Abstract
Biomedical and regenerative medicine has significantly contributed to developing new, specific techniques and technologies to improve patient care. A significant leap has been made with innovative materials, cell development, scaffold design, and fabrication in tissue engineering. The tissue engineering process focuses on regenerating tissues that have been lost accidentally or encounter defects such as osteosarcoma, osteoporosis, and osteoarthritis. India has a high number of people who suffer from bone diseases. An estimated 15–20% of the population suffers from osteoporosis. Bone scaffolds are proving to be an excellent treatment for osseous anomalies and defects. Scaffolds are porous, three-dimensional structures that enhance the growth of new tissues. Bone scaffolds are designed to facilitate osteoinductive cells’ growth, expansion, and migration on their surface. The purpose of this paper is to review possible polymeric materials for bone scaffolds and provide a suitable combination in terms of cost of material and cost of technology for tissue-engineered bone scaffolds.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
1.1 Tissue Engineering
The goal of tissue engineering is to enhance tissue function by combining engineering and life sciences. A tissue engineering procedure aims to regenerate damaged or diseased tissue in the body attached to the extracellular matrix (ECM). The ECM is made up of a network of carbohydrates and proteins. ECM configuration varies with tissue, and it also includes structural proteins like elastin, collagen, etc., adhesive proteins such as laminin or fibronectin, and proteoglycans. As proteins, proteoglycans carry molecules of sugar attached in the form of polysaccharide complexes (Kusindarta and Wihadmadyatami 2018; Zheng 2019; Biazar 2018). Two sugar units make up a polysaccharide called glycosaminoglycan (GAG). GAGs are commonly used as lubricants and shock absorbers in the body due to their ability to attract water. Based on the sugar type, GAG is categorized into five categories: chondroitin sulfate, epamin sulfate, heparin sulfate, and hyaluronic acid (Zhang et al. 2019).
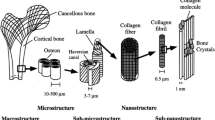
Collagen and hyaluronic acid are components of cartilage’s ECM and GAG. The ECM of bone is primarily composed of collagen and hydroxyapatite. In contrast, the ECM of the skin is composed of collagen, elastin, and proteoglycans (Chocholata et al. 2019).
A scaffold is a structure made of specific materials that can be manufactured artificially and implanted in the body. The scaffolding promotes cell interactions inside the body and helps to form new native functional tissues. The design of scaffolds focuses on having predefined porosity. In the same way that the native ECM in the body mimics the porous structure of the scaffold, it triggers cell proliferation, cell migration, and differentiation. Cells continuously reabsorb and sediment more ECM in the body. Bone, for example, grows in response to load. Healthy bones are also reabsorbed and sublimated. However, the resorption and sedimentation rates are roughly equal in normal bone. When a bone disease like osteoporosis occurs, this balance is disrupted. A tissue engineering scaffold is conventionally designed to degrade as the cells release enzymes within the body. Ideally, these scaffolds should be replaced with a natural ECM produced by the body’s cells (Abraham 2014; Wang 2020).
Tissue engineering was introduced in the 1980s and quickly became popular among researchers. In the early stage of the study, specific cells, such as stem cells, were mixed with chemicals to observe cell growth under controlled conditions (Chia and Wu 2015). Some implants have been used to treat human diseases since ancient times. Dentures and dental implants have been used in various cultures worldwide for centuries. The ancient Egyptians used gold wires to bind the surrounding teeth structures as early as 2500 BC. The French civilization mastered wrought iron implants on corpses around 200 AD. The Maya civilization made nacre teeth from calcium carbonate from seashells around 600 A.D.; the present-day process is called osseointegration (Abraham 2014). The Honduran dynasty made stone implants around 800 AD (Wang 2020). Almost every era in human history has used tissue engineering and implants in some form or another. It is a massive motivation for current researchers, and tissue engineering is on the rise due to the advancement of technology (Qi 2013). In vitro studies on scaffolds began around 25 years ago, focusing primarily on bone tissue engineering. Various materials and technologies have been used since then for bone scaffolding. The scaffolds are fabricated using electrospinning, solvent casting, porogen leaching, gas foaming, phase separation, fiber mesh, melt molding, bonding, membrane lamination, and freeze-drying, as well as rapid prototyping (Velasco et al. 2015).
1.2 Role of 3D Printing in Biomedical Applications
Tissue engineering has been given a significant boost with rapid prototyping. Although it is still in its infancy, it has already shown great potential in aerospace, medical, manufacturing, automobile, and construction owing to its rapid development.
Rapid prototyping is broadly considered an additive manufacturing technology. As of late, it has also been called 3D printing in a broad sense. The 3D printing is an important development in tissue engineering because of its ability to manufacture intricate shape parts very efficiently and precisely. Exoskeletons, jawbones, bones, and various tissues and organs are currently being created with 3D printing. This field has many possibilities, from making anatomical samples for study to creating a human organ that will function flawlessly inside the patient’s body (Zhang et al. 2019).
The process of 3D printing involves creating a three-dimensional (3D) model using a computer-aided design (CAD) package. Then, the 3D solid model is converted into a valid surface format (.stl or .obj). Slicing software then uses the surface file to create all inner details, including infill pattern, infill density, layer height, wall count, etc., to convert a surface file into a layer file. A 3D printer uses this layer definition to build the part layer by layer (Chocholata et al. 2019). All 3D printing technologies can create intricate geometric shapes, making them especially suitable for biomedical and tissue engineering applications. In general, porous scaffolds can be manufactured using various 3D printing technologies (Hospodiuk et al. 2017). Currently, there are approximately 40 different 3D printing technologies in existence. Each technology is based on a different approach (An et al. 2015).
2 Polymers in Tissue Engineering
In tissue engineering applications, polymer materials are used to fabricate scaffolds. Polymer material selection depends on various properties, such as molecular weight, shape, lubricity, chemistry, hydrophilicity, hydrophobicity, solubility, and biodegradability. The 3D printed polymer scaffolds have good mechanical strength, biodegradability, and porous structure. Natural polymers and synthetic polymers are the two main categories of polymers. Polymers are further classified into proteins, polysaccharides, and polynucleotides. The three types of synthetic polymers are copolymers, microbial polymers, and bioactive ceramics (see Fig. 1) (Dhandayuthapani et al. 2011).
2.1 Essential Properties Required in Polymers for 4D Printing
Pore sizes from 20 to 1500 µm are used primarily in tissue engineering applications to mimic the natural bone structure. For significant bone growth, it is recommended that the pore size be 80 to 120 mm. Pore sizes less than 80 µm affect the migration of cells, while larger pores, more than 500 µm, affect the attachment of cells as the specific surface area decreases. Pore size optimization facilitates many cell sites and increases bone ingrowth (Murphy and O’brien 2010).
Biocompatibility refers to a material’s ability to react with a specific host response in a given situation. Specifically, it refers to the suitability of the polymer material to the body and its fluids. Polymeric bone scaffolds should be biocompatible to increase bone tissue interaction with the scaffold material (Arif 2019).
Biodegradability results from a chemical process that produces a sharp division of covalent bonds. Polymers degrade by hydrolysis, which is typically a chemical process occurring in the presence of water molecules. When biology is concerned, biodegradable polymers refer to a material that degrades over time once it is implanted inside the patient (Ratner and Bryant 2004; Cameron and Kamvari-Moghaddam 2008).
Cytotoxicity is defined as the addition of foreign elements to the body that causes cells to become toxic. An implant material should not contain or exhibit cytotoxicity; the polymeric bone scaffold should be non-toxic (Gregor 2017).
Chemical bonding at surfaces is necessary for cell attachment, proliferation, and migration.
Mechanical strength, for a bone scaffold, is essential. In order to place the scaffold at an appropriate location within the patient’s body, the scaffold should resist a certain amount of compressive stress to support adjacent bones, if needed (Subia et al. 2010).
Printability refers to the ease with which a material can be 3D printed. A polymer material should be very printable (Dhandayuthapani et al. 2011).
2.2 Natural Polymers
Plants, animals, and microorganisms are all sources of natural polymers, also known as renewable resources. Different sources of these organisms possess complex structures with different physiological functions. Polymers of this type are typically created by adding or condensing groups together. Regular polymers possess remarkable properties. Material properties are often determined by the material’s structure rather than by molecular formation (Bassas-Galia et al. 2017). Composite polymers with enhanced properties are developed by studying and mimicking natural polymer structure and function. Natural polymers can frequently be developed from proteins. Many proteins, such as gelatin, soy protein, silk, casein, and keratin, have demonstrated excellent qualities. In combination with polymers, these proteins have improved shear and flexural strength, toughness, elasticity, and tensile modulus (Gupta and Nayak 2015).
An extended shackle of polymeric carbohydrate molecules, polysaccharides are composed of monosaccharides linked by o-glycosidic chains. Polysaccharides perform a variety of physiological functions. A few examples of polysaccharides are starch, cellulose, alginate, chitosan, glycosaminoglycans (GAGs), hyaluronic acid, pullulan, and dextran (Aravamudhan et al. 2014).
Polynucleotides are covalently bonded chains of nucleotide monomers. Polynucleotides include deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). Research is being conducted on gene therapy using bio-nano composite DNA and RNA (Noreen et al. 2020).
2.3 Synthetic Polymers
The advantages of synthetic polymers over natural polymers have been well documented. A synthetic polymer possesses desired mechanical properties, process control capabilities, and reliability. Synthetic polymers can be engineered to achieve the desired chemical bonding, cell interaction, porosity, and surface roughness characteristics. Synthetic polymers exhibit controlled resorption and biocompatibility, which are highly desirable properties when designing bone scaffolds (Gunatillake et al. 2003). They are more uniform and exhibit predictable responses to chemical and mechanical properties. Synthetic polymers can exhibit non-toxic behavior toward surrounding tissues, making them a preferred choice over natural polymers. In general, synthetic polymers can be classified according to their ability to degrade biologically and their inability to degrade biologically.
Synthetic polymer scaffolds can be created using a variety of methods, e.g., gas leaching, salt leaching, electrospinning, solvent casting, gas foaming, and 3D printing. The 3D printing is the most suitable technique for constructing bone scaffolds because it can deliver the desired shape and ensure adequate porosity (Bolívar-Monsalve 2021). In addition to providing cell attachment sites, 3D-printed bone scaffolds can mimic the shape of the bone. As this scaffold is implanted in a patient, it can be resorbed easily and promote bone growth. This section discusses highly suitable synthetic polymers in 3D printing using the fused deposition modeling (FDM) technique. FDM is used because it is cost-effective and can be used with biodegradable materials.
According to Table 1, polylactic acid (PLA) shows favorable chemical composition for bone scaffold application as its FTIR spectroscopy results. The contact angle of PLA is < 90° making it hydrophilic, which can provide enough cell sites for bone tissues to grow. PLA has a moderately high glass transition temperature in 45–60°C compared with the average human body temperature. When PLA is used as a bone scaffold, it can retain its solid form and provide adequate mechanical strength to the adjacent bones. The tensile modulus of PLA is between 0.35 and 3.5 GPa which ensures enough load-bearing capacity when used as a bone scaffold implant in the lower limb.
2.4 Fabrication of Polymer Composites Using FDM
Ranjan et al. demonstrated through in vitro studies that PLA-HAp-chitosan composite in proportions of 91-8-1 (by % of weight) was used to 3D print bone scaffold using the FDM technique. This composite bone scaffold exhibited good biocompatibility and bioactivity from the Ra profile and serum stability test (Ranjan 2020; Ranjan et al. 2018). Ales Gregor et al. 3D printed PLA scaffolds and performed in vitro studies to showcase porosity of 30–60% promotes cell attachment proliferation. It provides more cell sites for a natural ECM to grow (Gregor 2017). Ricardo Donate et al. suggested the use of additives which includes HAp, β-TCP, etc., to increase mechanical properties of PLA and enhance its osteoconductivity, use of surface treatments like alkali and plasma treatment to increase the hydrophilicity of PLA, and use of bioactive substances like chitosan, calcium phosphate, collagen, alginate, etc., to enhance cell bioactivity in PLA (Donate et al. 2020). Bruna Teixeira et al. suggested that the FDM technique is suitable to 3D print PLA scaffolds and exhibit structural properties comparable to cancellous bone (Marianna et al. 2016). To showcase good compressive strength, Zhang et al. created optimized PLLA (L-PLA)/nano-HA (nHA) composites with cost-effective FDM technology to 3D print PLLA/nHA porous bone scaffolds. It also exhibited good osteogenic properties when compared with HA ceramic scaffold and cancellous bone (Zhang 2021). Mazzanti et al. studied the mechanical properties of polymers like polyolefins when mixed with natural fillers and showcased significant improvement (Mazzanti et al. 2019).
FDM is the most widely used 3D printing technology across the globe. However, still, the cost of FDM 3D printers is comparatively higher. To overcome this barrier, open-source 3D printers have been manufactured in several parts of the world and getting more popular. These open-source FDM 3D printers work on open-source software that includes slicing software, e.g., Ultimaker Cura, slice 3r, etc., open-source community setup provides free access to users for different 3D models, designs, e.g., Thingiverse, Backster, etc. (Alagoz and Hasirci 2020). FDM is specifically useful in tissue engineering to print porous scaffolds used for bones. FDM can produce scaffolds with good mechanical properties and structural integrity in making bone scaffolds. It is possible to design patient-specific defects using MRI or C.T. scan data. Additionally, the infill structures can match the defect sites and encourage cell attachment and migration.
3 Conclusion
According to research, FDM outperforms all available 3D printing techniques in cost-effectiveness, a wide range of polymers, a harmless mode of operation, and specific tissue engineering applications, such as bone scaffolding. Materials such as PLA, PGA, and polylactic-co-glycolic acid (PLGA) are readily available in clinical grade. Several of these materials can be mixed with other natural polymers to produce biocompatible, biodegradable composite polymers; in vitro studies were conducted to determine cytotoxicity, biodegradation rate, percentage porosity, mechanical strength, and geometrical properties of these materials. In all of these studies, satisfactory results have been achieved concerning cell proliferation, migration, and differentiation. Additionally, functional scaffold prototypes were tested for statistical control and assembly applications, and the results were satisfactory. Using FDM, it is also possible to fabricate ceramic composites, which has been satisfactory. In conclusion, the material of the scaffold and fabrication techniques plays a significant role. A number of studies have demonstrated that FDM can be a cost-effective alternative in the 3D printing of bone scaffolds of various sizes and shapes.
4 Future Scope
The field of tissue engineering is experiencing rapid growth with the development of additive manufacturing. The development of new bio-printers equipped with increased efficiency, widening the variety of materials available, and improving accuracy. Despite all this progress, there remain some challenges to overcome. The high cost of these bio-printers and materials and technologies like SLA, SLS, and binder jet printing has a significant and somewhat limiting effect on the conduct of research. Moreover, most studies are in vitro studies, more emphasis should be placed on in vivo studies, and clinical trials should be carried out.
The field of multi-material printing is still nascent. Accelerating research toward this area is essential in developing multi-polymer printing, multi-metal printing, etc. Improved surface structures, layer adhesions, and cell interactions would allow customized implants to be used in surgeries.
References
Abraham CM (2014) A brief historical perspective on dental implants, their surface coatings and treatments. Open Dent J 8:50–55
Alagoz AS, Hasirci V (2020) 3D printing of polymeric tissue engineering scaffolds using open- source fused deposition modelling. Emerg Mater 3:429–439
An J, Teoh JEM, Suntornnond R, Chua CK (2015) Design and 3D printing of scaffolds and tissues. Engineering 1:261–268
Aravamudhan A, Ramos DM, Nada AA, Kumbar SG (2014) Natural polymers: polysaccharides and their derivatives for biomedical applications, natural and synthetic biomedical polymers. Elsevier, pp 67–89
Arif U (2019) Biocompatible polymers and their potential biomedical applications: a review. Curr Pharm Des 25:3608–3619
Bassas-Galia M, Follonier S, Pusnik M, Zinn M (2017) Natural polymers: a source of inspiration. In: Bioresorbable polymers for biomedical applications: from fundamentals to translational medicine
Benkaddour A, Jradi K, Robert S, Daneault C (2013) Grafting of polycaprolactone on oxidized nanocelluloses by click chemistry. Nanomaterials 3:141–157
Biazar E (2018) 3D bioprinting technology for body tissues and organs regeneration. J Med Eng Technol 42:187–202
Bolívar-Monsalve EJ (2021) Engineering bioactive synthetic polymers for biomedical applications: a review with emphasis on tissue engineering and controlled release. Mater Adv 2(14):4447–4478
Cai Z, Wan Y, Becker ML, Long YZ, Dean D (2019) Poly(propylene fumarate)-based materials: synthesis, functionalization, properties, device fabrication and biomedical applications. Biomaterials 208:45–71
Cameron RE, Kamvari-Moghaddam A (2008) Synthetic bioresorbable polymers. In: Degradation rate of bioresorbable materials: prediction and evaluation, pp 43–66
Chia HN, Wu BM (2015) Recent advances in 3D printing of biomaterials. J Biol Eng 9:1–14
Chieng BW, Ibrahim NA, Yunus WMZW, Hussein M (2014) Poly(lactic acid)/poly(ethylene glycol) polymer nanocomposites: effects of graphene nanoplatelets. Polymers 6:93–104
Chocholata P, Kulda V, Babuska V (2019) Fabrication of scaffolds for bone tissue regeneration. Materials 12:568
Dhandayuthapani B, Yoshida Y, Maekawa T, Kumar DS (2011) Polymeric scaffolds in tissue engineering application: a review. Int J Polymer Sci 2011. Article ID 290602, (19 pages)
Donate R, Monzón M, Alemán-Domínguez ME (2020) Additive manufacturing of PLA-based scaffolds intended for bone regeneration and strategies to improve their biological properties. E-Polymers 20:571–599
Gregor A (2017) Designing of PLA scaffolds for bone tissue replacement fabricated by ordinary commercial 3D printer. J Biol Eng 11:1–21
Gunatillake PA, Adhikari R, Gadegaard N (2003) Biodegradable synthetic polymers for tissue engineering. Eur Cells Mater 5:1–16
Gupta P, Nayak KK (2015) Characteristics of protein-based biopolymer and its application. Polym Eng Sci 55:485–498
Hospodiuk M, Dey M, Sosnoski D, Ozbolat IT (2017) The bio link: a comprehensive review on bioprintable materials. Biotechnol Adv 35:217–239
Jiang L, Nath C, Samuel J, Kapoor SG (2015) An enhanced microstructure-level finite element machining model for carbon nanotube-polymer composites. J Manuf Sci Eng Trans ASME 137(2):021009, (11 pages)
Karfarma M, Esnaashary MH, Rezaie HR, Javadpour J, Naimi-Jamal MR (2019) Poly(propylene fumarate)/magnesium calcium phosphate injectable bone composite: effect of filler size and its weight fraction on mechanical properties. Proc Inst Mech Eng Part H J Eng Med 33:1165–1174
Kharazmi A (2015) Structural, optical, opto-thermal and thermal properties of ZnS-PVA nanofluids synthesized through a radiolytic approach. Beilstein J Nanotechnol 6:529–536
Kusindarta DL, Wihadmadyatami H (2018) The role of extracellular matrix in tissue regeneration. Tissue Regen
Lee KW, Wang S, Yaszemski MJ, Lu L (2008) Physical properties and cellular responses to crosslinkable poly(propylene fumarate)/hydroxyapatite nanocomposites. Biomaterials. 29(19):2839–2848
Liu P, Chen W, Liu C, Tian M, Liu P (2019) A novel poly (vinyl alcohol)/poly (ethylene glycol) scaffold for tissue engineering with a unique bimodal open-celled structure fabricated using supercritical fluid foaming. Sci Rep 9:9534–9534
Marianna C, Bruna T, Daniel K, Mara TR (2016) Structural evaluation of PLA scaffolds obtained by 3D printing via fused deposition modelling (FDM) technique for applications in tissue engineering. Front Bioeng Biotechnol 4:995–997
Mazzanti V, Malagutti L, Mollica F (2019) FDM 3D printing of polymers containing natural fillers: a review of their mechanical properties. Polymers 11:1094
Mohammadi A, Barikani M, Barmar M (2015) Synthesis and investigation of thermal and mechanical properties of in situ prepared biocompatible Fe3O4/polyurethane elastomer nanocomposites. Polym Bull 72:219–234
Murphy CM, O’brien FJ (2010) Understanding the effect of mean pore size on cell activity in collagen-glycosaminoglycan scaffolds. Cell Adhes Migr 4:377–381
Narayanan G, Shen J, Boy R, Gupta BS, Tonelli AE (2018) Aliphatic polyester nano- fibres functionalized with cyclodextrins and cyclodextrin-guest inclusion complexes. Polymers 10:1–26
Noreen A, Sultana S, Sultana T, Tabasum S, Zia KM, Muzammil Z, Jabeen M, Lodhi AZ, Sultana S (2020) Natural polymers as constituents of bionanocomposites. In: Micro and Nano Technologies, Bionanocomposites. Elsevier, pp 55–85
Qi L (2013) Application of visible light-based projection stereolithography for live cell-scaffold fabrication with designed architecture. Biomaterials 34:331–339
Ranjan N (2020) On 3D printed scaffolds for orthopaedic tissue engineering applications. SN Appl Sci 2:1–8
Ranjan N, Singh R, Ahuja IPS, Singh J (2018) Fabrication of PLA-HAp-CS based biocompatible and biodegradable feedstock filament using twin-screw extrusion. In: Additive manufacturing of emerging materials, pp 325–345
Ratner BD, Bryant SJ (2004) Biomaterials: where we have been and where we are going. Annu Rev Biomed Eng 6:41–75
Subia B, Kundu J, Kundu SC (2010) Biomaterial scaffold fabrication techniques for potential tissue engineering applications [Internet]. Tissue Eng. InTech
Velasco MA, Narváez-Tovar CA, Garzón-Alvarado DA (2015) Design, materials, and mechanobiology of biodegradable scaffolds for bone tissue engineering. BioMed Res Int 2015. Article ID 729076, (21 pages)
Vrandečić NS, Erceg M, Jakić M, Klarić I (2010) Kinetic analysis of thermal degradation of poly(ethylene glycol) and poly(ethylene oxide)s of different molecular weight. Thermochim Acta 498:71–80
Wang Z (2020) Design and characterization of hydroxyapatite scaffolds fabricated by stereolithography for bone tissue engineering application. Proc CIRP 89:170–175
Wondu E, Lule Z, Kim J (2019) Thermal conductivity and mechanical properties of thermo- plastic polyurethane-/silane-modified Al2O3 composite fabricated via melt compounding. Polymers 11:1103
Zhang B (2021) 3D printed bone tissue regenerative PLA/HA scaffolds with comprehensive performance optimizations. Mater Des 201:109490–109490
Zhang L, Yang G, Johnson BN, Jia X (2019) Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater 84:16–33
Zhang G, Zheng G, Ren T et al (2020) Dopamine hydrochloride and carboxymethyl chitosan coatings for multifilament surgical suture and their influence on friction during sliding contact with skin substitute. Friction 8:58–69
Zheng XQ (2019) 3D bioprinting in orthopaedics translational research. J Biomater Sci Polym Ed 30:1172–1187
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Manohar, S.S., Das, C., Kakati, V. (2024). A Review of Materials Suitable for Tissue-Engineered Bone Scaffolds. In: Deka, J.K., Robi, P.S., Sharma, B. (eds) Emerging Technology for Sustainable Development. EGTET 2022. Lecture Notes in Electrical Engineering, vol 1061. Springer, Singapore. https://doi.org/10.1007/978-981-99-4362-3_3
Download citation
DOI: https://doi.org/10.1007/978-981-99-4362-3_3
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-4361-6
Online ISBN: 978-981-99-4362-3
eBook Packages: Computer ScienceComputer Science (R0)