Abstract
In bone cancer treatment, local delivery of chemotherapeutic agents is preferred compared to other routes of administration. Delivery of multiple drugs using biodegradable carriers improves the treatment efficiency and overcomes drug resistance and toxicity. With this approach, we have developed multilayer biodegradable core shell nanoparticles (NPs) using the electro-spraying technique to deliver methotrexate (MTX) and doxorubicin (DOX) for the treatment of osteosarcoma. These core-shell NPs with a mean particle size of 212 ± 41 nm consist of hydroxyapatite (HA) and DOX as core with the outer shell made of chitosan (CH) followed by polycaprolactone (PCL) with MTX. The encapsulation efficiency of MTX was around 85% and DOX was 38%. In vitro drug release studies were performed in phosphate buffered saline (PBS) at pH 5 and pH 7.4 for 8 days. Different release profiles were observed in both acidic and alkaline pH. The sequential release of MTX followed by DOX was observed in both pH in sustained manner. Human osteosarcoma MG 63 (OMG-63) cells lines were used to test the cytotoxicity of drug loaded NPs. Multi-drug encapsulated bioresorbable and biodegradable electro-sprayed core shell NPs will be promising as a bone substitute for the treatment of osteosarcoma.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Osteosarcoma is the most prevalent malignant bone tumor among the various skeletal diseases. It predominantly originates from the mesenchymal tissue and metastasizes to the other parts of the body [1, 2]. The annual rate of incidence is 1–3 cases per 1,000,000 people and 80% of people are in the age bracket of 10–25 years [3]. The standard treatment procedure for osteosarcoma comprises surgery, radiotherapy, hyperthermia and systemic or oral administration of chemotherapeutic agents [2, 4, 5]. To improve the treatment efficacy, combinational therapy with two or more therapeutic agents is preferred. Combinational therapy overcomes the drug resistance, prevents bone cancer metastasis and produces synergistic effects with decreased morbidity [6]. The main drawbacks of systemic or oral combinational chemotherapy are nephrotoxicity, cardiotoxicty, myelosuprression and ulcerative stomatitis [2]. To overcome these limitations, local delivery of drugs at high concentrations at the target site is preferred to improve the treatment outcome [1, 2, 7, 8].
Localized nanoparticle (NP) based carriers have unique advantages such as increase in retention period, minimal dose and controlled release over long periods. Different types of carriers were studied for the delivery of single or multiple chemo therapeutic agents for bone cancer treatment [1, 6, 8]. Various carriers such as gold NPs, polymeric NPs, liposomes, injectable hydrogels, mesoporous silica, quantum dots, silica, calcium phosphates and electrospun fibers have been studied [2, 9,10,11,12].
Electrospinning or electrospraying is also known as electro hydrodynamic atomization (EHDA) and is employed for the production of ultrafine fibers and particles in the micro or nano range [11,12,13]. Recently, the method has attracted considerable attention for biomedical and pharmaceutical applications to produce particles below 1μm with a narrow size distribution, less aggregation, low cost and higher drug loading efficiency [11,12,13].
Different electrospraying strategies such as coaxial, single emulsion, multiple emulsion and triple-axial techniques have been reported to produce NPs with core-shell architecture loaded with multiple drugs [14,15,16,17,18,19,20,21,22]. Cui et al. [17] developed poly(vinyl pyrrolidone) (PVP)/shellac core shell NPs for delivery of ferulic acid through coaxial electrospraying. Cao et al. [18] reported combinational delivery of DOX and combretastatin A4 (CA4) encapsulated PCL or PVP core and PLGA shell NPs by co-axial electrospraying. Using same core shell NPs, rhodamine B and naproxen were loaded and drug release profiles were studied by Cao et al. [19]. Yeh et al. [20] formulated core shell composite nanoparticles made of PLGA particles for delivery of budesonide and theophylline by dual capillary electrospray technique. A comprehensive review by Nguyen et al. [13] describes the importance and scope of electrospraying for pharmaceutical applications.
Kim et al. [21] developed triple layer electrospray system to synthesize multilayer capsules. Lee et al. [22] fabricated poly(styrene sulfonate) (PSS)/PLGA/PSS triple layer electrosprayed composite for multi-drug delivery of budesonide and epigallocatechin gallate (EGCG) through coaxial tricapillary electrospray method. Although coaxial is successful in the manufacture of core-shell architecture, due to the complexity of the instrument, spinning parameters, difficulties in controlling the viscoelasticity of different polymer solutions, great effort is needed to optimize the inner and outer layers [11].
In contrast, emulsion-based electrospray technique for producing core/shell particles has advantages such as simple experimental setup, less time consuming and it is possible to encapsulate both hydrophobic and hydrophilic drugs [11,12,13]. Wang et al. [23] fabricated chitosan NPs loaded PVP core shell micro/nano particles for dual delivery of naproxen and rhodamine B through emulsion electrospraying. Although emulsion-based core shell nanofibers (PEG-PLA, PLLA etc.) were well studied, there are only very few reports on nano/micro particles prepared by emulsion electrospray technique [24,25,26,27].
In this report, an attempt has been made to develop a hybrid HA-CH core and PCL shell NPs for dual delivery of MTX and DOX. HA was chosen as inner core material, because of its unique properties as a bone mineral that is biocompatible, bioresorbable and is an ideal carrier for delivery of drugs by diffusion controlled release kinetics [28]. MTX and DOX are first line anticancer agents for the treatment of osteosarcoma. Both shows high toxicity against osteosarcoma cells by inhibiting the DNA, RNA and protein synthesis via intercalating DNA and inhibiting dihydrofolate reductase to tetrahydrofolate reductase by MTX and DOX [2]. The model PCL-CH-HA core shell NPs were explored for combinational delivery of MTX and DOX to treat the osteosarcoma.
2 Materials and methods
2.1 Materials
PCL with average Mn 45,000 was purchased from Sigma Aldrich (Bangalore, India). Chitosan (CH, Mw ≈ 193400) was from SRL Chemicals (Mumbai, India). DOX and MTX were generously provided as gift from Celon Laboratories (Hyderabad, India) and Cipla Laboratories (Mumbai, India). Tween 20 was obtained from SRL Chemicals (Mumbai, India). Dulbecco’s Minimum Essential Medium (DMEM) and fetal bovine serum (FBS) were procured from Himedia Laboratories (Mumbai, India). Dimethyl sulfoxide (DMSO) cell culture grade was from MP Biomedicals, IIIkrich, France. The cell culture dishes were from Tarsons, Mumbai, India. Human osteosarcoma MG63 (OMG-63) cells were obtained from NCCS (National Center for Cell Science, Pune, India). All other chemicals employed were of analytical grade. Milli Q water was used in all experiments.
2.2 Instrumentation
Electrospraying was performed using ESPIN Nano system (Chennai, India). Fourier transform infrared spectra (FTIR) was performed by KBr pellet technique using a JASCO spectrophotometer (Germany). X-ray diffraction (XRD) was performed by Bruker instrument (Model D8, Discover, USA). UV-visible spectra were measured using a UV-Visible spectrophotometer (JASCO, Model V630, USA).Scanning Electron Microscopy (SEM) pictures were taken using a FEI Quanta400 instrument (Netherlands). Transmission electron microscopy (TEM) images were captured using JEOL (JSM-5600, Japan) microscope. Particle size analysis was carried out by Particle Size Analyzer (Micotrack, Germany). Micro plate reader were from Spectrum Max 5 (Molecular Devices, USA). HPLC was carried out in a Shimadzu instrument (Model Prominence, Japan).
2.3 Preparation of HA NPs
HA NPs were prepared by wet chemical precipitation technique as previously reported [29]. Briefly, diammonium hydrogen phosphate (0.5 M) solution was added slowly into calcium nitrate (0.5 M) solution in a beaker. After adding all the diammonium hydrogen phosphate solution, the pH of the mixture was maintained at 10.5–11.0 using ammonium hydroxide. The solution was stirred magnetically for 4 h at 40 °C and incubated at 40 °C in an oven overnight. The product was centrifuged for 30 min at 10,000 rpm, washed several times with water, dried in a hot air oven and was further heated at 800 °C in a muffle furnace for 1 h, and stored at room temperature.
2.4 DOX loaded HA NPs
DOX was encapsulated onto the HA NPs using a physical adsorption technique. In brief, 1% DOX solution in water was prepared and HA NPs (100 mg) was added to the solution and incubated for 24 h at 4 °C. The mixture was then centrifuged at 12000 rpm and DOX loaded NPs was separately collected. The quantity of DOX encapsulation in the NPs was measured by estimating the concentration of drug in the supernatant by UV Visible spectroscopy at 480 nm. The pellets were lyophilized and stored at 2–8 °C. The resultant NPs are named as DOX-HA NPs.
2.5 Preparation of electrospraying emulsion
Electrospraying emulsion was prepared using a modified procedure of Pal et al. [30]. Briefly, CH (5%) solution was prepared by dissolving the CH in 5% acetic acid. DOX-HA NPs was added to the CH solution at 1 mg/ml concentration. PCL (10%) solution was prepared by dissolving the PCL in chloroform/methanol (3:1). MTX (1 mg/ml) was mixed to the PCL solution and stirred magnetically at 1000 rpm until uniform dispersion. The CH solution containing DOX-HA NPs was introduced drop wise to the MTX containing PCL solution and magnetically stirred for different time intervals from 0 h to 24 h. Tween 20 (100 µl) was added to the emulsion as surfactant. Different ratios of CH/PCL (1:1, 1:2, 1:3, 2:1, 3:1) mixture was prepared and optimized to obtain a stable emulsion.
2.6 Formulation of core shell electrosprayed NPs
The standard electrospraying setup was used to formulate core shell NPs. Schematic representation of electrospraying setup is shown in Scheme 1. Briefly, the optimized emulsion was filled in a 2.5 ml syringe fitted with 23 G blunted needle. The emulsion was extruded through nozzle at 0.3 ml/h or 0.5 ml/h flow rate. The distance between the needle and the aluminum collector was maintained at 10 cm. For optimization, high voltage ranging from 10 kV to 25 kV was applied. The optimized electrosprayed NPs are named as MTX-PCL/CH-DOX-HA NPs.
2.7 Estimation of drug encapsulation
The amount of drug encapsulated in the NPs was calculated by using HPLC with a C18 column (150 × 4.6 mm, 5 µm) with SPD 20 A UV-visible detector at 254 nm (DOX) and 302 nm (MTX). The mobile phase was acetonitrile: water: acetic acid in the ratio of 48: 47.7: 4.3. The electrosprayed MTX-PCL/CH-DOX-HA NPs (5 mg) was added to 2 ml PBS and 3 ml of mobile phase and sonicated in a bath sonicator for 30 min. The solution was filtered using syringe filter (Acrodisc 0.2 µm, Pall Corporation, USA). About 20 µl was injected and the drug concentration was estimated by using the calibration plot of DOX and MTX.
2.8 In vitro drug release studies
MTX-PCL/CH-DOX-HA NPs (2 mg) was added to 10 ml PBS in 25 ml flat glass bottom vials and incubated at 37 °C in constant temperature water bath. Drug release studies were conducted in two different conditions, PBS pH 7 and pH 5 to mimic the acidic environment in tumor. At periodic intervals, one ml of supernatant solution was withdrawn and replaced with same amount of fresh PBS. MTX and DOX released in the media was determined by using dual wavelength in UV visible spectroscopy and sample absorbance was measured at 302 nm (MTX) and 480 nm (DOX).
2.9 Cell viability studies
2.9.1 Direct contact assay
A direct contact method was used for cell viability studies. MTX-PCL/CH-DOX-HA NPs (5 mg) was added to 5 ml DMEM and sonicated for 30 min until it uniformly dispersed. The NPs dispersed stock solution were further serially diluted to different concentration from 1000 µg/ml to 7.8 µg/ml. OMG-63 cells were cultured in DMEM with 10% FBS and 1% antibiotic and antimitotic solution. The dispersed NPs samples (100 µl) were added to the cells in 96 well plate with seeding density of 5000 cells per well. MTX and DOX were added as the positive controls with respect to the drug concentration in the NPs. The concentration of MTX was from 46 µg/ml to 7.8 µg/ml and DOX was from 22 µg/ml to 0.17 µg/ml. The plates were incubated at 37 °C with 5% CO2 in humidified atmosphere. After 24 and 72 h incubation, the media was changed with 10 µl of MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide) (5 mg/ml stock) and 90 μL of DMEM and incubated at 37 °C for 4 h. Consequently, cell culture grade DMSO (100 μL) was added to the wells. The plates were incubated for 5 min to dissolve the formazan crystals. The sample and control absorbance was measured at 570 nm using micro plate reader. The percentage cell viability were estimated using the ratio of sample absorbance to the ratio of control absorbance.
2.9.2 Indirect contact assay
Cytotoxicity assay were also performed using MTT by indirect contact method with OMG 63 cells [31]. Briefly, 0.25, 0.5 and 1 mg of MTX-PCL/CH-DOX-HA NPs were added to 1 mL DMEM and kept in an orbital shaker maintained at 150 rpm at 37 °C for 7 days. The samples were centrifuged and filtered using a 0.2 μm Acrodisc sterile syringe filter (PALL Corporation, USA). The extracted samples were stored at 4 °C and used for MTT assay. OMG 63 cells were seeded in 96 well plates with a density of 5000 cells/well. The extract (100 µL) was added to each well and incubated 37 °C with 5% CO2 for 24 and 72 h. MTX and DOX was added as positive controls. The concentration for MTX and DOX was approximately calculated based on the in vitro drug release experiments upto 7 days. The concentration of MTX was 2.8 µg/ml, 5.8 µg/ml and 11.5 µg/ml and DOX concentration was 0.69 µg/ml, 1.38 µg/ml and 2.76 µg/ml. The MTT assay was performed by the procedure described in Section 2.9.1.
3 Results
3.1 Electrospraying
Stable emulsion is a prerequisite in order to get uniform spherical particles via electrospray. To obtain the stable emulsion, different ratios of CH/PCL (1:1, 1:2, 1:3, 2:1, 3:1) were prepared without surfactant by stirring for 24 h and kept for observation. Among the different ratios of emulsion, 1:2 and 1:3 were stable upto 2 h and started separating after 2 h. Other ratios separated immediately. To obtain stable emulsion, Tween 20 (100 µl) was added and kept stirring for 24 h. The mixture of CH/PCL ratio 1:1, 2:1 and 3:1 with Tween 20 was stable but not electrosprayable due to viscosity and turbidity. Only the 1:2 ratio was stable and was electrosprayable. To achieve the uniform spherical core shell NPs various parameters were examined during the electrospraying. Table 1 lists the optimized parameters for getting spherical shape particles.
3.2 Characterization of NPs
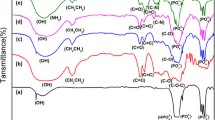
All the samples were characterized by FTIR spectroscopy. HA NPs showed distinct peaks at 3570 cm−1 (structural OH–), 3445 cm−1 (adsorbed water), 1098 cm−1 (HPO42–), 1057 cm−1 (PO43– bending), 962 cm−1 (PO43– bending), 632 cm−1(structural OH–), and 602 cm−1 and 569 cm−1 (PO43– bending) (Fig. 1a). DOX loaded HA NPs showed peaks at 3572 cm−1 (structural OH–), 3134 cm−1 and 2987 cm−1 (C–H stretching of CH2, CH3), 1678,1629 (N–H bending), 1400 cm−1 (C–H bending), 1191 cm−1 and 1102 cm−1 (N–H and PO43– bending), 962 cm−1 (PO43– bending), 752 cm−1, 656 cm−1, 644 cm−1 (structural OH–), 602 cm−1 (PO43– bending) (Fig. 1b). CH showed peaks at 3443 cm−1 (N–H stretching), 2925 cm−1 (CH2 stretching), 1631 cm−1 (C=O Stretching) (amide I), 1520 cm−1 (amide II), 1384 cm−1 (amide III), 1095 cm−1 (C–O–C bending) (Fig. 1c). PCL showed peaks at 2946 cm−1 (CH2 stretching), 2878 cm−1 (symmetrical CH2 stretching), 1723 cm−1 (C=O Stretching), 1367 cm−1, 1267 cm−1 (C–O–C stretching), 1161 cm−1 (C–O–C stretching), 1045 cm−1, 958 cm−1, 731 cm−1 (Fig. 1d). MTX–PCL–CH–DOX–HA NPs showed peaks at 3135 cm−1, 2926 cm−1 and 2869 cm−1 (C–H stretching of CH2, CH3), 1732 cm−1 (C=O Stretching), 1638 cm−1 (C=O Stretching) (amide I), 1469 cm−1 (aromatic C=C stretching), 1400 cm−1 (C–H bending), 1295 cm−1, 1242 cm−1 (C–O–C stretching), 1190 cm−1 and 1123 cm−1 (N–H and PO43–bending), 957 cm−1 (PO43– bending), 842 cm−1, 657 cm−1 and 602 cm−1 (PO43– bending) (Fig. 1e).
Crystalline nature and purity of HA NPs and all the other samples were characterized by XRD method. The synthesized HA NPs were compared with JCPDS standard (09432) (Fig. 2). The broad peak at 211 confirmed the synthesized HA NPs were at nanosize. The crystal size of the samples was measured using the Scherer formula,
where t is average crystal size (nm), λ is X-ray wavelength (λ = 1.5405 Å for Cu Kα radiation), K is shape factor, B is full width half maximum, and θ is Bragg’s diffraction angle. The crystal size of HANPs was found to be in the range of 18 to 32 nm. PCL showed semi crystalline nature (Fig. 2d) with a two distinct peaks and hump between 15° to 30° confirming the amorphous phase of CH (Fig. 2c). MTX-PCL-CH-DOX-HA NPs showed amorphous and semi crystalline nature in the range of 10° to 30° (Fig. 2e).
3.3 Microscopy
The morphology of the electrosprayed NPs were imaged using optical, SEM and TEM methods. After electrospraying the emulsion, the samples were observed in the optical microscope to check the morphology of the NPs. For attaining spherical shape core shell NPs, different parameters were optimized such as flow rate, voltage, distance and emulsion stability. Figure 3 shows the morphology of the samples at different parameters during electrospraying process. The optimized parameters to get spherical shape NPs were 16–13 kV, a flow rate of 0.5 ml/h and a distance of 10 cm. SEM images of MTX-PCL-CH-DOX-HA NPs are shown in Fig. 4.
To verify the core shell structure and NPs morphology, the samples were imaged by using TEM (Fig. 5). The particle size analysis of the optimized formulation MTX–PCL–CH–DOX–HA NPs showed mean particle size as 212.4 ± 41 nm (Fig. 6). The zeta potential of NPs was found to be +25.57 ± 6.7 mV with PDI in 1.14 ± 0.09. The red fluorescence was observed onto the NPs on imaging in fluorescence microscope which confirmed the presence of DOX (Fig. 7).
3.4 Drug encapsulation efficiency
The encapsulation efficacy of DOX loaded HA NPs was found to be 68.62 ± 4.58%. The drug encapsulation efficacy in electrosprayed MTX–PCL–CH–HA NPs was found to be 85 ± 5% (MTX) and 38 ± 4% (DOX).
3.5 In vitro drug release
The drug release experiments were conducted for 192 h and the amount of drug released from the NPs was estimated by UV-visible spectroscopy. Both MTX and DOX were sequentially released in a sustained manner (Fig. 8). At pH 7.4, MTX showed burst release of around 12% within 1 h followed by sustained release and a maximum release of 28% was observed after 8 days. In the case of DOX, there was no burst release, sustained release behavior was observed and maximum release was 13% (Fig. 8a). At pH 5, a maximum of 40% MTX was released at 8 days with a burst release of 13%. DOX showed maximum release of 20% in a sustained manner (Fig. 8b).
3.6 Cytotoxicity studies
Cell viability assays of MTX–PCL–CH–DOX–HA NPs was carried out using OMG-63 cells by direct contact method and by indirect contact method for 24 and 72 h. In direct contact assay, after 24 h incubation, drug loaded NPs showed 50% cell death at 1000 µg/ml and 40% cell death was observed from 500 µg/ml and 250 µg/ml (Fig. 9a). MTX does not show any significant difference between the control cells at lower concentrations (7.8 to 250 µg/ml) and 30% cell death was observed at higher concentrations (500 and 1000 µg/ml) while DOX showed around 40% cell death at higher concentrations (125 to 1000 µg/ml) (Fig. 9a). At 72 h incubation, all the concentrations of NPs and positive controls such as MTX and DOX showed more than 60% cell death. (Fig. 9b).
Indirect contact assay showed concentration dependent cell death for all the samples (NPs, MTX and DOX) at both 24 h and 72 h incubation (Fig. 10). At 24 h incubation, NPs showed more than 70% viability at all three concentrations (Fig. 10a). MTX and DOX showed 40% cell death only at 0.5 and 1 mg /ml concentrations. After 72 h of incubation, all samples showed concentration dependent cell death and 50% cell death was observed at 1 mg/ml. MTX and DOX showed less than 50% cell death and concentration dependent cytotoxicity. (Fig. 10b).
4 Discussion
The combination of anticancer drugs in localized cancer therapy is preferred to overcome drug resistance and to inhibit tumor growth effectively. For the treatment of bone cancer, localized chemotherapy using multiple drug combinations with different carriers showed improved treatment outcomes [2, 8, 32]. Ma et al. [2] delivered combination of MTX, DOX and cisplatin by thermosenstive hydrogel, which showed synergistic activity against osteosarcoma Soas-3 and MG 63 cells lines. Enhanced treatment efficiency was observed by co-delivery of genes and DOX in osteosarcoma [32, 33]. Li et al. [34] fabricated alginate/CaP core shell NPs for intracellular co-delivery of DOX and camptothecin and detected synergistic cell cycle arrest and apoptosis. In a nut shell, localized combinational delivery of chemotherapeutic agents through biodegradable carrier has been shown to be more effective for osteosarcoma treatment.
In this study, using biocompatible, biodegradable polymers (PCL, CH) and bioresorbable ceramics (HA), we have developed core shell electrosprayed NPs for combined delivery of MTX and DOX. HA NPs were successfully synthesized by wet chemical precipitation technique with a yield of 60–70%. DOX was physically loaded with an efficiency of 68.62 ± 4.58%. Zheng et al. [35] reported a loading efficiency of 43.2% DOX in rod shaped HA at 1 and 0.5 mg/ml concentrations. In situ method of DOX loading in HA nanorods showed around 41 mg/g payload as stated by Sun et al. [36]. In the current study, higher loading of DOX was observed compared to the previous reports. The loading of DOX into HA was confirmed by FTIR spectroscopy. A broad peak between 3500 cm−1 to 3000 cm−1 confirmed the NH and OH stretching vibrations of DOX and a peak at 2987 cm−1confirmed the CH stretching vibration of organic molecules. Appearance of additional peaks compared to the HA peaks at 1678, 1629 cm−1 (N–H bending), 1400 cm−1 (C–H bending) further confirmed the DOX incorporation into HA NPs.
From the XRD results, it was confirmed that the synthesized HA NPs were highly pure and crystalline. On comparing the XRD data of HA NPs with DOX–HA NPs, no significant change in crystallinity and peak changes was observed. PCL showed semi-crystalline nature with two strong peaks at 22.670 (110) and 24.451 (200). For the final formulation, MTX-PCL-CH-DOX-HA NPs, a small hump like peak and amorphous nature were observed between 10° to 30° compared to pure PCL and CH polymer. There was no change in the spectra with addition of drug molecules.
Production of spherical particles through emulsion electrospraying is a challenging task. Two possible mechanisms involved in the electrospraying technique are, i) evaporation of organic solvents leading to shrinkage of droplets followed by change in surface charge of droplets by Rayleigh limit which leads to the formation of micro or nanoparticles by Coulomb fission, ii) increase in surface charge density to reach the Rayleigh limit followed by Coulomb fission leads to disintegration of drops into smaller droplets [17, 23]. The optimized emulsions were electrosprayed using a flow rate of 0.3 and 0.5 ml/h at varying voltages from 10 to 25 kV and keeping the constant distance 10 cm throughout the experiment. The optimized parameter for getting core shell NPs was 0.5 ml/h, 10 cm distance and a voltage of 13–16 kV.
The alteration of voltage, distance and flow rate has an impact on morphology. It is clear from the SEM images (Fig. 3) that by using a flow rate of 0.3 ml or 0.5 ml and by decreasing the voltage from 25 kV to 20 kV, the morphology of fiber changes into beads. Further decrease in voltage upto 13 to 10 kV showed the formation of uniform spherical shape particles with rare fibers. SEM image of the MTX–PCL–CH–DOX–HA NPs (Fig. 4) clearly showed that electrosprayed particles were spherical in shape. Inner dark core with light gray color outer layer established the core shell architecture of the NPs by TEM (Fig. 5). TEM also clearly displayed the confinement of DOX-HA NPs in the CH core and outer PCL shell layer (Fig. 5). Apart from anticancer property, DOX have intrinsic fluorescence effect due to the presence of anthracycline chromophore moiety in the nucleus. This helps in visualization of DOX onto the living systems through fluorescence imaging [37]. In our study, the distribution of DOX in the NPs were analysed by fluorescence microscopy (Fig. 7). The confinement of red fluorescence in center of the NPs confirms the DOX loading onto the NPs.
Encapsulation of multiple drugs which are hydrophobic and hydrophilic in a single step is a major advantage of using single emulsion electrospraying. MTX (hydrophobic) and DOX (hydrophilic) encapsulated in core shell NPs exhibited different release profiles in acidic and alkali environment. Many factors are involved in the release behaviour of drugs from the drug delivery system such as structure, morphology, solubility, swelling, diffusibility in the release media and interaction between the carrier and drug molecules [19, 23]. As tumor microenvironment is in acidic pH 5–6 we have performed the drug release studies at pH 5 and compared the data with PBS pH 7.4. In the acidic environment, hydrophobic drug MTX showed burst release of 13% within 1 h followed by sustained release and a maximum of 40% was released at the end of 8 days (Fig. 8b). In case of DOX, a hydrophilic drug, a maximum release of 20% was observed in a sustained manner. In PBS pH 7.4, MTX showed maximum release of 28% with initial burst release of 12% and DOX showed maximum release of 13% at the end of the 8 days (Fig. 8a). At both acidic and alkaline pH, maximum MTX release was 40 and 28% respectively. There was slow and sustained release of MTX from the NPs, which may be due to the exterior hydrophobic nature of polymer shell PCL. More interestingly, DOX does not show any burst release throughout the study which could be due to inner CH layer retarding the release of DOX from the NPs or due to the diffusion controlled release kinetic properties of DOX from the HA NPs. Release of DOX from CH-PCL matrix is supposed to be pH dependent. At pH 5 acidic environment, DOX release is much faster due to high water solubility and DOX retains in its salt form as DOX HCl (pH 5) but in case of alkali environment (pH 7.4) it dissociates into neutral molecule and retards the release from the CH-PCL matrix.
Cytotoxic studies of both direct and indirect contact method showed concentration dependent activity in all time points. In the direct contact assay, NPs showed variable percentage of cell death at 24 and 72 h. It clearly indicates that cell death is dependent on the amount of drug released from the NPs. MTX–PCL–CH–DOX–HA NPs showed significant difference (50% cell death) at higher concentrations after 24 h of incubation due to released drug from the NPs (Fig. 9a). Positive controls MTX and DOX showed concentration-dependent activity and cytotoxic effect (50% cell death) at higher concentrations after 24 h. More interestingly, NPs showed syngersitic effect of both MTX and DOX compared to the positive controls’ cytotoxic profiles of MTX and DOX. In case of 72 h incubation, NPs and positive control of MTX and DOX showed very good activity in all the concentrations and showed a significant difference with respect to the control cells. When comparing the cell death profile of NPs with that of the controls, more than 70% of the cell death may be attributed to the combined effect of DOX and MTX (Fig. 9b).
Indirect contact assay was also performed in OMG 63 cells and the cells showed more than 70% viability for 24 h and concentration dependent cell death was observed at 24 and 72 h incubation (Fig. 10). No significant difference was observed between control and NPs after 24 h incubation. Meanwhile positive controls showed concentration dependent cell death and significant difference was observed at 0.5 and 1 mg /mL. On comparing the cell viability of NPs from lower to higher concentration, only higher concentration showed activity. The amount of drug encapsulated in the NPs are 46 µg (MTX) and 22 µg (DOX) per mg; while comparing the release profile of NPs, at end of 7 days around ~27% (~12 µg) of MTX and ~12% (~2 µg) of DOX was released. Approximately equal amounts of MTX and DOX were kept as positive controls which showed concentration dependent toxicity. In case of 72 h incubation, all the concentration of NPs showed concentration dependent toxicity and at 1 mg/mL, showed 50% cell death (Fig. 10b). The positive controls (MTX and DOX) also showed significant difference when compared with the control cells.
5 Conclusions
The core shell NPs of PCL/CH-HA fabricated using electrospray technique were explored for combinational delivery of MTX and DOX. TEM revealed the synthesized NPs were core shell in architecture with inner core consisting of DOX-HA NPs. DOX-HA NPs showed a loading efficiency of 69%. Core shell NPs showed encapsulation efficacy of 85% (MTX) and 38% (DOX). In vitro drug release studies displayed sequential release of both MTX and DOX for period of one week. Compared to alkaline pH, acidic pH showed increase in drug release from around 28 to 40% of MTX and from 13 to 20% of DOX. The concentration dependent cytotoxicity was observed in OMG 63 cell line. Dual drug loaded with bioresorbable HA core shell electrosprayed NPs will be a favorable and potential bone substitute material for the treatment of osteosarcoma.
References
Gu W, Wu C, Chen J, Xiao Y. Nanotechnology in the targeted drug delivery for bone diseases and bone regeneration. Int J Nanomed. 2013;8:2305–17.
Ma H, He C, Cheng Y, Yang Z, Zang J, Liu J, et al. Localized co-delivery of doxorubicin, cisplatin, and methotrexate by thermosensitive hydrogels for enhanced osteosarcoma treatment. ACS Appl Mater Interf. 2015;7:27040–8.
Li CJ, Liu XZ, Zhang L, Chen LB, Shi X, Wu SJ, et al. Advances in bone-targeted drug delivery systems for neoadjuvant chemotherapy for osteosarcoma. Orthop Surg. 2016;8:105–10.
Durfee RA, Mohammed M, Luu HH. Review of osteosarcoma and current management. Rheumatol Ther. 2016;3:221–43.
Bielack SS, Hecker-Nolting S, Blattmann C, Kager L. Advances in the management of osteosarcoma. F1000Research. 2016;5:2767.
Parhi P, Mohanty C, Sahoo SK. Nanotechnology-based combinational drug delivery: an emerging approach for cancer therapy. Drug Discov Today. 2012;17:1044–52.
Krukiewicz K, Zak JK. Biomaterial-based regional chemotherapy: Local anticancer drug delivery to enhance chemotherapy and minimize its side-effects. Mater Sci Eng C Mater Biol Appl. 2016;62:927–42.
Marques C, Ferreira JM, Andronescu E, Ficai D, Sonmez M, Ficai A. Multifunctional materials for bone cancer treatment. Int J Nanomed. 2014;9:2713–25.
Hossen S, Hossain MK, Basher MK, Mia MN, Rahman MT, Uddin MJ. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: a review. J Adv Res. 2019;15:1–18.
Bose S, Tarafder S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: a review. Acta Biomater. 2012;8:1401–21.
Zamani M, Prabhakaran MP, Ramakrishna S. Advances in drug delivery via electrospun and electrosprayed nanomaterials. Int J Nanomed. 2013;8:2997–3017.
Sridhar R, Ramakrishna S. Electrosprayed nanoparticles for drug delivery and pharmaceutical applications. Biomatter. 2013;19:e24281.
Nguyen DN, Clasen C, Van, den Mooter G. Pharmaceutical applications of electrospraying. J Pharm Sci. 2016;105:2601–20.
Li C, Yu DG, Williams GR, Wang ZH. Fast-dissolving core-shell composite microparticles of quercetin fabricated using a coaxial electrospray process. PLoS ONE. 2014;9:e92106.
Raheja A, Chandra TS, Natarajan TS. Design of a low cost spinneret assembly for coaxial electrospinning. Appl Phys Lett. 2015;106:254101.
Lee YH, Bai MY, Chen DR. Multidrug encapsulation by coaxial tri-capillary electrospray. Colloids Surf B, Biointerf. 2011;82:104–10.
Cui L, Liu Z-P, Yu D-G, Zhang S-P, Bligh SWA, Zhao N. Electrosprayed core-shell nanoparticles of PVP and shellac for furnishing biphasic controlled release of ferulic acid. Colloid Polym Sci. 2014;292:2089–96.
Cao Y, Wang B, Wang Y, Lou D. Polymer-controlled core–shell nanoparticles: a novel strategy for sequential drug release. RSC Adv. 2014;4:30430–9.
Cao Y, Wang B, Wang Y, Lou D. Dual drug release from core-shell nanoparticles with distinct release profiles. J Pharm Sci. 2014;103:3205–16.
Yeh HW, Chen DR. In vitro release profiles of PLGA core-shell composite particles loaded with theophylline and budesonide. Int J Pharm. 2017;528:637–45.
Kim W, Kim SS. Multishell encapsulation using a triple coaxial electrospray system. Anal Chem. 2010;11:4644–7.
Lee YH, Bai MY, Chen DR. Multidrug encapsulation by coaxial tri-capillary electrospray. Colloids Surf B, Biointerf. 2011;82:104–10.
Wang Y, Zhang Y, Wang B, Cao Y, Yu Q, Yin T. Fabrication of core–shell micro/nanoparticles for programmable dual drug release by emulsion electrospraying. J Nanopart Res. 2013;15:1726.
Zhang C, Feng F, Zhang H. Emulsion electrospinning: fundamentals, food applications and prospects. Trends Food Sci Technol. 2018;18:175–86.
Pal P, Dadhich P, Srivas PK, Das B, Maulik D, Dhara S. Bilayered nanofibrous 3D hierarchy as skin rudiment by emulsion electrospinning for burn wound management. Biomater Sci. 2017;5:1786–99.
Xu X, Chen X, Wang Z, Jing X. Ultrafine PEG-PLA fibers loaded with both paclitaxel and doxorubicin hydrochloride and their in vitro cytotoxicity. Eur J Pharm Biopharm. 2009;72:18–25.
McClellan P, Landis WJ. Recent applications of coaxial and emulsion electrospinning methods in the field of tissue engineering. Biores Open Access. 2016;5:212–27.
Yang L, Sheldon BW, Webster TJ. Nanophase ceramics for improved drug delivery. Am Ceram Soc Bull. 2010;89:24–32.
Zhang Y, Lu J. A simple method to tailor spherical nanocrystal hydroxyapatite at low temperature. J Nanopart Res. 2006;9:589–94.
Pal P, Srivas PK, Dadhich P, Das B, Maulik D, Dhara S. Nano-/microfibrous cotton-wool-like 3D scaffold with core–shell architecture by emulsion electrospinning for skin tissue regeneration. ACS Biomater Sci Eng. 2017;3:3563–75.
Prasad SR, Sampathkumar TS, Jayakrishnan A. Ceramic core with polymer corona hybrid nanocarrier for the treatment of osteosarcoma with co-delivery of protein and anti-cancer drug. Nanotechnology. 2018;29:015101.
Ta HT, Dass CR, Larson I, Choong PF, Dunstan DE. A chitosan hydrogel delivery system for osteosarcoma gene therapy with pigment epithelium-derived factor combined with chemotherapy. Biomaterials. 2009;30:4815–23.
Ma H, He C, Cheng Y, Li D, Gong Y, Liu J, et al. PLK1shRNA and doxorubicin co-loaded thermosensitive PLGA-PEG-PLGA hydrogels for osteosarcoma treatment. Biomaterials. 2014;35:8723–34.
Li WM, Su CW, Chen YW, Chen SY. In situ DOX-calcium phosphate mineralized CPT-amphiphilic gelatin nanoparticle for intracellular controlled sequential release of multiple drugs. Acta Biomater. 2015;15:191–9.
Zheng F, Wang S, Shen M, Zhu M, Shi X. Antitumor efficacy of doxorubicin-loaded electrospun nano-hydroxyapatite–poly(lactic-co-glycolic acid) composite nanofibers. Polym Chem. 2013;4:933–41.
Sun W, Fan J, Wang S, Kang Y, Du J, Peng X. Biodegradable drug-loaded hydroxyapatite nanotherapeutic agent for targeted drug release in tumors. ACS Appl Mater Interf. 2018;10:7832–40.
Mohan P, Rapoport N. Doxorubicin as a molecular nanotheranostic agent: effect of doxorubicin encapsulation in micelles or nanoemulsions on the ultrasound-mediated intracellular delivery and nuclear trafficking. Mol Pharm. 2010;27:1959–73.
Acknowledgements
The authors thank Dr. Anant Raheja and Dr. Rajashekhar Bijja, FIB SOL Life technologies, Chennai for electrospraying instrument and their support and guidance in electrospraying optimization. The authors thank Prof. Nitish R Mahapatra and Prof. Suresh Kumar Rayala for providing cell culture facility and Prof. Sakthi Kumar of Toyo University, Japan for the TEM. The authors also thank Sophisticated Analytical Instrument Facility (SAIF) and Central XRD Facility of IIT Madras for analytical support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Prasad, S.R., Jayakrishnan, A. & Kumar, T.S.S. Combinational delivery of anticancer drugs for osteosarcoma treatment using electrosprayed core shell nanocarriers. J Mater Sci: Mater Med 31, 44 (2020). https://doi.org/10.1007/s10856-020-06379-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-020-06379-5