Abstract
In forensic entomology, the presence of insects aids investigations by helping to understand the time since death. Insects are the first organisms that colonize and decompose the carrion. The main application of insect evidence is determining minimum post mortem interval (PMImin) either by analyzing insect developmental patterns or by estimating insect succession on the corpse. Age determination of insects has a pivotal role in the estimation of PMI. A range of techniques is available for estimating age, which provides accurate PMI in investigations. The present review critically discusses the applications of entomological evidence in PMI estimation and enlightens the factors influencing PMI calculations. Age estimation of insects is the primary task in PMI estimation. This review concentrates on methods used in age estimation, their strengths and weaknesses. Entomotoxicology analysis is a valuable tool in solving poisoning cases when dead bodies are recovered after a long period and this review also analyses the scope of entomotoxicology in the area of death investigations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
History of forensic entomology
The study of insects and arthropods in a legal context is known as forensic entomology and the origin of this discipline date back nearly 3,000 years back to Egypt (Smith 1951). The use of insects in a legal case was first recorded between 907 and 960 AD in the 10th century in China during a murder investigation (Ko 2002; Greenberg and Kunich 2002). The first publication on the use of insects for forensic purposes was during the thirteenth century when the Chinese scholar Song Ci (Sung Tz’u) commented on the value of evidence from flies in a murder investigation. Hsi Yiian Chi Lu (“The Washing Away of Wrongs”), Song Ci’s training manual for death scene investigators, addressed a case in which a murderer was caught when flies were attracted to the sickle he had used as his murder weapon (McKnight 1981). This field of study can be broadly divided into urban entomology, stored products entomology, and medico-legal entomology (Lord and Stevenson 1986). Medico-criminal entomology (Catts and Haskell 1990), which is the other name of medico-legal entomology, is invariably discussed and the subject of this review.
The application of entomology to a forensic case was published in the last half of the nineteenth century by Dr. Bergeret d’Arbois in a case related to a mummified infant’s body found in an apartment’s chimney 1855 (Leclercq 1969). The German physician, Hermann Reinhard, identified seven arthropod taxa in 1882 and it was the first taxonomic survey of the fauna of exhumed corpses (Goff 1993). Based on the treatise of the Paris morgue, the French army veterinarian and entomologist Pierre Megnin established entomology as a useful tool in legal applications. Megnin predicted the series of changes during the decomposition of the exposed corpse and the comprehensive account of the succession of specific arthropods (J.-P. Mégnin 1887; Mégnin 1894). Another French physician, G. P. Yovanovitch, published a thesis based on Megnin’s succession tables which contained 19 case reports spanning 30 years (Yovanovitch 1888). The limited forensic value of the succession tables of the carrion-associated flies and beetles was reported by Eduard Ritter von Niezabitowski through his studies on arthropod succession using stillborn human remains and carrion from several mammal species (Niezabitowski 1902).
Based on the observations of the succession of insects on corpses, two Canadian physicians, Wyatt Johnston and Georges Villeneuve, questioned the application of Megnin’s rules to other countries and climates even though they agreed with the successional sequence rules of Megnin (Johnston and Villeneuve 1897). In North America, Murray G. Motter established entomology in forensic cases by the end of the nineteenth century through his studies on 150 exhumed corpses in the Washington DC area (Motter 1898).
There was not much progress in forensic entomology in the first half of the 20th century. Various observational studies based on the exposure of the animal carcasses to different seasons and environments were done, which generated reports on species list, tables of succession, and description of decomposition only (Jaques 1915; Illingworth 1923; Fuller 1934; Bornemissza 1957; Reed Jr 1958). In these studies, there were no mechanisms proposed for the observed successional patterns. In late 1935, the first case using forensic entomology occurred in the UK, where police recovered butchered remains, and the estimation of time since death relied mainly on maggots (Glaister and Brash 1937). A chemical ecology study using domestic pig revealed the relationship between the cadaver ecosystem and silphine (Leclercq and Quinet 1949). Domestic pig has become the model corpse for carrion research since the study of Jerry A. Payne, who conducted a tandem field test of carrion exposed to insects at various field conditions (Payne et al. 1968; Payne 1965).
The field of forensic entomology was strengthened by the researches of two pioneers, Dr. Marcel Leclercq and Pekka Nuorteva (Dekeirsschieter et al. 2013). The determination of post mortem interval (PMI) through the application of forensic entomology was first introduced by Dr. Leclercq in the Europe and French-speaking regions (Leclercq 1978, 2008; Amendt et al. 2004). A new era was brought to forensic entomology researches in the 1980s after the establishment of the Anthropological Research Facility (ARF) at the University of Tennessee (Shirley et al. 2011). In India, interpretations relating to the maturation of blowfly stages in estimating time since the death of infested bodies were made (Kulshrestha and Chandra 1987). In another study in India, which was a comparative analysis, the entomological method was found to be statistically more reliable in the estimation of PMI (Kashyap and Pillay 1989).
Many countries used forensic entomology in legal investigations. The present review discusses the applications of entomological evidence in PMI estimation and enlightens the factors influencing PMI calculations. Age estimation of insects is the primary task in PMI estimation; the review also concentrates on methods used in age estimation and its strengths and weakness. Entomotoxicology is a growing subfield of forensic toxicology; this review also analyses the scope of entomotoxicology in the area of death investigations.
Significance of forensic entomology
Forensic entomology is the medicolegal or medico-criminal entomology area, focused on insects and other arthropods related to criminal events. It applied to events of felonies, trafficking, violent crimes, physical abuse and neglect, and animal poaching (Anderson 1999). The most fundamental application of forensic entomology involves estimating minimum post mortem interval (PMImin) for aiding in legal investigations. Insects and other arthropods are used as forensic tools for estimating time since death beyond 72 h when the death has no witness. Forensic entomologist identifies the insect specimen from the corpse and provides an objective estimation of time since death, the indication of corpse relocation, manner or cause of death, the association of suspect to the death scene, season of death, location of death, the specific site of injury on the body, etc. Forensic toxicologists also identify drugs or poisons mediated death with the help of insects and insect remnants as toxicological specimens (Catts and Goff 1992).
Flies and other insects are attracted to carrion as a source of food and shelter for their eggs or larvae. Carcass decomposition progression is closely linked with the insect colonization time, development time, and departure time (Harvey et al. 2016). The order of each forensically important species’ colonization on carrion is called succession. Temperature is the key factor governing insect development and by studying this development in reference with the thermal history, the age of a specimen can be determined. The rate of decomposition, insect succession, and seasonal availability is influenced by biogeoclimatic zone, temperature and humidity, type and physical state of carcass remains as well as habitat loss and fragmentation (Mashaly 2017). The succession studies provide insect colonization and development rate, which helps to estimate minimum post mortem interval (PMImin). Post mortem interval is the time between the death and discovery of carrion (Catts and Goff 1992). The range of PMI from one day to more than one month can be determined by the age of immature stages of insects found on the body. Age determination of unidentified larvae is a great challenge because the larvae of different species look extremely similar. Age determination of pupae is more complicated than larvae due to the lack of morphological changes (Bala and Sharma 2016).
Insect succession on carrions
On earth, 90% of the organic matter from the producers, the plants are not consumed. Only the remaining 10% organic matter is used by the animals and all the organic matter eventually returns to the detritus pool as excreta and carrion (Barton et al. 2013). In all ecosystems, the cycling of energy and matter occurs through the decomposition of organic matter. Carrion is a nutrient-rich resource that provides a temporary microhabitat for many facultative scavengers and predators ranging from bacteria and fungi to vertebrate scavengers, consuming dead animal matter and recycling its nutrients (Wilson and Wolkovich 2011). Arthropods are an indispensable component of the carrion food web, which are the most species-rich and abundant organisms found at carrion that contribute to the consumption, recycling, and dispersion of nutrients (Parmenter and MacMahon 2009). Chemicals are a valuable tool to communicate with other species (intraspecific) and within the species of the same community (interspecific). Insects locate the decomposition site through the chemical stimuli and colonize it (Giao and Godoy 2007). Also, insects use gustatory, auditory, and visual stimuli to locate carrion. Similarly, carrion itself emits volatile organic compounds (VOCs), which help insects reach decomposing vertebrates from miles away (Tomberlin et al. 2011).
The chronological sequence of insect attraction towards the specific stages of decomposition is called insect succession, first proposed by Megnin, 1894 and is considered to occur predictably (Mégnin 1894). Many biotic and abiotic factors are contributing to insects asses, leading to the decomposition process. Abiotic factors which delay insect colonization include weather, temperature, precipitation events, tornados, altitude, and time of the day. Biotic factors include predation of blowfly eggs and larvae by other organisms (Anderson et al. 2001; Campobasso et al. 2001). Previous arthropod species created an environment suitable for colonizing species during the arthropod succession after that on carrion (D. B. Rivers and G. A. Dahlem 2014). Arthropods found on carrion are categorized into four groups depending upon their ecological roles: necrophagous species, predators and parasites, omnivores species, and adventive species (Sawyer 2017).
Necrophagous species are arthropods that feed directly on carrion in their adult or larval stages. These include many species of order Diptera (True Flies) and some species of the order Coleoptera (beetles). Two major dipterans located on carrion are blowflies (Caliphorids) and flesh flies (Sarcophagidae), commonly called filth flies. The insects, including the family Calliphoridae, Sarcophagidae, Muscidae, are first to arrive and colonize at the site of decomposing remains. These species are strong indicators of the length of time after an organism has been dead (D. B. Rivers and G. A. Dahlem 2014). Other notable flies such as scuttle flies (Phoridae), cheese skippers (Piophilidae), and soldier flies also lay eggs in the decomposing matter. Necrophagous species of Coleoptera, such as carpet beetles (Dermestidae) are often late colonizers and they are strongly attracted during the active stage of decomposition, consuming leathery flesh and cartilage (Hall and Gerhardt 2002).
Predators and parasites are second-most forensically essential insects, attracted to vertebrate decomposition, but not feeding on the carrion. They lay eggs in the larvae of other insects, which kill the host. They include beetles from the family Staphylinidae (Rove beetles) and Siliphidae (Carrion beetles). Parasitoid wasps from the order Hymenoptera (Family Braconida) are another type of insect that attacks the dipteran larvae at carrion (Rivers and Dahlem 2014). Omnivore species consume carrions as well as carrion-associated insects, usually necrophagous species. Carrion beetles, Hymenopterans such as bees, wasps, and ants are the most abundant of this group. Ants colonies are established rapidly near carrion, which are forensically important categories (de Moretti et al. 2011). Adventive species or non-associated species may or may not play a significant role in carrion decomposition. Example of non-associated arthropods includes Acari, spiders (Chelicerata: Araneae), Isopods (Crustacea: Isopoda) and field crickets (Hexapoda: Orthoptera) (Sawyer 2017).
The decomposition rate of vertebrate species, their stages, and events are described in Table 1 (Parmenter and MacMahon 2009). Decomposition rate is significantly high in buried carcasses than placed on a surface. Among seasons, higher decomposition is seen at spring subsequently decreased in summer, autumn and winter, with mass loss demonstrated a linear relationship with ambient temperature. In insects, the temperature has a tremendous effect on the development and fecundity and the appearance or dynamics of insects population in the field (Ratte 1984). Corpse manner of decomposition is also influenced the species composition found on them. Scuttle flies (Diptera: Phoridae) regularly colonize buried cadavers, and Muscina species prefer colonizing on shallow burials. Blow flies are attracted to cadaver through decomposition odours; hence there are colonizing on exposed corpses (Gaudry 2009). Delayed Diptera colonization on carrion could affect forensic entomology applications, making errors while estimating the minimum time of insect colonization based on the entomological evidence (Pechal et al. 2014).
Estimation of post mortem interval
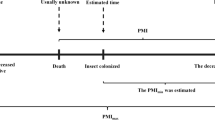
In forensic studies, the most common applications of entomological evidence are the estimation of time since death or post-mortem interval (PMI) for decomposing remains, either human or animal. Carrion-associated arthropod investigation helps to determine the cause and manner of death (includes detection of drugs/toxins), determination of death location, placement of body after death and identification of wound sites, estimation of post-mortem interval (PMI) (Hall and Huntington 2001). In homicide investigation, post-mortem interval (PMI) or time elapsed since death gives valid information about the death (Fig. 1). The physical and chemical changes of the corpse along with the succession of arthropod species found on and within the body are the methods used to determine the PMI. Through entomological evidence, there are two ways to estimate the PMI.
One method is to measure insect succession found on the body, and another method is by estimating the development of the oldest maggot feeding on the corpse (Catts and Goff 1992). For accurate estimation of minimum post mortem interval require species-specific insect development data. For generating the insect development data, specimens were caught from fields generally from single geographical locations, and they were reared in the laboratory settings under a range of temperature regimes. A growth curve is produced based on the thermal history of specimens in the field, which helps determine the reared species’ development time (Byrd and Butler 1997).
Developmental data for many forensically important species are sourced from a single location. This reference location is often different from the crime scene location, making it difficult in PMI estimation. Same blowfly species located in geographically separated regions show different responses to the same temperature condition and have different developmental data (Owings et al. 2014). This difference indicates genetic variation between populations of the same species. Population differences due to environmentally induced changes are called phenotypic plasticity. Forensic entomologist identifies the corpse relocation through the study of genetic diversity of species. When the corpse relocated from the original place, the insects found on the body have different genotypes than the population from that relocated place. Several factors cause genetic variation between populations, including natural selection, mutation, genetic drift, migration, or gene flow (Wells et al. 2009).
Carrion-associated insects visit on carcass show a unique pattern of succession (Greenberg 1991). This pattern is used for the estimation of the post-mortem intervals (PMI). The information such as recognition of species and pattern of succession and the subsequent emergence of immature stages (eggs, larvae, and pupae) obtained are needed for the accurate PMI estimation. Minimum PMI (PMImin) is estimated by calculating the age of developing insects on a body and it is possible to calculate the time of colonization. During carrion decomposition, insect activity is a continuous process; hence accurate minimum PMI estimation has to be made up to several months after death depending on the circumstance (Campobasso et al. 2001). Forensic entomologist uses a three-step process for calculating the age of a sampled insects, which are (1) accurately identify the species found on a corpse, (2) reconstructing crime scene temperature, and (3) modelling the rate of development of the immature insects found on a corpse. Insect identification is crucial in forensic entomology made with the help of museums and universities by insect taxonomy experts. Species identification depends upon morphological characters of larvae is complex, and the lack of taxonomic experts is also a challenge towards identification. Molecular taxonomy is the alternative and efficient tool for identifying forensically important species and this technique is based on the sequencing of certain genes, which is species-specific. Mitochondrial cytochrome oxidase-I is a well-established gene that is widely used for this purpose, and reference sequences are available in the genebank (Zehner et al. 2004).
Methods for age estimation of insects
In forensic entomology, insect specimen age determination is an essential goal. This can introduce considerable error also into associated PMI estimates. Primarily, insect morphology analysis is done for age estimation. Insect development has egg, larval, pupal, and adult stages, which are morphologically distinct. Insect larvae size (length, width, or height) indicates a relative period of development. However, these measures are influenced by some other factors such as drugs, competition, temperature, preservation, and measurement errors. Pupal age determination is much difficult and hence internal morphology of pupae is also used for more accurate age estimation (Harvey et al. 2016). By using histological analysis, internal morphological changes such as histolysis and histogenesis of tissues, organs transitions, and thoracic flight muscles recognition are analyzed, creating a chronology of development. Optical tomography and hyperspectral imaging help in age estimation of live stages of insects without the need for destructive analysis. Also, scanning electron microscopy and micro-computed tomography are used to visualize the morphological development of preserved specimens (Bala and Sharma 2016).
Cuticular hydrocarbons are lipid wax layer found on the insect surface, comprised of alkenes, n-alkanes, internally and terminally branched monomethyl alkanes and polymethyl alkanes. Serving as pheromones or kairomones and also limiting water loss are their functions (Lockey 1988; Harvey et al. 2016). Cuticular hydrocarbon is a useful compound that has many applications in the area of forensic entomology which includes species identification, determination of the geographical origin of the specimen, and estimating the age of individual insects for calculating PMImin by using gas chromatography with flame-ionization detection (GC-FID) and gas chromatography-mass spectrometry (GC-MS) (Bala and Sharma 2016).
Similarly, steroidogenesis measures quantitative levels of specific steroids such as ecdysteroids produced throughout insect development. This method provides valuable information regarding morphological development (Gaudry et al. 2006). Another method is pteridine fluorescence analysis which is a biochemical technique used to determine the age of insects. A pteridine is a fluorescent pigment that is a degradation product of purine metabolism and accumulates in the compound eye, increases concentration linearly with chronological age. Pteridine level in laboratory individuals linearly increases with age, rearing temperature, and ambient light level but are independent of sex and nutrition of adults. Pteridine aging method was developed in 1983 to determining the age of Stomoxy calcitrans adults, and for determining the age of many insets this method is widely used (Mail et al. 1983; Lehane et al. 1986).
Gene expression studies are an emerging technique used in forensic entomology to determine the age of developing individuals. Gene expression pattern varies with the age of maturing insects, though monitoring the number of specific gene products gives a time scale for age estimation (Tarone et al. 2007; Boehme et al. 2013). Cuticular banding pattern analysis is another age determination method in which the difference in the deposition of apodeme layers in the cuticle is used to measure the age in days since eclosion. The apodemes are internal cuticular projections of the exoskeleton. Under ultraviolet light, the apodeme layers autofluorescences brightly and dimly in an alternating pattern. This method gives an precise estimation of the chronological age of young insects of both sexes and can use in materials that have been dried, pinned, frozen, or preserved (Bala and Sharma 2016).
Volatile organic compounds (VOCs) released from flies, and their immature stages are used to evaluate the chronological age to establish a PMI. Different fly species release different VOCs in various developmental stages. The composition and quantity of VOCs also vary with the age of larvae or pupae. Branched and unbranched hydrocarbons, alcohols, esters, and acids are identified by headspace solid-phase microextraction, followed by gas chromatography-mass spectroscopy. Two factors that affect chemical composition and emission of VOCs include (1) genetic factors such as age and gender of fly, (2) environmental factors such as diet and temperature (Frederickx et al. 2012).
Modelling of insect development
Age of immature insects calculated by available measures such as size (weight, length) and developmental events (instars, or pupation) was then summarised in one or more developmental models, mainly isomorphen and isomegalen diagrams, thermal summation model, and curvilinear model/ ExLAC (Sharma et al. 2015).
Isomorphen and isomegalen diagrams
Isomorphen diagram is a simple basic model representing the duration of developmental events (Egg hatching, ecdysis, onset of wandering, pupation, adult eclosion) on the X-axis against a constant temperature range Y-axis. This method is very simple to use, but this simplicity compromises the accuracy of the output because, in cases, the environment temperature fluctuates. Isomorphen diagram considers all the morphological stages from oviposition to eclosion. In the graph, the area between lines shows the morphological stages of the fly. Isomorphen diagram based on the last recorded live developmental stage shows no gradation between events, this resulting in an underestimate of PMImin (Sharma et al. 2015).
Isomegalen diagram is advanced, which can calculate PMImin using dead larvae, and this can be used in analyzing fluctuating environment temperature. Isomegalen diagram is a 3D contour plot representing larval size (length, weight, or width) as a measure of age rather than life stage on the Z-axis against temperature on the Y-axis and time on the X-axis. Growth between developmental events shows a significant larval size difference, which provides a higher resolution and gives a more accurate estimate of PMImin (Amendt et al. 2011). Isomegalen diagram has several criticisms because it depends on the larval size, which is not always an accurate measure of age. In some insects, e.g., blowflies, larvae are shrinking size before entering the subsequent developmental events. Similarly, stunted larval and pupal forms are found commonly because of the scarcity of food sources, competition, etc. The preservation of larvae also leads to shrinkage or expansion of larvae depending on different preservatives and duration of preservation. These changes in larval size directly influence the calculation of age and estimation of PMImin (Campobasso and Introna 2001; Adams and Hall 2003).
Thermal summation model and curvilinear/ExLAC model
The thermal summation model is the most sophisticated method for modelling insect development, representing both size and developmental events in fluctuating environmental conditions. Usually, this method is used in pest control to calculate the developmental threshold. In forensic entomology, this method is used to calculate PMImin (Amendt et al. 2011). This method, also called the accumulated degree hours (ADH) model, applies linear regression analysis to a positive relationship between fluctuating temperature and insect development. Insect development is measured between a range of temperature, and a graph is plotted, where the degree of development compared to temperature results in a sigmoid curve. At extreme temperature insect development slowdown which corresponds to an upper and lower threshold. There is a linear relationship between development and upper- lower developmental thresholds, species-specific (Harvey et al. 2016). X intercept (lower developmental threshold, TL) and inverse of the slope of the linear regression (thermal summation constant, K) were determined through linear regression, which predicts the development time from the thermal history of a specimen. The disadvantage of this model is that the high variance in the upper and lower developmental temperature extremes significantly influences the regression coefficient leads to effects on PMImin accuracy. The non-linear or curvilinear model known as ExLAC is an alternative to linear thermal summation modelling, more precisely describe the association between development and temperature for insect populations. This model is based on the duration of life stages during the development of insects with the association of temperature (Briere et al. 1999).
Entomotoxicology
Entomotoxicology is the branch of forensic entomology, study about insects and other arthropods as toxicological samples in death investigations (Chophi et al. 2019). For detecting the presence of drugs and toxins from badly decomposed bodies, alternative samples such as insects and pupae from the body are used. These are considered more reliable biosamples without decomposition interference (Nolte et al. 1992). Empty pupal cases and beetle exuviae and faecal material also can be used as a substrate for analysis of drugs and toxins (Pien et al. 2004). Dipetrans and coleopterans are frequently using toxicological samples. Sample collection and preservation is the first step of entomotoxicological analysis. Various methods of killing and preservation include adding specimens directly into preservatives such as 10% formalin, 80% ethanol, and 95% ethanol or applying hot water (80 or 100 °C) killing (Adams and Hall 2003). Sample extraction is the second step of xenobiotic detection. Liquid-liquid extraction and solid-phase extraction are the best organic toxicant purification methods. Immunoassay, high-performance liquid chromatography (HPLC), liquid chromatography-mass spectrometry (LC-MS), and gas chromatography-mass spectrometry (GC-MS) are the frequently used analytical techniques for the identification of xenobiotics (Karampela et al. 2015). Due to human wildlife conflicts, the practices of deliberate wildlife poisoning cases are increasing. The effect of these poisoning in the local necrophagous and associated insect communities is being studied in wildlife and environmental forensic investigations (Verón et al. 2021).
The action of drugs in the body of insects depends upon the developmental stages and feeding activity of each species. Insect larvae actively feed cadaver tissue, and xenobiotics such as drugs and other toxins transfer to the metabolic system of larvae. These xenobiotics transfer to the food chain through the predation of cadaver-eating larvae by predatory and parasitic insects that colonize on the cadaver. Drugs or toxins ingested during the larval development were metabolized and eliminated in the growth course. Hence, the actively feeding maggots have a significantly high concentration of drugs than their post-feeding larvae (Wolff et al. 2001). Drug concentration varies on different tissues, and site-to-site variability in the same organ is also recognized. Since fly’s larval development rate varies depending upon the tissues they occupy, a detailed study of drugs metabolism and excretion in different developmental stages of insects is needed to determine which insect phase is most interesting to detect drugs, which tissue has the highest drug concentration and which insect tissue have most changes due to the drug (Sadler et al. 1995). Some drugs accelerated the developmental events, some drugs delay the colonization of insects for several days, which make changes in insect development significantly and alter the PMI estimates (Introna et al. 1990). Hence there should be a multidisciplinary approach in entomotoxicology (Fig. 2).
Conclusion and future aspects
It is evident from the preceding discussions that insects and other arthropods are reliable specimens for routine forensic entomological analysis, which provides vital information for the investigators regarding the time of death. Entomological evidence was used for the estimation of post-mortem interval (PMI). Two basic approaches for estimating PMI include analyzing the degree of insect development formed from the first egg deposited on the corpse and analyzing the succession pattern of insects colonize on the corpse. For PMI estimation, initially examine the assemblage of insect present, followed by accurate identification, then the time of death can be calculated based on the knowledge of developmental rate and ambient temperature. Many factors affect PMI calculations, but temperature is the fundamental and most studied factor. Insect age determination is a crucial task in forensic entomology. In recent times, several advanced approaches are proposed, and this provides accuracy in age determination. Whereas insect modelling methods still have several limitations, this challenges the reliability of PMI estimation.
Questionably, reference data for age determination of many important species are not available to date, including insect categories such as beetles. Regional genetic variations between populations of the same species show different developmental times when reared at the same temperature, making critical objection towards forensic entomologists for arriving developmental reference data of insect species. The development of a species varies in fluctuating temperature, whereas the reference data of a species generated using constant temperature and from a single location. Nowadays, developmental data of a species from a single location in a constant temperature is applied to estimate PMI of the same species from different locations, which makes significant errors in PMI estimation. Along with temperature, factors such as humidity, photoperiod, and nutrition also influence insect development. At the same time, these factors were ignored in most studies documenting the development of forensically important species, which impact the accuracy of minimum post-mortem interval. More research works are required in this field to overcome such limitations.
References
Adams ZJ, Hall MJ (2003) Methods used for the killing and preservation of blowfly larvae, and their effect on post-mortem larval length. Forensic Sci Int 138(1–3):50–61
Amendt J, Krettek R, Zehner R (2004) Forensic entomology. Naturwissenschaften 91(2):51–65
Amendt J, Richards CS, Campobasso CP, Zehner R, Hall MJ (2011) Forensic entomology: applications and limitations. Forensic Sci Med Pathol 7(4):379–392
Anderson GS (1999) Wildlife forensic entomology: determining time of death in two illegally killed black bear cubs. J Forensic Sci 44(4):856–859
Anderson GS, Byrd J, Castner J (2001) Insect succession on carrion and its relationship to determining time of death. Forensic entomology: the utility of arthropods in legal investigations 143:76
Bala M, Sharma A (2016) Review of some recent techniques of age determination of blow flies having forensic implications. Egypt J Forensic Sci 6(3):203–208
Barton PS, Cunningham SA, Lindenmayer DB, Manning AD (2013) The role of carrion in maintaining biodiversity and ecological processes in terrestrial ecosystems. Oecologia 171(4):761–772
Boehme P, Spahn P, Amendt J, Zehner R (2013) Differential gene expression during metamorphosis: a promising approach for age estimation of forensically important Calliphora vicina pupae (Diptera: Calliphoridae). Int J Legal Med 127(1):243–249
Bornemissza G (1957) An analysis of Arthropod succession in Carrion and the effect of its decomposiion on the soil fauna. Australian J Zool 5(1):1–12
Briere J-F, Pracros P, Le Roux A-Y, Pierre J-s (1999) A novel rate model of temperature-dependent development for arthropods. Environ Entomol 28(1):22–29
Byrd JH, Butler JF (1997) Effects of temperature on Chrysomya rufifacies (Diptera: Calliphoridae) development. J Med Entomol 34(3):353–358
Campobasso CP, Di Vella G, Introna F (2001) Factors affecting decomposition and Diptera colonization. Forensic Sci Int 120(1–2):18–27
Campobasso CP, Introna F (2001) The forensic entomologist in the context of the forensic pathologist’s role. Forensic Sci Int 120(1–2):132–139
Catts EP, Goff ML (1992) Forensic entomology in criminal investigations. Ann Rev Entomol 37(1):253–272
Catts EP, Haskell NH (1990) Entomology & death: a procedural guide. Forensic Entomology Assocs
Chophi R, Sharma S, Sharma S, Singh R (2019) Forensic entomotoxicology: current concepts, trends and challenges. J Forensic Leg Med 67:28–36
de Moretti C, Giannotti T, Thyssen E, Solis PJ, Godoy WAC (2011) Bait and habitat preferences, and temporal variability of social wasps (Hymenoptera: Vespidae) attracted to vertebrate carrion. J Med Entomol 48(5):1069–1075
Dekeirsschieter J, Frederickx C, Verheggen F, Boxho P, Haubruge E (2013) Forensic entomology investigations from Doctor Marcel Leclercq (1924–2008): a review of cases from 1969 to 2005. J Med Entomol 50(5):935–954
Frederickx C, Dekeirsschieter J, Brostaux Y, Wathelet J-P, Verheggen F, Haubruge E (2012) Volatile organic compounds released by blowfly larvae and pupae: New perspectives in forensic entomology. Forensic Sci Int 219(1–3):215–220
Fuller ME (1934) The insect inhabitants of carrion: a study in animal ecology. Bulletin of the Council for Scientific and Industrial Research, Australia( 82)
Gaudry E (2009) The insects colonisation of buried remains. Current concepts in forensic entomology. Springer, pp 273–311
Gaudry E, Blais C, Maria A, Dauphin-Villemant C (2006) Study of steroidogenesis in pupae of the forensically important blow fly Protophormia terraenovae (Robineau-Desvoidy)(Diptera: Calliphoridae). Forensic Sci Int 160(1):27–34
Giao JZ, Godoy WAC (2007) Ovipositional behavior in predator and prey blowflies. J insect Behav 20(1):77–86
Glaister J, Brash JC (1937) Medico-legal aspects of the Ruxton case. W. Wood
Goff ML (1993) Estimation of postmortem interval using arthropod development and successional patterns. Forensic Sci Rev 5:81–81
Greenberg B (1991) Flies as forensic indicators. J Med Entomol 28(5):565–577
Greenberg B, Kunich JC (2002) Entomology and the law: flies as forensic indicators. Cambridge University Press
Hall D, Gerhardt R (2002) Flies (Diptera); in medical and veterinary entomology. Mullen G. L. Academic Press, San Diego, Durden
Hall RD, Huntington T (2001) Introduction: perceptions and status of forensic entomology. Forensic entomology: the utility of arthropods in legal investigations,1–15
Harvey ML, Gasz NE, Voss SC (2016) Entomology-based methods for estimation of postmortem interval. Res Rep forensic Med Sci 6:1–9
Illingworth J Insects attracted to carrion in Southern California. In Proc Hawaii Entomol Soc, 1926 (Vol. 6, pp. 397–401)
Introna F, Dico CL, Caplan YH, Smialek JE (1990) Opiate analysis in cadaveric blowfly larvae as an indicator of narcotic intoxication. J Forensic Sci 35(1):118–122
Jaques HE (1915) The fish-feeding coleoptera of Cedar Point
Johnston W, Villeneuve G (1897) On the medico-legal application of entomology, vol 45315. sn, Sl
Karampela S, Pistos C, Moraitis K, Stoukas V, Papoutsis I, Zorba E et al (2015) Development and validation of a LC/MS method for the determination of ∆9-tetrahydrocannabinol and 11-carboxy-∆9-tetrahydrocannabinol in the larvae of the blowfly Lucilia sericata: Forensic applications. Sci Justice 55(6):472–480
Kashyap V, Pillay V (1989) Efficacy of entomological method in estimation of postmortem interval: A comparative analysis. Forensic Sci Int 40(3):245–250
Ko C (2002) Cases in the history of Chinese trials [English translation of Zhe yu gui jian bu] Lu Shih China. Cambridge University Press, Cambridge
Kulshrestha P, Chandra H (1987) Time since death: an entomological study on corpses. Am J Forensic Med Pathol 8(3):233–238
Leclercq J (2008) Marcel Leclercq (1924–2008), médecin, diptériste, parasitologue et pionnier de l’entomologie forensique (Part. 1). Entomologie faunistique-Faunistic Entomology
Leclercq M (1969) Entomological parasitology. The relations between entomology and the medical sciences. Entomological parasitology. The relations between entomology and the medical sciences.
Leclercq M (1978) Entomologie et médecine légale. Datation de la mort (Vol. 614.1 L459e): París, FR: Masson
Leclercq M, Quinet L (1949) Quelques cas d’application de l’entomologie a la deÂtermination de l’eÂpoque de mort Several cases concerning the application of entomology on determination of postmortem interval. Ann Med LeÂg 29:324–326
Lehane M, Chadwick J, Howe M, Mail T (1986) Improvements in the pteridine method for determining age in adult male and female Stomoxys calcitrans (Diptera: Muscidae). J Econ Entomol 79(6):1714–1719
Lockey KH (1988) Lipids of the insect cuticle: origin, composition and function. Comp Biochem Physiol Part B: Comp Biochem 89(4):595–645
Lord W, Stevenson J (1986) American registered professional entomologists. Washington DC: Chesapeake,42
Mail T, Chadwick J, Lehane M (1983) Determining the age of adults of Stomoxys calcitrans (L.)(Diptera: Muscidae). Bull Entomol Res 73(3):501–525
Mashaly AMA (2017) Carrion beetles succession in three different habitats in Riyadh, Saudi Arabia. Saudi J Biol Sci 24(2):430–435
McKnight B (1981) the washing away of wrongs: forensic medicine in thirteenthcentury China. University of Michigan, Ann Arbor
Mégnin J-P (1887) La faune des tombeaux. Gauthier-Villars
Mégnin P (1894) La faune des cadavres: application de l’entomologie à la médecine légale. Gauthier-Villars
Motter MG (1898) A contribution to the study of the fauna of the grave. A study of on hundred and fifty disinterments, with some additional experimental observations. J New York Entomol Soc 6(4):201–231
Niezabitowski E (1902) Experimentelle Beitrage zur Lehre von der Leichenfauna. Vjschr fg M 23:44–50
Nolte KB, Pinder RD, Lord W (1992) Insect larvae used to detect cocaine poisoning in a decomposed body. J Forensic Sci 37(4):1179–1185
Owings CG, Spiegelman C, Tarone AM, Tomberlin JK (2014) Developmental variation among Cochliomyia macellaria Fabricius (Diptera: Calliphoridae) populations from three ecoregions of Texas, USA. Int J Legal Med 128(4):709–717
Parmenter RR, MacMahon JA (2009) Carrion decomposition and nutrient cycling in a semiarid shrub–steppe ecosystem. Ecol Monogr 79(4):637–661
Payne JA (1965) A summer carrion study of the baby pig Sus scrofa Linnaeus. Ecology 46(5):592–602
Payne JA, King EW, Beinhart G (1968) Arthropod succession and decomposition of buried pigs. Nature 219(5159):1180–1181
Pechal JL, Benbow ME, Crippen TL, Tarone AM, Tomberlin JK (2014) Delayed insect access alters carrion decomposition and necrophagous insect community assembly. Ecosphere 5(4):1–21
Pien K, Laloup M, Pipeleers-Marichal M, Grootaert P, De Boeck G, Samyn N et al (2004) Toxicological data and growth characteristics of single post-feeding larvae and puparia of Calliphora vicina (Diptera: Calliphoridae) obtained from a controlled nordiazepam study. Int J Legal Med 118(4):190–193
Ratte HT (1984) Temperature and insect development. Environmental physiology and biochemistry of insects. Springer, pp 33–66
Reed H Jr (1958) A study of dog carcass communities in Tennessee, with special reference to the insects.American midland naturalist,213–245
Rivers D, Dahlem G (2014) Biology, taxonomy, and natural history of forensically important insects. West Sussex, UK, Wiley & Sons, Ltd., pp 69–94
Rivers DB, Dahlem GA (2014) The science of forensic entomology. John Wiley & Sons
Sadler D, Fuke C, Court F, Pounder D (1995) Drug accumulation and elimination in Calliphora vicina larvae. Forensic Sci Int 71(3):191–197
Sawyer SJ (2017) Effects of Carrion Decomposition. on Arthropod Community Structure and Habitat Seeking Behavior
Sharma R, Garg RK, Gaur J (2015) Various methods for the estimation of the post mortem interval from Calliphoridae: A review. Egypt J Forensic Sci 5(1):1–12
Shirley NR, Wilson RJ, Jantz LM (2011) Cadaver use at the University of Tennessee’s anthropological research facility. Clin Anat 24(3):372–380
Smith S (1951) History and development of forensic medicine. BMJ 1(4707):599
Tarone AM, Jennings KC, Foran DR (2007) Aging blow fly eggs using gene expression: a feasibility study. J Forensic Sci 52(6):1350–1354
Tomberlin JK, Benbow ME, Tarone AM, Mohr RM (2011) Basic research in evolution and ecology enhances forensics. Trends Ecol Evol 26(2):53–55
Verón IF, Zorrilla I, Richards NL (2021) Is deliberate pesticide poisoning of wildlife impacting local insect communities? Wildlife and environmental forensic investigations in southern Spain present an opportunity for collaborative entomological monitoring.Journal of Insect Conservation,1–9
Wells JD, Stevens J, Byrd J, Castner J (2009) Molecular methods for forensic entomology. JH Castner and JL Byrd (éds.), Forensic Entomology: the Utility of Arthropods in Legal Investigations, 2, 437–452
Wilson EE, Wolkovich EM (2011) Scavenging: how carnivores and carrion structure communities. Trends Ecol Evol 26(3):129–135
Wolff M, Uribe A, Ortiz A, Duque P (2001) A preliminary study of forensic entomology in Medellın, Colombia. Forensic Sci Int 120(1–2):53–59
Yovanovitch GP (1888) Entomologie appliquée à la médicine légale. Ollier-Henry
Zehner R, Amendt J, Schütt S, Sauer J, Krettek R, Povolný D (2004) Genetic identification of forensically important flesh flies (Diptera: Sarcophagidae). Int J Legal Med 118(4):245–247
Funding
No funds, grants, or other support was received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest/ competing interests
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Siva Prasad, M.S., Aneesh, E.M. Tools and techniques in forensic entomology- A critical review. Int J Trop Insect Sci 42, 2785–2794 (2022). https://doi.org/10.1007/s42690-022-00823-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-022-00823-5