In this study we analyzed the ovipositional behavior of C. albiceps, C. megacephala and L. eximia in response to previous presence of larvae of different species, both predator and prey. The preference for substrates that previously had had no larvae was predominant for all species. However, the experiments showed that C. megacephala and L. eximia avoid laying eggs principally in patches with previous presence of C. albiceps larvae. The implications of these results for the necrophagous Diptera community dynamics are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Blowflies are carrion breeders and lay eggs usually in batches of 100–300, frequently among eggs of several different species (Smith, 1986). The distribution of immature individuals in discrete breeding sites occurs with the random dispersal of adult females (Blackith and Blackith, 1990) and might influence the level of competition for food and space among the former, with consequences for the viability of resultant adults (De Jong, 1979, 1982) and with a marked effect upon population dynamics, since insect density may differ between patches (Ives and May, 1985; Turchin, 1998).
Chrysomya (Calliphoridae) is a genus of blowflies that includes about thirty abundant and economically important species (Dear, 1985), with distribution restricted to the Old World and Australasia before 1975 (Guimarães et al., 1978; Baumgartner and Greenberg, 1984; Dear, 1985). Chrysomya megacephala, C. putoria, C. albiceps and C. rufifacies are the most well studied species on account of their introduction to the Americas thirty years ago, in addition to their rapid dispersal and colonization process (Guimarães et al., 1978, 1979; Baumgartner and Greenberg, 1984; Greenberg, 1988). The introduction of these species has influenced the Brazilian necrophagous Diptera fauna, as shown by the displacement of Cochliomyia macellaria (Guimarães et al., 1978, 1979; Prado and Guimarães, 1982).
Interspecific interactions have been studied in blowflies, with results showing strong influences of those introduced on native species with respect to competition for food (Wells and Greenberg, 1992a,b,c; Reis et al., 1999). Larval predation in experimental blowfly populations has also been investigated by Faria et al. (1999), Andrade et al. (2002) and Reigada and Godoy (2005). They observed that C. albiceps third instar larvae attacked C. macellaria, C. putoria and C. megacephala third instar larvae, indicating the negative impact of the predator species on the survival of the prey species.
Although the studies focused on interspecific interactions have clarified several important points related to larval behavior, the ovipositional behavior of blowflies has received little attention, principally in the context of interspecific interactions. Choice of ovipositional patches and dispersion of eggs by adult insects can vary among host species or substrate type, among individuals within a host population, or among individuals of a particular host population (Dukas et al., 2001; Holland et al., 2004). Oviposition is a very important biological process for flies, because it determines the potential population size for successive generations (Ullyett, 1950; Smith, 1986).
Especially in taxonomic groups in which the predator-prey relationship is predominant, the choice among ovipositional patches can influence the population positively or negatively in terms of dynamics and persistence (Waage and Greathead, 1986). Generalist predators frequently have complex behavioral mechanisms involving the search for, and the choice and consumption of prey, habits which may influence ovipositional behavior (Hagen, 1987; Scott and Barlow, 1990; Hagen et al., 1999).
Despite intensive research focusing on ovipositional behavior in predator species (Albuquerque et al., 1997; Bargen et al., 1998; Hagen et al., 1999; Sadeghi and Gilbert, 2000), few studies have evaluated the consequences of patch preference on predators that consume different prey species and on the population dynamics of prey (Petersen and Hunter, 2002). Preference for ovipositional patches occurs generally in response to several factors such as suitability of food for offspring, decrease of prey-predator encounters, minimization of risks between natural enemies or prevention of high densities in ephemeral substrates that can be insufficient to support high population sizes (Atkinson and Shorrocks, 1981; Petersen and Hunter, 2002).
In Calliphoridae, studies focusing on ovipositional behavior have not been frequent. The principal studies performed until now focused on patch dynamics and distribution of Diptera in different food substrates (Hanski, 1987; D’Almeida and Almeida, 1998). We believe that the blowfly ovipositional behavior occurs in a random manner. However, there has been no study evaluating the influence of the previous presence of different species, principally in case of larval competitor or predator species. The principal reason to investigate this point is because ovipositional behavior may indirectly affect individual fitness, population dynamics, and community structure (Blaustein, 1999).
In the present study we analyzed the ovipositional behavior of C. albiceps, C. megacephala and L. eximia in response to previous presence of different species in the larval stage in both predator and prey. With this experimental design we intend to show how the previous presence of predator and prey larvae can influence or not the ovipositional behavior in blowflies.
METHODS
Specimens of C. albiceps, C. megacephala and L. eximia were collected in the vicinity of the Campus of Universidade Estadual Paulista, Botucatu, São Paulo, Brazil. Adults were maintained in the laboratory, in cages (30×30×30 cm) covered with nylon at 25±1°C and were fed water and sugar ad libitum. Adult females were fed fresh beef liver to permit the complete development of the gonotrophic cycle (Linhares, 1988). Newly hatched larvae of C. albiceps, C. megacephala and L. eximia were obtained from eggs of adult flies kept at constant temperature (25°C) and 70% relative humidity, and raised in vials containing ground beef in excess.
Experimental Setting for Ovipositional Patch Choice Behavior
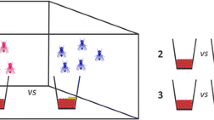
Cylindrical vials (15×13 cm) received three C. albiceps females with developed gonotrophic cycle. Three vials (4×4 cm) were introduced into the larger cylindrical vials to permit oviposition by females of C. albiceps (Fig. 1). Each small vial had 20 g of ground beef with two of them also receiving blowfly larvae. One hundred hatched larvae of L. eximia were introduced into the vial and one hundred larvae of C. megacephala into the other (Fig. 1). This procedure was adopted to permit different options for oviposition by C. albiceps. Thus, C. albiceps females could choose among three treatments: ground beef only, ground beef plus L. eximia larvae and ground beef plus C. megacephala larvae. The number of eggs found in each vial was recorded after 24, 48 and 72 h. Twenty replicates were performed for each treatment. The same experimental protocol was employed to investigate the ovipositional patch choice behavior by C. megacephala, with previous presence of C. albiceps and L. eximia larvae, and to evaluate the patch choice behavior by L. eximia, with previous presence of C. albiceps and C. megacephala.
Statistical Analysis
Comparison of frequency distribution of eggs among ovipositional patches was performed by using the G homogeneity test (Sokal and Rohlf, 1981). The frequency distribution of C. albiceps, C. megacephala and L. eximia eggs was fitted to the Negative binomial and Poisson distributions in order to determine whether the distribution of eggs was clumped or random. The k parameter in the Negative binomial distribution was estimated by the maximum likelihood method (Bliss and Fisher, 1953; Ludwig and Reynolds, 1988) and the fits of the Negative binomial and Poisson distributions were tested by the Pearson χ2 statistic (Sokal and Rohlf, 1981). K values close to zero describe the maximum aggregation level. In the Negative binomial distribution the null hypothesis was that the egg distribution of C. albiceps, C. megacephala and L. eximia exhibits a clumped distribution pattern. In the Poisson distribution the null hypothesis was that the egg distribution follows a random pattern.
RESULTS AND DISCUSSION
Most of the eggs laid by C. albiceps, L. eximia and C. megacephala were found 24 h after the exposure to ovipositional patches. Females of C. albiceps produced two egg batches after 24 h, one in the patch which had ground beef plus C. megacephala larvae and the other in the patch with only ground beef. Females of C. megacephala laid two egg batches in the patch with only ground beef and two in the patch which had ground beef plus L. eximia larvae. Females of L. eximia produced three egg batches in the patch with only ground beef and one in the patch with ground beef plus C. albiceps. The G test was employed to analyze the difference among the numbers of eggs found in the three ovipositional patches, through a period of 72 h. The results suggest that the number of eggs laid by the three species differs significantly among the three ovipositional patches (Table I).
Females of C. albiceps exhibited preference for the patch in which there were no larvae (Fig. 2). Comparing only the ovipositional patches where there were larvae, the difference between the number of C. albiceps eggs laid in each patch was not significant (Table I). With respect to ovipositional habit of C. albiceps, our findings indicate a preference for patches containing no larvae, suggesting that C. albiceps look for empty patches in which to lay eggs, avoiding the presence of other larvae. This result suggests that the risk of competition for food may be influencing the decision of C. albiceps.
Females of L. eximia also showed preference for patches that had no larvae (Fig. 2). However, where there were larvae of C. albiceps the number of L. eximia eggs was clearly lower than in the other patches (Fig. 2). Compared to the ovipositional patches where there were larvae, the difference between the number of eggs of L. eximia also was not significant (Table I). Females of C. megacephala exhibited a slight preference for the ovipositional patch that had only ground beef, followed by the patch with ground beef plus L. eximia larvae (Fig. 2). There was a significant difference between the two ovipositional patches with larvae (Table I). Egg distribution by C. albiceps, C. megacephala and L. eximia in the ovipositional patches trended to a clumped pattern (P<0.05), independent of patch content; and the Negative binomial model seemed to provide a good fit for the data exhibiting K values between 0.01 and 0.07.
It is interesting to note that, although all species showed a preference for patches where there was only ground beef, C. albiceps appears to have been a species that rejected more substrates in which other species had been present previously. In these cases its ovipositional behavior resulted in no more than 321 eggs, versus a higher number of eggs laid by the other species in patches with previous presence of larvae (Fig. 2). Both C. megacephala and L. eximia laid significantly fewer eggs where C. albiceps larvae were previously present, suggesting an ovipositional inhibition when their females faced C. albiceps larvae.
On the other hand, when C. megacephala females faced L. eximia larvae or L. eximia faced C. megacephala larvae, the number of eggs laid was not excessively low. It is possible that the ovipositional avoidance occurred in response to the movement of C. albiceps larvae, which during the third larval instar move vigorously on the substrate searching for food or prey larvae (Faria et al., 1999; Reigada and Godoy, 2005). Hence, our results suggest that the previous presence of C. albiceps larvae can influence the ovipositional behavior of L. eximia and C. megacephala.
Ovipositional behavior has been studied in Diptera, but the emphasis given to previous presence of predator and prey has not been directed to Calliphoridae species (Solar and Ruiz, 1992; Prokopy and Reynolds, 1998; Dukas et al., 2001; Joachim-Bravo et al., 2001). Although these species belong to other taxonomic groups, the results of these studies suggest that the risk of predation can influence the ovipositional behavior of Diptera females.
Of all Calliphoridae species C. albiceps has been the most abundant species in Brazil in recent years (Souza et al., 1997; Carvalho et al., 2001, 2004). We believe that the highly seasonal and spatial abundance of C. albiceps in fact is related to its predatory habit since the larval predation is an interaction capable of influencing the necrophagous fauna composition (Faria et al., 1999). Confronting the results recently found in previous investigations performed in Brazil with other countries, it is possible to conclude that C. albiceps is a species, which has exhibited a remarkable performance in terms of abundance. In this sense we also suppose that the ovipositional behavior of prey species mentioned herein also contributes to the results recorded in the several studies.
This is the first time that ovipositional behavior in Diptera (Calliphoridae) has been evaluated by taking into account the previous presence of prey and predator. Several studies have focused on prey-predator interaction in Calliphoridae, but all were designed to analyze larval interactions (Faria et al., 1999; Faria et al., 2004a,b; Rosa et al., 2004; Reigada and Godoy, 2005). Larval predation in blowflies frequently results in important consequences for community dynamics of necrophagous Diptera since it may significantly influence the abundance of several species (Faria et al., 1999). In addition, the necrophagous fauna composition appears to exhibit expressive variations with respect to diversity, making the interspecific interaction a very important factor to be considered in forensic entomology (Grassberger et al., 2003). In this sense, the focus of the present study, evolving adult behavior, makes an important contribution to the database applied to population ecology and forensic science, since the population potential for future generations essentially depends on ovipositional behavior of the flies.
REFERENCES
Albuquerque, G. S., Tauber, M. J., and Tauber, C. A. (1997). Life-history adaptations and reproductive costs associated with specialization in predacious insects. J. Anim. Ecol. 66: 307–317.
Andrade, J. B., Rocha, F. A., Rodrigues, P., Rosa, G. S., Faria, L. D. B., Von Zuben, C. J., Rossi, M. N., and Godoy, W. A. C. (2002). Larval dispersal and predation in experimental populations of Chrysomya albiceps and Cochliomyia macellaria (Diptera: Calliphoridae). Mem. Inst. Oswaldo Cruz 97: 1137–1140.
Atkinson, W. D., and Shorrocks, B. (1981). Competition on divided and ephemeral resources: A simulation model. J. Anim. Ecol. 50: 461–471
Bargen, H., Saudhof, K., and Poehling, H.-M. (1998). Prey finding by larvae and adult females of Episyrphus balteatus. Entomol. Exp. Appl. 87: 245–254.
Baumgartner, D. L., and Greenberg, B. (1984). The genus Chrysomya (Diptera: Calliphoridae) in the New World. J. Med. Entomol. 21: 105–113.
Blackith, R. E., and Blackith, R. M. (1990). Insect infestation of small corpeses. J. Nat. Hist. 24: 699–709.
Blaustein, L. (1999). Oviposition patch selection in response to risk of predation: Evidence from aquatic habitats and consequences for population dynamics and community structure. In Wasser, S. P. (ed.), Evolutiorary theory and process: Modern perspectives. Dorbrecht, Kluwer, pp. 441–456.
Bliss, C. I., and Fisher, R. A. (1953). Fitting the negative binomial distribution to biological data. Biometrics 9: 176–200.
Carvalho, L. M. L., Linhares, A. X., and Trigo, J. R. (2001). Determination of drug levels and the effect of diazepam on the growth of necrophagous flies of forensic importance in southeastern Brazil. For. Sci. Int. 120: 140–144.
Carvalho, L. M. L., Thyssen, P. J., Goff, M. L., and Linhares, A. X. (2004). Observations on the succession patterns of necrophagous insects onto a pig carcass in an urban area of Southeastern Brazi. Aggrawal’s Int. J. For. Med. Toxicol. 5: 33–39.
D’almeida, J. M., Almeida, J. R. (1998). Nichos tróficos em dípteros caliptrados, no Rio de Janeiro, RJ. Rev. Brasil. Biol. 58: 563–570.
Dear, J. P. (1985). A revision of the New World Chrysomyini (Diptera: Calliphoridae). Revta. Bras. Zool. 3: 109–169.
De Jong, G. (1979). The influence of the distribution of juveniles over patches of food on the dynamics of a population. Neth. J. Zool. 29: 33–51.
De Jong, G. (1982). The influence of dispersal pattern on the evolution of fecundity. Neth. J. Zool. 32: 1–30.
Dukas, R., Prokopy, R. J., Papaj D. R., Duan, J. J. (2001). Egg laying behavior of Mediterranean fruit flies (Diptera: Tephritidae): Is social facilitation important? Florida Entomologist 84: 665–671.
Faria, L. D. B., Orsi, L., Trinca, L. A., and Godoy, W. A. C. (1999). Larval predation by Chrysomya albiceps on Cochliomyia macellaria, Chrysomya megacephala and Chrysomya putoria. Ent. Exp. App. 90: 149–155.
Faria, L. D. B., Godoy, W. A. C., and Trinca, L. A. (2004a). Dynamics of handling time and functional response by larvae of Crysomya albiceps (Dip., Calliphoridae) on different prey species. J. Appl. Entomol. 128: 432–436.
Faria, L. D. B., Godoy, W. A. C., Reis, S. F. (2004b). Larval predation on different instars blowfly populations. Braz. arch. biol. technol. 47: 887–894.
Grassberger, M., Friedrich, E., Reiter, C. (2003). The blowfly Chrysomya albiceps (Wiedemann) (Diptera: Calliphoridae) as a new forensic indicator in Central Europe. Int. J. Leg. Med. 117: 75–81.
Greenberg, B. (1988). Chrysomya megacephala (F.) (Diptera: Calliphoridae) collected in North America and notes on Chrysomya species present in the New World. J. Med. Entomol. 25: 199–200.
Guimarães, J. H., Prado, A. P., and Linhares, A. X. (1978). Three newly introduced blowfly species in Southern Brazil (Diptera: Calliphoridae). Rev. Bras. Ent. 22: 53–60.
Guimarães, J. H., Prado, A. P., and Buralli, M. (1979). Dispersal and distribution of three newly introduced species of Chrysomya Robineau-Desvoidy in Brasil (Diptera, Calliphoridae). Rev. bras. Entomol. 23: 245–255.
Hagen, K. S. (1987). Nutritional ecology of terrestrial insects predators. In: Slansky, F., and Rodriguez, J. G. (eds.), Nutritional ecology of insects, mites, spiders. Related Invertebrates. Wiley, N.Y.
Hagen, K. S., Mills, N. J., Gordh, G., and McMurtry, J. A. (1999). Terrestrial arthropod predators of insect and mite pests. In Bellows, T. S., and Fisher, T. W. (eds.), Handbook of biological control. principles and applications of biological control. San Diego, Academic Press, pp. 383–503.
Hanski, I. (1987). Carrion fly community dynamics: Patchiness, seasonality and coexistence. Ecol. Ent. 12: 257–266.
Holland, J. N., Buchanan, A. L., and Loubeau, R. (2004). Oviposition choice and larval survival of an obligately pollinating granivorous month. Evol. Ecol. Res. 6: 607–618.
Ives, A. R., and May, R. M. (1985). Competition within and between species in a patchy environment—relations between microscopic and macroscopic models. J. Theor. Biol. 115: 65–92.
Joachim-Bravo, J. S., Fernades, O. A., De Bortoli, S. A., and Zucoloto, F. S. (2001). Ecology, behavior and bionomics. Oviposition behavior of Ceratitis capitata Wiedemann (Diptera: Tephitidae): Association between oviposition preference and larval performace in individual females. Neotrop. Entomol. 30: 559–564.
Linhares, A. X. (1988). The gonotrophic cycle of Chrysomya megacephala (Diptera, Calliphoridae) in the laboratory. Revta. Bras. Ent. 32: 383–392.
Ludwig, J. A., and Reynolds, J. F. (1988). Statistical ecology. A primer on methods and computing. New York, John Wiley & Sons.
Petersen, M. K., and Hunter, M. S. (2002). Ovopositional preference and larval—early adult performance of two generalist lacewing predators of aphids in pecans. Biol. Control 25: 101–109.
Prado, A. P., and Guimarães, J. H. (1982). Estado atual da dispersão e distribuição do gênero Chrysomya Robineau-Desvoidy na região neotropical (Diptera, Calliphoridae). Rev. bras. Ent. 26: 225–231.
Prokopy, R. J., and Reynolds A. H. (1998). Ovipositional enhancement through socially facilitated behavior in Rhagoletis pomonella flies. Entomologia Experimentalis et Applicata 86: 281–286.
Reigada, C., and Godoy, W. A. C. (2005). Dispersal and predation behavior in larvae of Chrysomya albiceps and Chrysomya megacephala (Diptera: Calliphoridae). J. Ins. Beh. 18: 543–555.
Reis, S. F., Von Zuben C. J., and Godoy, W. A. C. (1999). Larval aggregation for food in experimental population of Chrysomya putoria (Wied.) and Cochliomyia macellaria (F.) (Dipt. Calliphoridae). J. App. Ent. 123: 485–489.
Rosa, G. S., Carvalho, L. R., and Godoy, W. A. C. (2004). Survival rate, body size and food abundance in pure and mixed blowfly densities. Afr. Entomol. 12: 97–105.
Sadeghi, H., and Gilbert, F. (2000). Oviposition preferences of aphidophagous hoverflies. Ecol. Entomol. 25: 91–100.
Scott, S. M., and Barlow, C. A. (1990). Effect of hunger on the allocation of time among pea plants by the larvae of an aphidophagous hover fly Eupeodes corollae (Dipt.: Syrphidae). Entomophaga 35: 163–172.
Smith, K. G. V. (1986). A manual of forensic entomology. Oxford, Univ. Print. House.
Sokal, R. R., and Rohlf, F. J. (1981). Biometry. NY, W. H. Freeman & Company.
Solar, E., and Ruiz, G. (1992). Behavioral analysis of the choice of oviposition patch by single females of D. melanogaster (Diptera: Drosophilidae). Insect Behav. 5: 571–581.
Souza, A. M., and Linhares, A. X. (1997) Diptera and Coleoptera of potencial forensic importance in Southeastern Brazil: Relative abundance and sasonality. Med. Vet. Entomol. 11: 8–12.
Turchin, P. (1998). Quantitative analysis of movement: measuring and modeling population redistribution in animals and plants. Sinauer and Associates, Sunderland, Massachussetts, USA.
Ullyett, G. C. (1950). Competition for food and allied phenomena in sheep-blowfly populations. Phil. Trans. Royal Soc. London 234: 77–174.
Waage, J., and Greathead, D. (1986). Insect parasitoids. London, Academic Press.
Wells, J. D., and Greenberg, B. (1992a). Rates of predation by Chrysomya rufifacies (Macquart) on Cochliomyia macellaria (Fabr.) (Diptera: Calliphoridae) in the laboratory: Effect of predator and prey development. Pan-Pacific Entomologist 68: 12–14.
Wells, J. D., and Greenberg, B. (1992b). Laboratory interaction between introduced Chrysomya rufifacies and native Cochliomyia macellaria (Diptera: Calliphoridae). Environ. Entomol. 21: 640–645.
Wells, J. D., and Greenberg, B. (1992c). Interaction between Chrysomya rufifacies and Cochlyiomyia macellaria (Diptera: Calliphoridae): The possible consequences of an invasion. Bull Ent. Res. 82: 133–137.
ACKNOWLEDGMENTS
JZG has been supported by scholarships from FAPESP (01/02667-5) and WACG from CNPq. We thank two anonymous reviewers for useful comments and suggestions, which improved the clarity and content of the manuscript. The authors also thank James Welsh for reviewing the English of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gião, J.Z., Godoy, W.A.C. Ovipositional Behavior in Predator and Prey Blowflies. J Insect Behav 20, 77–86 (2007). https://doi.org/10.1007/s10905-006-9064-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10905-006-9064-x