Abstract
Mountains are the preferred sites for studying long-range atmospheric transportation and deposition of heavy metals, due to their isolation and steep temperature decrease that favors cold trapping and condensation of particulate forms of heavy metals. Any enrichment of heavy metals in mountains is presumed to primarily occur through atmospheric deposition. In this particular study, we assessed the status of 27 subsurface soils collected along two elevation gradients of Mt. Kenya using enrichment factors (EFs) as the ecological risk assessments. The collected soils were analyzed for total organic carbon, zinc (Zn), iron (Fe), manganese (Mn), and copper (Cu). The mean concentration of Mn, Fe, Zn, and Cu was 0.376 mg/kg, 47.6 mg/kg, 12.3 mg/kg, and 4.88 mg/kg in Chogoria and 0.560 mg/kg, 113 mg/kg, 12.7 mg/kg, and 2.70 mg/kg in Naro Moru respectively. These concentrations were below the US-EPA maximum permissible levels for soils, implying that the levels recorded had low toxicity. Meanwhile, the mean enrichment factors for Mn, Cu, and Zn were 0.447, 131, and 78.8 in Chogoria and 0.463, 38.9, and 53.0 in Naro Moru respectively. This implied that Zn and Cu in Chogoria sites were extremely enriched, while in Naro Moru, enrichment levels ranged from significant to extreme. However, Mn was found to have minimal enrichment in all the sites. Lower montane forest and bamboo zone recorded relatively high enrichment due to distance from source of pollution. Ericaceous zone also had high mean enrichment due to influence of wind which favors higher deposition at mid-elevations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Particulate forms of heavy metals originating from power plants, smelting activities, residential heating, motor vehicle emissions, and industries are emitted into urban atmosphere, thereafter transported to other areas away from source of emission depending on wind patterns (Sharma et al. 2008). While light pollutants such as persistent organic pollutants are transported for longer distance and preferentially trapped at high latitudes (Tremolada et al. 2008), fast deposition processes favor heavier pollutants to get trapped along mountain slopes (Wania and Westgate 2008). The elevated land area, together with the decreasing temperatures as one moves up a mountain slope, allows for cold trapping and cold condensation of heavy metals (Bing et al. 2016; Bacardit and Camarero 2010; Wania and Westgate 2008).
Mountain regions were initially thought to be clean because they are found in isolated areas away from human activities (Wu et al. 2011). However, heavy metal pollution through long-range atmospheric transportation and deposition has been witnessed along mountain slopes (Wu et al. 2011; Bacardit and Camarero 2010; Gerdol and Bragazza 2006). Due to their remote locations and steep elevation, mountains are isolated from local pollution sources, therefore becoming the preferred sites for studying short- and long-range atmospheric transportation and deposition. Further, most mountains are protected zones free from human activities because of their unique flora and rich biodiversity. Any enrichment of heavy metals along mountain slopes is therefore presumed to primarily occur through atmospheric deposition (Battarbee et al. 2002).
Monitoring of heavy metal deposition in mountains has been done before using mosses, for the reason that they primarily depend on atmospheric deposition to get mineral nutrients (Gerdol and Bragazza 2006; Zechmeister 1995; Sun et al. 2009; Lucaciu et al. 2010; Viet et al. 2010). Other studies have explored deposition using surface snow and glacier (Bacardit and Camarero 2010; Zongxing Li et al. 2010; Yeo and Langley-Turnbaugh 2010), while other studies have considered soil as a media of deposition (McGee and Vallejo 1996; Bing et al. 2016; Steinnes et al. 1989; Yeo and Langley-Turnbaugh 2010). However, we note that unlike soils, surface snow and mosses are able to undergo rapid transformation over a short period of time, therefore only representing recent deposition activities (Wania and Westgate 2008). Meanwhile, soils are comparatively stable and are capable of holding a series of accumulations and depositions that have occurred for several years (Bing et al. 2016; Bacardit and Camarero 2010).
Heavy metals deposited in high altitude soils are likely to be transported downstream through surface runoff, eventually finding their way into fluvial systems. This may cause adverse health risks associated with heavy metals to the dependent populations (Duruibe et al. 2007; Zhiyuan Li et al. 2014). Considering the fact that mountains are more sensitive to global warming compared with lowland areas (Bacardit and Camarero 2010; Zongxing Li et al. 2010), and that mountains have been reported to be warming significantly faster than other regions in the world (Rangwala and Miller 2012), then the predicted ice and snow melting may transport heavy metals downstream and pose the adverse effects sooner than expected, hence the need for continues monitoring.
In this particular study, we assessed enrichment levels of four heavy metals (zinc (Zn), copper (Cu), manganese (Mn), and iron (Fe)) in subsurface soils collected along two elevation gradients of tropical Mt. Kenya. Total organic carbon (TOC) was also analyzed in the soils. We focused more on biologically active trace elements which tend to display a thin line between sufficient, deficient, and toxic amounts (Brady and Weil 2000). High concentration of heavy metals in soils does not necessarily equate to anthropogenic pollution as it may result from natural pedogenetic processes (Hernandez et al. 2003). The use of enrichment factors (EFs) has therefore proven to be a vital tool for detecting any enrichment from anthropogenic activities, by contrasting current levels with pre-industrial levels (Loska and Wiechuła 2003). This approach is widely used because it considers concentration of heavy metal of interest, in relation to the availability of immobile lithogenic element with no significant anthropogenic source (Blaser et al. 2000).
Materials and methods
Study area
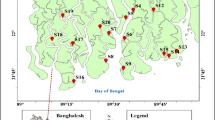
Figure 1 demonstrates the location of the study area and sampling points. Volcanic Mt. Kenya lies between 0.1512°S and 37.30710°E having the highest peak in Kenya at 5199 m above sea level (asl). The mountain harbors various endemic plant and animal species and as a result, it was listed as a world heritage by UNESCO in 1997 (Badman and Bomhard 2008). Due to this, Mt. Kenya is protected by two government agencies, the Kenya Wildlife Service (KWS) and the Kenya Forest Service (KFS). Mt. Kenya also serves as a water catchment area for the longest river in Kenya, the Tana River, which holds 33.5% and 23.8% of Kenya’s total surface water and underground water respectively (Botzen et al. 2015). Thus, it provides the water used in industries, commercial towns, and agricultural farms. Precipitation is influenced by effects of monsoon and trade winds (Mutai and Ward 2000), and ranges from 2500 mm per annum at foot of the mountain to about 700 mm at altitudes exceeding 4500 m that occurs in form of snow or mist, depending on the season. Temperatures also vary in the same manner from 21 to 4 °C at altitudes of above 4800 m with slight fluctuations occurring at night (Thompson 1966). Variations in temperature and precipitation categorize vegetation into five zones: lower montane forests (2000 to 3500 m), bamboo zones (2200 to 3200 m), upper montane forest (2500 to 3500 m), ericaceous zone (3500 to 4000 m), and moorland zone (4000 to about 4800 m) above which bare rocks and glacier occurs. As a result of the high precipitation, agriculture is the main activity in surrounding areas. Windward sides support cash crops like tea and coffee growing, while on the leeward side, wheat and cattle rearing become the major activities. As a result, several industrial and commercial towns have developed, including Embu, Meru, Nyeri, Karatina, and Nanyuki, some of which may significantly contribute to heavy metal enrichment in the environment.
Sampling strategy
Field sampling was conducted in May 2017 during the onset of rainy season. A total of 27 subsurface soils were collected at a depth of 10–15 cm. Only the undisturbed altitudes of Mt. Kenya, which are characterized by minimal or no anthropogenic activities, were sampled. Sampling ranged from the lowest protected lower montane forest to the highest accessible point in the moorland zone. During the sampling process, two climbing routes leading to the Mt. Kenya summit were followed, that is, Naro Moru to the west and Chogoria to the east. For the Chogoria route, sampling begun at 1996 m asl and concluded at 4041 m asl, while for the Naro Moru route, samples were collected from 2217 m asl to 4279 m asl, covering a total altitude of 2045 m and 2062 m respectively. This ensured a 100–200-m altitude interval from one sampling point to another. However, logistical constraints prevented us to sample beyond the moorland. Information regarding the elevation of each site was recorded and thereafter, samples were taken to laboratories at the Kenya Agricultural and Livestock Research Organization (KALRO) for elemental analysis. The samples were prepared by drying in an oven at 40 °C, grounding, and passing through a < 2-mm sieve to obtain the appropriate particle size distribution ready for analysis.
Sample analysis
Mehlich double acid method (Mehlich 1984) was used to extract manganese. Soil samples were extracted in a ratio of 1:5 (w/v) with a mixture of 0.1 N HCL and 0.025 N H2SO4.The digests were filtered through a 0.45-μm filter and put into a 50-mL bottle. One milliliter of concentrated solution of cesium chloride (CsCl) and lanthanum chloride (LaCl3) was added. The mixture was then topped using deionized water and analysis conducted using a UV/VIS spectrophotometer (SPEKOL 1500 Analytik Jena, Germany). For Fe, Zn, and Cu determination, acid hydroxylamine method by Wang et al. (1987) was used, where the soil samples were extracted in a 1:10 ratio (w/v) using 0.1 M HCl. Elements were determined with AAS (atomic absorption spectrophotometer; Analyst 100, PerkinElmer, USA). Calorimetric method (Bisutti et al. 2004) for TOC analysis was applied for this study. Extraction was done using a mixture of potassium dichromate (K2Cr2O2) and concentrated H2SO4 heated at 150 °C for 30 min to ensure complete oxidation. Barium chloride was added to the cool digests and allowed to stay overnight; the digests were filtered and analyzed using the spectrophotometer (SPEKOL 1500 Analytik Jena, Germany) at 600 nm wavelength.
Calculating enrichment factors
As aforementioned, enrichment factor is a vital tool for detecting any heavy metal enrichment from anthropogenic activities. The enrichment factor was calculated using the below formula proposed by Sinex and Helz (1981).
where CM is the content of the element studied and CX is the content of the immobile element. Generally, the immobile heavy metal that is naturally available at high concentrations, e.g., Al, Mn, and Fe, is used as the conservative element (Jain 2004). However, Fe is the most widely used element for normalization due to its immobility (Ghrefat and Yusuf 2006; Çevik et al. 2009; Tippie 1984). Consequently, Fe enrichment was not determined. Due to lack of information on background values for Kenyan soils, we therefore used the background levels in the earth’s crust as determined by Soto-Jiménez et al. (2003). These concentrations are 32 mg/kg, 720 mg/kg, 127 mg/kg, and 3.09% for Cu, Mn, Zn, and Fe respectively. Five contamination categories were recognized on the basis of the enrichment factor: minimal enrichment (< 2), moderate enrichment (2–5), significant (5–20), very high (20–40), and extreme enrichment (> 40) (Sutherland 2000; Sinex and Helz 1981).
Data analysis
Descriptive statistics (maximum, minimum, and mean values) were computed for the heavy metals and TOC. We employed Pearson’s correlation analysis to explore relationships among the heavy metals and TOC along the elevation gradient. Further, an independent-samples t test was conducted to compare the heavy metal distribution between Chogoria and Naro Moru route soil samples. Statistical Package for the Social Sciences version 23 was used for all statistical analysis.
Results and discussion
Heavy metal concentrations in soils from Mt. Kenya
Presented in Table 1 are the minimum, maximum, mean, and standard deviation statistics involving TOC, Fe, Zn, Cu, and Mn in the subsurface soils from the two Mt. Kenya slopes. The mean concentration of Mn, Fe, Zn, and Cu was 0.376 mg/kg, 47.6 mg/kg, 12.3 mg/kg, and 4.88 mg/kg in Chogoria and 0.560 mg/kg, 113 mg/kg, 12.7 mg/kg, and 2.70 mg/kg in Naro Moru respectively. According to independent t test results, there was a significant difference in the distribution of Mn between Chogoria (mean = 0.38, standard deviation = 0.24) and Naro Moru (mean = 0.56, standard deviation = 0.48) conditions (t (25) = − 1.23, p = 0.023). Another significant difference was in the distribution of Fe between Chogoria (mean = 47.6, standard deviation = 25.6) and Naro Moru (mean = 113.2, standard deviation = 93.4) conditions (t (25) = − 2.45, p = 0.035). However, Zn and Cu did not show any significant difference between the two routes.
In comparison with other regions, Mt. Kenya soils had the lowest Mn, Cu, and Zn concentrations when compared with the high Mountain Tibetan plateaus (Bing et al. 2016) and Mt. Everest part of China (Yeo and Langley-Turnbaugh 2010) as shown in Table 2. Because of the extreme cold temperatures on the temperate China mountains, and the fact that China is more developed in terms of industries and cities, more heavy metal deposition is expected. In contrast with Mt. Kilimanjaro in Africa (Mathew et al. 2016), Fe, Zn, and Mn were relatively similar. Apart from being formed from same volcanic activity, the two mountains are close to one another, having almost similar climatic and geological features. This could partly explain the similarity in Fe, Zn, and Mn concentration.
Cu and Zn concentrations were also comparable to previously studied agricultural soils from central Kenya (Ndungu et al. 2019). The range of Cu, Fe, and Zn concentration in sugarcane-growing soils of Kakamega County in Kenya (Akenga et al. 2014) was also comparable to that of Mt. Kenya soils. Agricultural lands may accumulate heavy metals mainly from fertilizer, fungicide, and pesticide application. The similarity in concentration of Mt. Kenya soils with agricultural soils can serve as evidence of a series of atmospheric depositions in Mt. Kenya soils. As expected, urban soils from Nairobi city in Kenya (Katana et al. 2013) and Varanasi city in India (Sharma et al. 2008) had highest levels of Zn and Cu among other land use, and this was closely related to more motor vehicle and industrial waste pollution. Nevertheless, both Cu and Zn concentrations from our study area did not exceed the permissible levels set by United States Environmental Protection Agency (US-EPA) for evaluation of soil contamination.
Heavy metals and elevation intercorrelation
As elevation increased, TOC increased significantly in Chogoria route, where the coefficient of correlation was 0.599 at p < 0.05 (Table 3). Positive relationship between TOC and elevation increase is quite rare, and it may be attributed to the ability of cushion plants (low laying plants at high altitudes) to modify their microenvironment. By trapping heat underneath them, cushion plants are able to increase soil temperatures and influence nutrient availability, therefore enhancing decomposition of organic matter (Chen et al. 2015; Butterfield et al. 2013). In Naro Moru, the only significant correlation occurred between Zn and Mn (r = 0.646 at p < 0.05), while in Chogoria, Cu showed a significant correlation with Mn and TOC. According to Makokha et al. (2016), heavy metals that show strong correlations have similar sources and comparable characteristics or the availability of one element is influenced by the other. In this study therefore, Zn availability in the Naro Moru route was dependent on Mn availability, whereas abundance of Cu in Chogoria was dependent on TOC and Mn availability.
Ecological assessment
As shown in Table 4, the mean enrichment factors for Mn, Cu, and Zn in Chogoria were 0.447, 131, and 78.8 respectively, while in Naro Moru, the values were 0.463, 38.9, and 53.0 for Mn, Cu, and Zn respectively. Compared with Zn and Cu, we found Mn to be less enriched in all the samples. Notably, deposition of Mn on surface and subsurface soils is generally lower than that of Zn and Cu (Bing et al. 2016). This is because human activities such as coal burning, tire abrasion, and car breaking systems, which are some of the main urban sources of particulate forms of heavy metals, discharge higher Cu and Zn amounts into various ecosystems compared with Mn (Harrison et al. 2003; Sun et al. 2009). Based on the five contamination categories above, Mn enrichment was categorized as minimally enriched in all sites, except in site 9 in Naro Moru which recorded an EF value of 2.10. However, all sites in Chogoria were found to be extremely enriched with Cu. Only three sites in Chogoria were not extremely enriched with Zn. In Naro Moru, enrichment levels of both Cu and Zn ranged from moderate to extreme.
Generally, metals originating solely from parent rock have EFs ≤ 1, whereas EFs > 1 usually indicates other sources besides natural input (Soto-Jiménez and Páez-Osuna 2001; Wu et al. 2011). A contrasting study by Bacardit and Camarero (2010) suggests that enrichment factors < 10 should indicate crustal or natural sources, while those > 10 are an indication of anthropogenic sources. This study further categorizes EF values < 10 as moderate enrichment while those between 10 and 500 as highly enriched. These two studies reflect confusion that may arise when interpreting enrichment factors; thus, caution is recommended. Nevertheless, the EF values in Mt. Kenya slopes indicated minimal Mn enrichment originating from natural sources. On the other hand, EF values concluded that Cu and Zn were from anthropogenic sources. Motor vehicle emissions from heavy traffic in commercial and industrial towns around Mt. Kenya, coal using in tea and coffee factories during the fixing and drying stages, and spray of agriculture-based chemicals such as pesticides and fungicides could account for the high Zn and Cu enrichment on the slopes of Mt. Kenya, and their enrichment was closely associated with atmospheric deposition. Similarly, high Cd enrichment of up to 144.5 in Gongga Mountain in China (Wu et al. 2011), high Cu and Pb enrichment in Swiss forest soils (Blaser et al. 2000), and high Cd and Pb, as well as moderate Cu and Zn, in French forest soils (Hernandez et al. 2003) were also attributed to atmospheric deposition.
Cu was the only heavy metal that decreased significantly with elevation increase, and did so in Chogoria route only r = − 0.846 at p < 0.001 (Table 3). Similarly, the concentration of Cu along the High Mountain in Eastern Tibetan Plateau was significantly higher in the lower elevations than that in the higher elevations (Bing et al. 2016). This was attributed to vegetation playing an important role in the distribution of heavy metals and the close distance from pollution sources. Thus, Cu enrichment in our study area could be resulting from short-range atmospheric deposition. Other heavy metals did not correlate positively with elevation increase. The results were contrary to our expectation and results from the northern slope of Qomolangma Mountain in China (Zhang et al. 2007). This is because although decreasing temperature is the major driver of atmospheric deposition (Wania and Westgate 2008), an interplay of multiple climatic factors, including wind, precipitation, and canopy cover, shapes the distribution and deposition patterns of heavy metals along an elevation gradient (Mathew et al. 2016; Vitousek et al. 1990; Wilcke et al. 2008; Zechmeister 1995). These factors are, however, not similar on all mountain slopes and are not uniformly distributed along elevation gradient. For instance, while atmospheric temperature decreases uniformly by 6 °C for every kilometer increase in elevation (Barry 2008), precipitation pattern, which is a major factor affecting wet deposition and leaching of heavy metals, differs from one mountain to another and even from one slope to another due to proximity to water bodies or slope direction (McCain and Grytnes 2010). With such multi-variations, atmospheric deposition is expected to vary considerably across elevation, slopes, and vegetation types (Bacardit and Camarero 2010). Unfortunately, wind, annual temperature, and annual precipitation data along elevation gradient of Mt. Kenya is insufficient, as only a few weather stations, including one in the Naro Moru route, have been set up and are not along the elevation gradient. Therefore, correlating heavy metal concentration with environmental factors such as wind, temperature, or precipitation data proved difficult. However, we note that the eastern slope (Chogoria) receives more precipitation than the western slope (Naro Moru) (Niemelä and Pellikka 2004).
The lower montane forest separated agricultural farms with other forest vegetation types. Consequently, lower montane forest and bamboo zone showed relatively higher EF values than the upper montane forest and moorland (Fig. 2). Notably, when sampling sites are close source of pollution, more heavy metal enrichment is expected (Tremolada et al. 2008; Bing et al. 2016). Ericaceous zone also had higher mean enrichment of Zn and Cu compared with upper montane forests and bamboo and moorland zones. We note that under the influence of wind, middle elevations have been found to be the ideal zones for heavy metal deposition (Yeo and Langley-Turnbaugh 2010). Further, reduction of canopy cover may have facilitated higher atmospheric deposition in ericaceous zone due canopy filtering effect (Gandois et al. 2010).
Conclusion
The enrichment factor proved once again to be a useful tool capable of detecting the slightest heavy metal enrichment propagated by human activities. Further, it provided evidence of short- and long-range atmospheric deposition of heavy metals, particularly Zn and Cu, in the subsurface soils along the elevation gradient of Mt. Kenya. However, atmospheric deposition did not increase linearly with elevation increase as earlier thought. This study also noted that deposition of Mn on surface soils was lower than that of Zn and Cu, as main anthropogenic activities responsible for heavy metal discharge in the atmosphere release higher Cu and Zn amounts compared with Mn. Meanwhile, the recorded concentrations were below the US-EPA maximum permissible levels for soils, implying a less negative impact on the mountain ecosystem. We therefore recommended further monitoring to determine enrichment of other heavy metals as well as evaluating their exact sources around Mt. Kenya area.
References
Akenga, P., Salim, A., Onditi, A., Yusuf, A., & Waudo, W. (2014). Determination of selected micro and macronutrients in sugarcane growing soils at Kakamega North District, Kenya. IOSR Journal of Applied Chemistry, 7(7), 34–41.
Bacardit, M., & Camarero, L. (2010). Atmospherically deposited major and trace elements in the winter snowpack along a gradient of altitude in the Central Pyrenees: the seasonal record of long-range fluxes over SW Europe. Atmospheric Environment, 44(4), 582–595.
Badman, T., & Bomhard, B. (2008). World heritage and protected areas. Gland (IUCN).
Barry, R. (2008). Mountain weather and climate. (Cambridge University Press: Cambridge, UK).
Battarbee, R. W., Thompson, R., Catalan, J., Grytnes, J.-A., & Birks, H. (2002). Climate variability and ecosystem dynamics of remote alpine and arctic lakes: the MOLAR project. Journal of Paleolimnology, 28(1), 1–6.
Bing, H., Wu, Y., Zhou, J., Li, R., Luo, J., & Yu, D. (2016). Vegetation and cold trapping modulating elevation-dependent distribution of trace metals in soils of a high mountain in eastern Tibetan Plateau. Scientific Reports, 6, 24081.
Bisutti, I., Hilke, I., & Raessler, M. (2004). Determination of total organic carbon–an overview of current methods. TrAC Trends in Analytical Chemistry, 23(10–11), 716–726.
Blaser, P., Zimmermann, S., Luster, J., & Shotyk, W. (2000). Critical examination of trace element enrichments and depletions in soils: As, Cr, Cu, Ni, Pb, and Zn in Swiss forest soils. Science of the Total Environment, 249(1–3), 257–280.
Botzen, W., Eiselin, M., Kamau, P., Lange, K., van Maanen, E., Mogoi, S., et al. (2015). The economics of ecosystem services of the Tana River Basin.
Brady, N. C., & Weil, R. R. (2000). Elements of the nature and properties of soils. Upper River: Prentice Hall.
Butterfield, B. J., Cavieres, L. A., Callaway, R. M., Cook, B. J., Kikvidze, Z., Lortie, C. J., et al. (2013). Alpine cushion plants inhibit the loss of phylogenetic diversity in severe environments. Ecology Letters, 16(4), 478–486.
Çevik, F., Göksu, M. Z. L., Derici, O. B., & Fındık, Ö. (2009). An assessment of metal pollution in surface sediments of Seyhan Dam by using enrichment factor, geoaccumulation index and statistical analyses. Environmental Monitoring and Assessment, 152(1–4), 309.
Chen, J., Yang, Y., Stöcklin, J., Cavieres, L. A., Peng, D., Li, Z., et al. (2015). Soil nutrient availability determines the facilitative effects of cushion plants on other plant species at high elevations in the South-Eastern Himalayas. Plant Ecology and Diversity, 8(2), 199–210.
Duruibe, J. O., Ogwuegbu, M., & Egwurugwu, J. (2007). Heavy metal pollution and human biotoxic effects. International Journal of physical sciences, 2(5), 112–118.
Gandois, L., Tipping, E., Dumat, C., & Probst, A. (2010). Canopy influence on trace metal atmospheric inputs on forest ecosystems: speciation in throughfall. Atmospheric Environment, 44(6), 824–833.
Gerdol, R., & Bragazza, L. (2006). Effects of altitude on element accumulation in alpine moss. Chemosphere, 64(5), 810–816.
Ghrefat, H., & Yusuf, N. (2006). Assessing Mn, Fe, Cu, Zn, and Cd pollution in bottom sediments of Wadi Al-Arab Dam, Jordan. Chemosphere, 65(11), 2114–2121.
Harrison, R. M., Tilling, R., Romero, M. A. S. C., Harrad, S., & Jarvis, K. (2003). A study of trace metals and polycyclic aromatic hydrocarbons in the roadside environment. Atmospheric Environment, 37(17), 2391–2402.
Hernandez, L., Probst, A., Probst, J. L., & Ulrich, E. (2003). Heavy metal distribution in some French forest soils: evidence for atmospheric contamination. Science of the Total Environment, 312(1–3), 195–219.
Jain, C. (2004). Metal fractionation study on bed sediments of river Yamuna, India. Water Research, 38(3), 569–578.
Katana, C., Jane, M., & Harun, M. (2013). Speciation of zinc and copper in open-air automobile mechanic workshop soils in Ngara area-Nairobi Kenya. Resources and Environment, 3(5), 145–154.
Li, Z., He, Y., Yang, X., Theakstone, W. H., Jia, W., Pu, T., et al. (2010). Changes of the Hailuogou Glacier, Mt. Gongga, China, against the background of climate change during the Holocene. Quaternary International, 218(1–2), 166–175.
Li, Z., Ma, Z., van der Kuijp, T. J., Yuan, Z., & Huang, L. (2014). A review of soil heavy metal pollution from mines in China: pollution and health risk assessment. Science of the Total Environment, 468, 843–853.
Loska, K., & Wiechuła, D. (2003). Application of principal component analysis for the estimation of source of heavy metal contamination in surface sediments from the Rybnik Reservoir. Chemosphere, 51(8), 723–733.
Lucaciu, A., Motoc, C., Jelea, M., & Jelea, S. G. (2010). Survey of heavy metal deposition in Romania: Transylvanian Plateau and western Carpathians Mountains. University "Politehnica" of Bucharest Scientific Bulletin, Series A: Applied Mathematics and Physics, 72(2), 171–178.
Makokha, V. A., Qi, Y., Shen, Y., & Wang, J. (2016). Concentrations, distribution, and ecological risk assessment of heavy metals in the East Dongting and Honghu Lake, China. Exposure and Health, 8(1), 31–41.
Mathew, M. M., Majule, A. E., Marchant, R., & Sinclair, F. (2016). Variability of soil micronutrients concentration along the slopes of Mount Kilimanjaro, Tanzania. Applied and Environmental Soil Science.
McCain, C. M., & Grytnes, J. A. (2010). Elevational gradients in species richness. Encyclopedia of life sciences (ELS), 1–10.
McGee, E., & Vallejo, V. (1996). Long range transport and soil interception of atmophile elements on a transect across the Pyrenees. In Proceedings of EUROTRAC Symposium (Vol. 96, pp. 307–314).
Mehlich, A. (1984). Mehlich 3 soil test extractant: a modification of Mehlich 2 extractant. Communications in Soil Science and Plant Analysis, 15(12), 1409–1416.
Mutai, C. C., & Ward, M. N. (2000). East African rainfall and the tropical circulation/convection on intraseasonal to interannual timescales. Journal of Climate, 13(22), 3915–3939.
Ndungu, A. W., Yan, X., Makokha, V. A., Githaiga, K. B., & Wang, J. (2019). Occurrence and risk assessment of heavy metals and organochlorine pesticides in surface soils, Central Kenya. Journal of Environmental Health Science and Engineering, 1–11.
Niemelä, T., & Pellikka, P. (2004). Zonation and characteristics of the vegetation of Mt. Kenya. In P. Pellikka, Ylhäisi, & B. Clark (Eds.), Taita Hills and Kenya, Expedition reports of the Department of Geography (Vol. 40, pp. 26–30). University of Helsinki.
Rangwala, I., & Miller, J. R. (2012). Climate change in mountains: a review of elevation-dependent warming and its possible causes. Climatic Change, 114(3–4), 527–547.
Sharma, R. K., Agrawal, M., & Marshall, F. M. (2008). Atmospheric deposition of heavy metals (Cu, Zn, Cd and Pb) in Varanasi City, India. Environmental Monitoring and Assessment, 142(1–3), 269–278.
Sinex, S., & Helz, G. (1981). Regional geochemistry of trace elements in Chesapeake Bay sediments. Environmental Geology, 3(6), 315–323.
Soto-Jiménez, M., & Páez-Osuna, F. (2001). Cd, Cu, Pb, and Zn in lagoonal sediments from Mazatlán harbor (SE Gulf of California): bioavailability and geochemical fractioning. Bulletin of Environmental Contamination and Toxicology, 66(3), 350–356.
Soto-Jiménez, M., Páez-Osuna, F., & Ruiz-Fernández, A. (2003). Geochemical evidences of the anthropogenic alteration of trace metal composition of the sediments of Chiricahueto marsh (SE Gulf of California). Environmental Pollution, 125(3), 423–432.
Steinnes, E., Solberg, W., Petersen, H. M., & Wren, C. D. (1989). Heavy metal pollution by long range atmospheric transport in natural soils of southern Norway. Water, Air, and Soil Pollution, 45(3–4), 207–218.
Sun, S.-Q., Wang, D.-Y., He, M., & Zhang, C. (2009). Monitoring of atmospheric heavy metal deposition in Chongqing, China—based on moss bag technique. Environmental Monitoring and Assessment, 148(1–4), 1–9.
Sutherland, R. (2000). Bed sediment-associated trace metals in an urban stream, Oahu, Hawaii. Environmental Geology, 39(6), 611–627.
Thompson, B. (1966). The mean annual rainfall of Mount Kenya. Weather, 21(2), 48–49.
Tippie, V. K. (1984). An environmental characterization of Chesapeake Bay and a framework for action. In The estuary as a filter (pp. 467–487). Elsevier.
Tremolada, P., Villa, S., Bazzarin, P., Bizzotto, E., Comolli, R., & Vighi, M. (2008). POPs in mountain soils from the Alps and Andes: suggestions for a ‘precipitation effect’ on altitudinal gradients. Water, Air, and Soil Pollution, 188(1–4), 93–109.
Viet, H. N., Frontasyeva, M. V., Thi, T. M. T., Gilbert, D., & Bernard, N. (2010). Atmospheric heavy metal deposition in northern Vietnam: Hanoi and Thai Nguyen case study using the moss biomonitoring technique, INAA and AAS. Environmental Science and Pollution Research, 17(5), 1045–1052.
Vitousek, P. M., Field, C. B., & Matson, P. A. (1990). Variation in foliar δ 13 C in Hawaiian Metrosideros polymorpha: a case of internal resistance? Oecologia, 84(3), 362–370.
Wang, C., Schuppli, P., & Ross, G. (1987). A comparison of hydroxylamine and ammonium oxalate solutions as extractants for Al, Fe and Si from spodosols and spodosol-like soils in Canada. Geoderma, 40(3–4), 345–355.
Wania, F., & Westgate, J. N. (2008). On the mechanism of mountain cold-trapping of organic chemicals. Environmental Science & Technology, 42(24), 9092–9098.
Wilcke, W., Oelmann, Y., Schmitt, A., Valarezo, C., Zech, W., & Homeier, J. (2008). Soil properties and tree growth along an altitudinal transect in Ecuadorian tropical montane forest. Journal of Plant Nutrition and Soil Science, 171(2), 220–230.
Wu, Y., Bin, H., Zhou, J., Luo, J., Yu, D., Sun, S., et al. (2011). Atmospheric deposition of Cd accumulated in the montane soil, Gongga Mt., China. Journal of Soils and Sediments, 11(6), 940–946.
Yeo, B., & Langley-Turnbaugh, S. (2010). Trace element deposition on Mount Everest. Soil Horizons, 51(3), 72–78.
Zechmeister, H. G. (1995). Correlation between altitude and heavy metal deposition in the Alps. Environmental Pollution, 89(1), 73–80.
Zhang, Y., Wang, Z., Luo, K., Ding, M., Zhang, W., Lin, X., et al. (2007). The spatial distribution of trace elements in topsoil from the northern slope of Qomolangma (Everest) in China. Environmental Geology, 52(4), 679–684.
Funding
This project was funded by Sino-Africa Joint Research Center, Chinese Academy of Sciences (SAJC201601), and National Natural Science Foundation of China (313111079).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Githaiga, K.B., Njuguna, S.M., Makokha, V.A. et al. Assessment of Cu, Zn, Mn, and Fe enrichment in Mt. Kenya soils: evidence for atmospheric deposition and contamination. Environ Monit Assess 192, 167 (2020). https://doi.org/10.1007/s10661-020-8123-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-020-8123-7