Abstract
As a bone scaffold, meeting all basic requirements besides dealing with other bone-related issues—bone cancer and accelerated regeneration—is not expected from traditional scaffolds, but a newer class of scaffolds called multifunctional. From a clinical point of view, being a multifunctional scaffold means reducing in healing time, direct costs—medicine, surgery, and hospitalization—and indirect costs—loss of mobility, losing job, and pain. The main aim of the present review is following the multifunctional bone scaffolds trend to deal with both bone regeneration and cancer therapy. Special consideration is given to different fabrication techniques which have been applied to yield these materials spanning from traditional to modern ones. Moreover, the hierarchical structure of bone plus bone cancers and available medicines to them are introduced to familiarize the potential reader of review with the pluri-disciplinary essence of the field. Eventually, a brief discussion relating to the future trend of these materials is provided.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bone is perceived as a responsive tissue to its surrounding medium with a complex structure; it is in charge of many important functions throughout the human body—structural support of internal organs, locomotion, and producing blood cells [1]. Bone is composed of an inorganic phase called hydroxyapatite (~ 70%) and the rest attributes to proteins mainly collagen (type I) [2]. Bearing in mind, although aging is to blame for increasing the rate of musculoskeletal diseases, high active routines of youth cause the risk of musculoskeletal-related injuries to soar [3]. Taking a glance at statistics is enough to realize that the total costs including both direct and indirect ones are astronomical [4]. Among different types of musculoskeletal-related issues, the majority of recorded cases belong to bone defects caused by tumors, trauma, etc. When a bone defect goes beyond a small size, which can be repaired through an endogenous regenerate process spontaneously, it is called a critical-sized defect requiring an external intervention [5].
Bone tumors are recognized as one of the culprits leaving behind a critical-sized defect after being removed, but before that, an introduction to bone cancer is of great significance here. So far, it is reported that 45 types of bone tumors exist and the most important one is called osteosarcoma [6]. It is noteworthy that compared to other types of cancer which is prevalent among old people, young people are prone to osteosarcoma. The most practical treatments to deal with bone cancer are surgery followed by chemotherapy and radiotherapy [7]. To fight other types of cancer, some unconventional methods like hyperthermia, photothermal therapy, and targeted drug delivery are introduced and being applied clinically [8,9,10]. On the upside, theoretically, the multifunctional nanoparticles are injected through the bloodstream and either actively or passively they find their way through a tumor to get their job done [11]. However, in the case of bone cancer, the situation is different; after removal of a bone tumor, medical practitioners do not have any other choice instead of filling up the defect with an osteoconductive material. In this case, the risk of recurrence of tumors is reported to be high, and some bone substitutes capable of doing multiple tasks like eradication of cancerous cells and bone regeneration are required [12]. Complicating matters when a bone substitute should be designed to not only possess all the basic requirements of load-bearing applications, but it must also address cancerous tissues; this class of bone substitutes is called multifunctional.
As a multidisciplinary field of study, tissue engineering and regenerative medicine (TERM) brings together experts of different fields including materials science, chemistry, mechanical engineering, and medicine under one roof to come up with startling discoveries—restoring and improving the function of the tissues [13]. Bone tissue engineering (BTE) is considered as a subcategory of TERM, and when it comes to a critical-sized bone defect, BTE has made a revelation that traditional bone grafts, which carry a huge cost and pain on the patients, should be gradually replaced with 3D multifunctional bone scaffolds [14]. Based on the reported studies, at the first stage, a 3D bone scaffold intended for load-bearing applications should be biocompatible, highly porous, mechanically matched with the host tissue, and biodegradable in a rate close to the regeneration process [15,16,17]. On the other hand, besides the mentioned basic requirements, a 3D multifunctional bone scaffold has one or more functions—prevention of bone infection [14], treatment of inflammation [18], extirpating cancerous cells [19], etc. It is critical to bear in mind that the design and fabrication route are pivotal factors to yield a desirable multifunctional bone scaffold.
Up to now, there is a variety of techniques adopted to yield 3D multifunctional bone scaffolds—polymer sponge [20], freeze-drying [21], gel casting [22], etc. Although the mentioned methodologies have their advantages, they are regarded conventional mostly due to their incapability to be shaped close to the complex form of defect. Therefore, other techniques like rapid prototyping or 3D printing and electrospinning with more precise control over the design and microstructure of the final product have attracted more attention in recent years in BTE [23].
In recent years, some reviews relating to multifunctional bone scaffolds for bone cancer therapy have been published [19, 24], but none of which has reviewed the field from the fabrication techniques viewpoint and their subsequent effect on the applicability of the scaffolds. The aim of the present review is to cover multifunctional ceramic-based and composite scaffolds for both bone cancer therapy and regeneration. In the first stage, the structure of bone and its hierarchical nature will be introduced to make the potential reader familiar with its biology. Next, bone cancer, its types, and available medicine to treat them will be discussed followed by introducing bone scaffolds with desirable properties for BTE. The emphasis of this review is on the multifunctional bone scaffolds fabricated through traditional and more up-to-date routes and their advantages and disadvantages; such multifunctional scaffolds are of great concern because they are able to address both eradication of bone tumors and subsequent regeneration process effectively. Eventually, conclusions and a brief future perspective will be outlined as well.
Bone structure and microstructure
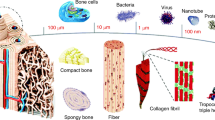
Bone as a connective tissue has an exceptionally mineralized extracellular matrix giving it desirable mechanical compressive and tensile strengths required for daily activities. Besides its mechanical support, which is the most recognized role of bone, it is responsible to protect the internal organs from any potential danger and provide an appropriate substrate for muscle adhesion. Moreover, bone maintains calcium ions balance throughout the body (homeostasis) and controls the formation of blood cells (hematopoiesis) [25]. The anatomy of bone is hierarchical spanning from macroscopic level to the nanoscale. From a macroscopic viewpoint, there are two types of bone tissue—spongy (trabecular) and cortical; these tissues are not different in composition, but their microstructure, function, and distribution [26]. Approximately, up to 80% of total bone mass attributes to the cortical bone that is highly dense with low porosity; these characteristics make this tissue mechanically strong (compressive strength). On the contrary, trabecular or spongy bone has an interconnected porous structure (~ 50–90%) causing this tissue to have a compressive strength one-tenth that of cortical bone; it is critical to notice that the porous structure of trabecular bone provides a high surface area which means more contact with blood cells followed by controlling over hematopoiesis and homeostasis [27]. Throughout the human body, there are long and short bones, and to exemplify both trabecular and cortical tissues, it is important to introduce different regions of a long bone as an example. Long bone has three main regions including a cylindrical dense shaft made of cortical bone called diaphysis and two parts at the end of the shaft made of trabecular bone called metaphysis (Fig. 1). It is interesting to mention that the tube-like essence of the shaft increases the bending resistance of bone without making the bone heavy [25].
A schematic showing the macroscopic anatomy of a long bone. Reproduced with permission from Ref. [25]
As mentioned earlier, as a composite material, bone is formed by organic and inorganic components—collagen (type I, ~ 20%), and the remained belongs to carbonated hydroxyapatite and water. It is noteworthy that the majority of the organic part of bone relates to type I collagen, whereas the rest attributes to the other types of collagen; as a fibrous protein, collagen consists of nearly 1000 amino acids and it has a length of about 300 nm with a rope-like morphology. Collagens are normally synthesized throughout the human body spontaneously by osteoblasts. The precursors (procollagen molecules) are first secreted through osteoblast cells, and these molecules are gradually congregated and form collagen fibrils; collagen fibrils are made of two α1 and an α2 polypeptide chains. The deposition of mainly calcium phosphate nanocrystals through collagen fibrils leads to the formation of bone composite-like unit cells called osteons yielding the final bone structure [28] (Fig. 2).
Step-by-step formation of the bone’s hierarchical structure from collagen molecules and nanohydroxyapatite crystals. Reproduced with permission from Ref. [29]
Bone cancer and available medications
The occurrence of bone cancer is more probable in youth rather than old people [6]. The very first action after diagnosis of a bone tumor is resection of the tumor through surgery; surgery plays an integral part in treating bone cancers, combined with other methods [30]. Limb-sparing surgery is generally accomplished when cancer has just affected the patient. Only the affected bone is removed, along with the surrounding cells. It is reported that even after the resection process, there will be remained cancerous tissues in the bone defect’s wall raising the risk of tumor recurrence [31]. If the limb-sparing surgery were unavailable, the next solution would be an amputation. This might be needed in the following cases [19]:
-
If the cancer has affected not just the bone but vital blood vessels and nerves.
-
In the occurrence of an infection because of the limb-sparing surgery leading to the removal of the graft or prosthetics.
-
When cancer develops in a part of the body where the limb-sparing surgery may not be possible such as the ankle.
Through Table 1, different bone cancer types, formation and growth, symptoms, causes, and risk factors are summarized. Although injecting massive chemotherapeutic drugs in the case of bone cancer therapy has catastrophic effects on healthy tissues, there are some drugs which are still being used clinically which are introduced in Table 2. Systemic chemotherapy has failed to address the issue effectively due to the weak bloodstream of bone tissue [32]. Therefore, attention is focused on localized treatment through different methods—localized drug delivery, photothermal therapy, and hyperthermia therapy. Through novel drug delivery systems, the pharmaceutical community has focused on two fields to deal with bone cancer—finding new drugs and enhancing the performance of available drugs. From the early of 1990 to now, different kinds of drug delivery systems—polymeric and composite—have been developed and tried among which PLGA/doxorubicin (DOX) [33], chitosan/paclitaxel [34], polyurethane/curcumin [35], gelatin/DOX [36], and collagen/hydroxyapatite/cisplatin [37] can be enumerated. The idea of applying ceramic-based composites is of particular interest because treating bone cancer and bone regeneration should occur simultaneously, and applying drug-loaded hydroxyapatite, which has a similar structure to the inorganic phase of bone, would be killing two birds with one stone.
Multifunctional bone scaffolds
Before getting into multifunctional bone scaffolds, it is of great significance to get familiar with a bone scaffold’s design and basic requirements. As a bone scaffold, it is intended to provide temporal support for accelerating bone tissue regeneration, whereas the scaffold finally will be degraded without triggering any cytotoxicity to its surrounding medium. This biodegradable bone scaffold must be biocompatible, highly porous, and mechanically strong to withstand mechanical loads and its degradation rate should be matched with the tissue regeneration [53]. Besides the mentioned properties, having appropriate surface chemistry provides an ideal surface for the cell’s attachment and proliferation [54]. Various materials have been used as bone scaffolds to fulfill the aforementioned requirements including polymers, ceramics, metals, and their composites. This information is summarized in Fig. 3.
That was in the early 1990s that Langer et al. [55] made a revelation discovery by which tissues could be reconstructed through cell-seeded synthetic materials. From that moment on, a huge explosion has happened in the scientific community of TERM and different researchers around the globe came up with different scaffolds made of various materials and fabrication techniques. However, most of them were eliminated by the death valley toward commercialization. From a clinical point of view, generally, there are four parameters when it comes to commercialization of a bone scaffold—form, function, formation, and fixation; the very first one (form) refers to designing and fabrication of a bone scaffold based on the complex form of defect. Function means the tolerance of scaffold—mechanical loads—every day until the regeneration is reached. The formation, briefly, means providing a structural scaffold to accelerate the regeneration process through releasing biological materials, and finally, fixation relates to making all of the requirements available in a package for easy implantation in clinical practices [56, 57].
It is critical to bear in mind that the structural properties of 3D bone scaffolds are a key factor resulting in the future success of these materials; the aim is to mimic the hierarchical structure of bone consisting different length scales and so fabricating a 3D bone scaffold in harmony with the natural bone [26]. In the former paragraph, the importance of mechanical properties is introduced and now the effects of total porosity, pore size, and interconnectivity of these pores will be discussed. To ensure effective nutrients penetration and waste removal, reaching more than 60% total porosity with the interconnected porous structure in the size of 100–1000 µm is indispensable. Pores with a size smaller than 1 µm improve not only the interaction of proteins with the scaffold, but they also increase the surface area leading to more ions exchange with the surrounding medium and faster formation of carbonated hydroxyapatite. All the properties of an ideal bone scaffold are represented in Fig. 4.
It is reported that the newly formed carbonated hydroxyapatite is capable of making a strong bond with the host bone and so a desirable fixation (bioactivity). Having pores in the range of 1–20 µm provides an appropriate surface on which the cells’ attachment, proliferation, and differentiation could be eased [58, 59]. Through Fig. 5, it is observable that a multifunctional bone scaffold with hierarchical porosity is designed and fabricated through the polymer sponge technique. The scaffold’s composition consists of a magnetic nanocomposite, and then, it is coated with a polymer-ceramic composite to address the issues related to bone cancer therapy and regeneration [60].
Multifunctional nanocomposite scaffolds’ microstructure including non-coated, polymer-coated, and polymer-ceramic composite-coated scaffolds having a hierarchical porous structure from macro to nano. Reproduced with permission from Ref. [60]
Hyperthermia therapy
When it comes to eradication of a bone tumor followed by repairing the defect left behind after surgery, there are many impediments to traditional bone grafts; the remained cancerous cells in the defect have a high potential to form a new tumor in the site again. Therefore, there is a great need to apply multifunctional scaffolds to fight the cancerous cells on the one hand and supporting the bone regrowth on the other hand. Generally, there are three main approaches toward making a multifunctional bone scaffold for bone cancer therapy—being responsive to an external magnetic field (EMF) (hyperthermia therapy) [61], catalytically active to raise the heat locally (photothermal therapy) [30], and localized release of chemotherapeutic drugs [62]. Through hyperthermia-based therapy, generally, there is a magnetic material by which a scaffold is fabricated or a magnetic agent is introduced to a non-magnetic bone scaffold; when the magnetic bone scaffold is exposed to EMF, the hysteresis and relaxation loss are governing factors to generate heat. If the magnetic agent is superparamagnetic, there is no hysteresis and so the heat generation is controlled through the relaxation loss—Neel and Brown losses. When a single-domain particle rotates, which leads to raising in the local heat, it is called Brown relaxation, and an increase in the heat through rotation of magnetic moments is called Neel relaxation [63]. Based on the applied temperature, there are three categories relating to hyperthermia-based therapy—T ≤ 41 °C, 42 ≤ T ≤ 46 °C, and T ≥ 46 °C [64]. The first category has either small effects or nothing on the cancerous cells, and its application mainly falls into treating osteoarthritis. Applying a temperature in the range of 42–46 °C is of particular interest for bone cancer therapy because applying this range can kill the cancerous cells, and on the other side, there is no side effect for healthy tissues. The last category belongs to temperatures above 46 °C which is called thermoablation; there is no doubt that the generated heat is high enough to extirpate the cancerous cells, but it severely affects the healthy tissues on the downside. It is important to notice that taking control over the released heat is essential and based on the previous studies, the applied magnetic field and frequency are two governing factors by which the released heat from magnetic bone scaffolds can be controlled [20, 65, 66]. Through Fig. 6, a schematic of how a magnetic-responsive bone scaffold can treat remained bone cancerous cells is indicated.
A schematic illustrating how a bone tumor is treated through hyperthermia-based therapy by multifunctional bone scaffolds. Reproduced with permission from Ref. [67]
Photothermal therapy
Photothermal therapy as a method with minimal invasiveness has received tremendous attention in recent years. In this method, a near-infrared laser is adopted to excite a catalyst that is embedded in the tumor’s site through shedding light on it. The absorption of light is synchronized with releasing heat in the tumor’s site [68]. Three parameters are affecting the effectiveness of photothermal therapy—the catalyst, the light’s wavelength, and the delivery mode of laser light which can be either interstitial or non-invasive. It is noteworthy that all laser delivery modes focus on raising the local temperature in a manner which is destructive to cancerous cells without any negative effect on the surrounded healthy tissues. It is well known that increase in the tumor’s temperature up to 41 °C is ineffective, and through photothermal therapy, it is reported that raising the temperature of the tumor’s center up to 50 °C to reach complete necrosis of cancer cells is required [69, 70]. Taking control over this high temperature to prevent any potential harm to healthy tissues is one of the main challenges of photothermal therapy [71]. Figure 7 illustrates a multifunctional 3D bone scaffold composed of tri-calcium phosphate modified with LaB6 to deal with both bone cancer cells and regeneration [72].
The application of LaB6-modified TCP scaffolds for bone tumor therapy through photothermal therapy followed by bone defect repair. Reproduced with permission from Ref. [72]
Localized drug delivery
Through great advances which have been made in drug delivery systems in recent years, the idea of targeted drug delivery or localized controlled release has opened many doors to unsolved issues; ignoring the type of cancer, destroying the cancerous cells while keeping the healthy tissues intact is the main aim of targeted drug delivery systems [12, 73]. The liberation of chemotherapeutic drugs in the targeted site has some advantages—preserving prolonged release and so improving the efficiency, decreasing the side effects of chemotherapeutic drugs on the surrounded cells and organs, and finally, the applied dose of the drug will be comparatively less than the systemic administration [74, 75]. Following the traditional drug delivery methods, in the case of bone disease, a massive drug dose is required due to poor bloodstream through bone tissue compared to soft tissues [32, 76]. Therefore, it is clear that when a chemotherapeutic drug in massive concentrations is injected directly through the bloodstream, the effects on healthy organs would be devastating [61, 77]. Up to now, various methods are adopted to equip a bone scaffold with a drug delivery capability—loading drug molecules in the interparticle porosity of bioceramic scaffolds [20], loading the drug into a polymer matrix [78], surface coating of some porous or mesoporous nanoparticles which have a great potential for high drug loading followed by prolonged release [18, 60]. Figure 8 indicates a 3D bone scaffold with meso-macroporosity for both bone tissue regeneration and localized drug delivery applications.
A 3D-printed bone scaffold composed of ordered mesoporous silicate making the scaffold ideal to load huge amounts of drug molecules followed by releasing them in a sustained manner (localized drug delivery applications). Reproduced with permission from Ref. [79]
Fabrication techniques of bone scaffolds
There is quite number of fabrication techniques applied to yield 3D bone scaffolds spanning from traditional routes—polymer sponge (replica) [20], leaching [80], freeze-drying [81], space holder [82], foaming [83], etc.—to the up-to-dated ones including 3D printing and electrospinning capable of taking precise control over the final microstructure [84, 85]. In any case, the key aspects that should be accounted for are biocompatibility, hierarchical structure, porosity, mechanical properties, and surface properties. In the following section, a brief explanation of each technique is carried out as follows:
Foaming methods entail a foaming agent in the form of direct injection gases, gas incorporation through agitation, gas produced with chemical reactions, or thermal decomposition of peroxides. The foaming techniques have generally an easy process allowing an acceptable control of total porosity, pore size, and also pore shape through changing in the technique’s parameters—temperature and the concentration of the reactant agents [86]. However, common issues with these techniques are lack of interconnected porous structure, a non-uniform surface layer, and poor mechanical properties [87].
The space holder technique is characterized by the inclusion of sacrificial particles in the green body that is sintered. The porogen particles are polymers, either synthetic such as polyethylene, or natural such as starch or rice husk [88]. This cheap technique allows to obtain good mechanical properties and to control the level of porosity. However, the level of total porosity is difficult to exceed over 70% in volume and the pores interconnectivity tends to be low [89].
Polymer sponge technique is ideal to obtain scaffolds similar to the trabecular bone stemming from the sacrificial polymer foam’s structure. It is possible to achieve totally interconnected pores, with a level of the void that can reach 90% in volume while maintaining the desired mechanical properties close to the values of trabecular bone [90]. The architecture of scaffolds can be controlled, and the technique is compatible with various materials as well allowing the production of scaffolds made of glass [91], ceramics [20], metals [92], and composites [60]. This technique suffers from a lack of reproducibility, and also yielding a scaffold with complex shapes is difficult to obtain.
The freeze-drying technique involves the sudden and directional freezing, and it is pioneered by Fukasawa et al. [93] to yield porous ceramics; the technique takes the advantage of ice crystals to form columnar porous structures instead of inclusion of an organic matter. Once the formation of long and oriented ice crystals is carried out, the crystals will be sublimated followed by exposing the scaffold to a high temperature to be consolidated [94]. Due to the oriented structure of the crystals, the mechanical properties of the resulting scaffold are excellent, but the process is both energy and time-consuming [59].
Solvent casting and particulate leaching techniques are versatile fabrication routes for both polymeric and polymer/ceramic composite scaffolds. The techniques revolve around pouring a polymeric/slurry (polymer-ceramic) solution into a mold followed by the consolidation of solution/slurry through lyophilization or evaporation. As the result, a nanoporous structure is obtained, but in the case of yielding a macroporous structure, preferably, a water-soluble agent is introduced in the polymer/slurry solution before complete consolidation; the final bulk material will then be immersed into the water leading to the removal of the agents and yielding a macroporous scaffold [95, 96]. On the upside, fabrication of scaffolds with total porosity over even 90% is possible, the whole process is cost-effective and also based on the particle size and morphology of leaching agents, the final pore size and shape can be altered. On the downside, the limitation of the final shape, cytotoxicity of the leaching agents remained after immersion into the solvent, and achieving an interconnected porous structure are some drawbacks that should be taken into consideration.
Electrospinning is a versatile and simple fabrication technique which adopts an electric field to construct fibrous scaffolds in nano- and microscales; through using this technique, it is possible to produce scaffolds from both polymer and polymer/ceramic solutions [97, 98]. One of the greatest advantages of electrospinning, which has made this technique so popular, is its high surface area resembling the fibrous protein structure found in the natural extracellular matrix [99, 100]. Electrospinning has some fabrication-related parameters—the flow rate, applied voltage, the solution concentration, collector distance, the solution’s conductivity, and volatility—by which the textural properties of the final scaffold such as pore size, total porosity, and fiber shape can be manipulated [101].
3D printing is formed by a group of techniques, and through their applicability and compatibility with different kinds of materials, they are being applied to fabricate different bone scaffolds [56, 84, 102]. Generally, 3D printing works based on a programmed 3D image and it fabricates a bone scaffold through the deposition of layers [103]. This technique has two important advantages—reproducibility and precise control on the porosity, pore size, pore shape, and mechanical properties which make this technique stand head and shoulders above the rest. Specifically, in the case of bone defects, 3D printing endows the clinical practitioners to design a scaffold based on the complex shape of the defect; once the scan is finished, a bone scaffold will be printed. Through Table 3, the advantages and disadvantages of the mentioned fabrication techniques are summarized. In the next section, four techniques—polymer sponge and space holder as traditional fabrication routes and both electrospinning and 3D printing as more up-to-dated techniques applied to produce multifunctional bone scaffolds for bone regeneration and cancer therapy, are chosen to be discussed more comprehensively.
Fabrication techniques of multifunctional bone scaffolds for bone cancer
Polymer sponge technique
Polymer sponge technique was initially expanded in 1963 [109], and from that moment on, this technique has been widely applied to construct porous ceramic-based foams for different applications—refractory [110], separation [111], biomedical engineering [112], etc. Through this process, there is a polymeric foam (normally polyurethane sponge (PUS)) which is impregnated in a ceramic slurry; there are other materials including a binder and dispersant besides ceramic particles and water to form an appropriate and stable slurry. After impregnation of the foam, a little squeeze is required to remove additional slurry to prevent clogging the macropores. The PUS plays a sacrificial role, and after being into a furnace followed by raising the temperature, it will be burned out. It is important to notice that a multistep process for the sintering of these materials is required. The sacrificial agent should be slowly and gradually removed, and if not, the green body would be ruined. Therefore, a heating rate of about 1–2 °C min−1 is applied up to normally 600 °C plus preserving the scaffold in this temperature for about an hour to remove the backbone of sacrificial agent followed by raising the temperature to the sintering temperature of applied ceramic (normally above 1000 °C) [20]. Figure 9 shows the process of the polymer sponge process. As mentioned earlier, the PUS foam perfectly resembles the porous structure of trabecular bone and that is the reason why this technique has attracted so much attention in bone tissue engineering. Moreover, the polymer sponge technique gives an opportunity to design and fabricate bone scaffolds with adjustable pore dimensions, and based on the bone’s defect shape, this technique allows producing scaffolds with irregular shapes [60]. On the other side, the highly porous structure of scaffolds produced by this technique is a double-edged sword. The compressive strength of these scaffolds normally is not high enough to withstand load-bearing applications and so the scaffolds require complete sintering plus a reinforcement agent (normally a polymer) to improve both the natural brittleness of ceramic-based scaffolds and other properties—compressive and tensile strengths.
In 2011, Wu et al. [91] fabricated a multifunctional bone scaffold made of mesoporous bioactive glass (MBG) through the polymer sponge technique. In this study, Fe ions with different concentrations are introduced in the crystal structure of MBG particles as a new way of making the MBG particles magnetic-responsive without ruining the mesoporous structure. It is well known that the incorporation of different ions into the crystal structure of a silica-based mesoporous material negatively alters the ordered mesoporous structure [113]. Wu et al. have obtained a meso-macroporous scaffold successfully and the magnetization saturation (Ms) reached nearly 1 emu g−1; dexamethasone (DEX) is used as a drug model to assess the controlled release capability of scaffolds as well. Based on their results, the Fe-incorporated MBG scaffolds can be applied for both hyperthermia-based bone cancer therapy and localized chemotherapeutic drug release. Although they were successful to fabricate a meso-macroporous multifunctional bone scaffold with nearly 80% total porosity and promising cell compatibility, the compressive strength is poor limiting its application. In a similar study, Zhu et al. [114] have worked on the same composition reported in the Wu’s study, but through the newer study, it is mentioned that due to the calcination of Fe-incorporated MBG scaffolds under an air atmosphere, an undesirable phase of α-Fe2O3 is formed and caused the Ms to reduce dramatically in the Wu’s study. Therefore, Zhu et al. have applied a modified calcination process in which the scaffolds are first calcined at 700 °C (heating rate of 2 °C min −1) for 8 h followed by reducing the scaffolds in 10% (H2)/90% (Ar) at 400 °C (3 h). The calcination process was successful to prevent the α-Fe2O3 formation, and as a result of that, a superparamagnetic property is achieved with the Ms of 1.75 emu g−1. The heat generation capacity of scaffolds is scrutinized as well through applying an EMF whose magnetic field strength and frequency are chosen to be 1.47 kA m−1 and 232 kHz, respectively. It is observed that the applied field strength and frequency caused the temperature to rise from 36 °C to 44.25 °C after 25 min; in a parallel manner, a vial without any scaffold is also exposed the same EMF and frequency and it turned out that the temperature just increased from 36 °C to 36.5 °C after the same time.
In 2017, Farzin et al. [62] developed a bifunctional magnetic calcium-zinc-silicate scaffold through polymer sponge to address bone regeneration and cancer therapy simultaneously. The substitution of Fe3+ instead of Ca2+ was the way by which the magnetism property has been added to the scaffolds. The results show that the obtained Ms reached nearly 1 emu g−1 without the appearance of any Fe-based phase—magnetite or maghemite. The compressive strength reported being in the range of 1.8–2.5 MPa which is in the range of trabecular bone. Nonetheless, through applying a magnetic field strength and frequency of 0.8 kA m−1 and 750 kHz, respectively, the temperature generated from the scaffold has reached 50 °C after 350 s. It is noteworthy that the drug delivery of scaffolds is assessed through-loading cisplatin followed by evaluation of its release rate; the results indicate that a sustained release is obtained after an initial burst release during the first 24 h. In 2019, a study is reported by Bigham et al. [20] in which a multifunctional bone scaffold is made of a core–shell nanocomposite. The nanocomposite consists of CoFe2O4 as the core and Mg2SiO4 as the shell. The scaffold made of the nanocomposite has shown 84% total porosity with a compressive strength of 2 MPa. The Ms of scaffold is reported to be 15 emu g−1 which is higher than that of other similar studies; through hyperthermia assessment in vitro, a range of magnetic fields with different strengths including 7.95, 9.95, 11.94, and 15.91 kA m−1 along with a constant frequency of 200 kHz is adopted. The results reveal that a progressive increase in the magnetic field results in an increase in temperature up to 500 s. Nevertheless, the drug delivery potential of a nanostructured scaffold is assessed as well through loading an antibiotic, and the related results evidence that the multifunctional Mg2SiO4-CoFe2O4 scaffold has a promising potential to keep drug availability for about 100 h. In a complementary study carried out by Bigham et al. [60], the surface coating of a multifunctional Mg2SiO4-CoFe2O4 scaffold is carried out to not only reinforce the mechanical properties but also take better control over the release rate of the scaffold. The scaffold is coated with poly-3-hydroxybutyrate (P3HB)-ordered mesoporous magnesium silicate (OMMS) composite. The polymeric phase has significantly improved the compressive strength of the scaffold up to 3 MPa and the OMMS microparticles by having ordered mesoporous structure (4 nm) provided a carrier to load high amounts of drug molecules followed by liberating the molecules in a controlled way. It is important to remind that coating of the scaffold has decreased the Ms, but through applying a magnetic field of 19.9 kA m−1, that shortcoming was reported to be compensated.
Space holder technique
Space holder technique can be regarded as a derivative of the traditional powder sintering process in which metal or ceramic powder particles are compacted followed by sintering at high temperatures; the porous structure is formed as the result of rearrangement of particles during sintering procedure [115, 116]. The final pore size and shape totally depend on the morphology of initial powders, but through applying the powder sintering process, a maximum total porosity of 35% can be achieved [117]. Space holder technique adopts different temporary pore-forming agents, and these agents are embedded through either ceramic or metal starting particles; elimination of the agents either before or during the sintering process forms a porous structure in the final ceramic/metal material [118, 119]. To fabricate a bone scaffold through space holder technique, the following steps are generally applied [120, 121] (Fig. 10):
-
1.
Preparation of a mixture of the starting powder (ceramic/metal) and pore-forming agents.
-
2.
Compaction of the obtained mixture.
-
3.
Elimination of the pore-forming agents through the compacted material.
-
4.
Consolidation of the scaffold through the sintering process.
It is critical to bear in mind that choosing an appropriate space holder agent has direct effects on the textural and mechanical properties of the scaffold and it may indirectly have the potential to trigger cytotoxicity in the case of incomplete removal. Therefore, some parameters including the agent’s biocompatibility, chemical stability, and removal capability are of great importance in this technique. Up to now, various pore-forming agents are used in space holder technique—sodium chloride (NaCl) [122], carbamide [117], ammonium hydrogen carbonate [123], etc. To minimize the negative effects of remained pore-forming agents on cell compatibility, some researchers have used food-grade powders—cornstarch [124], NaCl [125], and saccharose [126].
In 2018, Abdellahi et al. [105] developed a novel composite scaffold composed of diopside (CaMgSi2O6)-magnetite (Fe3O4) to be applied in bone cancer therapy and regeneration. Different amounts of Fe3O4 are used in the scaffold’s structure, and their effects on the bioactivity, heat generation capability, and mechanical properties of scaffolds are assessed in vitro. Figure 11 exhibits the scaffolds with different amounts of Fe3O4. The results related to the scaffolds’ microstructure reveal that the increase in the Fe3O4 content results in the improvement in the pore’s interconnectivity and heat generation capability. Increasing the porous structure is a double-edged sword because on the one side, these pores ensure penetration of nutrients and waste removal, and on the other side, the mechanical properties weaken significantly. In the same year, Najafinezhad et al. [127] reported a research job in which a hexaferrite composition (SrFe12O19) was mixed with hydroxyapatite to fabricate a multifunctional bone scaffold for hyperthermia-based therapy. It is worth mentioning that applying hard ferrites in hyperthermia have always been debatable due to their high anisotropy. The effects of SrFe12O19 contents plus gelatin coating on the heat generation capacity and compressive strength of hydroxyapatite scaffolds are assessed. Based on the reported results, the HA-20 wt% SrFe12O19 scaffold had a larger pore size leading to the absorption of more gelatin and better compressive strength compared to other scaffolds.
The microstructure of diopside-magnetite scaffolds with different magnetite contents: a 10 wt%; b 20 wt% c 30 wt%. Reproduced with permission from Ref. [105]
In 2020, Sahmani et al. [128] designed and fabricated a multifunctional Ha-Fe3O4 bone scaffold through space holder technique with different Fe3O4 contents—0 wt%, 5 wt%, 10 wt%, and 15 wt%. Next, a coating containing ibuprofen and gelatin is developed on the scaffolds to monitor the controlled release rate along with the hyperthermia capability. The overall results show that the addition of Fe3O4 reduces the scaffolds’ interconnected porous structure on the downside, but it improves the degradation rate and effectiveness of hyperthermia therapy on the upside. Moreover, the release trend of ibuprofen is reported to depend on the Ha content.
Electrospinning
Through applying high voltages, electrospun macro-/nanofibers are fabricated from electrified jets; due to a repulsive interaction of the applied solvent and surface charge, fibers are being elongated continuously during the fabrication process [129]. As mentioned earlier, yielding a fibrous scaffold with a high surface area similar to the extracellular matrix is considered as one of the most important features of the electrospinning technique [130]. Moreover, the compatibility of technique with various materials—natural and synthetic polymers, ceramics, metals, and also their combinations—has made it an easy-to-use technique [131]. Although electrospinning has numerous advantages, designing and fabrication of densely packed fibers with the entangled structure were difficult to achieve [132]. Therefore, the rotating collectors are applied in the technique to compensate for the setback [133]. Figure 12 indicates a typical electrospinning system adopting a high voltage to obtain macro-/nanofibrous scaffolds.
A typical electrospinning system yielding fibrous scaffolds for different applications. Reproduced with permission from Ref. [59]
As it is well known that the scaffold’s topography directly affects the cell adhesion and proliferation, electrospinning is capable of taking control over the size and shape of the fibers, and total porosity as well [134]. There are some criteria when it comes to designing electrospun fibers for a specific application in tissue engineering—choosing an appropriate material based on the desired need, total porosity, surface modification, and fibers orientation (aligned vs. random) [135]. Through controlling the fiber size and orientation, the final pore size and total porosity will be optimized for an intended application [136]. Moreover, after the fabrication process, because of the high surface area of scaffolds, the surface can be modified through different kinds of bioactive molecules [137]. Applying single-phase electrospun scaffolds (normally a natural/synthetic polymer) has expansively assessed for different applications, but recently, obtaining an electrospun composite scaffold consists of a bioceramic and a polymer for bone tissue regeneration is of particular interest; different bioceramics including hydroxyapatite, bioactive glass, etc. have been incorporated into the matrix of various polymers—PCL, gelatin, PVA, etc. [98, 138,139,140,141].
Designing an electrospun multifunctional scaffold comes back to 2013 when Gloria et al. [142] developed a magnetic scaffold composed of PCL/Fe-doped hydroxyapatite for advanced bone tissue engineering. The main aim of their study was to fabricate a fully degradable multifunctional scaffold to deal with different bone-related issues—cancer and regeneration. The results show that the fibrous nanocomposite has a superparamagnetic character and Ms is directly related to the Fe-doped hydroxyapatite content and the heat generation ability of scaffolds for hyperthermia-based therapy is evident; the osteogenic differentiation is also accelerated through applying an EMF. In the same year, Amarjargal et al. [143] studied the effects of Fe3O4 nanoparticles on the physical and chemical properties of polyurethane fibers. The novelty of this work revolves around the immobilization of Fe3O4 nanoparticles on the fibers through a simple immersion of the fibrous scaffolds into a polyol solution. The addition of Fe3O4 nanoparticles led to obtaining the Ms of 33.12 emu g−1 that is suitable for hyperthermia-based therapy. The thermal activity of nanocomposite is very promising, and the sample containing the highest amounts of Fe3O4 nanoparticles called MNF2 raised the temperature around 55 °C in 600 s when it exposed to an EMF. The SEM and TEM micrographs of polyurethane and polyurethane-Fe3O4 nanoparticles along with the related VSM and hyperthermia studies are exhibited in Fig. 13.
The FESEM and TEM micrographs of the electrospun membranes: (a, a′, and a″) PU; (b, b′, and b″) MNF0.5; (c, c′, and c″) MNF1; (d, d′, and d″) MNF2; magnetic hysteresis loops of Fe3O4 and MNF2 membrane (e); heat generation vs. time curves of the membranes in the exposure of an EMF (f). Reproduced with permission from Ref. [143]
Another composite fibrous scaffold containing polylactic acid (PLA), hydroxyapatite, and γ-Fe2O3 is developed by Meng et al. [144] through electrospinning technique. The Ms scaffold is found to be 0.049 emu g−1, and the scaffold is implanted into a bone defect of white rabbit; the rabbit models are permanently exposed to an EMF during the test to assess whether the magnetic property of scaffold accelerates the bone regeneration rate. The results reveal that the magnetic scaffold has a promising potential to regenerate the bone defect and alleviate the postsurgery period. In 2018, a doxorubicin hydrochloride-loaded electrospun scaffold composed of chitosan/cobalt ferrite/titanium oxide was fabricated for both chemotherapy and hyperthermia therapy by Radmansouri et al. [145]. It turned out that the fiber size of the composite scaffold is in the range of 90–110 nm. The thermal activity of the scaffold is assessed in vitro in the exposure of an EMF and the temperature reached 44.7 °C after 900 s (strength of magnetic field = 1 kA m−1, frequency = 290 kHz). The drug release study is performed in both the presence and absence of EMF up to 72 h, and the results exhibit that after a burst release in the initial hours of soaking, there is a steady rate up to 72 h. Moreover, the magnetic field applied in the release study caused the liberation trend to become faster stemming from the vibration-related effects of magnetism on the magnetic materials.
3D printing
Nowadays, 3D printing technologies have changed the traditional prostheses and presented a sufficient method to replace tumors with patient-matched devices. Figure 14 shows a hypothetical model for a working plan to produce patient-specific devices. Besides theoretical and laboratory models, 3D-printed prostheses have earned the approval for successful clinical trials. It should be noted that the most important feature about 3D-printed prostheses is that their contour should be able to accurately fit the injured bone. In addition, 3D printing can help in producing porous devices. Porous devices not only promote bone ingrowth but also reduce the modulus, which can prevent prolonged mechanical issues such as fracture and loosening. Porous 3D-printed scaffolds have shown to possess a high potential for bone-related problems. In practice, interconnected networks are necessary to provide enough space for cell bioactivity, cell feeding, and interactions. In the meantime, the 3D-printed surface with a specified design explicitly affects cell interactions and formation. Therefore, producing a porous scaffold by the 3D printing method is of great interest and importance for developing bone formation. Figure 15 shows a 3D-printed prosthesis for a 13-year-old girl, who showed a diagnosis of osteosarcoma.
The path of making patient-specific devices via 3D printing. Reproduced with permission from Ref. [146]
a X-ray and MRI image, b 3D model of the tumor and bone plate, c patient-matched, d tumor and bone defect, e the matched contour to the patient, f One month after surgery. Reproduced with permission from Ref. [147]
3D printing provides a unique feature to produce an architecturally complicated design with high reproducibility. Regarding conventional methods, a wide range of polymers have been printed using techniques which are mostly based on extrusion of materials, for instance, fused filament fabrication to make scaffolds and devices with controllable porosity. The good point about them is that they can offer high mechanical properties. However, the cons about them are related to the low resolution and cell culture has to be done after fabrication. Moreover, printing materials are limited and the high printing temperatures during fused filament fabrication do not allow for the incorporation of cells and biomolecules during manufacturing. On the contrary, 3D bioprinting techniques provide in situ incorporation of cells such as direct-ink writing and inkjet printing. However, there are some issues about utilizing inkjet printing which is needle clogging for viscous inks and exposing cells to severe shear forces. Respecting other useful methods which can incorporate cell at the time of printing, stereolithography should be mentioned which shows great resolution as well as favorable properties. It should be noted that the toxicity related to monomers and utilizing UV radiation for curing may cause some viability problems for cells in long times. Table 4 demonstrates available additive manufacturing methods used for bone scaffold production including their advantages and disadvantages [18]. There are five different approaches in additive manufacturing of bone scaffolds—direct-ink writing, laser-assisted bioprinting, selective laser sintering, stereolithography, and fused deposition modeling.
Direct-ink writing technique refers to applying a slurry-based ink which is highly concentrated, and it should be able to carry its weight during the assembly while not being deformed; the composition should be carefully chosen and its viscoelastic properties must be taken into account.
Laser-assisted bioprinting has vast applicability in tissue engineering rooting in its physical mechanisms giving an ample opportunity to print liquid materials plus cells. The technique is very promising because it allows the engineers to control the cell density carefully and also organizing the 3D tissue construct.
Selective laser sintering through adopting a CO2 laser beam applies heat during the printing process to consolidate the layers; the technique is compatible with polymers, ceramics, and the combinations.
The stereolithography technique is based on using a light-responsive polymer which means after or during the deposition of a layer, light is being emitted on the polymer resulting in the solidification of layers.
Fused deposition modeling is based on using a thermoplastic filament; before the extrusion of filament, it exposes to heat through a liquefier-head, and then it will be extruded through a nozzle to form the layers.
Inkjet printing is one of the most useful 3D printing techniques for the fabrication of bone scaffolds. In this technique, a ceramic suspension is pumped and the droplets of the suspension are accumulated on the printing bed to form the final design of the product.
Generally, after printing scaffolds, it is needed to culture cells on the printed device prior to implantation in the body. Hence, it should be noted that some of these approaches, such as direct-ink writing and stereolithography, are able to incorporate medicine, cells, and biomolecules while printing the scaffold. However, each of these methods has some limitations which are completely discussed in Table 4.
Designing and applying 3D-printed multifunctional ceramic bone scaffolds for simultaneous bone cancer therapy and regeneration traces back to 2016 when Ma et al. [159] developed a 3D-printed tri-calcium phosphate scaffold modified with graphene oxide to deal with bone tumors through photothermal therapy. Besides possessing appropriate microstructural and mechanical properties, it is found that based on the graphene oxide concentration, the heat generation of the modified scaffold can be altered in the range of 40–90 °C, whereas the unmodified scaffold did not raise the temperature in the presence of near-infrared laser. Moreover, the modified scaffold is turned out to successfully stimulate rabbit bone mesenchymal stem cells toward osteogenic cells (Fig. 16). The same group accomplished another work on 2016 [160] in which a calcium-silicate-phosphate called Nagelschmidite (Ca7Si2P2O16) is printed followed by a surface modification on the scaffolds consisting of Ca-P/polydopamine. The in vitro and in vivo studies together suggest that the surface-modified scaffold is a promising candidate for large bone defects caused by the removal of bone tumors to not only accelerate the regeneration process but also reducing the risk of tumor recurrence. In 2017, Khandan et al. [161] prepared a 3D-printed nanocomposite bone scaffold composed of Ca7MgSi4O16-Fe3O4 for hyperthermia-based bone cancer therapy. Based on the results, incorporation of 30% wt Fe3O4 leads to a significant improvement in the physical, chemical, and biological-related properties of Ca7MgSi4O16 scaffold and the magnetic nanoparticles endow hyperthermia capability to the scaffold as well. Up to now, various modifiers being responsive to near-infrared laser have been investigated for bone cancer therapy such as CuFeSe2 [30], SrFe12O19 [162], LaB6 [72], carbon [108], and MoS2 [163]. Although all of them were well established and successfully getting the osteosarcoma cells killed, a sufficient and easy way is introduced by Liu et al. [164, 165] which is doping metallic elements into the crystal structure of a bioactive ceramic-like bioglass. They have developed 3D-printed Cu, Fe, Mn, and Co-doped bioactive glass–ceramic scaffolds for bone cancer therapy. Besides, some of these elements are reported to have angiogenesis and antibacterial properties which are added values to take into consideration [65, 66, 166,167,168]. The added elements show an excellent photothermal ability besides playing another key role which is being a sintering aid increasing the mechanical properties. The overall results exhibit that this approach is a smart strategy to develop bone scaffolds with multiple capabilities—bone cancer therapy, desired mechanical properties, accelerated bone regeneration, antibacterial activity, and angiogenesis (Fig. 17).
a A schematic showing 3D printing and surface modification of the scaffolds followed by applying for bone regeneration and cancer therapy; b digital photographs relating to 3D-printed TCP (left) and surface-modified TCP scaffolds (right) scaffolds; the microstructure of c TCP and d surface-modified TCP scaffolds. Reproduced with permission from Ref. [159]
Below each digital photograph, there is the related SEM micrograph of scaffolds doped with different elements. Reproduced with permission from Ref. [165].
Conclusions
Due to a high risk of bone tumor recurrence, filling up a large-sized bone defect caused by a tumor removal with a multifunctional bone scaffold is killing two birds with one stone. The multifunctional scaffolds are able to address the remained bone cancerous cells in the defect’s wall through hyperthermia, photothermal, and localized drug delivery besides regenerating the bone tissue. Various fabrication techniques are applied in recent years to yield multifunctional scaffolds with desirable properties like polymer sponge, space holder, electrospinning, and 3D printing. Despite yielding 3D scaffolds with a porous structure similar to bone, polymer sponge and space holder techniques are suffering from a lack of precise control over microstructure and reproducibility. Modern techniques—electrospinning and 3D printing—are proven to be practically applicable when it comes to designing a bone scaffold based on the complex shape of a bone defect. Through this review, we aimed to shed light on the multifunctional bone scaffolds potential for both bone cancer therapy and regeneration, in particular, different fabrication techniques adopted to construct these scaffolds are reviewed.
With the advent of 3D printing in bone tissue engineering, this field has undergone a fundamental change since then. Therefore, it is clear that 3D printing’s excellent properties—precise control on morphology and reproducibility—have made the researchers put aside the traditional techniques. Despite the advantages of 3D printing compared to traditional techniques, it requires permanent progress in its accuracy and resolution. Moreover, working with up-to-dated 3D printing systems is costly and so cooperation between different fields to reduce the costs is necessary. It is worth mentioning that 3D-printed ceramic scaffolds are much more difficult to obtain than polymeric ones; preparation of ceramic-based slurries with high flowability for 3D printing requires an appropriate binder, dispersant, and solvent in the first stage. Next, to be mechanically strong for load-bearing applications, it needs to be sintered at high temperatures leading to shrinkage followed by the appearance of cracks. Therefore, it can be expected that through the near future, we will witness vast progress in the 3D printing techniques resulting in more structurally accurate multifunctional scaffolds for bone cancer therapy.
References
Pérez-Amodio S, Engel E (2014) Bone biology and regeneration. Bio-Ceram Clin Appl. https://doi.org/10.1002/9781118406748.ch11
Reznikov N, Shahar R, Weiner S (2014) Bone hierarchical structure in three dimensions. Acta Biomater 10:3815–3826. https://doi.org/10.1016/j.actbio.2014.05.024
Lohkamp M, Kromer TO, Schmitt H (2017) Osteoarthritis and joint replacements of the lower limb and spine in ex-professional soccer players: a systematic review. Scand J Med Sci Sports 27:1038–1049. https://doi.org/10.1111/sms.12846
Salmon JH, Rat AC, Sellam J, Michel M, Eschard JP, Guillemin F, Jolly D, Fautrel B (2016) Economic impact of lower-limb osteoarthritis worldwide: a systematic review of cost-of-illness studies. Osteoarthr Cartil 24:1500–1508. https://doi.org/10.1016/j.joca.2016.03.012
Pawelec KM (2019) Introduction to the challenges of bone repair. In: Pawelec KM, Planell E (eds) Woodhead publishing series in biomaterials, 2nd edn. Woodhead Publishing, Sawston, pp 1–13. https://doi.org/10.1016/B978-0-08-102451-5.00001-9
Fraumeni JF Jr (1967) Stature and malignant tumors of bone in childhood and adolescence. Cancer 20:967–973. https://doi.org/10.1002/1097-0142(196706)20:6%3c967:AID-CNCR2820200606%3e3.0.CO;2-P
Purushotham S, Ramanujan RV (2010) Thermoresponsive magnetic composite nanomaterials for multimodal cancer therapy. Acta Biomater 6:502–510. https://doi.org/10.1016/j.actbio.2009.07.004
Gupta R, Bajpai AK (2011) Magnetically guided release of ciprofloxacin from superparamagnetic polymer nanocomposites. J Biomater Sci Polym Ed 22:893–918. https://doi.org/10.1163/092050610X496387
Xu R, Ma J, Sun X, Chen Z, Jiang X, Guo Z, Huang L, Li Y, Wang M, Wang C, Liu J, Fan X, Gu J, Chen X, Zhang Y, Gu N (2009) Ag nanoparticles sensitize IR-induced killing of cancer cells. Cell Res 19:1031–1034. https://doi.org/10.1038/cr.2009.89
Gallego Ó, Puntes V (2006) What can nanotechnology do to fight cancer? Clin Transl Oncol 8:788–795. https://doi.org/10.1007/s12094-006-0133-6
Vallet-Regí M, Ruiz-Hernández E (2011) Bioceramics: from bone regeneration to cancer nanomedicine. Adv Mater 23:5177–5218. https://doi.org/10.1002/adma.201101586
Gu W, Wu C, Chen J, Xiao Y (2013) Nanotechnology in the targeted drug delivery for bone diseases and bone regeneration. Int J Nanomed 8:2305–2317. https://doi.org/10.2147/IJN.S44393
Qu H, Fu H, Han Z, Sun Y (2019) Biomaterials for bone tissue engineering scaffolds: a review. RSC Adv 9:26252–26262. https://doi.org/10.1039/C9RA05214C
Vallet-Regí M, Lozano D, González B, Izquierdo-Barba I (2020) Biomaterials against bone infection. Adv Healthc Mater. https://doi.org/10.1002/adhm.202000310
Hutmacher DW (2000) Scaffolds in tissue engineering bone and cartilage. Biomater Silver Jubil Compend. https://doi.org/10.1016/B978-008045154-1.50021-6
Bigham A, Kermani S, Saudi A, Aghajanian A, Rafienia M (2020) On the bioactivity and mechanical properties of gehlenite nanobioceramic: a comparative study YR—2020/4/1. J Med Signals Sens 10:105–112. https://doi.org/10.4103/jmss.JMSS_41_19
Rafienia M, Bigham A, Saudi A, Rahmati S (2018) Gehlenite nanobioceramic: sol–gel synthesis, characterization, and in vitro assessment of its bioactivity. Mater Lett. https://doi.org/10.1016/j.matlet.2018.04.094
Bigham A, Hassanzadeh-Tabrizi SA, Rafienia M, Salehi H (2016) Ordered mesoporous magnesium silicate with uniform nanochannels as a drug delivery system: the effect of calcination temperature on drug delivery rate. Ceram Int. https://doi.org/10.1016/j.ceramint.2016.08.009
Marques C, Ferreira JMF, Andronescu E, Ficai D, Sonmez M, Ficai A (2014) Multifunctional materials for bone cancer treatment. Int J Nanomed 9:2713–2725. https://doi.org/10.2147/IJN.S55943
Bigham A, Aghajanian AH, Behzadzadeh S, Sokhani Z, Shojaei S, Kaviani Y, Hassanzadeh-Tabrizi SA (2019) Nanostructured magnetic Mg2SiO4–CoFe2O4 composite scaffold with multiple capabilities for bone tissue regeneration. Mater Sci Eng, C 99:83–95. https://doi.org/10.1016/j.msec.2019.01.096
Lu Y, Li L, Zhu Y, Wang X, Li M, Lin Z, Hu X, Zhang Y, Yin Q, Xia H, Mao C (2018) Multifunctional copper-containing carboxymethyl chitosan/alginate scaffolds for eradicating clinical bacterial infection and promoting bone formation. ACS Appl Mater Interfaces 10:127–138. https://doi.org/10.1021/acsami.7b13750
Zhang Q, Mochalin VN, Neitzel I, Hazeli K, Niu J, Kontsos A, Zhou JG, Lelkes PI, Gogotsi Y (2012) Mechanical properties and biomineralization of multifunctional nanodiamond-PLLA composites for bone tissue engineering. Biomaterials 33:5067–5075. https://doi.org/10.1016/j.biomaterials.2012.03.063
Tamay DG, Dursun Usal T, Alagoz AS, Yucel D, Hasirci N, Hasirci V (2019) 3D and 4D printing of polymers for tissue engineering applications. Front Bioeng Biotechnol 7:164. https://doi.org/10.3389/fbioe.2019.00164
Ma H, Feng C, Chang J, Wu C (2018) 3D-printed bioceramic scaffolds: from bone tissue engineering to tumor therapy. Acta Biomater 79:37–59. https://doi.org/10.1016/j.actbio.2018.08.026
Fuchs RK, Thompson WR, Warden SJ (2019) Bone biology. In: Pawelec KM, Planell E (eds) Woodhead publishing series in biomaterials, 2nd edn. Woodhead Publishing, Sawston, pp 15–52
Wegst UGK, Bai H, Saiz E, Tomsia AP, Ritchie RO (2015) Bioinspired structural materials. Nat Mater 14:23–36. https://doi.org/10.1038/nmat4089
Koester KJ, Ager JW, Ritchie RO (2008) The true toughness of human cortical bone measured with realistically short cracks. Nat Mater 7:672–677. https://doi.org/10.1038/nmat2221
Rho J-Y, Kuhn-Spearing L, Zioupos P (1998) Mechanical properties and the hierarchical structure of bone. Med Eng Phys 20:92–102. https://doi.org/10.1016/S1350-4533(98)00007-1
Eliaz N, Metoki N (2017) Calcium phosphate bioceramics: a review of their history, structure, properties, coating technologies and biomedical applications. Materials. https://doi.org/10.3390/ma10040334
Dang W, Li T, Li B, Ma H, Zhai D, Wang X, Chang J, Xiao Y, Wang J, Wu C (2018) A bifunctional scaffold with CuFeSe2 nanocrystals for tumor therapy and bone reconstruction. Biomaterials 160:92–106. https://doi.org/10.1016/j.biomaterials.2017.11.020
Samavedi S, Joy N (2017) 3D printing for the development of in vitro cancer models. Curr Opin Biomed Eng 2:35–42. https://doi.org/10.1016/j.cobme.2017.06.003
Mouriño V, Boccaccini AR (2010) Bone tissue engineering therapeutics: controlled drug delivery in three-dimensional scaffolds. J R Soc Interface 7:209–227. https://doi.org/10.1098/rsif.2009.0379
Wadajkar AS, Bhavsar Z, Ko C-Y, Koppolu B, Cui W, Tang L, Nguyen KT (2012) Multifunctional particles for melanoma-targeted drug delivery. Acta Biomater 8:2996–3004. https://doi.org/10.1016/j.actbio.2012.04.042
Ruel-Gariépy E, Shive M, Bichara A, Berrada M, Le Garrec D, Chenite A, Leroux J-C (2004) A thermosensitive chitosan-based hydrogel for the local delivery of paclitaxel. Eur J Pharm Biopharm 57:53–63. https://doi.org/10.1016/S0939-6411(03)00095-X
Nagarajan S, Reddy BSR, Tsibouklis J (2011) In vitro effect on cancer cells: synthesis and preparation of polyurethane membranes for controlled delivery of curcumin. J Biomed Mater Res, Part A 99A:410–417. https://doi.org/10.1002/jbm.a.33203
Fan H, Dash AK (2001) Effect of cross-linking on the in vitro release kinetics of doxorubicin from gelatin implants. Int J Pharm 213:103–116. https://doi.org/10.1016/S0378-5173(00)00651-7
Andronescu E, Ficai A, Albu MG, Mitran V, Sonmez M, Ficai D, Ion R, Cimpean A (2013) Collagen-hydroxyapatite/cisplatin drug delivery systems for locoregional treatment of bone cancer. Technol Cancer Res Treat 12:275–284. https://doi.org/10.7785/tcrt.2012.500331
Mann S, Khawar S, Moran C, Kalhor N (2019) Revisiting localized malignant mesothelioma. Ann Diagn Pathol 39:74–77. https://doi.org/10.1016/j.anndiagpath.2019.02.014
Sabino MAC, Luger NM, Mach DB, Rogers SD, Schwei MJ, Mantyh PW (2003) Different tumors in bone each give rise to a distinct pattern of skeletal destruction, bone cancer-related pain behaviors and neurochemical changes in the central nervous system. Int J Cancer 104:550–558. https://doi.org/10.1002/ijc.10999
Kaliki S, Rathi SG, Palkonda VAR (2018) Primary orbital Ewing sarcoma family of tumors: a study of 12 cases. Eye 32:615–621. https://doi.org/10.1038/eye.2017.278
Goyal S, Roscoe J, Ryder WDJ, Gattamaneni HR, Eden TOB (2004) Symptom interval in young people with bone cancer. Eur J Cancer 40:2280–2286. https://doi.org/10.1016/j.ejca.2004.05.017
Corre I, Verrecchia F, Crenn V, Redini F, Trichet V (2020) The osteosarcoma microenvironment: a complex but targetable ecosystem. Cells. https://doi.org/10.3390/cells9040976
Glass AG, Fraumeni JF Jr (1970) Epidemiology of bone cancer in children. JNCI J Natl Cancer Inst 44:187–199. https://doi.org/10.1093/jnci/44.1.187
Atallah JP, Elshenawy MA, Ali Badran A, Alata MK, Gad A, Alharbi AH, Alquaydheb HN, Alshamsan BI (2020) The outcome of vincristine, dactinomycin D, ifosfamide and doxorubicin (VAIA) as first-line therapy for adult-patients with metastatic ewing sarcoma; a single-center experience. J Clin Oncol 38:e23501–e23501. https://doi.org/10.1200/JCO.2020.38.15_suppl.e23501
Scarborough JA, McClure E, Anderson P, Dhawan A, Durmaz A, Lessnick SL, Hitomi M, Scott JG (2020) Identifying states of collateral sensitivity during the evolution of therapeutic resistance in Ewing’s sarcoma. BioRxiv. https://doi.org/10.1101/2020.02.11.943936
Miles DT, Voskuil RT, Dale W, Mayerson JL, Scharschmidt TJ (2020) Integration of denosumab therapy in the management of giant cell tumors of bone. J Orthop 22:38–47. https://doi.org/10.1016/j.jor.2020.03.020
Sun J, Wei Q, Zhou Y, Wang J, Liu Q, Xu H (2017) A systematic analysis of FDA-approved anticancer drugs. BMC Syst Biol 11:87. https://doi.org/10.1186/s12918-017-0464-7
Prasad SR, Jayakrishnan A, Kumar TSS (2020) Combinational delivery of anticancer drugs for osteosarcoma treatment using electrosprayed core shell nanocarriers. J Mater Sci Mater Med 31:44. https://doi.org/10.1007/s10856-020-06379-5
Plichta Z, Horák D, Mareková D, Turnovcová K, Kaiser R, Jendelová P (2020) Poly[N-(2-hydroxypropyl)methacrylamide]-modified magnetic γ-F2O3 nanoparticles conjugated with doxorubicin for glioblastoma treatment. ChemMedChem 15:96–104. https://doi.org/10.1002/cmdc.201900564
Ahmadi D, Zarei M, Rahimi M, Khazaie M, Asemi Z, Mir SM, Sadeghpour A, Karimian A, Alemi F, Rahmati-Yamchi M, Salehi R, Jadidi-Niaragh F, Yousefi M, Khelgati N, Majidinia M, Safa A, Yousefi B (2020) Preparation and in vitro evaluation of pH-responsive cationic cyclodextrin coated magnetic nanoparticles for delivery of methotrexate to the Saos-2 bone cancer cells. J Drug Deliv Sci Technol 57:101584. https://doi.org/10.1016/j.jddst.2020.101584
Kinch MS, Hoyer D, Patridge E, Plummer M (2015) Target selection for FDA-approved medicines. Drug Discov Today 20:784–789. https://doi.org/10.1016/j.drudis.2014.11.001
Bao Y, Kong X, Yang L, Liu R, Shi Z, Li W, Hua B, Hou W (2014) Complementary and alternative medicine for cancer pain: an overview of systematic reviews, evidence-based complement. Altern Med 2014:170396. https://doi.org/10.1155/2014/170396
Hutmacher DW (2000) Scaffolds in tissue engineering bone and cartilage. In: Williams JC (ed) Biomaterials. Elsevier Science, Oxford, pp 175–189. https://doi.org/10.1016/B978-008045154-1.50021-6
Bigham A, Saudi A, Rafienia M, Rahmati S, Bakhtiyari H, Salahshouri F, Sattary M, Hassanzadeh-Tabrizi SA (2019) Electrophoretically deposited mesoporous magnesium silicate with ordered nanopores as an antibiotic-loaded coating on surface-modified titanium. Mater Sci Eng, C 96:765–775. https://doi.org/10.1016/j.msec.2018.12.013
R. Langer, J.P. Vacanti, Tissue engineering, Science (80-.). 260 (1993) 920 LP – 926. https://doi.org/10.1126/science.8493529
Hollister SJ (2005) Porous scaffold design for tissue engineering. Nat Mater 4:518–524. https://doi.org/10.1038/nmat1421
Hollister SJ (2009) Scaffold design and manufacturing: from concept to clinic. Adv Mater 21:3330–3342. https://doi.org/10.1002/adma.200802977
Vallet-Regí M, González-Calbet JM (2004) Calcium phosphates as substitution of bone tissues. Prog Solid State Chem 32:1–31. https://doi.org/10.1016/j.progsolidstchem.2004.07.001
Izquierdo-Barba I (2014) Scaffold designing. Bio-Ceram Clin Appl. https://doi.org/10.1002/9781118406748.ch10
Bigham A, Aghajanian AH, Saudi A, Rafienia M (2020) Hierarchical porous Mg2SiO4–CoFe2O4 nanomagnetic scaffold for bone cancer therapy and regeneration: surface modification and in vitro studies. Mater Sci Eng, C 109:110579. https://doi.org/10.1016/j.msec.2019.110579
Ansari M, Bigham A, Hassanzadeh Tabrizi SA, Abbastabar Ahangar H (2018) Copper-substituted spinel Zn–Mg ferrite nanoparticles as potential heating agents for hyperthermia. J Am Ceram Soc. https://doi.org/10.1111/jace.15510
Farzin A, Fathi M, Emadi R (2017) Multifunctional magnetic nanostructured hardystonite scaffold for hyperthermia, drug delivery and tissue engineering applications. Mater Sci Eng, C 70:21–31. https://doi.org/10.1016/j.msec.2016.08.060
Iqbal Y, Bae H, Rhee I, Hong S (2016) Control of the saturation temperature in magnetic heating by using polyethylene-glycol-coated rod-shaped nickel-ferrite (NiFe2O4) nanoparticles. J Korean Phys Soc 68:587–592. https://doi.org/10.3938/jkps.68.587
Sun C, Lee JSH, Zhang M (2008) Magnetic nanoparticles in MR imaging and drug delivery. Adv Drug Deliv Rev 60:1252–1265. https://doi.org/10.1016/j.addr.2008.03.018
Ansari M, Bigham A, Ahangar HA (2019) Super-paramagnetic nanostructured CuZnMg mixed spinel ferrite for bone tissue regeneration. Mater Sci Eng, C 105:110084. https://doi.org/10.1016/j.msec.2019.110084
Bigham A, Aghajanian AH, Allahdaneh S, Hassanzadeh-Tabrizi SA (2019) Multifunctional mesoporous magnetic Mg2SiO4–CuFe2O4 core-shell nanocomposite for simultaneous bone cancer therapy and regeneration. Ceram Int 45:19481–19488. https://doi.org/10.1016/j.ceramint.2019.06.205
Bigham A, Foroughi F, Motamedi M, Rafienia M (2018) Multifunctional nanoporous magnetic zinc silicate-ZnFe2O4 core-shell composite for bone tissue engineering applications. Ceram Int. https://doi.org/10.1016/j.ceramint.2018.03.264
Hsiao C-W, Chuang E-Y, Chen H-L, Wan D, Korupalli C, Liao Z-X, Chiu Y-L, Chia W-T, Lin K-J, Sung H-W (2015) Photothermal tumor ablation in mice with repeated therapy sessions using NIR-absorbing micellar hydrogels formed in situ. Biomaterials 56:26–35. https://doi.org/10.1016/j.biomaterials.2015.03.060
Chen Q, Xu L, Liang C, Wang C, Peng R, Liu Z (2016) Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat Commun 7:13193. https://doi.org/10.1038/ncomms13193
Zhang C, Bu W, Ni D, Zuo C, Cheng C, Li Q, Zhang L, Wang Z, Shi J (2016) A polyoxometalate cluster paradigm with self-adaptive electronic structure for acidity/reducibility-specific photothermal conversion. J Am Chem Soc 138:8156–8164. https://doi.org/10.1021/jacs.6b03375
Zhou F, Li X, Naylor MF, Hode T, Nordquist RE, Alleruzzo L, Raker J, Lam SSK, Du N, Shi L, Wang X, Chen WR (2015) InCVAX—a novel strategy for treatment of late-stage, metastatic cancers through photoimmunotherapy induced tumor-specific immunity. Cancer Lett 359:169–177. https://doi.org/10.1016/j.canlet.2015.01.029
Dang W, Ma B, Huan Z, Lin R, Wang X, Li T, Wu J, Ma N, Zhu H, Chang J, Wu C (2019) LaB6 surface chemistry-reinforced scaffolds for treating bone tumors and bone defects. Appl Mater Today 16:42–55. https://doi.org/10.1016/j.apmt.2019.04.015
Mehrafzoon S, Hassanzadeh-Tabrizi SA, Bigham A (2018) Synthesis of nanoporous Baghdadite by a modified sol-gel method and its structural and controlled release properties. Ceram Int. https://doi.org/10.1016/j.ceramint.2018.04.244
Wang L, Cao J, Lei DL, Cheng XB, Zhou HZ, Hou R, Zhao YH, Cui FZ (2010) Application of nerve growth factor by gel increases formation of bone in mandibular distraction osteogenesis in rabbits. Br J Oral Maxillofac Surg 48:515–519. https://doi.org/10.1016/j.bjoms.2009.08.042
Bigham A, Hassanzadeh-Tabrizi SA, Khamsehashari A, Chami A (2018) Surfactant-assisted sol–gel synthesis and characterization of hierarchical nanoporous merwinite with controllable drug release. J Sol–Gel Sci Technol 87:618–625. https://doi.org/10.1007/s10971-018-4777-9
Foroughi F, Hassanzadeh-Tabrizi SA, Bigham A (2016) In situ microemulsion synthesis of hydroxyapatite-MgFe2O4 nanocomposite as a magnetic drug delivery system. Mater Sci Eng, C. https://doi.org/10.1016/j.msec.2016.07.028
Ansari M, Bigham A, Hassanzadeh-Tabrizi SA, Abbastabar Ahangar H (2017) Synthesis and characterization of Cu0.3Zn0.5Mg0.2Fe2O4 nanoparticles as a magnetic drug delivery system. J Magn Magn Mater 439:67–75. https://doi.org/10.1016/j.jmmm.2017.04.084
Abdal-hay A, Sheikh FA, Lim JK (2013) Air jet spinning of hydroxyapatite/poly(lactic acid) hybrid nanocomposite membrane mats for bone tissue engineering. Colloids Surf B Biointerfaces 102:635–643. https://doi.org/10.1016/j.colsurfb.2012.09.017
Izquierdo-Barba I, Colilla M, Vallet-Regí M (2008) Nanostructured mesoporous silicas for bone tissue regeneration. J Nanomater 2008:106970. https://doi.org/10.1155/2008/106970
Roy TD, Simon JL, Ricci JL, Rekow ED, Thompson VP, Parsons JR (2003) Performance of degradable composite bone repair products made via three-dimensional fabrication techniques. J Biomed Mater Res, Part A 66A:283–291. https://doi.org/10.1002/jbm.a.10582
Deville S, Saiz E, Nalla RK, Tomsia AP (2006) Freezing as a path to build complex composites. Science 311:515–518. https://doi.org/10.1126/science.1120937
Aghajanian AH, Bigham A, Khodaei M, Hossein Kelishadi S (2019) Porous titanium scaffold coated using forsterite/poly-3-hydroxybutyrate composite for bone tissue engineering. Surf Coat Technol 378:124942. https://doi.org/10.1016/j.surfcoat.2019.124942
Almirall A, Larrecq G, Delgado JA, Martı́nez S, Planell JA, Ginebra MP (2004) Fabrication of low temperature macroporous hydroxyapatite scaffolds by foaming and hydrolysis of an α-TCP paste. Biomaterials 25:3671–3680. https://doi.org/10.1016/j.biomaterials.2003.10.066
Yeong W-Y, Chua C-K, Leong K-F, Chandrasekaran M (2004) Rapid prototyping in tissue engineering: challenges and potential. Trends Biotechnol 22:643–652. https://doi.org/10.1016/j.tibtech.2004.10.004
Huang Z-M, Zhang Y-Z, Kotaki M, Ramakrishna S (2003) A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos Sci Technol 63:2223–2253. https://doi.org/10.1016/S0266-3538(03)00178-7
Yoon JJ, Kim JH, Park TG (2003) Dexamethasone-releasing biodegradable polymer scaffolds fabricated by a gas-foaming/salt-leaching method. Biomaterials 24:2323–2329. https://doi.org/10.1016/S0142-9612(03)00024-3
Baino F, Novajra G, Vitale-Brovarone C (2015) Bioceramics and scaffolds: a winning combination for tissue engineering. Front Bioeng Biotechnol 3:202. https://doi.org/10.3389/fbioe.2015.00202
Abdellahi M, Najafinezhad A, Ghayour H, Saber-Samandari S, Khandan A (2017) Preparing diopside nanoparticle scaffolds via space holder method: simulation of the compressive strength and porosity. J Mech Behav Biomed Mater 72:171–181. https://doi.org/10.1016/j.jmbbm.2017.05.004
Najafinezhad A, Abdellahi M, Nasiri-Harchegani S, Soheily A, Khezri M, Ghayour H (2017) On the synthesis of nanostructured akermanite scaffolds via space holder method: the effect of the spacer size on the porosity and mechanical properties. J Mech Behav Biomed Mater 69:242–248. https://doi.org/10.1016/j.jmbbm.2017.01.002
Miao X, Lim W-K, Huang X, Chen Y (2005) Preparation and characterization of interpenetrating phased TCP/HA/PLGA composites. Mater Lett 59:4000–4005. https://doi.org/10.1016/j.matlet.2005.07.062
Wu C, Fan W, Zhu Y, Gelinsky M, Chang J, Cuniberti G, Albrecht V, Friis T, Xiao Y (2011) Multifunctional magnetic mesoporous bioactive glass scaffolds with a hierarchical pore structure. Acta Biomater 7:3563–3572. https://doi.org/10.1016/j.actbio.2011.06.028
Aghajanian AH, Khazaei BA, Khodaei M, Rafienia M (2018) Fabrication of porous Mg–Zn scaffold through modified replica method for bone tissue engineering. J Bionic Eng 15:907–913. https://doi.org/10.1007/s42235-018-0077-x
Fukasawa T, Ando M, Ohji T, Kanzaki S (2001) Synthesis of porous ceramics with complex pore structure by freeze-dry processing. J Am Ceram Soc 84:230–232. https://doi.org/10.1111/j.1151-2916.2001.tb00638.x
Fukasawa T, Deng Z-Y, Ando M, Ohji T, Kanzaki S (2002) Synthesis of porous silicon nitride with unidirectionally aligned channels using freeze-drying process. J Am Ceram Soc 85:2151–2155. https://doi.org/10.1111/j.1151-2916.2002.tb00426.x
Rezwan K, Chen QZ, Blaker JJ, Boccaccini AR (2006) Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 27:3413–3431. https://doi.org/10.1016/j.biomaterials.2006.01.039
Niu Y, Guo L, Liu J, Shen H, Su J, An X, Yu B, Wei J, Shin J-W, Guo H, Ji F, He D (2015) Bioactive and degradable scaffolds of the mesoporous bioglass and poly(l-lactide) composite for bone tissue regeneration. J Mater Chem B 3:2962–2970. https://doi.org/10.1039/C4TB01796J
Ma Z, Kotaki M, Inai R, Ramakrishna S (2005) Potential of nanofiber matrix as tissue-engineering scaffolds. Tissue Eng 11:101–109. https://doi.org/10.1089/ten.2005.11.101
Enayati MS, Behzad T, Sajkiewicz P, Rafienia M, Bagheri R, Ghasemi-Mobarakeh L, Kolbuk D, Pahlevanneshan Z, Bonakdar SH (2018) Development of electrospun poly (vinyl alcohol)-based bionanocomposite scaffolds for bone tissue engineering. J Biomed Mater Res, Part A 106:1111–1120. https://doi.org/10.1002/jbm.a.36309
Yin Z, Chen X, Chen JL, Shen WL, Hieu Nguyen TM, Gao L, Ouyang HW (2010) The regulation of tendon stem cell differentiation by the alignment of nanofibers. Biomaterials 31:2163–2175. https://doi.org/10.1016/j.biomaterials.2009.11.083
Li D, Xia Y (2004) Electrospinning of nanofibers: reinventing the wheel? Adv Mater 16:1151–1170. https://doi.org/10.1002/adma.200400719
Agarwal S, Wendorff JH, Greiner A (2009) Progress in the field of electrospinning for tissue engineering applications. Adv Mater 21:3343–3351. https://doi.org/10.1002/adma.200803092
Leong KF, Cheah CM, Chua CK (2003) Solid freeform fabrication of three-dimensional scaffolds for engineering replacement tissues and organs. Biomaterials 24:2363–2378. https://doi.org/10.1016/S0142-9612(03)00030-9
Butscher A, Bohner M, Hofmann S, Gauckler L, Müller R (2011) Structural and material approaches to bone tissue engineering in powder-based three-dimensional printing. Acta Biomater 7:907–920. https://doi.org/10.1016/j.actbio.2010.09.039
Ghomi H, Jaberzadeh M, Fathi MH (2011) Novel fabrication of forsterite scaffold with improved mechanical properties. J Alloys Compd 509:L63–L68. https://doi.org/10.1016/j.jallcom.2010.10.106
Abdellahi M, Karamian E, Najafinezhad A, Ranjabar F, Chami A, Khandan A (2018) Diopside-magnetite; a novel nanocomposite for hyperthermia applications. J Mech Behav Biomed Mater 77:534–538. https://doi.org/10.1016/j.jmbbm.2017.10.015
Naeimi M, Rafienia M, Fathi M, Janmaleki M, Bonakdar S, Ebrahimian-Hosseinabadi M (2016) Incorporation of chitosan nanoparticles into silk fibroin-based porous scaffolds: chondrogenic differentiation of stem cells. Int J Polym Mater Polym Biomater 65:202–209. https://doi.org/10.1080/00914037.2015.1099103
Toloue EB, Karbasi S, Salehi H, Rafienia M (2019) Potential of an electrospun composite scaffold of poly (3-hydroxybutyrate)-chitosan/alumina nanowires in bone tissue engineering applications. Mater Sci Eng, C 99:1075–1091. https://doi.org/10.1016/j.msec.2019.02.062
Fu S, Hu H, Chen J, Zhu Y, Zhao S (2020) Silicone resin derived larnite/C scaffolds via 3D printing for potential tumor therapy and bone regeneration. Chem Eng J 382:122928. https://doi.org/10.1016/j.cej.2019.122928
Schwartzalder K, Somers AV (1963) Method of making a porous shape of sintered refractory ceramic articles. https://patents.google.com/patent/US3090094A/en
Bartonickova E, Ptacek P, Opravil T, Soukal F, Masilko J, Novotny R, Svec J, Havlica J (2015) Mullite-based refractories fabricated by foam casting. Ceram Int 41:14116–14123. https://doi.org/10.1016/j.ceramint.2015.07.032
Deng X, Wang J, Huang Z, Zhao W, Li F, Zhang H (2015) Research progress in preparation of porous ceramics. Interceram Int Ceram Rev 64:100–103. https://doi.org/10.1007/BF03401108
Bretcanu O, Misra S, Roy I, Renghini C, Fiori F, Boccaccini AR, Salih V (2009) In vitro biocompatibility of 45S5 Bioglass®-derived glass–ceramic scaffolds coated with poly(3-hydroxybutyrate). J Tissue Eng Regen Med 3:139–148. https://doi.org/10.1002/term.150
Khamsehashari N, Hassanzadeh-Tabrizi SA, Bigham A (2018) Effects of strontium adding on the drug delivery behavior of silica nanoparticles synthesized by P123-assisted sol-gel method. Mater Chem Phys 205:283–291. https://doi.org/10.1016/j.matchemphys.2017.11.034
Zhu Y, Shang F, Li B, Dong Y, Liu Y, Lohe MR, Hanagata N, Kaskel S (2013) Magnetic mesoporous bioactive glass scaffolds: preparation, physicochemistry and biological properties. J Mater Chem B 1:1279–1288. https://doi.org/10.1039/C2TB00262K
Güden M, Çelik E, Hızal A, Altındiş M, Çetiner S (2008) Effects of compaction pressure and particle shape on the porosity and compression mechanical properties of sintered Ti6Al4V powder compacts for hard tissue implantation. J Biomed Mater Res Part B Appl Biomater 85B:547–555. https://doi.org/10.1002/jbm.b.30978
Oh I-H, Nomura N, Masahashi N, Hanada S (2003) Mechanical properties of porous titanium compacts prepared by powder sintering. Scr Mater 49:1197–1202. https://doi.org/10.1016/j.scriptamat.2003.08.018
Niu W, Bai C, Qiu G, Wang Q (2009) Processing and properties of porous titanium using space holder technique. Mater Sci Eng, A 506:148–151. https://doi.org/10.1016/j.msea.2008.11.022
Dunand DC (2004) Processing of titanium foams. Adv Eng Mater 6:369–376. https://doi.org/10.1002/adem.200405576
Arifvianto B, Zhou J (2014) Fabrication of metallic biomedical scaffolds with the space holder method: a review. Materials (Basel) 7:3588–3622. https://doi.org/10.3390/ma7053588
Singh R, Lee PD, Dashwood RJ, Lindley TC (2010) Titanium foams for biomedical applications: a review. Mater Technol 25:127–136. https://doi.org/10.1179/175355510X12744412709403
Wen CE, Mabuchi M, Yamada Y, Shimojima K, Chino Y, Asahina T (2001) Processing of biocompatible porous Ti and Mg. Scr Mater 45:1147–1153. https://doi.org/10.1016/S1359-6462(01)01132-0
Torres Y, Pavón JJ, Rodríguez JA (2012) Processing and characterization of porous titanium for implants by using NaCl as space holder. J Mater Process Technol 212:1061–1069. https://doi.org/10.1016/j.jmatprotec.2011.12.015
Laptev A, Bram M, Buchkremer HP, Stöver D (2004) Study of production route for titanium parts combining very high porosity and complex shape. Powder Metall 47:85–92. https://doi.org/10.1179/003258904225015536
Gligor I, Soritau O, Todea M, Berce C, Vulpoi A, Marcu T, Cernea V, Simon S, Popa C (2013) Porous c.p. titanium using dextrin as space holder for endosseous implants. Part Sci Technol 31:357–365. https://doi.org/10.1080/02726351.2012.749556
Zhao X, Sun H, Lan L, Huang J, Zhang H, Wang Y (2009) Pore structures of high-porosity NiTi alloys made from elemental powders with NaCl temporary space-holders. Mater Lett 63:2402–2404. https://doi.org/10.1016/j.matlet.2009.07.069
Jakubowicz J, Adamek G, Dewidar M (2013) Titanium foam made with saccharose as a space holder. J Porous Mater 20:1137–1141. https://doi.org/10.1007/s10934-013-9696-0
Najafinezhad A, Abdellahi M, Saber-Samandari S, Ghayour H, Khandan A (2018) Hydroxyapatite-M-type strontium hexaferrite: a new composite for hyperthermia applications. J Alloys Compd 734:290–300. https://doi.org/10.1016/j.jallcom.2017.10.138
Sahmani S, Khandan A, Saber-Samandari S, Mohammadi Aghdam M (2020) Effect of magnetite nanoparticles on the biological and mechanical properties of hydroxyapatite porous scaffolds coated with ibuprofen drug. Mater Sci Eng, C 111:110835. https://doi.org/10.1016/j.msec.2020.110835
Sill TJ, von Recum HA (2008) Electrospinning: applications in drug delivery and tissue engineering. Biomaterials 29:1989–2006. https://doi.org/10.1016/j.biomaterials.2008.01.011
Rezvani Ghomi E, Khalili S, Nouri Khorasani S, Esmaeely Neisiany R, Ramakrishna S (2019) Wound dressings: current advances and future directions. J Appl Polym Sci 136:47738. https://doi.org/10.1002/app.47738
Ramakrishna S, Fujihara K, Teo W-E, Yong T, Ma Z, Ramaseshan R (2006) Electrospun nanofibers: solving global issues. Mater Today 9:40–50. https://doi.org/10.1016/S1369-7021(06)71389-X
Teo W-E, Ramakrishna S (2006) A review on electrospinning design and nanofibre assemblies. Nanotechnology 17:R89. https://doi.org/10.1088/0957-4484/17/14/R01
Kim TG, Park S-H, Chung HJ, Yang D-Y, Park TG (2010) Microstructured scaffold coated with hydroxyapatite/collagen nanocomposite multilayer for enhanced osteogenic induction of human mesenchymal stem cells. J Mater Chem 20:8927–8933. https://doi.org/10.1039/C0JM01062F
Dalby MJ, Gadegaard N, Tare R, Andar A, Riehle MO, Herzyk P, Wilkinson CDW, Oreffo ROC (2007) The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater 6:997–1003. https://doi.org/10.1038/nmat2013
Pramanik S, Pingguan-Murphy B, Abu Osman NA (2012) Progress of key strategies in development of electrospun scaffolds: bone tissue. Sci Technol Adv Mater 13:43002. https://doi.org/10.1088/1468-6996/13/4/043002
Neisiany RE, Enayati MS, Kazemi-Beydokhti A, Das O, Ramakrishna S (2020) Multilayered bio-based electrospun membranes: a potential porous media for filtration applications. Front Mater 7:67. https://doi.org/10.3389/fmats.2020.00067
Khosravi F, Nouri Khorasani S, Rezvani Ghomi E, Kichi MK, Zilouei H, Farhadian M, Esmaeely Neisiany R (2019) A bilayer GO/nanofibrous biocomposite coating to enhance 316L stainless steel corrosion performance. Mater Res Express 6:086470. https://doi.org/10.1088/2053-1591/ab26d5
Kim H-W, Song J-H, Kim H-E (2005) Nanofiber generation of gelatin-hydroxyapatite biomimetics for guided tissue regeneration. Adv Funct Mater 15:1988–1994. https://doi.org/10.1002/adfm.200500116
Stanishevsky A, Chowdhury S, Chinoda P, Thomas V (2008) Hydroxyapatite nanoparticle loaded collagen fiber composites: microarchitecture and nanoindentation study. J Biomed Mater Res, Part A 86A:873–882. https://doi.org/10.1002/jbm.a.31657
Fu S, Wang X, Guo G, Shi S, Liang H, Luo F, Wei Y, Qian Z (2010) Preparation and characterization of nano-hydroxyapatite/poly(ε-caprolactone) − poly(ethylene glycol) − poly(ε-caprolactone) composite fibers for tissue engineering. J Phys Chem C 114:18372–18378. https://doi.org/10.1021/jp106488t
Khosravi F, Nouri Khorasani S, Khalili S, Esmaeely Neisiany R, Rezvani Ghomi E, Ejeian F, Das O, Nasr-Esfahani HM (2020) Development of a highly proliferated bilayer coating on 316L stainless steel implants. Polymers 12:1022. https://doi.org/10.3390/polym12051022
Gloria A, Russo T, D’Amora U, Zeppetelli S, D’Alessandro T, Sandri M, Bañobre-López M, Piñeiro-Redondo Y, Uhlarz M, Tampieri A, Rivas J, Herrmannsdörfer T, Dediu VA, Ambrosio L, De Santis R (2013) Magnetic poly(ε-caprolactone)/iron-doped hydroxyapatite nanocomposite substrates for advanced bone tissue engineering. J R Soc Interface 10:20120833. https://doi.org/10.1098/rsif.2012.0833
Amarjargal A, Tijing LD, Park C-H, Im I-T, Kim CS (2013) Controlled assembly of superparamagnetic iron oxide nanoparticles on electrospun PU nanofibrous membrane: a novel heat-generating substrate for magnetic hyperthermia application. Eur Polym J 49:3796–3805. https://doi.org/10.1016/j.eurpolymj.2013.08.026
Meng J, Xiao B, Zhang Y, Liu J, Xue H, Lei J, Kong H, Huang Y, Jin Z, Gu N, Xu H (2013) Super-paramagnetic responsive nanofibrous scaffolds under static magnetic field enhance osteogenesis for bone repair in vivo. Sci Rep 3:2655. https://doi.org/10.1038/srep02655
Radmansouri M, Bahmani E, Sarikhani E, Rahmani K, Sharifianjazi F, Irani M (2018) Doxorubicin hydrochloride—loaded electrospun chitosan/cobalt ferrite/titanium oxide nanofibers for hyperthermic tumor cell treatment and controlled drug release. Int J Biol Macromol 116:378–384. https://doi.org/10.1016/j.ijbiomac.2018.04.161
Mishra R, Bishop T, Valerio IL, Fisher JP, Dean D (2016) The potential impact of bone tissue engineering in the clinic. Regen Med 11:571–587. https://doi.org/10.2217/rme-2016-0042
Lu Y, Chen G, Long Z, Li M, Ji C, Wang F, Li H, Lu J, Wang Z, Li J (2019) Novel 3D-printed prosthetic composite for reconstruction of massive bone defects in lower extremities after malignant tumor resection. J Bone Oncol 16:100220. https://doi.org/10.1016/j.jbo.2019.100220
Ke X, Qiu J, Wang X, Yang X, Shen J, Ye S, Yang G, Xu S, Bi Q, Gou Z, Jia X, Zhang L (2020) Modification of pore-wall in direct ink writing wollastonite scaffolds favorable for tuning biodegradation and mechanical stability and enhancing osteogenic capability. FASEB J 34:5673–5687. https://doi.org/10.1096/fj.201903044R
Chen Y, Han P, Vandi L-J, Dehghan-Manshadi A, Humphry J, Kent D, Stefani I, Lee P, Heitzmann M, Cooper-White J, Dargusch M (2019) A biocompatible thermoset polymer binder for Direct Ink Writing of porous titanium scaffolds for bone tissue engineering. Mater Sci Eng, C 95:160–165. https://doi.org/10.1016/j.msec.2018.10.033
Kanczler JM, Wells JA, Gibbs DMR, Marshall KM, Tang DKO, Oreffo ROC (2020) Chapter 50—Bone tissue engineering and bone regeneration. In: Lanza R, Langer R, Vacanti JP, Atala E (eds) Principles of tissue engineering, 5th edn. Academic Press, Cambridge, pp 917–935. https://doi.org/10.1016/B978-0-12-818422-6.00052-6
Kondiah PPD, Choonara YE, Kondiah PJ, Marimuthu T, du Toit LC, Kumar P, Pillay V (2020) Recent progress in 3D-printed polymeric scaffolds for bone tissue engineering. In: du Toit LC, Kumar P, Choonara YE, Pillay TE (eds) Woodhead publishing series in biomaterials. Elsevier, Amsterdam, pp 59–81. https://doi.org/10.1016/B978-0-12-818471-4.00003-0
MorenoMadrid AP, Vrech SM, Sanchez MA, Rodriguez AP (2019) Advances in additive manufacturing for bone tissue engineering scaffolds. Mater Sci Eng, C 100:631–644. https://doi.org/10.1016/j.msec.2019.03.037
Emre Ö, Mirigul A (2019) Effect of structural hybrid design on mechanical and biological properties of CoCr scaffolds fabricated by selective laser melting. Rapid Prototyp J 26:615–624. https://doi.org/10.1108/RPJ-07-2019-0186
Qu H (2020) Additive manufacturing for bone tissue engineering scaffolds. Mater Today Commun 24:101024. https://doi.org/10.1016/j.mtcomm.2020.101024
Esslinger S, Gadow R (2020) Additive manufacturing of bioceramic scaffolds by combination of FDM and slip casting. J Eur Ceram Soc 40:3707–3713. https://doi.org/10.1016/j.jeurceramsoc.2019.10.029
Choi WJ, Hwang KS, Kwon HJ, Lee C, Kim CH, Kim TH, Heo SW, Kim J-H, Lee J-Y (2020) Rapid development of dual porous poly(lactic acid) foam using fused deposition modeling (FDM) 3D printing for medical scaffold application. Mater Sci Eng, C 110:110693. https://doi.org/10.1016/j.msec.2020.110693
Kholgh Eshkalak S, Rezvani Ghomi E, Dai Y, Choudhury D, Ramakrishna S (2020) The role of three-dimensional printing in healthcare and medicine. Mater Des 194:108940. https://doi.org/10.1016/j.matdes.2020.108940
Ji K, Wang Y, Wei Q, Zhang K, Jiang A, Rao Y, Cai X (2018) Application of 3D printing technology in bone tissue engineering. Bio-Des Manuf 1:203–210. https://doi.org/10.1007/s42242-018-0021-2
Ma H, Jiang C, Zhai D, Luo Y, Chen Y, Lv F, Yi Z, Deng Y, Wang J, Chang J, Wu C (2016) A bifunctional biomaterial with photothermal effect for tumor therapy and bone regeneration. Adv Funct Mater 26:1197–1208. https://doi.org/10.1002/adfm.201504142
Ma H, Luo J, Sun Z, Xia L, Shi M, Liu M, Chang J, Wu C (2016) 3D printing of biomaterials with mussel-inspired nanostructures for tumor therapy and tissue regeneration. Biomaterials 111:138–148. https://doi.org/10.1016/j.biomaterials.2016.10.005
Khandan A, Ozada N, Saber-Samandari S, Ghadiri Nejad M (2018) On the mechanical and biological properties of bredigite-magnetite (Ca7MgSi4O16–Fe3O4) nanocomposite scaffolds. Ceram Int 44:3141–3148. https://doi.org/10.1016/j.ceramint.2017.11.082
Lu J-W, Yang F, Ke Q-F, Xie X-T, Guo Y-P (2018) Magnetic nanoparticles modified-porous scaffolds for bone regeneration and photothermal therapy against tumors. Nanomed Nanotechnol Biol Med 14:811–822. https://doi.org/10.1016/j.nano.2017.12.025
Wang H, Zeng X, Pang L, Wang H, Lin B, Deng Z, Qi ELX, Miao N, Wang D, Huang P, Hu H, Li J (2020) Integrative treatment of anti-tumor/bone repair by combination of MoS2 nanosheets with 3D printed bioactive borosilicate glass scaffolds. Chem Eng J 396:125081. https://doi.org/10.1016/j.cej.2020.125081
Liu Y, Lin R, Ma L, Zhuang H, Feng C, Chang J, Wu C (2020) Mesoporous bioactive glass for synergistic therapy of tumor and regeneration of bone tissue. Appl Mater Today 19:100578. https://doi.org/10.1016/j.apmt.2020.100578
Liu Y, Li T, Ma H, Zhai D, Deng C, Wang J, Zhuo S, Chang J, Wu C (2018) 3D-printed scaffolds with bioactive elements-induced photothermal effect for bone tumor therapy. Acta Biomater 73:531–546. https://doi.org/10.1016/j.actbio.2018.04.014
Wu C, Zhou Y, Xu M, Han P, Chen L, Chang J, Xiao Y (2013) Copper-containing mesoporous bioactive glass scaffolds with multifunctional properties of angiogenesis capacity, osteostimulation and antibacterial activity. Biomaterials 34:422–433. https://doi.org/10.1016/j.biomaterials.2012.09.066
Wu C, Zhou Y, Fan W, Han P, Chang J, Yuen J, Zhang M, Xiao Y (2012) Hypoxia-mimicking mesoporous bioactive glass scaffolds with controllable cobalt ion release for bone tissue engineering. Biomaterials 33:2076–2085. https://doi.org/10.1016/j.biomaterials.2011.11.042
Hadidi M, Bigham A, Saebnoori E, Hassanzadeh-Tabrizi SA, Rahmati S, Alizadeh ZM, Nasirian V, Rafienia M (2017) Electrophoretic-deposited hydroxyapatite-copper nanocomposite as an antibacterial coating for biomedical applications. Surf Coat Technol. https://doi.org/10.1016/j.surfcoat.2017.04.055
Author information
Authors and Affiliations
Contributions
AB, FF, and ERG summarized the literature and wrote a major part of the manuscript. MR, REN, and SR conducted the deep review, editing, guidance, and supervision. All authors have read and approved the article for publication.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Rights and permissions
About this article
Cite this article
Bigham, A., Foroughi, F., Rezvani Ghomi, E. et al. The journey of multifunctional bone scaffolds fabricated from traditional toward modern techniques. Bio-des. Manuf. 3, 281–306 (2020). https://doi.org/10.1007/s42242-020-00094-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42242-020-00094-4