-YEM agar. The quantification of total fungi was conducted on PDA using serial dilution spread technique at regular intervals (0, 10, 20, and 40-days post-transplantation) before the application of ZnONPs. The calculation of soil dehydrogenase enzyme activity was followed according to the methodology by Meena and Rao in 2021. For microbial respiration analysis, soil samples were incubated in Erlenmeyer flasks, each containing 50 g of soil. A 15 ml screwcap vial with cork cap containing 5 ml of KOH solution (0.2 mol/L) was incubated in dark at 25 ± 2 °C, and experiments were conducted in triplicates. After 24 h, the KOH solutions were extracted and analysed for CO2 using the titration method, as described by Li et al. in 2008. The activities of dehydrogenase enzyme and soil microbial respiration were recorded at the initial stage, as well as 20 and 40 days after transplantation, prior to the application of ZnONPs suspensions.
2.5 Photosynthetic Parameters
Chlorophyll measurements were conducted at the midpoint of every leaf blade using a chlorophyll meter (SPAD-502, Minolta Camera Co. Osaka, Japan) for measuring the fully extended index leaves per pot at 0, 10, 20, and 40 days after transplanting (DAT) and experiment was performed in triplicate. These dimensionless values were recorded based on transmittance ratios utilizing 650/940 nm wavelengths.
2.6 Growth and Yield Characters
Vegetative growth parameters i.e. shoot length including both fresh and dry weight, and root mass (dry weight, volume, and fresh), were analysed. Additionally, yield-contributing characteristics such as the total number of grains per plant, and grain weight were analysed at 40 days after transplantation (DAT) and during the harvesting period, respectively.
2.7 Plant Nutrient Status
Samples of shoot were collected from 1 cm above the soil surface at the end of the experiment using a stainless-steel blade and washed with double-distilled water. On Whatman filter paper no. 1 these samples were placed, air-dried, and later oven dried at 65 ± 2 °C until a constant weight was obtained and analysed for micro and macronutrient contents. Quantification of nitrogen content in root and pea samples was carried out using the Kjeldahl method. The samples were digested with 10 ml of concentrated sulfuric acid in the presence of a digestion mixture. This process, as per Kjeldahl’s method, facilitates the release and subsequent measurement of nitrogen compounds for accurate analysis. For the determination of phosphorus and potassium content in peas and roots, a di-acid mixture (HNO3 and HClO4 in a ratio of 4:1) was utilized to treat the samples. Phosphorus content was analysed using the Vanadomolybdate method, generating a yellow colour in an HNO3 system, while potassium content was measured via Flame Photometry. For micronutrients (Mn, Cu, Fe, Zn) estimation, oven-dried pea samples, as well as roots were wet digested in a di-acid mixture of 15 ml HNO3 and HClO4 (3:1). The measurement was conducted using Atomic Absorption Spectrophotometry, following the methodology of Wheal et al. in 2011.
2.8 Statistical Analysis
The results were statistically analysed by utilizing generalized linear model through SAS software (version 9.2). The means were differentiated by using the least significant difference (LSD) at a significance level of p ≤ 0.05.
3 Results
3.1 Synthesis and Characterization of Zinc Oxide Nanoparticles
The ZnONPs were synthesized successfully by sol-gel method. The change of solution colour to off-white confirmed the synthesis of ZnONPs. The solution was placed at 176 °F for oven drying overnight and the pellet was oven-dried to acquire pure nanoparticles.
The UV-Vis spectroscopy analysis of the synthesized ZnONPs showed absorption peak at 380 nm (Fig. 1a). The sharp absorption of ZnONPs, compared to bulk Zinc, confirmed the presence of monodispersed nanoparticles. The FTIR transmission spectrum revealed a significant peak at 3200/cm (Fig. 1b), with peaks at 898.24/cm, 422.52/cm, 3421.36/cm, 2921.41/cm, 1631.26/cm, 1416.23/cm corresponding to C-C, Cl-C-O, NH/OH, C-H, C-O, C-N stretching of different functional groups, respectively. The hexagonal ZnO wurtzite structure of the nanoparticles was confirmed by diffraction peaks in the XRD pattern (Fig. 1c). The EDX analysis determined the percentage composition of the synthesized nanoparticles, with zinc accounting for 45.9% and oxygen for 54.05% (Fig. 1d). SEM images illustrated that the synthesized nanoparticles had a wurtzite structure characteristic of ZnO nanoparticles, with a mean size of 37 nm (Fig. 1e).
3.2 Soil Chemical Properties
3.2.1 Soil pH, Organic Carbon (OC), and Electrical Conductivity (EC)
The initial analysis of soil chemical properties, including organic carbon, electrical conductivity, pH, and micro and macronutrient contents, is presented in Table 1. Initially, the soil exhibited an alkaline pH with low levels of organic carbon and zinc. After treatment, the duration and concentration of ZnONPs significantly influenced soil pH, organic carbon, and electrical conductivity, as outlined in Table 2. In the days following treatment, there was a notable increase in organic carbon (0.34 vs. 0.26%) and electrical conductivity (0.26 vs. 0.24 dS/m), with the values at 40 DAT being significantly higher than those at 0 DAT in the soil (Table 2).
The application of ZnONP to the soil, a minor decrease in soil pH was observed. The most significant pH reduction (8.260) occurred in the 1.0 g/L treatment, followed by treatments with 5.0 g/L (8.34) and 0.5 g/L (8.35), in contrast to the control (8.37) at 40 DAT (Table 2). The highest organic carbon content (0.339%) was observed in the 5.0 g/L ZnONPs treatment, highlighting organic carbon as a crucial biofertility indicator. This value was significantly higher compared to other ZnONPs treatments and the control (0.302%) organic carbon (Table 2). The soil electrical conductivity, representing the concentration of soluble salts and provides direct measurement of salinity, was minimally affected by ZnONP application, as observed in other soil parameters. The recorded values did not exceed the crucial limit of 4.0 dS/m, as indicated by the study. The treatment with 1.0 g/L ZnONPs showed the highest electrical conductivity (0.34 dS/m), followed by treatments with 5.0 g/L and 0.5 g/L, while the control showed the minimum value (0.25 dS/m) (Table 2).
3.3 Soil Macronutrients
The concentration of days after treatment (DAT) and ZnONPs showed a significant impact on soil macronutrients, specifically nitrogen (N), phosphorus (P), and potassium (K). Over the treatment period, there was an increase in nitrogen (33 vs. 25 mg/kg) at 40 DAT compared to 0 DAT. However, for phosphorus (17.30 vs. 13.60 kg/ha) and potassium (224.30 vs. 121.50 kg/ha), the highest values were observed at 0 DAT in the soil (Table 2).
Among the treatments, the highest nitrogen availability was observed at 5.0 g/L (33 mg/kg) and 1.0 g/L (32.0 mg/kg), followed by 0.5 g/L (31.0 mg/kg) (Table 2). In contrast, the phosphorus content in the soil was observed to decrease with the increasing ZnONPs application rate. The highest soil phosphorus content was recorded in the control (17.30 kg/ha), followed by the ZnONPs treatment with 0.5 g/L (15.73 kg/ha) at 40 DAT (Table 2).
The highest potassium value was registered in the 1.0 g/L treatment (173.13 kg/ha), which was comparable to the control (172.58 kg/ha) and other ZnONPs treatments (Table 2). However, the interactions post-treatments across the days also had a significant effect on the availability of phosphorus and nitrogen content related to ZnONPs concentration. These results indicate that the available soil macronutrients changes with the application of ZnONPs treatments and across various DATs.
3.4 Soil Micronutrients
The soil zinc micronutrient contents were significantly influenced by both the days after treatment (DAT) and the concentration of ZnONPs. The application of ZnONPs had a notable effect on soil zinc content. Over the treatment period, the soil zinc concentration at 0 DAT was notably higher than that at 40 DAT (0.35 vs. 0.27 mg/kg) (Table 2). The highest recorded soil zinc content (0.38 mg/kg) was observed in the treatment of ZnONPs at 5.0 g/L (Table 2). The relationship between DAT and treatment with ZnONPs, as indicated by DAT × C, had a significant impact on soil zinc content. This suggests variations in soil levels across different DATs and ZnONPs treatments.
Similarly, the micronutrients manganese (Mn), copper (Cu), and iron (Fe) were significantly affected by both DAT and various concentrations of ZnONPs. Within the DATs, the soil micronutrient values (Mn, Cu, Fe) at 40 DAT were notably higher than those at 0 DAT (Table 2). High values of Mn and Cu were observed among the treatments compared to the soil without applied ZnONPs. However, the soil treated with ZnONPs exhibited higher Fe content compared to the control (Table 2). There was a relationship between DAT and ZnONPs concentration, significantly impacting soil Zn, Cu, and Mn.
3.5 Soil Microbial Counts
The microbial soil count showed a significant impact due to the interaction between days after treatment (DAT) and the concentration of ZnONPs. This indicates variations in microbial soil count among different DATs (Table 3). The highest microbial count was observed at 35 DAT, suggesting a greater count of microbes among the different DATs.
The application of ZnONPs had a significant effect on soil microbial count. In terms of bacteria, a higher microbial count was observed for pseudomonads, fungi, and rhizobia with the 5.0 g/L treatment of ZnONPs. However, the count of actinomycetes count was remained either low or was comparable to the control treatment among various ZnONP treatments.
3.6 Soil Enzyme Activities
A significant improvement in soil microbial respiration and dehydrogenase activity was observed in treatments of ZnONPs, indicating a certain influence on the enzyme activities of soil microbes. The highest values of dehydrogenase activity (6.18 µg TPF/g/h) and microbial respiration of the soil (0.036 mg/L) were recorded for 5.0 g/L treatment of ZnONPs (Table 4).
3.7 Growth Characters
3.8 Shoot Length and Biomass
Increasing concentrations of application of ZnONPs resulted in a significant increase in the length of the shoot and biomass (Table 5). The maximum plant height (76.40 cm), fresh weight (13.50 g), and dry weight (9.90 g) were noted with the application at 5 g/L ZnONPs, showed higher values than different treatments and the control (Table 5).
3.9 Root Biomass
Root biomass was significantly affected by the application of ZnONPs through at various concentrations (Table 5). Root volume (27.72 mm3), fresh weight (19.28 g), and dry weight (5.10 g) showed a significant increase with ZnONP application at a concentration of 1.00 g/L compared to other ZnONPs treatments besides control (Table 5).
3.10 Chlorophyll Content
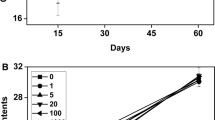
The content of chlorophyll serves as a marker for net photosynthesis and plant growth. Various treatments of ZnONPs application resulted in significant differences in chlorophyll content, with significant changes observed among different concentrations of ZnONPs and days after treatment (DAT) (Table 5). At 35 DAT, the maximum chlorophyll was recorded, showing a significant increase compared to the chlorophyll levels at 0 DAT (Table 5). The highest chlorophyll content was observed in treatment with ZnONPs at a concentration of 5.0 g/L (53.84 for SPAD) (Table 5). The chlorophyll content in leaves changed based on the interaction effects, particularly influenced by DAT and the ZnONPs concentration (Table 5).
3.11 Nutrient Uptake by Shoot, Root, and Grain
ZnONPs treatments had a significant impact on Zn content in the root, shoot, and peas, (Table 6). In control plants, low Zn concentrations were observed in the shoot, root, and grain, while high Zn contents were recorded in plants treated with ZnONPs. The highest Zn content in the shoot (123.93 mg/kg), root (91.85 mg/kg), and peas (20.00 mg/kg) was observed in the 5.0 g/L treatment. Other micronutrients (Mn, Fe, and Cu) were also affected by the application of ZnONPs (Table 6). Enhanced Mn, Fe, and Cu contents were observed in the shoot by the application of ZnONPs, Mn and Cu in the grain, and Mn in the root. Thus, these nanoparticles modulated the shoot, root, and grain micronutrient contents in a differential manner.
A significant changing pattern was observed in in macronutrient N and P levels in the root, shoot, and peas resulted from the concentration of ZnONPs. In the 5.0 g/L treatment, a higher uptake of N by the shoot (106.0 mg/kg) and peas (156.0 mg/kg) was observed, while for the root (112.0 mg/kg), it was recorded in the 1.0 g/L treatment, in comparison with the control (Table 6). The nitrogen absorption increased with the rise in ZnONPs concentration, except for root N levels, indicating the adverse impact of higher ZnONPs concentrations on root parameters. The highest phosphorous value in the shoot (19.00 mg/kg), root (29.00 mg/kg), and peas (18.0 mg/kg) was noted in the control treatment.
3.12 Grains Weight and Number
The foliar application of ZnONPs at various concentrations significantly affected yield-related characteristics, such as the total number of grains per plant and the grain weight, compared to the untreated control. These yield-related characteristics improved as the concentration of ZnONPs increased. The highest number of grains per plant (362) and the highest grain weight (29.09 g) were observed in the treatment with 5 g/L ZnONPs (Table 7).
4 Discussion
Zinc oxide nanoparticles (ZnONPs) serve a crucial role in fertilizers by enhancing plant growth and development. ZnONPs provide an efficient means of delivering zinc, an essential micronutrient, to plants, to combat zinc deficiency in soils. By improving nutrient uptake and utilization, ZnONPs contribute to increased crop yield and overall plant health. Their controlled release properties can minimize environmental impact, ensuring optimal zinc availability without the risk of over-fertilization. Additionally, ZnONPs support soil health by aiding enzymatic processes and microbial activity (Mi et al. 2023).
In the present study, effect of ZnONPs was analysed as a nonfertilizer on the growth of pea plants. The ZnONPs were synthesized successfully by sol-gel method and change in colour confirmed the process of synthesis. The maximum absorption peak at 380 nm was observed in the UV visible spectroscopy of the synthesized ZnONPs (Fig. 1a). The presence of monodispersed nanoparticles was confirmed through the sharp absorption of ZnO nanoparticles compared to bulk Zinc. FTIR transmission spectra showed intense peak at 3200 cm − 1 (Fig. 1b). The peak at 3422.63 cm − 1, 2920.48 cm − 01, 898.23 cm − 1, 1632.62 cm − 1, 1413.37 cm − 1, 420.15 cm − 01 corresponds to NH/OH, C-H, C-C, C-O, C-N, Cl-C-O showing various functional groups respectively. Hexagonal ZnONPs structure was confirmed by the diffraction peaks present in XRD pattern (Fig. 1c). EDX analysis was used to analyse the percentage composition of metal in the synthesized nanoparticles. The synthesized nanoparticle was subjected to EDX analysis, revealing a zinc composition of 45.9% and oxygen composition of 54.05% (Fig. 1d). The images showed by SEM that the synthesized ZnONPs were having mean size of 37 nm (Fig. 1e).
Under zinc deficiency conditions in the soil, application of ZnONPs on pea plants resulted in improvements in vegetative parameters. This enhancement is likely due to zinc’s crucial role in processes such as cell elongation, membrane function, and protein synthesis. The observed increase in shoot biomass with ZnONPs application is supported by the findings of Burman et al. (2013) and Moradi et al. (2022) with SiO2 and TiO2 nanoparticles.
The improvement in root growth attributed to ZnONPs through stomatal openings uptake and subsequent mobilization to reach the roots via apoplast/symplast showed a negative impact below and above the optimum concentration of 1.0 g/L. This adverse effect on roots aligns with similar findings reported by Rajput et al. (2021) in spring barley tissues and Raliya and Tarafdar (2013) in Cyamopsis tetragonoloba, where the application of ZnONPs controlled root growth at higher concentrations. The inhibition of root growth is likely linked to structural alterations on the root surface.
The crop’s chlorophyll content represents another significant vegetative characteristic vital for photosynthesis. A deficiency in micronutrients can inhibit chlorophyll formation, leading to a reduction in protein synthesis, as reported by Li et al. (2021). The increase in chlorophyll content observed in the present study is in accordance with the reports of Sawati et al. (2022) on Brassica napus, where varying concentrations of ZnONPs led to a significant increase in chlorophyll content. This study highlights the potential of ZnONPs application to positively influence chlorophyll levels, addressing micronutrient deficiencies and promoting crop growth.
The improved yield due to the application of ZnONPs results from increase in yield-contributing characteristics of the treated plants. This improvement may be attributed to enhanced physiological and biochemical activities, ensuring better crop growth in addition to improved photosynthesis. These benefits can be linked to zinc’s role as an important enzyme cofactor. Furthermore, the easy solubility, ultra-small size, and diffusible nature of ZnONPs enable them to penetrate the leaf surface, crossing the cuticle and releasing zinc ions. Additionally, the increased weight of peas indicates an increase in individual pea size, possibly due to an increased activity of the hormone cytokinin, as reported by Sosnowski et al. (2023). The results described by Sharma et al. (2022) and Prasad et al. (2012) further support these findings, as they observed an increased length of tillers per plant with ZnONPs application.
The application of ZnONPs resulted in improved zinc nutrient levels in the shoot, root, and peas, aligning with the findings of Srivastav et al. (2021) and Hussain et al. (2018), who observed increased zinc contents in the grain and shoot of test crops with the application of ZnONPs. Additionally, ZnONPs affected the levels of other micronutrients in the crop plants. This study demonstrated an improvement in iron (Fe) content following ZnONPs application, suggesting potential Zn-macronutrient interactions affecting nitrogen content. However, the application of Zn led to a decrease in pea phosphorus (P) contents, possibly because of an antagonistic association between Zn and P, as observed by Aboyeji et al. (2020).
Furthermore, the application of ZnONPs significantly increased soil microbial viable cell counts. These results align with the findings of Raliya and Tarafdar (2013), Mohd Yusof et al. (2019) and Saberi and Moradi (2021) for foliar application of ZnONPs. The dehydrogenase activity of the soil was also improved, indicating the impact of ZnONPs application on soil microbes has no negative effect. Soil enzyme activities increased due to ZnONPs have also been reported by Srivastav et al. (2021) and Ur Rehman et al. (2023). The application of ZnO NPs also influenced the chemical properties of the soil, such as pH, electrical conductivity (EC), and organic carbon (OC). These chemical parameters of the soil play a crucial role in adsorption-desorption reactions of Zn, regulating the solubility of Zn in the soil and its fractionation. Various published data has reported different reactions of rice to Zn fertilizers, influenced by factors such as the source, application period, method of application, and soil’s chemical properties (Yuan et al. 2013).
The macronutrients of the soil, specifically the available nitrogen (N) content, showed improvement with the application of ZnONPs. These findings are in accordance with the results of Wang et al. (2023), who reported a significant increase in nitrogen content among various rice varieties due to the foliar application of zinc compared to the control. The content of available potassium in the soil remained unaffected by ZnONPs application; however, Wang et al. (2023) identified a relationship between Zn application and the total percentage of potassium in the soil, observing an impact on the overall percentage of potassium at reaping time based on various methods of Zn application. Similarly, the micronutrient contents of the soil were affected by ZnONPs treatment, showing increased soil zinc content compared to the control (Sattar et al. 2022). Wang et al. (2023) reported different results in rice cultivars in response to Zn fertilizers, dependent on factors such as source, application time, application method, and chemical properties of the soil, suggesting their potential role in increasing soil zinc content.
In conclusion, present study highlights the impact of zinc oxide nanoparticles on pea plants and soil health. The application of ZnONPs under zinc deficiency conditions significantly improved vegetative parameters, including shoot biomass, root growth, and chlorophyll content, contributing to enhanced crop yield. The study also demonstrated the positive influence of ZnONPs on zinc and iron content in plant tissues, while representing the potential antagonistic relationship with phosphorus. Moreover, ZnONPs positively affected soil microbial activity, dehydrogenase activity, and chemical properties, such as pH, EC, and organic carbon. The findings emphasize the potential of ZnONPs to address micronutrient deficiencies, promote crop growth, and enhance soil health, offering a sustainable and controlled approach to zinc applications in agriculture. However, careful consideration of concentration levels and potential interactions with other nutrients is essential for optimizing the benefits of ZnONPs application in crop management practices.
5 Conclusion
The application of zinc oxide nanoparticles (ZnONPs) effectively addresses zinc deficiency in soil and enhances the growth and yield of pea plants. ZnONPs significantly improve vegetative parameters such as plant height, leaf area, and biomass accumulation, leading to increased crop yield. Furthermore, ZnONPs increase the zinc content in peas and positively influence soil health by boosting microbial counts and dehydrogenase enzyme activity.
This study highlights the dual benefits of ZnONPs: promoting plant growth and improving soil fertility. These findings suggest that ZnONPs can be a valuable tool for sustainable agriculture, addressing micronutrient deficiencies while enhancing overall soil health.
However, the long-term effects of ZnONP application on soil health and plant growth need further exploration, particularly under field conditions. Future research should focus on the long-term impacts, optimizing application rates, and minimizing potential environmental risks to maximize the benefits of ZnONPs in agricultural practices.
Data Availability
Not applicable.
References
Aboyeji CM, Dunsin O, Adekiya AO, Suleiman KO, Chinedum C, Okunlola FO, Joseph A, Ejue SW, Adesola OO, Olofintoye TA, Owolabi IO (2020) Synergistic and antagonistic effects of soil applied P and zn fertilizers on the performance, minerals, and heavy metal composition of groundnut. Open Agric. https://doi.org/10.1515/opag-2020-0002
Al Jabri H, Saleem MH, Rizwan M, Hussain I, Usman K, Alsafran M (2022) Zinc oxide nanoparticles and their biosynthesis: overview. Life. https://doi.org/10.3390/life12040594
Bala R, Kalia A, Dhaliwal SS (2019) Evaluation of efficacy of ZnO nanoparticles as remedial zinc nanofertilizer for rice. J Soil Sci Plant Nutr 15:379–389
Burman U, Saini M, Kumar P (2013) Effect of zinc oxide nanoparticles on growth and antioxidant system of chickpea seedlings. Toxicol Environ Chem. https://doi.org/10.1080/02772248.2013.803796
Dhaliwal SS, Sharma V, Kaur J, Shukla AK, Hossain A, Abdel-Hafez SH, Gaber A, Sayed S, Singh VK (2021) The pedospheric variation of DTPA-extractable Zn, Fe, Mn, Cu and other physicochemical characteristics in major soil orders in existing land use systems of Punjab, India. Sustainability 14:29
Hussain A, Ali S, Rizwan M, ur Rehman MZ, Javed MR, Imran M, Chatha SA, Nazir R (2018) Zinc oxide nanoparticles alter the wheat physiological response and reduce the cadmium uptake by plants. Environ Pollut. https://doi.org/10.3390/su14010029
Li ZG, Luo YM, Teng Y (2008) Research methods on soil and environmental microorganisms. Science, Beijing
Li J, Cao X, Jia X, Liu L, Cao H, Qin W, Li M (2021) Iron deficiency leads to chlorosis through impacting chlorophyll synthesis and nitrogen metabolism in Areca catechu L. Front Plant Sci. https://doi.org/10.3389/fpls.2021.710093
Meena A, Rao KS (2021) Assessment of soil microbial and enzyme activity in the rhizosphere zone under different land use/cover of a semiarid region, India. https://doi.org/10.1186/s13717-021-00288-3. Ecological Processes
Mi K, Yuan X, Wang Q, Dun C, Wang R, Yang S, Yang Y, Zhang H, Zhang H (2023) Zinc oxide nanoparticles enhanced rice yield, quality, and zinc content of edible grain fraction synergistically. Front Plant Sci. https://doi.org/10.3389/fpls.2023.1196201
Mohd Yusof H, Mohamad R, Zaidan UH, Abdul Rahman NA (2019) Microbial synthesis of zinc oxide nanoparticles and their potential application as an antimicrobial agent and a feed supplement in animal industry: a review. J Anim Sci Biotechnol. https://doi.org/10.1186/s40104-019-0368-z
Moradi Pour M, Saberi Riseh R, Ranjbar-Karimi R, Hassanisaadi M, Rahdar A, Baino F (2022) Microencapsulation of Bacillus velezensis using alginate-gum polymers enriched with TiO2 and SiO2 nanoparticles. https://doi.org/10.3390/mi13091423. Micromachines
Nisab CM, Sahu M, Ghosh GK (2020) Distribution of DTPA-extractable micronutrient cations (Zn, Fe, Mn, and Cu) and its relationship with physico-chemical properties in soils of Birbhum district, West Bengal. https://doi.org/10.22271/chemi.2020.v8.i3d.9236
Prasad TNVKV, Sudhakar P, Sreenivasulu Y, Latha P, Munaswamy V, Reddy KR, Sreeprasad TSP, Sajanlal R, Pradeep T (2012) Effect of nanoscale zinc oxide particles on the germination, growth, and yield of peanut. J Plant Nutr. https://doi.org/10.1080/01904167.2012.663443
Quintieri L, Nitride C, De Angelis E, Lamonaca A, Pilolli R, Russo F, Monaci L (2023) Alternative protein sources and Novel foods: benefits, Food Applications and Safety issues. Nutrients. https://doi.org/10.3390/nu15061509
Rajput VD, Minkina T, Fedorenko A, Chernikova N, Hassan T, Mandzhieva S, Sushkova S, Lysenko V, Soldatov MA, Burachevskaya M (2021) Effects of zinc oxide nanoparticles on physiological and anatomical indices in spring barley tissues. Nanomaterials 11:1722
Raliya R, Tarafdar JC (2013) ZnO nanoparticle biosynthesis and its effect on phosphorous-mobilizing enzyme secretion and gum contents in cluster bean (Cyamopsis tetragonoloba L). Agric Res. https://doi.org/10.3390/nano11071722
Rashid A, Ram H, Zou CQ, Rerkasem B, Duarte AP, Simunji S, Yazici A, Guo S, Rizwan M, Bal RS, Wang Z (2019) Effect of zinc-biofortified seeds on grain yield of wheat, rice, and common bean grown in six countries. J Plant Nutr Soil Sci. https://doi.org/10.1002/jpln.201800577
Reshma Z, Meenal K (2022) Foliar application of biosynthesised zinc nanoparticles as a strategy for fertifortification by improving yield, zinc content and zinc use efficiency in amaranth. https://doi.org/10.1016/j.heliyon.2022.e10912. Heliyon
Rudani K, Vishal P, Kalavati P (2018) The importance of zinc in plant growth-A review. Int Res J Nat Appl Sci 5:38–48
Saberi RR, Gholizadeh VM, Hassanisaadi M, Skorik YA (2023) Micro-/nano-carboxymethyl cellulose as a promising biopolymer with prospects in the agriculture sector: a review. https://doi.org/10.3390/polym15020440. Polymers
Saberi Riseh R, Moradi Pour M, Ait Barka E, Agronomy (2022) https://doi.org/10.3390/agronomy12030655
Saberi-Riseh R, Moradi‐Pour M (2021) A novel encapsulation of Streptomyces fulvissimus Uts22 by spray drying and its biocontrol efficiency against Gaeumannomyces Graminis, the causal agent of take‐all disease in wheat. Pest Manag Sci. https://doi.org/10.1002/ps.6469
Sato JH, Figueiredo CC, Marchão RL, Madari BE, Benedito LE, Busato JG, Souza DM (2014) Methods of soil organic carbon determination in Brazilian savannah soils. Scientia Agricola. https://doi.org/10.1590/0103-9016-2013-0306
Sattar A, Wang X, Ul-Allah S, Sher A, Ijaz M, Irfan M, Abbas T, Hussain S, Nawaz F, Al-Hashimi A, Al Munqedhi BM (2022) Foliar application of zinc improves morpho-physiological and antioxidant defense mechanisms, and agronomic grain biofortification of wheat (Triticum aestivum L.) under water stress. Saudi J Biol Sci. https://doi.org/10.1016/j.sjbs.2021.10.061
Sawati L, Ferrari E, Stierhof YD, Kemmerling B, Mashwani ZU (2022) Molecular effects of biogenic zinc nanoparticles on the growth and development of Brassica napus L. revealed by proteomics and transcriptomics. Front Plant Sci. https://doi.org/10.3389/fpls.2022.798751
Sharma S, Kaur P, (2022) Gaikwad K Role of cytokinins in seed development in pulses and oilseed crops: current status and future perspective. Front Genet. https://doi.org/10.3389/fgene.2022.940660
Sosnowski J, Truba M, Vasileva V (2023) The impact of Auxin and Cytokinin on the growth and development of selected crops. https://doi.org/10.3390/agriculture13030724. Agriculture
Srivastav A, Ganjewala D, Singhal RK, Rajput VD, Minkina T, Voloshina M, Srivastava S, Shrivastava M (2021) Effect of ZnO nanoparticles on growth and biochemical responses of wheat and maize. https://doi.org/10.3390/plants10122556. Plants
Umair Hassan M, Aamer M, Umer Chattha M, Haiying T, Shahzad B, Barbanti L, Nawaz M, Rasheed A, Afzal A, Liu Y, Guoqin H (2020) The critical role of zinc in plants facing the drought stress. https://doi.org/10.3390/agriculture10090396. Agriculture
Ur Rehman F, Paker NP, Khan M, Zainab N, Ali N, Munis MF, Iftikhar M, Chaudhary HJ (2023) Assessment of application of ZnO nanoparticles on physiological profile, root architecture and antioxidant potential of Solanum lycopersicum. Biocatal Agric Biotechnol. https://doi.org/10.1016/j.bcab.2023.102874
Wang R, Mi K, Yuan X, Chen J, Pu J, Shi X, Yang Y, Zhang H, Zhang H (2023) Zinc oxide nanoparticles foliar application effectively enhanced zinc and aroma content in Rice (Oryza sativa L.) grains. https://doi.org/10.1186/s12284-023-00653-0. Rice
Wheal MS, Fowles TO, Palmer LT (2011) A cost-effective acid digestion method using closed polypropylene tubes for inductively coupled plasma optical emission spectrometry (ICP-OES) analysis of plant essential elements. Analytical Methods. https://doi.org/10.1039/C1AY05430A
Wu DT, Li WX, Wan JJ, Hu YC, Gan RY, Zou L (2023) A comprehensive review of pea (Pisum sativum L.): chemical composition, processing, health benefits, and food applications. Foods. https://doi.org/10.3390/foods12132527
Yuan L, Lianghuan W, Chunlei Y, Qian LV (2013) Effects of iron and zinc foliar applications on rice plants and their grain accumulation and grain nutritional quality. J Sci Food Agric. https://doi.org/10.1002/jsfa.5749
Zeng H, Wu H, Yan F, Yi K, Zhu Y (2021) Molecular regulation of zinc deficiency responses in plants. J Plant Physiol. https://doi.org/10.1016/j.jplph.2021.153419
Zhou XQ, Hayat Z, Zhang DD, Li MY, Hu S, Wu Q, Cao YF, Yuan Y (2023) Zinc oxide nanoparticles: synthesis, characterization, modification, and applications in food and agriculture. Processes. https://doi.org/10.3390/pr11041193
Acknowledgements
The authors extend their appreciation to the Researchers Supporting Project number (RSP2024R293) King Saud University, Riyadh, Saud Arabia.
Funding
The funding for this project was provided by the Researchers Supporting Project number (RSP2024R293) King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by [Irshad Arshad], [Samia Nazir], [Sobia Hafeez Kiani], [Wahidah H. Al-Qahtani], [Akram A. Alfuraydi], [Ibrahim A. Saleh] and [Mostafa A Abdel-Maksoud]. The first draft of the manuscript was written by [Bushra Hafeez Kiani] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
No ethical approvals were required to conduct research with plant material, in accordance with institutional/local regulations.
Consent for Publication
Not applicable.
Competing Interests
The author reports no conflicts of interest in this work. The author (s) declare no competing interests. This original manuscript has not been submitted for publication in another journal or elsewhere.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kiani, B.H., Arshad, I., Nazir, S. et al. Innovative Approaches for Sustainable Zinc Nutrition and Crop Yield Enhancement in Pea Plants Using Zinc Oxide Nanoparticles. J Soil Sci Plant Nutr (2024). https://doi.org/10.1007/s42729-024-01944-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42729-024-01944-1