Abstract
This study illustrates the inconsistencies that may result from using different background values and conservative elements in the calculation of enrichment factors. By using 75 soil samples collected from agricultural lands adjacent to Mau and Aberdare forests, Zn and Cu enrichments was assessed using enrichment factor. The geochemical background values used for this study were concentrations of the upper continental crust (UCC), logarithmized UCC concentrations and concentrations of elements in soils from the forested lands as the local background values. Calcium, Mn, and Fe were used as the conservative elements. In addition, Cu and Zn geoaccumulation index was determined for comparison purpose. When UCC values were applied, the average Cu enrichment was 0.24, 1.42 and 15.3 when Mn, Ca and Fe were used as the conservative elements, respectively. The average Zn enrichment was 0.6 when Mn was used, and increased to 4.53 and 31.3 Ca and Fe was used, respectively. Compared to Mn and Ca, Fe resulted to higher enrichments. Application of local background values significantly reduced variation in Zn and Cu enrichments, as the average Zn and Cu enrichments were relatively similar regardless of the conservative element used. Based on this, Zn enrichment ranged from minimal to extreme while Cu enrichment ranged from minimal to high. Cu Igeo ranged from − 1.96 to 2.49 while Zn Igeo ranged from − 2.74 to 2.11, implying that the soils ranged from not polluted to moderately polluted. Zn and Cu contamination levels in the agricultural soils was best evaluated using local geochemical baselines.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Enrichment factor (EF) is a common tool used to identify geogenic versus anthropogenic element sources in the environment, and its application in environmental studies dates back to 1980s [1, 2]. The enrichment factor was initially developed to detect metals in the atmosphere, precipitation, or seawater [3], but it was gradually extended to include different environmental media, including soils and sediments [4,5,6]. EF is defined as the factor by which the concentration of a metal in an environmental media is greater than the average concentration of that metal in the earth’s crust [7]. Apart from being a relatively simple to use method [8], enrichment factor is considered superior to other such indices [9], as it evaluates the concentration of metal of interest in relation to the concentration of lithogenic element with no significant anthropogenic source [10].
However, despite being extensively used to assess metal contamination, several studies have raised credible concerns regarding its suitability due to a number of shortcomings [5, 6, 11, 12]. For instance, there has been discrepancies in deciding what values to use as the background or geochemical reference values [9]. While some countries have already established geochemical background values, e.g. China [13], researchers mainly from countries lacking established geochemical background values may choose to use the established values of a different country, background values derived from other researchers, or the average concentrations of elements in the earth’s crust [9]. This can systematically distort EF values, as the background values may not be a true representative of the study area due to spatial variability [14]. There is also evidence that geochemical baselines from the earth’s crust are not uniform in all regions [15,16,17]. To circumvent this problem, other researchers choose to use the measured concentration of elements in a deeper soil horizon as the local background values [4, 12, 18]. While this may correctly represent metal enrichment of the study area, many of such studies fail to account for metal weathering, volatilization and vertical transport (leaching) into the deep soil horizons [11]. The discrepancies regarding geochemical baselines not only affect EF but other pollution indices as well, including geoaccumulation index (Igeo) [19]. As a result, selecting proper reference values is requisite to accurately determine metals contamination.
Another intrinsic flaw of using enrichment factor is the difficulty of choosing the conservative element [18]. Though a major flaw, little attention is given regarding the compositional differences of conservative elements [14]. Generally, the metal that is naturally available at high concentrations originating almost exclusively from the earth’s crust is used as the conservative element [20,21,22]. The elements include aluminum (Al), zirconium (Zr), iron (Fe), scandium (Sc), titanium (Ti), manganese (Mn) and calcium (Ca) [5, 7, 10, 23]. Application of different conservative elements results to large variations in EF results. For instance, a study by Poh and Tahir [5] demonstrated that the choice of conservative element could easily alter EF results by more than fourfolds. In a similar study, Bern et al.[9] noted that using Fe as the conservative elements gave 84% higher EFs than when using Al. In addition, Reimann and Caritat [6] also noted that use of certain conservative elements led to high enrichment of the metals under investigation, regardless of where the samples were taken. Clearly, these studies suggest that the choice of conservative element is important in finding the correct enrichment of a metal, and a poor choice could lead to serious misinterpretation of assessment results.
Although not common, EF results may also be misinterpreted due to different classifications of EFs available today. Sinex and Helz [1] identified five contamination categories on the basis of the enrichment factor. The lowest category entails EF values < 2, which denotes depletion to minimal enrichment. This lowest category is often misinterpreted. For instance, several studies have come up with their own classification, suggesting that EF value of < 1 may at times indicate depletion of that metal in soils [7, 11], while EFs > 1.5 may show significant portion of metals originating from anthropogenic activities [4]. Further, the impact of biogeochemical processes or natural sources is often neglected in the above classifications [5]. In attempt to account for natural sources of metals, Bacardit and Camarero [24] suggests that enrichment factors < 10 should indicate crustal or natural sources, while those > 10 are an indication of anthropogenic sources. This clearly shows that misinterpretation of results may occur, and as such, care should be taken when interpreting EF results [22]. Due to these shortcomings, several studies are of the opinion that application of EF in environmental studies should be avoided [12]. However, this study set to find out how some of the shortcomings can be reduced.
Here, copper (Cu) and zinc (Zn) enrichment in agricultural lands adjacent to Mau and Aberdare forests in Kenya was investigated. Although Cu and Zn deficiency is common in agricultural soils [25, 26], their enrichment in agricultural soils due to human activities has been reported in various studies [27,28,29,30,31,32]. Copper, for instance, is specifically related to long-term application of commercial fertilizers, copper-based pesticides and fungicides [27, 33]. As for Zn, its variability in agricultural soils is controlled mainly by the application of both mineral fertilizers and animal manure [34]. The two metals are included in the list of potentially toxic elements [35]. Considering the fact that agricultural activities are the major sources of water pollution [36], metal accumulation in cultivated lands should be monitored regularly to prevent possible adverse effects on aquatic ecosystems and the dependent human population.
The principal objective of the study was to determine how application of three different conservative elements and different geochemical baselines may affect EF results for metals. In this particular study, Fe, Mn and Ca were used as the conservative elements, while the concentrations of elements in the upper continental crust (UCC) by Wedepohl [37], and the concentration of elements in forest soils were used as the geochemical baselines. In addition, logarithmized UCC values were also used in attempt to reduce variability that might occur when different conservative elements are applied [8]. Moreover, Cu and Zn geoaccumulation index in the agricultural soils was determined in order to compare with EF results. The agricultural lands adjacent to Mau and Aberdare forests were transformed nearly 40 years ago from the forest lands. Converted forest soils usually undergo several changes, the common one being reduced soil fertility due to nutrient mining [38,39,40]. Without proper soil management practices, parameters such as soil organic carbon (SOC), total nitrogen (TN), soil pH and phosphorous (P) have been reported to decline over time [40, 41]. For the metals however, particularly Zn and Cu, accumulation due to addition of fertilizers, pesticides, herbicides, animal manure and fungicides often occurs [7, 42]. Application of EF was thus important to determine the extent of Zn and Cu contamination as a result of extensive agriculture in these areas.
2 Materials and Methods
2.1 Study Areas
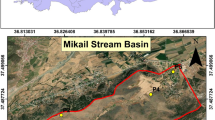
Figure 1 shows the location of study area and study sites. Mau Forest is found between 0° 36′ 35″ S and 35° 44′ 08″ E. It is part of the largest montane forest ecosystem in Eastern Africa, having an area of 400,000 ha [43]. Mau forest serves as the largest drainage basin in the country and origin of several major rivers [43, 44]. On the other hand, the Aberdare Forest is located in central Kenya between 0° 37′ 40″ S and 36° 42′ 30″ E. The forest stretches over 125 km from Nyahururu in the North to Limuru in the South. It also hosts the third highest mountain in Kenya, with two main peaks, Oldonyo Lesatima (also known as Sattima) and Kinangop, which reach altitudes of 4001 and 3906 m, respectively [45]. The Aberdare Forest is the main catchment for Sasumua and Ndakaini dams, and contributes towards the largest river in Kenya, the Tana River [45]. According to Geological Map of Kenya (Fig. 2), the Mau and Aberdare forests are majorly composed of tertiary volcanic deposits (pyroclastics and sediments), although quaternary volcanic deposits can also be found in Narok and Nakuru areas. In general, volcanic soils have lower bulk density, higher ability of retaining water, and form strong associations between minerals and organic matter [46]. This makes the soils fertile and desirable for crop cultivation. Unfortunately, the two forests are increasingly being exploited for cattle grazing, human settlement, agriculture and charcoal burning [47, 48]. In the Mau Forest, for instance, significant land use changes have occurred in less than 40 years. Specifically, 25.5% of forest land was lost in the period 1973–2013, with much of it lost to smallholder and commercial agriculture, and only 273,300 ha currently remain as a closed-canopy forest [49].
For the Aberdare forest, most of it is mostly lost to tea plantations, where it is estimated that 1.4% of the forest land is lost annually [50]. As aforementioned, forest conversion may lead to accumulation of metals, particularly Zn and Cu in the agricultural lands, primarily due to extensive agricultural practices [7]. If not properly monitored, accumulated metals in the agricultural lands can be transported to aquatic ecosystems through soil erosion, where they are capable of bioaccumulating and biomagnifying.
2.2 Soil Sampling
A total of 75 surface soil samples (0–15 cm depth) were collected in farms adjacent to Mau and Aberdare Forests. The sampling points were carefully selected to cover large geographical area and different agricultural lands with varying agricultural techniques, and the lands sampled included tea, maize, wheat and mixed farms. In addition, three undisturbed forest soil samples were collected from both Mau and Aberdare forests to act as background values in the calculation of enrichment factors. From each study site, information regarding the global positioning system (GPS) was recorded. The collected samples were put in polythene bags and taken to Crop Nutrition Laboratory Services Ltd, Nairobi-Kenya for elemental analysis. The soils were then oven dried to remove moisture, grinded and passed through a < 2 mm sieve for homogeneity.
2.3 Sample Extraction and Analysis
The metals Fe, Mn, Cu, Zn and Ca were extracted using aqua regia digestion method. This was achieved by using concentrated HCl and HNO3 in an open vessel in a temperature controlled block according to ISO 11466 [51]. The extracted metals were then analyzed simultaneously using emission spectroscopy (ICP-OES Perkin Elmer Inc). An elaborate quality control procedure incorporating both internal and external quality control was employed. Blanks, 1 control sample and 2 reference samples at the beginning and after every 15 samples were run. To monitor equipment drift, initial and continuous verification at intervals of 10 runs were used. For the calibration standards, the solutions were National Institute of Standards and Technology (NIST) traceable and obtained from Inorganic Ventures Company, USA. Water for dilution was deionised and during analysis had a conductivity of 2 µS/cm. All acids were acquired from Sigma Aldrich. The limit of detection for Cu and Zn was 0.2 mg/kg, while that of Ca, Mn and Fe was 0.5 mg/kg. The rate of recovery of all the metals was above 99%. In addition, soil pH and total organic carbon (TOC) were also analyzed to explore any relationships between them and the metals. Walkley–Black chromic acid wet oxidation method was used to determine the available total organic carbon (TOC) in soils. Oxidizable matter in the soil was oxidized by 1 N potassium dichromate (K2Cr2O7) solution, and the reaction was assisted by the heat generated when two volumes of sulfuric acid (H2SO4) were mixed with one volume of the dichromate. The dichromate was then titrated with ferrous sulphate, where the titer was inversely related to the amount of organic carbon present in the soil sample. Alternatively, the amount of chromic acid (Cr3+) produced was determined colorimetry at 600 nm. For Soil pH, soil samples were mixed with deionized water in a ratio of 1:2 soil–water, and the pH then measured using a high impedance voltmeter (Inolab WTW 9430), which measures the potential between two electrodes on a soil suspension.
2.4 Calculating Enrichment Factors
Zn and Cu enrichment was calculated using a formula proposed by Sinex and Helz [1].
where CM is the content of studied element and CX is the content of the conservative element used for normalization purposes. In the first case, the concentrations of elements in the upper continental crust (UCC) by Wedepohl [37] were used as the geochemical baseline values. These were 14.3 mg/kg, 527 mg/kg, 52 mg/kg and 3.09% and 2.95% for Cu, Mn, Zn, Fe and Ca respectively. These values have been extensively used worldwide to evaluate metal enrichment [23], including in several studies in Kenya [52, 53]. In the second case, same reference values of elements in the UCC were used, only that this time they were logarithmized. This is a modified formula meant to reduce variability that might occur when different conservative elements are used [8]. In the third case, local baseline values were applied, which were the average concentrations of elements obtained from forests soils: Ca = 2987.33 mg/kg, Fe = 202.80 mg/kg, Mn = 208.67 mg/kg, Cu = 0.520 mg/kg, Zn = 4.81 mg/kg. This was under the assumptions that forests soils were not enriched since there was no human interference inside the forests, and that all the agricultural soils were once the forest soils before being converted. Notably, our local geochemical baselines varied from those determined by Wedepohl [37], Kabata-Pendias and Mukherjee [54] (Fe = 4.5%, Mn = 437 mg/kg, Cu = 25 mg/kg and Zn = 52 mg/kg), and Rudnick et al. [55] (Mn = 438.59 mg/kg, Zn = 67 mg/kg, Cu = 28 mg/kg). As mentioned before, geochemical background values are not uniform but they vary within a region and between regions [15], and deviation from local backgrounds was therefore expected [18]. In all the three cases, Fe, Mn and Ca were used as conservative elements as they are considered to have little anthropogenic influences, and are generally found at high concentrations in soils [5, 10, 56, 57]. Five degrees of contamination were recognized on the basis of the enrichment factor: < 2 as minimal, 2–5 as moderate, 5–20 as significant, 20–40 as very high and > 40 as extremely enriched [1].
2.5 Geoaccumulation Index
The geoaccumulation index (Igeo) was also applied to determine the level of pollution. Igeo was calculated using the formular proposed by Muller [58].
where CM is the content of studied element and Bn is the background value. Similar to EF, UCC values and local baselines were both applied. A factor of 1.5 is usually introduced to reduces possible variation due to lithogenic effects [19]. Igeo values were classified into seven classes: Not polluted (≤ 0), slight pollution (0–1), moderate pollution (1–2), moderate to high pollution (2–3), high pollution (3–4), high to very high pollution (4–5), and extreme pollution (≥ 5) [58].
All data was analyzed using SPSS Version 24 while maps were drawn using ArcMap 10.5.
3 Results
3.1 Descriptive Statistics
Results for the concentration of elements and soil pH are delineated in Table 1. The median concentration of Ca, Mn, Fe, Zn, TOC and Cu were 2190 mg/kg, 193 mg/kg, 170 mg/kg, 7.13 mg/kg, 5.49% and 0.66 mg/kg respectively arranged in decreasing order. The concentrations of Ca, Fe and Mn in the soils was higher compared to Cu and Zn, and possibly originating explicitly from natural sources. This made Ca, Mn and Fe be considered as good conservative elements to be used in the calculation of enrichment factors. Ca, Cu, and Mn were more distributed in the North Eastern parts of Aberdare Forest, but Fe distribution was similar to that of Zn, and were found in relatively higher concentrations in the southern parts of Mau Forest (Fig. 3). Cu was highest in site 25 (4.37 mg/kg), and this was a vegetable cultivated land near a river, therefore there were high chances of Cu contamination in water. On the other hand, Zn was highest in site 53 (31.2 mg/kg), which was a small maize cultivated farm in Elburgon, and animal manure may be the probable reason for the relatively high Zn. Soil pH ranged from 3.78 to 7.88, and its median was 5.51. Notably, 94.6% of the study sites had a pH < 7, thus most agricultural lands adjacent to Mau and Aberdare forests were considered to have acidic soils. This was attributed to high annual precipitation in the tropical area [59], as well as addition of nitrogen-based fertilizers that have been linked to increased soil acidity in agricultural soils [60,61,62]. The concentrations of TOC were lower than the recommended levels in Kenyan agricultural soils [63], probably due to removal of organic matter in terms of produce (organic matter mining).
In comparison with other studies in Kenya, the average Zn (8.86 mg/kg) and Cu (1.02 mg/kg) concentrations in our study area were lower than those recorded in Mai Mahiu and Juja area in Kenya by Mungai et al. [64] (Zn = 247.4 mg/kg, Cu = 88.59 mg/kg), and in West Pokot by Wanjala et al. [65] (Zn = 73.49 mg/kg, Cu = 46.91 mg/kg). This was attributed to longer time of exposure to the metals due to agricultural activities in Juja, Mai Mahiu and West Pokot. Soils from central part of Kenya had relatively similar Zn and Cu concentrations with our study (Zn = 12.7 mg/kg, Cu = 2.734 mg/kg) [66], while those from Kapsabet and Eldoret had even lower Zn and Cu concentrations that averaged to 0.87 and 2.3 for Zn, and 0.04 and 0.34 for Cu in Kapsabet and Eldoret, respectively [66, 67]. However, Zn and Cu recorded in agricultural soils in Europe were higher [68], possibly due to more intensive agriculture in Europe. Nevertheless, Zn and Cu concentrations in our study were below the set guidelines by World Health Organization guidelines for agricultural soils (Zn = 300 mg/kg, Cu = 100 mg/kg) [69], and the Canadian Council of Ministers of the Environment, soil quality guidelines for agricultural soils (Zn = 250 mg/kg, Cu = 63 mg/kg) [70].
To identify significant relationships between and among the soil variables, that is soil pH, Ca, TOC and the metals, Pearson’s correlation analysis was conducted. Data skewness ranged between + 2/− 2 for all the parameters (Table 1), and Shapiro–Wilk and Kolmogorov–Smirnov normality tests found that Ca, Fe, Cu, and Zn were normally distributed, but Mn, pH and TOC were not normally distributed. Therefore, data was normalized through log transformation before correlation analysis was conducted as recommended by Reimann et al. [71]. As shown in Table 2, soil pH correlated strongly and positively with Ca and Cu (r = 0.866 and 0.659 at p < 0.01 respectively), meaning that soil pH may have influenced Cu and Ca concentration through sorption and desorption reactions [72]. Mn–Fe, Fe–Zn, Mn–Cu correlations were significant but weak, to indicate that the three metals had some comparable characteristics.
3.2 Zn and Cu Enrichment Factors
Results for Cu and Zn enrichment using UCC concentrations as the background values are given in Table 3. When Mn was used as the conservative element, the EF values of Cu ranged from 0.03 to 1.10 with a mean of 0. 24. According to Sinex and Helz [1], this translated to all the study sites being classified as depleted or minimal enriched. Specifically, only one study site, site 13, had an EF > 1. The site was located in an agricultural land close to a tarmac road, and vehicular emission, especially particles from car brakes, could have contributed to the Cu enrichment [73]. Thus, based on enrichment classifications by Desaules [11] and Loska [7], 98.7% of the study sites were categorized as depleted. In comparison, Cu EF ranged from 0.23 to 8.19 with a mean of 1.42 when Ca was used as the conservative element, meaning that the average Cu EF had increased by almost a sixfold. The sites were categorized to vary from minimal to significantly enriched. A similar case was observed when Fe was used as the conservative element, where the mean enrichment for Cu increased significantly by a larger margin of 63-fold in relation to Mn. Based on these results, Cu EF ranged from moderate to extreme enrichment. 20% of the sites were moderately enriched, 59% significantly enriched, 16% highly enriched and 5% as extremely enriched.
For Zn metal, its enrichment in the agricultural soils ranged from 0.06 to 2.41, with a mean of 0.6 when Mn was used as the conservative element. This was categorized to range from depletion to moderate enrichment. Zn enrichment also ranged from 0.23 to 49.0 when using Ca and from 4.47 to 182.4 when using Fe. It was apparent that average enrichment of Zn was 7.5 times higher when Ca was used and 52 times higher when Fe was used. In a similar study conducted in cultivated lands of Southern Poland, extremely high enrichment for arsenic was recorded when Ca was used as the conservative element. When chromium was used however, arsenic enrichment in soils were all < 20, indicating significant enrichment [8]. Notably, using Fe as the conservative element led to even higher Cu and Zn enrichments compared to using Mn and Ca. This was similar to a study by Abrahim and Parker [18], which showed that using Fe as a conservative element gave higher enrichment values for Cu, Zn, cadmium (Cd) and lead (Pb). In Nigeria, Cu and Zr were highly enriched in all sites when Fe was used as the reference element but poorly enriched in all the locations when Ti was used instead [74]. Our results also agrees with a study by Bern et al. [9], which noted that using Fe as the conservative elements gave 84% higher EFs than when using Al.
When concentrations of the upper continental crust were standardized by log transformation, very low Zn and Cu EFs were recorded, all < 1. The maximum recorded Cu EFs were 0.07, 0.015 and 0.301, while the maximum Zn EFs were 0.378, 0.225 and 0.806 when Mn, Ca and Fe were used as the conservative elements, respectively (Table 3). All the study sites were therefore classified as minimal or depleted. Mean Cu enrichment was lowest when using Ca, and increased by fivefold and tenfold when using Mn and Fe as the conservative elements, respectively. Similarly, mean Zn was lowest when Ca was used as the conservative element, and increased by fourfold and sixfold when Mn and Fe were used, respectively. It was apparent that using standardized background values reduced the variations by large margins, especially the variation caused by Fe. Our results are in agreement with Loska et al. [8], noting that application of logarithmized geochemical baselines rather than unlogarithmized values reduced vast differences in EFs, thus enabling a normal distribution of results. However, logarithmized background values led to extremely low EFs, which may not be a true reflection of our study site.
When forest soils were used as the baseline values, Cu and Zn enrichments were relatively similar, regardless the element used as a conservative element. The mean enrichment of Cu was 2.58, 3.95 and 2.76 while mean enrichment factor for Zn was 2.58, 4.97 and 2.23 when Mn, Ca and Fe were used as the conservative elements, respectively. Cu enrichment ranged from 0.281 to 30.19, representing minimal/depletion to high enrichment. On the other hand, Zn EF ranged from 0.249 to 53.7, also representing minimal/depletion to extreme enrichment. Application of local or study-specific background values significantly reduced the variation caused by the use of different conservative elements. Cu registered highest enrichment when Fe was used, a value of 30.2, while Zn reported highest enrichment when Ca was used as the conservative element.
3.3 Zn and Cu Igeo Index
Results from geoaccumulation index are given in Table 4. When UCC values were used, the geoaccumulation index for Zn and Cu in all the study sites was < 0, and therefore all the sites were classified as not polluted. However, when local baselines were used, Cu Igeo ranged from − 1.96 to 2.49 while Zn Igeo ranged from − 2.74 to 2.11. Accordingly, 53.3% of the study sites were categorized as not polluted with Cu, 18.7% as slightly polluted, 26.7% as moderately polluted and only 1.3% as moderately to highly polluted with Cu. On the other hand, 50.7% of the sites were categorized as not polluted with Zn, 32% as slightly polluted, 16% as moderately polluted and 1.3% as moderately to highly polluted with Zn. Although the 1.5 factor was meant to reduce variation caused by lithogenic effects, we still recorded different results when two geochemical baselines were used. This was expected, especially because application of different geochemical baselines leads to varying geoaccumulation index results [18, 19].
3.4 Comparing EF and Igeo Indices
Here, only results obtained when local baselines were applied were compared. Cu Igeo correlated positively with Cu EF when Mn and Fe were used as the conservative elements; r = 0.574 and 0.643 at p < 0.01, respectively. Similar results were found for Zn, where Zn Igeo correlated positively with Zn EF when Mn and Fe were used as the conservative elements; r = 0.682 and 0.728 at p < 0.01, respectively. Cu and Zn Igeo correlation with Cu and Zn EF when using Ca was low and insignificant. These results imply that Ca was not a good conservative element as the results did not correlate with Igeo values. Notably, Zn and Cu Igeo had stronger relationship with Cu and Zn EF when Fe was used as the conservative element compared to Mn. Among the three conservative elements, it may be suggested that application of Fe might result to more accurate Cu and Zn enrichments. However, more research on this is needed to make a solid conclusion.
As seen from this study, deciding on which geochemical background values to use is very important in the application of enrichment factor and geoaccumulation index. Our results indicate that application of local baselines may be the best approach, and this finding has been echoed by other studies as well [18]. Using the average concentrations of the upper continental crust disregards natural geochemical variation, and as noted by past researchers, background values of elements in soils are subject to variations [14, 15]. Local background values may be derived from regions suspected to have minimal human interference, such as deep soil horizons, mountain soils and forest soils [56, 75]. When done correctly, more precise enrichment factors in a region can be determined. However, care should also be taken, as in some cases, metal contamination has been recorded in such places, mainly due to atmospheric deposition and downward leaching of metals [22, 76].
4 Conclusion
Current study revealed that there was significant variation in Zn and Cu enrichments when Fe, Mn and Ca were used as conservative elements. Notably, use of Fe gave higher enrichments compared to Mn and Ca. Similarly, using different geochemical background values also resulted to varying Zn and Cu enrichments. Concentrations of upper continental crust gave highest enrichments while logarithmized background values gave the lowest EFs. However, application of study-specific or local background values was recommended, as they resulted to less variation in Cu and Zn enrichment regardless of the conservative element used. Based on average EF values when local baselines were used, Cu enrichment in the agricultural soils ranged from minimal to high enrichment, while Zn ranged from minimal to extreme enrichment. On the other hand, geoaccumulation index concluded that the soils ranged from not polluted to highly polluted with Cu and Zn. When Igeo and EF results were compared, a stronger relationship was observed when Fe was used as the conservative element. This study was able to demonstrate the major flaws of using enrichment factors, and noted that use of local geochemical baselines may be a more realistic approach towards the correct use of enrichment factor and the geoaccumulation index.
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Sinex S, Helz G (1981) Regional geochemistry of trace elements in Chesapeake Bay sediments. Environ Geol 3:315–323. https://doi.org/10.1007/BF02473521
Hakanson L (1980) An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res 14:975–1001. https://doi.org/10.1016/0043-1354(80)90143-8
Qingjie G, Jun D, Yunchuan X, Qingfei W, Liqiang Y (2008) Calculating pollution indices by heavy metals in ecological geochemistry assessment and a case study in parks of Beijing. J China Univ Geosci 19:230–241. https://doi.org/10.1016/S1002-0705(08)60042-4
Barbieri M (2016) The importance of enrichment factor (EF) and geoaccumulation index (Igeo) to evaluate the soil contamination. J Geol Geophys 5:1–4. https://doi.org/10.4172/2381-8719.1000237
Poh S-C, Tahir NM (2017) The common pitfall of using enrichment factor in assessing soil heavy metal pollution. Malays J Anal Sci 21:52–59. https://doi.org/10.17576/mjas-2017-2101-07
Reimann C, Caritat Pd (2000) Intrinsic flaws of element enrichment factors (EFs) in environmental geochemistry. Environ Sci Technol 34:5084–5091. https://doi.org/10.1021/es001339o
Loska K, Wiechuła D, Pelczar J (2005) Application of enrichment factor to assessment of zinc enrichment/depletion in farming soils. Commun Soil Sci Plant Anal 36:1117–1128. https://doi.org/10.1081/CSS-200056880
Loska K, Wiechula D, Barska B, Cebula E, Chojnecka A (2003) Assessment of arsenic enrichment of cultivated soils in Southern Poland. Pol J Environ Stud 12:187–192
Bern CR, Walton-Day K, Naftz DL (2019) Improved enrichment factor calculations through principal component analysis: examples from soils near breccia pipe uranium mines, Arizona, USA. Environ Pollut 248:90–100. https://doi.org/10.1016/j.envpol.2019.01.122
Blaser P, Zimmermann S, Luster J, Shotyk W (2000) Critical examination of trace element enrichments and depletions in soils: As, Cr, Cu, Ni, Pb, and Zn in Swiss forest soils. Sci Total Environ 249:257–280. https://doi.org/10.1016/S0048-9697(99)00522-7
Desaules A (2012) Critical evaluation of soil contamination assessment methods for trace metals. Sci Total Environ 426:120–131. https://doi.org/10.1016/j.scitotenv.2012.03.035
Reimann C, de Caritat P (2005) Distinguishing between natural and anthropogenic sources for elements in the environment: regional geochemical surveys versus enrichment factors. Sci Total Environ 337(1–3):91–107. https://doi.org/10.1016/j.scitotenv.2004.06.011
Shifaw E (2018) Review of heavy metals pollution in China in agricultural and urban soils. J Health Pollut 8:180607. https://doi.org/10.5696/2156-9614-8.18.180607
Anderson RH, Kravitz MJ (2010) Evaluation of geochemical associations as a screening tool for identifying anthropogenic trace metal contamination. Environ Monit Assess 167:631–641. https://doi.org/10.1007/s10661-009-1079-2
Reimann C, Garrett RG (2005) Geochemical background—concept and reality. Sci Total Environ 350(1–3):12–27. https://doi.org/10.1016/j.scitotenv.2005.01.047
de Caritat P, Reimann C, Smith DB, Wang X (2018) Chemical elements in the environment: multi-element geochemical datasets from continental-to national-scale surveys on four continents. Appl Geochem 89:150–159. https://doi.org/10.1016/j.apgeochem.2017.11.010
Reimann C, de Caritat P (2017) Establishing geochemical background variation and threshold values for 59 elements in Australian surface soil. Sci Total Environ 578:633–648. https://doi.org/10.1016/j.scitotenv.2016.11.010
Abrahim G, Parker R (2008) Assessment of heavy metal enrichment factors and the degree of contamination in marine sediments from Tamaki Estuary, Auckland, New Zealand. Environ Monit Assess 136:227–238. https://doi.org/10.1007/s10661-007-9678-2
Kowalska JB, Mazurek R, Gąsiorek M, Zaleski T (2018) Pollution indices as useful tools for the comprehensive evaluation of the degree of soil contamination—a review. Environ Geochem Health 40:2395–2420. https://doi.org/10.1007/s10653-018-0106-z
Jain C (2004) Metal fractionation study on bed sediments of River Yamuna, India. Water Res 38:569–578. https://doi.org/10.1016/j.watres.2003.10.042
Thuong NT, Yoneda M, Shimada Y, Matsui Y (2015) Assessment of trace metal contamination and exchange between water and sediment systems in the To Lich River in inner Hanoi, Vietnam. Environ Earth Sci 73:3925–3936. https://doi.org/10.1007/s12665-014-3678-7
Githaiga KB, Njuguna SM, Makokha VA, Wang J, Gituru RW, Yan X (2020) Assessment of Cu, Zn, Mn, and Fe enrichment in Mt. Kenya soils: evidence for atmospheric deposition and contamination. Environ Monit Assess 192:167. https://doi.org/10.1007/s10661-020-8123-7
Bourennane H, Douay F, Sterckeman T, Villanneau E, Ciesielski H, King D, Baize D (2010) Mapping of anthropogenic trace elements inputs in agricultural topsoil from Northern France using enrichment factors. Geoderma 157:165–174. https://doi.org/10.1016/j.geoderma.2010.04.009
Bacardit M, Camarero L (2010) Atmospherically deposited major and trace elements in the winter snowpack along a gradient of altitude in the Central Pyrenees: the seasonal record of long-range fluxes over SW Europe. Atmos Environ 44:582–595. https://doi.org/10.1016/j.atmosenv.2009.06.022
Alloway BJ (2008) Zinc in soils and crop nutrition, 2nd edn. IZA and IFA, Brussels
Reimann C, Fabian K, Flem B, Englmaier P (2019) The large-scale distribution of Cu and Zn in sub-and topsoil: separating topsoil bioaccumulation and natural matrix effects from diffuse and regional contamination. Sci Total Environ 655:730–740. https://doi.org/10.1016/j.scitotenv.2018.11.248
Kelepertzis E (2014) Accumulation of heavy metals in agricultural soils of Mediterranean: insights from Argolida basin, Peloponnese, Greece. Geoderma 221:82–90. https://doi.org/10.1016/j.geoderma.2014.01.007
Keshavarzi A, Kumar V (2019) Ecological risk assessment and source apportionment of heavy metal contamination in agricultural soils of Northeastern Iran. Int J Environ Health Res 29:544–560. https://doi.org/10.1080/09603123.2018.1555638
He ZL, Yang XE, Stoffella PJ (2005) Trace elements in agroecosystems and impacts on the environment. J Trace Elem Med Biol 19:125–140. https://doi.org/10.1016/j.jtemb.2005.02.010
Fagnano M et al (2020) Copper accumulation in agricultural soils: Risks for the food chain and soil microbial populations. Sci Total Environ 734:139434. https://doi.org/10.1016/j.scitotenv.2020.139434
Li X et al (2020) Status of copper accumulation in agricultural soils across China (1985–2016). Chemosphere 244:125516. https://doi.org/10.1016/j.chemosphere.2019.125516
Baran A, Wieczorek J, Mazurek R, Urbański K, Klimkowicz-Pawlas A (2018) Potential ecological risk assessment and predicting zinc accumulation in soils. Environ Geochem Health 40:435–450. https://doi.org/10.1007/s10653-017-9924-7
Acosta J, Faz A, Martínez-Martínez S, Arocena J (2011) Enrichment of metals in soils subjected to different land uses in a typical Mediterranean environment (Murcia City, southeast Spain). Appl Geochem 26:405–414. https://doi.org/10.1016/j.apgeochem.2011.01.023
Nicholson FA, Smith SR, Alloway B, Carlton-Smith C, Chambers B (2003) An inventory of heavy metals inputs to agricultural soils in England and Wales. Sci Total Environ 311:205–219. https://doi.org/10.1016/S0048-9697(03)00139-6
Reimann C, Fabian K, Birke M, Filzmoser P, Demetriades A, Négrel P, Oorts K, Matschullat J, de Caritat P, Albanese S (2018) GEMAS: Establishing geochemical background and threshold for 53 chemical elements in European agricultural soil. Appl Geochem 88:302–318. https://doi.org/10.1016/j.apgeochem.2017.01.021
Bhat MM, Narain K, Shukla R, Yunus M (2013) Apportionment of pollution loads arising from catchments in pond water bodies. Adv Appl Sci Res 4:436–441
Wedepohl KH (1995) The composition of the continental crust. Geochim Cosmochim Acta 59:1217–1232. https://doi.org/10.1016/0016-7037(95)00038-2
Saviozzi A, Levi-Minzi R, Cardelli R, Riffaldi R (2001) A comparison of soil quality in adjacent cultivated, forest and native grassland soils. Plant Soil 233:251–259. https://doi.org/10.1023/A:1010526209076
Adejuwon J, Ekanade O (1988) A comparison of soil properties under different land use types in a part of the Nigerian cocoa belt. CATENA 15:319–331
Balota EL, Yada IFU, Amaral HF, Nakatani AS, Hungria M, Dick RP, Coyne MS (2015) Soil quality in relation to forest conversion to perennial or annual cropping in southern Brazil. Rev Bras Ciênc Solo 39:1003–1014. https://doi.org/10.1590/01000683rbcs20140675
Evrendilek F, Celik I, Kilic S (2004) Changes in soil organic carbon and other physical soil properties along adjacent Mediterranean forest, grassland, and cropland ecosystems in Turkey. J Arid Environ 59:743–752. https://doi.org/10.1016/j.jaridenv.2004.03.002
Xu Y, Yu W, Ma Q, Zhou H (2013) Accumulation of copper and zinc in soil and plant within 10-year application of different pig manure rates. Plant Soil Environ 59:492–499. https://doi.org/10.17221/121/2013-PSE
Were K, Singh BR, Dick ØB (2016) Spatially distributed modelling and mapping of soil organic carbon and total nitrogen stocks in the Eastern Mau Forest Reserve, Kenya. J Geog Sci 26:102–124. https://doi.org/10.1007/s11442-016-1257-4
Olang LO, Kundu PM (2011) Land degradation of the Mau forest complex in Eastern Africa: a review for management and restoration planning. Environ Monit. https://doi.org/10.5772/28532
Lambrechts C, Woodley B, Church C, Gachanja M (2003) Aerial survey of the destruction of the Aberdare Range forests Division of Early Warning and Assessment, UNEP
Aksa D, Tanaka N, Ohta S, Effendi S (2000) Characteristics of soils developed on volcanic parent materials in the Barong Tongkok Area. In: Rainforest ecosystems of East Kalimantan. Springer, pp 89–96. https://doi.org/10.1007/978-4-431-67911-0_8.
Bewernick T (2016) Mapping forest degradation in the Mau Forest Complex using NDFI time series. Wageningen University and Research Centre, The Netherlands.
Louppe D, Depommier D, Kabengi M (2009) Rehabilitation of the Aberdare forest ecosystem. A project implemented by the Green Belt Movement and supported by the French Agency for Development: a report of the mid term review mission (CIRAD Consultancy team, Nairobi, September, 17–October 02, 2009)
Swart R (2016) Monitoring 40 years of land use change in the Mau Forest Complex, Kenya. A land use change driver analysis
Kaniaru MP (2013) The Effect of tea growing on forest degradation in Chinga area of The Aberdare Ranges in Nyeri County, Kenya Kenyatta University
ISO (1995) International Standard Organization (ISO) 11466: Soil quality-Extraction of trace elements soluble in aqua regia. ISO Genebra
Maina CW, Sang JK, Raude JM, Mutua BM (2019) Geochronological and spatial distribution of heavy metal contamination in sediment from Lake Naivasha, Kenya. J Radiat Res Appl Sci 12:37–54. https://doi.org/10.1080/16878507.2019.1593718
Olando G, Olaka LA, Okinda PO, Abuom P (2020) Heavy metals in surface sediments of Lake Naivasha, Kenya: spatial distribution, source identification and ecological risk assessment. SN Appl Sci 2:279. https://doi.org/10.1007/s42452-020-2022-y
Kabata-Pendias A, Mukherjee AB (2007) Trace elements from soil to human. Springer Science & Business Media. https://doi.org/10.1007/978-3-540-32714-1
Rudnick R, Gao S, Holland H, Turekian K (2003) Composition of the continental crust. In: Holland HD, Turekian KK (eds) Treatise on Geochemistry. The crust, vol 3, pp 1–64. https://doi.org/10.1016/B0-08-043751-6/03016-4.
Bounouira H et al (2018) Study of heavy metal assessment in the Gharb plain along Sebou river (Morocco) using k0-NAA method at the Moroccan Triga Mark II research reactor. Ann Agrar Sci 16:376–388. https://doi.org/10.1016/j.aasci.2018.08.002
Chen H, Teng Y, Lu S, Wang Y, Wang J (2015) Contamination features and health risk of soil heavy metals in China. Sci Total Environ 512:143–153. https://doi.org/10.1016/j.scitotenv.2015.01.025
Muller G (1969) Index of geoaccumulation in sediments of the Rhine River. GeoJournal 2:108–118
Slessarev E, Lin Y, Bingham N, Johnson J, Dai Y, Schimel J, Chadwick O (2016) Water balance creates a threshold in soil pH at the global scale. Nature 540:567–569. https://doi.org/10.1038/nature20139
Han J, Shi J, Zeng L, Xu J, Wu L (2015) Effects of nitrogen fertilization on the acidity and salinity of greenhouse soils. Environ Sci Pollut Res 22:2976–2986. https://doi.org/10.1007/s11356-014-3542-z
Zhou J, Xia F, Liu X, He Y, Xu J, Brookes PC (2014) Effects of nitrogen fertilizer on the acidification of two typical acid soils in South China. J Soils Sediments 14:415–422. https://doi.org/10.1007/s11368-013-0695-1
Gerald A, Zeynab W (2019) Kenya's soils are too acidic for growing crops,study reveals. Nation media Group, Nairobi. p 10
Akenga P, Salim A, Onditi A, Yusuf A, Waudo W (2014) Determination of selected micro and macronutrients in sugarcane growing soils at Kakamega North District, Kenya. IOSR J Appl Chem 7:34–41
Mungai TM, Owino AA, Makokha VA, Gao Y, Yan X, Wang J (2016) Occurrences and toxicological risk assessment of eight heavy metals in agricultural soils from Kenya, Eastern Africa. Environ Sci Pollut Res 23:18533–18541. https://doi.org/10.1007/s11356-016-7042-1
Wanjala F, Hashim N, Otwoma D, Nyambura C, Kebwaro J, Ndege M, Bartilol S (2020) Environmental assessment of heavy metal pollutants in soils and water from Ortum, Kenya. Environ Monit Assess 192:1–15. https://doi.org/10.1007/s10661-020-8070-3
Ndungu AW, Yan X, Makokha VA, Githaiga KB, Wang J (2019) Occurrence and risk assessment of heavy metals and organochlorine pesticides in surface soils, Central Kenya. J Environ Health Sci Eng 17:63–73. https://doi.org/10.1007/s40201-018-00326-x
Ngure V, Sitati N, Shisia S, Kinuthia G (2015) Assessment of heavy metals pollution in urban soils and the implications to consumers health Assessment 5
Tóth G, Hermann T, Da Silva M, Montanarella L (2016) Heavy metals in agricultural soils of the European Union with implications for food safety. Environ Int 88:299–309. https://doi.org/10.1016/j.envint.2015.12.017
Kamunda C, Mathuthu M, Madhuku M (2016) Health risk assessment of heavy metals in soils from Witwatersrand Gold Mining Basin, South Africa. Int J Environ Res Public Health 13:663. https://doi.org/10.3390/ijerph13070663
CCME (1999) Canadian Soil Quality Guidelines for the Protection of Environmental and Human Health
Reimann C, Filzmoser P, Hron K, Kynčlová P, Garrett R (2017) A new method for correlation analysis of compositional (environmental) data—a worked example. Sci Total Environ 607:965–971. https://doi.org/10.1016/j.scitotenv.2017.06.063
Caporale AG, Violante A (2016) Chemical processes affecting the mobility of heavy metals and metalloids in soil environments. Curr Pollut Rep 2:15–27. https://doi.org/10.1007/s40726-015-0024-y
Thorpe A, Harrison RM (2008) Sources and properties of non-exhaust particulate matter from road traffic: a review. Sci Total Environ 400:270–282. https://doi.org/10.1016/j.scitotenv.2008.06.007
Ediagbonya T (2016) Enrichment factor of atmospheric trace metal using zirconium titanium iron and copper as reference element. Niger J Technol 35:785–795. https://doi.org/10.4314/njt.v35i4.13
Gałuszka A (2007) A review of geochemical background concepts and an example using data from Poland. Environ Geol 52:861–870. https://doi.org/10.1007/s00254-006-0528-2
Sucharovà J, Suchara I, Hola M, Marikova S, Reimann C, Boyd R, Filzmoser P, Englmaier P (2012) Top-/bottom-soil ratios and enrichment factors: what do they really show? Appl Geochem 27(1):138–145. https://doi.org/10.1016/j.apgeochem.2011.09.025
Acknowledgements
This work was supported by Sino-Africa Joint Research Center (number: SAJC202102), Chinese Academy of Sciences and National Natural Sciences of China, (number: NSFC 31361140360) and the Chinese Academy of Science—The World Academy of Science (CAS-TWAS) presidential fellowship.
Funding
This work was supported by Sino-Africa Joint Research Center (Number: SAJC202102), Chinese Academy of Sciences and National Natural Sciences of China, (Number: NSFC 31361140360) and the Chinese Academy of Science—The World Academy of Science (CAS-TWAS) presidential fellowship.
Author information
Authors and Affiliations
Contributions
Project idea and proposal writing by KBG and XY; project approval and funds acquisition by XY; fieldwork and data collection by KBG and SMN; data analysis by KBG and SMN; manuscript writing by KBG; manuscript review and correction by all the authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Githaiga, K.B., Njuguna, S.M. & Yan, X. Local Geochemical Baselines Reduce Variation Caused by the Use of Different Conservative Elements in Predicting Cu and Zn Enrichment in Agricultural Soils, Kenya. Chemistry Africa 4, 869–880 (2021). https://doi.org/10.1007/s42250-021-00256-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-021-00256-6