Abstract
The assessment of groundwater geochemistry and characterization is an important aspect of groundwater studies. For this study, a total of 20 groundwater samples were collected from hand-dug well and analyzed using the America Public Health Association standard (APHA) method. Result obtained from the study revealed that Ec, pH, and TDS range from 78.2 to 850 µS/cm, 3.34 to 7.09 and 0.8 to 71.58 mg/L, respectively. From geochemical model, it was observed that findings from End-member plots showed that silicate and carbonate weathering is the major process that influences groundwater. While plot of Ca2+/(HCO3− + SO42−) versus Na+/Cl− revealed that 92% of groundwater within the area falls within the natural state. The plot of Mg/ca against Mg/Na and Parson plot revealed that 73% of groundwater is influenced by rock–water interaction. Further findings from Diamond field plots showed that groundwater fell within High Ca + Mg and SO4 + Cl, 98% of groundwater fell within Ca–Mg–Cl water type, respectively. The plot of TDS against TH revealed that groundwater fell within soft-fresh and moderate–hard category, Chadba’s plot revealed that groundwater was classified to be of Na+ + HCO3− water type. Gibbs plot and Soltan classification revealed that larger percentage of groundwater is not influenced by the three major factors in Gibbs plots, groundwater within the study area is of deep meteoric type respectively. Findings obtained from ion exchange revealed that groundwater is influenced by mostly silicate weathering. The evaluation of groundwater seepage in heavy metals loads against pH revealed that heavy metals were classified under acid, high-metal, near-neutral high-metal, acid low metal, and near-neutral low metal. Results obtained from the study revealed that groundwater within the study area is influenced by geogenic and anthropogenic activities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Enyigba area is known to host large deposit of Lead–Zinc (Pb–Zn) minerals (Nnabo 2011, 2015; Ezeh et al. 2007; Eyankware et al. 2020a). It is also one of the major mining hubs in southeastern Nigeria, where mining activity is carried out on daily basis in small, medium and large scale. The occurrence of these minerals has made the communities and clusters around the mines commercial hubs hosting crowd all day; this has led to tremendous increase in the demand for freshwater due to growth in population (Eyankware et al. 2020a). According to Obasi et al. (2019), it was observed that Pb–Zn mining has contributed drastically to economical development of Ebonyi State through taxes generated from various companies that are involved in the process. The major kind of mining carried out within the study area is open cast mining. This type of mining tends to harbor mine water, all year round, but it is mostly pronounced during the rainy season.
Mine waters from these open pits are directly discharged into nearby streams or infiltrate into groundwater. In most cases, strict laws that tend to avert this process have not been enforced, thereby making the miner dig mining pits indiscriminately. This mining process in most cases tends to lead to the release of heavy metals into water and soil in and around the mines (Igwe et al. 2020, 2021). Several authors believed that if these heavy metals occur in large quantities above the specific standard, it is considered harmful to humans, plants, and aquatic organisms (Obasi and Akudinobi 2020; Eyankware et al. 2020a; Igwe et al. 2020). Regrettably, hydro-chemical parameters, such as major ionic species and heavy elements, strongly influence the management of water resources, as well as their potability for various use. Additionally, over the last past 20 years, there has been a series of reports by various scholars that groundwater quality is on the decline due to illegal mining activities around active and abandoned mining sites across Ebonyi State, Nigeria (Eyankware et al. 2018a, 2020a; Obasi et al. 2021). According to Eyankware et al. (2018a, b) one the major geogenic factor that influences groundwater geochemistry is rock–water interaction, especially within the Asu River Group of the Southern Benue Trough. Similar study conducted by Ighalo et al. (2020) reported that in Nigeria, 25% of groundwater pollution can be linked to hydrogeology, the hydrogeology process is known as rock–water interaction. Rock–weathering interaction often mobilizes elements into the water surface, while the other infiltrates into groundwater leading to distribution and dispersion along the geochemical cycle in these aquifers (Eyankware et al. 2018a; Akakuru et al. 2021b; Ighalo and Adeniyi 2020).
Interactions among water–rock–sediment–soil and the mineral composition of the aquifer materials through which water moves play an important role in groundwater chemistry variation and groundwater quality variation. In most cases, groundwater quality is often degraded by anthropogenic activities (Arumugam and Elangovan 2009; Eyankware et al. 2020b; Kelepertsis et al. 2006; Akakuru et al. 2017; Skeppstrom and Olofsson 2007; Simsek 2008; Omo-Irabor et al. 2018). Although several studies have been carried out around various mining sites within Ebonyi State, Nigeria. But detailed study has not been varied to thoroughly characterize major factors that influence groundwater geochemistry and how heavy metals seep into groundwater within the study area. Previous studies in the area include notable works done by Okogbue and Ukpai (2013a, b), Obiora et al. (2016) and Obasi (2020) and these studies done were to evaluate groundwater for drinking, irrigation and study occurrence of heavy metals in soil and water within various mining and other area of Ebonyi state (Okolo et al. 2018; Eyankware et al. 2020a, b; Nnabo 2015, 2016; Igwe et al. 2020, 2021). For example, Eyankware et al. (2020a) conducted a holistic study on the effect of mining activities across Ebonyi State on groundwater resources around the mining hub of the state.
They concluded that certain physicochemical parameters, such as Na+, K+, SO42−, NO3− and Ca2+, were within WHO permissible limit at various sampling points, while Mg2+ and Cl− concentrations were above WHO drinking water quality standard at various mine and quarry sites. Nnabo (2015) studied the occurrence of heavy metals in water resources (mine water, surface water, and borehole water) around the mining areas of Enyigba, Ebonyi State, Nigeria; physicochemical results obtained from his study were compared to (WHO 2011) permissible limit and contamination factor was also used to evaluate the occurrence of heavy metals in water resources. Findings on this study using contamination factor suggested that cadmium was found to have the highest concentration in analyzed heavy metals found in surface water and borehole water and when compared to WHO, permissible limit. The contamination of heavy metals in water resources is in order Cd >>> As >> Pb > Ni > Zn > Cu. In surface water, the order is Cd >>> As >> Pb > Ni > Zn > Cu; in mine ponds, it is Cd >>> Pb >> As > Ni > Zn > Cu, and in borehole water, the order is Cd >>> As >> Pb > Zn > Ni > Cu. And that contamination factor showed contamination status of Cd, Pb, and As in sampled water resources. In the same vein, Nnabo (2016) studied the occurrence of heavy metals in surface water resources around Enyigba mining district, using metal enrichment and pollution indices.
Reports from his findings revealed that heavy metals, such as arsenic, cadmium, lead, and nickel, were found to be high in surface water around the mine areas. A similar study conducted by Opoke and Osayande (2018) assessed the presence of heavy metals in surface and groundwater in the vicinity of the Pb–Zn mining area at Ameri, Ameka, Enyigba Ebonyi States, SE Nigeria. Findings from their study revealed that surface water showed a higher concentration of HMs when compared to groundwater. They reported that the risk of HMs leaching and groundwater contamination from the mine wastes is very high with considerable likelihood of HM transport by water percolating through the wastes/soils. On the other hand, Obasi and Akudinobi (2020) studied potential health risk and levels of heavy metal in water resources of the Pb–Zn mining area of Enyigba and its environs during the dry and season, report from their study revealed that the concentration of heavy metals in water resources tends to be high in the dry season than in the rainy season. They recommended constant monitoring of water resources around mine locations within Ebonyi State to avert health-related diseases associated with drinking contaminated water. Currently, no research has been carried out within the study area to characterize and also determine heavy metal seepage into groundwater resources within the study area. The objectives of this study are to assess the different mechanisms controlling groundwater chemistry and to identify the potential sources for the presence of heavy metals at Enyigba mining district of Ebonyi State Nigeria.
Location, climate, vegetation, and physiography
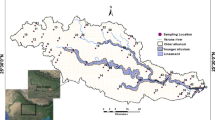
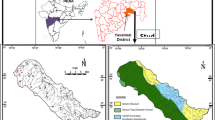
The study area is located in the southern part of Abakaliki, Ebonyi State, southeastern Nigeria. It lies between longitudes 6° 03′ E to 6° 21′ E and latitudes 8° 03′ N to 8° 21′ N. The major river that drained the study area is the Ebonyi River. The most ephemeral tributaries of this river generally flow in the N–S direction into the Ebonyi River and exhibit dendritic drainage patterns. The area falls completely within the Guinea Savannah vegetation belt, characterized by scattered trees, shrubs, and bushes. It experiences two distinct seasons: the wet season which spans from May to October and the dry season that spans from November to April in each climatic cycle. During the wet and dry seasons, the study area experiences mean precipitation of 2125 mm and 250 mm, respectively. The average temperature ranges from 27 to 28 °C and the atmospheric pressure varies between 1010 and 1016 millibars.
Geology and hydrogeological settings
The study area lies within the Abakaliki anticlinorium of the southern Benue Trough and is marked by the undulating range of shale outcrops of the Abakaliki shales of the Asu River Group (Albian age) which serve as the host rock for Pb–Zn mineral deposits. The shale underlying the area is predominantly dark gray to black, calcareous, hard, indurated and fractured, fissile, laminated, and interbedded with siltstone and mudstones. The shale outcrops which are highly indurated trend mostly NE–SW with dip direction in NW–SE and dip amounts ranging from 5° to 42°. In addition to the shale rock underlying the area is the alluvium sand exposed mainly along the river and stream channels. The study area is underlain by fractured shales of the Asu River Group which constitutes the only known aquifer in the area. The quality of water determines its potability and usefulness for other purposes. Environmental factors that determine the quality of a water supply source are related either to anthropogenic activities or geogenic processes (Eyankware et al. 2018a). Groundwater constitutes the main source of water supply for drinking and domestic purposes, while surface water supply is mainly for mining purposes in the area (Eyankware et al. 2020a).
Methods and materials
Twenty (20) water samples were randomly collected from hand-dug wells as shown in Fig. 1. Sampling was carried out during the dry season when there was a decrease in the water level and the concentration of cations, anions, and trace elements were more stable. Precautionary measures were taken by washing the bottles with clean water, and cleaning reagents and thoroughly rinsing with distilled, de-ionized water before collection of water samples from the site. The samples were analyzed for the following parameters; pH, electrical conductivity, total dissolved solids, total hardness, magnesium, calcium, chloride, nitrate, sulfate, potassium, sodium, bicarbonate, iron, cobalt, lead, zinc, manganese, mercury, cadmium, and silver following (APHA 2012) standard (Table 1).
Soltan classification
The sources of groundwater have been classified into two types by Soltan (1999). The classifications are based on base-exchange indices (r1) and meteoric genesis indices (r2) as presented in Eqs. (1a) and (1b).
Chadha classification
Gibbs plots
Gibbs (1970) proposed a diagram that shows the relationship between the chemical components of water from their respective aquifer lithologies using Eqs. (3a) and (3b) below.
For cations:
For anions:
Total Hardness (TH)
Potential geochemical factors influencing groundwater
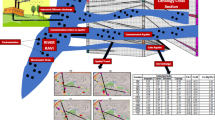
There are lots of natural processes that influence the geochemistry of groundwater. These include evaporation, precipitation, ion exchange, oxidation–reduction, weathering, and dissolution of minerals among others. A broad classification of the mechanisms controlling the groundwater chemistry is shown in Figs. 2, 3, 4, 5, 6, 7, 8 and 9. Figure 4 shows the relationship between Ca2+/(HCO3− + SO42−) versus Na+/Cl−.
A plot of Ca2+/(HCO3 − + SO4 2−)meg/L
From Fig. 4, it was observed that the spatial distribution of samples indicates samples ENY/01, 02, 03, 05, 06, 08, 09, 11, 12, 13, 15 and 20 were categorized within natural state. This implies that natural salt dissolutions, such as carbonates rock and silicate, are the main processes influencing geochemical constituent of within the study area. This is in line with findings carried out by Akakuru et al. (2017), and Eyankware et al. (2018b) which stated that it is influenced by the water–rock deduction, such as follows: dolomite-type weathering, gypsum-type weathering, alkaline and alkaline-type weathering. Further findings from Fig. 2 revealed that samples ENY/04, 10 and 19 fell within reverse cation exchange, and lastly ENY/07, 14, 16, 17, and 18 are considered to not be influenced by any process.
End-member diagram
The End-member diagram is used to analyze and determine the types of rock-weathering sources related to the hydro-chemical characteristics of groundwater (Gaillardet et al. 1999). Gaillardet et al. (1999) stated that the chemical composition of Ca2+/Na+ = 0.35 ± 0.15, Mg2+/Na+ = 0.24 ± 0.12 and HCO3−/Na+ = 2 ± 1 is for the silicate end member, and Ca2+/Na+ = 50, Mg2+/Na+ = 10 and HCO3−/Na+ = 120 is for the carbonate end member. Findings from Fig. 3 revealed that the 99.9% geochemistry of groundwater composition originates from the weathering of carbonate and silicate. That implies that silicate and carbonate weathering influences groundwater geochemistry as shown in Fig. 3.
A plot of Mg2+/Na+ versus Mg2+/Ca2+
The plot Mg2+/Na+ versus Mg2+/Ca2+ was used to determine the major factor that influences the geochemistry of groundwater within the study area. From Fig. 4 below, it was observed that rock weathering, Ca-salt leaching, and evaporation were the main mechanisms controlling the chemical constituents of groundwater within the study area. An important process of water–rock interaction is mineral dissolution (Li 2018; Akakuru and Akudinobi 2018) and the assessment of ionic meq/L ratios can indicate which minerals are likely to have the largest influence on groundwater quality.
A plot of HCO3 + CO3 versus Na + K
The water classification of hydro-geochemical facies of Piper diagram was reconstructed by a group of researchers in the new reconstructed diamond field (Fig. 5). It classified water into various zones based on various reactions occurring in the groundwater aquifer system. The reconstructed diamond field revealed that groundwater fell within High Ca + Mg + SO4 + Cl hydro-geochemical facies as shown in Fig. 5. This implies that, Ca and Mg, are the primary causes of hardness in water. Any water with high Ca + Mg is said to be hard. This is in line with a previous study conducted by Eyankware (2019) which stated that groundwater within the Asu River Group of the southern Benue Trough is generally hard. Alsuhaimi et al. (2019) reported that host rock in most terrain tends to weather, and even the residence overtime of groundwater in a certain aquifer giving rise to groundwater hardness respectively.
Parson plot
From Fig. 6, it was observed that groundwater within study area fell within three categories of water type Ca–Mg–Cl, Na–Cl and Ca–Mg–SO4 water type. It was observed that 98% of sampled groundwater within the study area fell within Ca–Mg–Cl, water type, thus is classified to be permanently hard water. Such water contains high amount of calcium, magnesium and some other cations. This kind of hardness can only be reduced with the aid of hard water softener. One of the major disadvantages of permanently hard water is that it can leave one skin dry to deposition of scale on appliances, which can later cause serious damage (Ravikumar and Somashekar 2015). This can be attributed to the evolutionary dissolution of calcium and magnesium from the calcic-rich rocks shales, limestone and calcareous sandstones in the area (Eyankware 2019).
Plot of Total Dissolved Solid (TDS) versus Total Hardness (TH)
The value of TH was estimated using Eq. (4). From Fig. 7, it was observed that sampled groundwater fell within soft, fresh, and moderately hard freshwater types. Generally, an increase in TDS in groundwater may be connected to an increase in pumping of water directly increases the concentration of TDS in groundwater. Figure 7 revealed that groundwater at sample locations ENY/01, 05, 06, 07, 08, 12, and 17; ENY/03, 04, 09, 10, 11, 12, 13, 19 and 20; ENY/02, 14, 15, and 18 fell within moderately hard fresh water, hard fresh, and very hard fresh water categories, respectively.
Chadha plot
Chadha plot was used to classify groundwater geochemistry of the study area as shown in Fig. 8. The rectangular field of the plot describes the primary character of the water including the permanent and temporary hardness domain and was determine using Eqs. (2a) and (2b). The rectangular field is divided into eight sub-fields, each of which represents a water type and hardness domain (Fig. 8), which are as follows: (1) alkaline earths exceed alkali metals. (2) Alkali metals exceed alkaline earths. (3) Weak acidic anions exceed strong acidic anions. (4) Strong acidic anions exceed weak acidic anions. (5) Alkaline earths and weak acidic anions exceed both alkali metals and strong acidic anions, respectively. The positions of data points in this domain represent Ca2+–Mg2+–HCO3− water type. (6) Alkaline earths exceed alkali metals and strong acidic anions exceed weak acidic anions. Such water has permanent hardness and does not deposit residual sodium carbonate in irrigation use. The positions of data points in this domain represent Ca2+–Mg2+–Cl− type of waters. (7) Alkali metals exceed alkaline earths and strong acidic anions exceed weak acidic anions. Such water generally creates salinity problems both in irrigation and drinking. The positions of data points in this domain represent Na+–Cl− type and Na+ + SO42− type of waters. (8) Alkali metals exceed alkaline earths and weak acidic anions exceed strong acidic anions. From Fig. 8, it was observed groundwater sample fell within Na+ + HCO3− water type; such water type deposits residual Na2CO3 during irrigation and is considered saline. Elsewhere in the Niger Delta Basin of Nigeria, Eyankware et al. (2020b) noted that groundwater samples that fell within Na+ + HCO3−such water type exhibit traces of acidic anions and weak acidic anion.
Gibb’s plot
Characteristics of cations and anions in groundwater display certain physicochemical attributes caused by the groundwater’s interaction with soil and rock, while flowing through the aquifer (Obasi et al. 2021). Aquifer shows attributes of water bodies with various chemical compositions (Eyankware et al. 2018b). Such attributes can be linked to the hydro-chemical facies of groundwater. The distributions of anions (Cl−, HCO3−) and cations (Na+, Ca2+) as well as the TDS value as shown in (Eqs. 3a and 3b), were used to plot the Gibbs diagram in other to show the major process that has an influence on groundwater within the study area, such as rock dominance, evaporation dominance, precipitation dominance. Gibbs plot helps in interpreting the influence of hydro-geochemical processes, such as precipitation, rock–water interaction mechanism, and evaporation on groundwater geochemistry. The functional sources of dissolved ions can be broadly assessed by plotting the samples, according to the variation in the ratio of Na+/(Na+ + Ca2+) and Cl−/(Cl− + HCO3−) as a function of TDS (Gibb’s 1970) as shown in Fig. 9. From Fig. 9, it was observed that groundwater is not influenced by factors, such as rock, evaporation crystallization, and precipitation dominance. 97% of groundwater is influenced other factors aside the three aforementioned factors, while the remaining 3% is influenced by rock and precipitation dominance, see Fig. 9. Findings obtained from Fig. 9 contradict findings obtained from Fig. 10a–e which showed groundwater is largely influenced by weathering process and rock–water interaction. This could attribute to several anthropogenic activities, such as mining, seepage from mine water into groundwater among others.
pH
Eyankware et al. (2020b) stated that pH is a term used universally to express the intensity of the acid or alkaline condition of a solution. According to Rao and Rao (1991), pH has no direct effect on human; all biochemical reactions are influenced by variation of pH value. The pH value of the study area ranges from 3.34 to 7.09, see Table 2. The variation of pH value in analyzed groundwater samples may be due to te composition of aquifer, geological and seasonal variation (Chanderaseker et al. 2013; Adeyeye et al. 2021) From Table 2, 80% of groundwater samples fell within the acidic category except for samples ENY/08, 12, 13, and 14. From Table 2, it was obsrved that sample locations ENY/02 and 06 showed low pH values, low pH in groundwater can lead to corrosion or dissolving of metals and other substances in groundwater, thereby making such water unsafe for drinking and irrigation use (Akakuru et al. 2021). Low pH can be attributed to rainwater that percolates through the soil; it reacts with the carbon dioxide produced during the microbial oxidation of organic matter in the unsaturated zone and forms carbonic acid. Dissolution of carbon dioxide increases the total inorganic carbon concentrations in the aquifer and decreases pH through the dissociation of the carbonic acid formed (Henderson 1985; Eyankware et al. 2021b). The pH of water is affected by several factors, such as bedrock and soil composition, through the water moves, both in its bed and groundwater, another factor that influences groundwater pH is the amount of plant growth and inorganic materials in water bodies.
Soltan classification
Soltan (1999) classified groundwater into two types, which are base-exchange indices (r1) and meteoric genesis indices (r2) as shown in Eqs. (1a) and (1b), where Na+, K+, Cl−, and SO42− concentrations are expressed in meq/L (Table. 1). If r1 < 1 and r2 < 1, the groundwater sources are of Na+–SO42− and deep meteoric type, respectively, while r1 > 1 and r2 > 1 indicate the sources are of Na+–HCO3− and shallow meteoric type. From Table 3, sampling points were categorized under Na+–SO42− water type.
Ion exchange
Factor controlling groundwater chemistry was also evaluated using bivariate diagrams, such as Na/Cl ratio versus EC, Mg versus Ca + Mg, Na versus Ca, Cl versus Na, and Ca + Mg versus HCO3 + SO4 respectively. According to Etteieb et al. (2017), bivariate plots are used to identify the origin of dissolved ions in groundwater, weathering process that influences the geochemistry of groundwater. The plot of Na/Cl against Ec suggested that the trend line is inclined with the increasing Ec, indicating that the evaporation is not the dominant process (Fig. 10a). From Fig. 10a, it was observed that a large number of samples fell below the equiline and the plot of Na/Cl against Ec is > 1. From Fig. 10b, the plot of Mg against Ca + Mg ratio is > 1 and a larger percentage of sample points was below equiline, indicating that silicate weathering influences groundwater. The plot of Na against Ca revealed that 98% of groundwater samples fell below the equiline, indicating that weathering process influences groundwater (Fig. 10c). From a detailed review of water resources in Nigeria by Ighalo and Adeniyi (2020), it was observed that the major factor that influences groundwater is hydrogeology. This usually occurs as an interaction between host rock and groundwater. This interaction alters groundwater geochemistry. Figure 10d showed that most of the groundwater sampling points recorded Na against Cl ratio > 1. This is in confirmation that Na+ concentration is above that of Cl−. If Na against Cl is equal to 1, it indicates halite dissolution; further, the silicate-weathering process is responsible for Na+ production if the ratio is > 1 and is an indication of the ion-exchange process (Meybeck 1987; Egbueri et al. 2019). The plot of Na against Cl indicates that 98% of samples are below the equiline suggesting silicate weathering. From Fig. 10e, it was observed that the plot of (HCO3 + SO4) vs. (Ca + Mg) revealed that the majority of groundwater samples fell above the equiline indicating that although silicate weathering influences groundwater chemistry, carbonate weathering influences tend to be higher when compared to silicate weathering. Elsewhere a similar study conducted by Ogwah and Eyankware (2020) within the vicinity of abandoned Okpara coal mine in Enugu state of the Anambra Basin, Nigeria suggested that silicate and carbonate weathering influences the geochemistry of groundwater.
Heavy metal in groundwater
Heavy metal contaminants, such as cobalt (Co), manganese (Mn), iron (Fe), silver (Ag), mercury (Hg), zinc (Zn), cadmium (Cd), and lead (Pb), are generally more persistent than organic contaminants. They can be mobile in soils and leach into aquifers (Sha et al. 2017). A fraction of heavy metal contaminants may lead to severe poisoning if they pollute the groundwater that is used for drinking.
Cadmium: The concentration of cadmium ranges 0.00 to 0.88 mg/L and its concentrations were below (WHO 2011) at sample locations ENY/02, 05, 06, 07, 08, 09, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 and 20, while sample locations ENY/ 01, 03, and 04 were below (WHO 2011) permissible limit for drinking. Friberg et al. (1986) stated that in natural waters, cadmium is found mainly in bottom sediments and suspended particles. Iron: is the second-most abundant metal in the earth's crust, of which it accounts for about 5% (Igwe et al. 2021). The concentration of Fe ranges from 0 to 0.08 mg/L, see Table 4a and b. It was observed that all sample points were below (WHO 2011) permissible limit for drinking water. Silver: In most cases, it occurs as a result of natural wearing down of silver-bearing rocks and soil by the wind and rain also releases large amounts of silver into the water bodies and environment (Obasi et al. 2015). Its concentration ranges from 0 to 0.91 mg/L, it was observed sampled points were below the (WHO 2011) permissible limit for drinking water except samples ENY/01, 02 03 and 04, see Table 4a and b. Obasi et al. (2015) further stated that the presence of Ag in water is also associated with lead, copper, and zinc ores. A recent study conducted by Obasi and Akundiobi (2020) within the Pb–Zn mining communities of Abakaliki, southeastern Nigeria revealed that high concentrations of Ag were not observed around mining sites, rather high concentrations of Ag were observed around (mine dump sites) the Umuoghara and Abakaliki mine waste dumpsites. This is in line with our research as analysis on Ag in groundwater for is study is placed around the mining area; result obtained from this showed the concentrations of Ag were below WHO (2011) permissible limit, Mercury: Mercury is found naturally in rock deposits as a native element, and bound to other elements in minerals. Mercury value ranges from 0 to 2.3 mg/L; Table 4a and b showed that samples ENY/01, 02, 03, 05, 06, 07, 08, 09, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 and 20 were above (WHO 2011) permissible limit for drinking water. Similar study conducted by Obasi and Akundiobi (2020) and Obasi et al. (2015) within Pb–Zn mining areas of Ebonyi State, Nigeria, suggested that high concentrations of mercury were reported in mining areas of Ameka, Amanchara, and Mkpuma Akpatakpa and that 60% of groundwater showed high trace of mercury in groundwater. Anthropogenic activities, such as mining and burning of fossil fuel, are believed to trigger an increase in concentration of mercury in groundwater (Dikinya and Areola 2010).
On the other hand, sample, ENY/04 was below the set limit (Table 4a), although result obtained from sample location ENY/04 at Ezza-Abia revealed that groundwater showed a low concentration of mercury. This could due to the geology of the terrain as groundwater exists in fractures. These fractures sometimes prevent high permeability, thereby reducing the inflow of heavy metals (Eyankware and Obasi 2021). High exposures to mercury may result in damage to the gastrointestinal tract, the nervous system, and the kidneys (Obasi et al. 2015). Manganese: The concentration of manganese ranges from 0 to 12.1 mg/L, samples ENY/01, 02, 03, 04, 05, 07, 18, 19, and 20 were above (WHO 2011) permissible limit for drinking water. While samples ENY/06, 08, 09, 10, 11, 12, 13, 14, 15 and 17 were observed to be below the set limit, see Table 4a and b. It often occurs, naturally together, through the weathering of rocks and minerals may also be present in underground water due to pollution sources (Obasi et al. 2015). Cobalt: Its concentration ranges from 0 to 0.15 mg/L. Most cobalt resources are present in nickel-bearing laterite deposits, with the remainder occurring primarily in nickel–copper sulfide deposits present in mafic and ultramafic rocks and sedimentary copper deposits (Obasi et al. 2015). Lead: Obasi et al. (2015) reported in occurrence of lead in groundwater within vicinity of various mining sites across the state can be attributed to mining activities, lead infiltrates into groundwater through leaching process. The concentration of lead for this study ranges from 0 to 0.80 mg/L as shown in Table 4a and b. The sampling points were below the (WHO 2011) permissible limit, except for samples ENY/ 06, 11, 12, 13, 14, 15 and 20 were above the set limit, see Table 4a and b, respectively. According to Obasi and Akundiobi (2020), a high lead in groundwater is usually high around activity active mine areas. Report from this study is in line with the report from Obasi and Akundiobi (2020) which showed that for ENY/06, 11, 12, 13, 14, 15, and 20, Enyigba, Achara Ndiagu, Ebiom, Echara, Nduia Igbagu, respectively, were mining activities tending to show a high trace of lead in groundwater. The amount of dissolved lead in groundwater depends on pH and the concentration of dissolved salts and the types of mineral surfaces present. In surface water and ground-water systems, a significant fraction of lead is undissolved and occurs as precipitates [PbCO3, Pb2O, Pb(OH)2, PbSO4], absorbed ions or surface coatings on minerals, or as suspended organic matter (Evanko and Dzombak 1997). Zinc: can be introduced into water naturally by erosion of minerals from rocks and soil. However, since zinc ores are only slightly soluble in water. Zinc is only dissolved at relatively low concentrations. High natural levels of zinc in water are usually associated with higher concentrations of other metals, such as lead and cadmium. Zinc is an essential nutrient for body growth and development; however, drinking water containing high levels of zinc can lead to stomach cramps, nausea, and vomiting. The concentration of zinc ranges from 0 to 2.09 mg/L samples ENY/01, 02, 04, 10, 11, 12, 13, 17, 18, 20 and were above (WHO 2011) permissible limit for drinking water; Li et al. (2019) and Obasi and Akundiobi (2020) reported that a high concentration of zinc in water leads to its dissolution, thereby increasing the groundwater acidity. In the same vein, Igwe et al (2020) and Obasi et al (2021) stated that acidic water is peculiar with mine areas and mine water discharge, while samples ENY/03, 05, 06, 07, 08, 09, 14, 15, 16, and 19 were below the (WHO 2011) permissible limit, regrettable 50% of analysed groundwater samples were above the set limit as shown in Table 4a and b. Studies have shown that high concentration of zinc in groundwater can be attributed to anthropogenic activities, such as industrial combustion, mining activities. When the concentration of zinc in groundwater is above set limit, it could result to age-related macular degeneration in human, chronic disease, such as diabetes and high blood pressure, (Obasi and Akundiobi 2020).
Evaluation of groundwater seepage around the study area
Ficklin diagram was used to evaluate mine seepage to groundwater within the study area. Groundwater pH and metal load (mg/L) (Ficklin et al. 1992), show that the samples ENY/01 and 11 fall within acid high metal, ENY/04 falls within near-neutral high metals, ENY/02, 05, 06, 07, and 10 fall with acid-low metals, and lastly samples ENY/03, 08, 09, 12, 13, 14, 15, 16, 17, 18, 19 and 20 as shown in Fig. 11. Rao and Rao (1991), reported that pH is major parameter that influences the dissolution of heavy metal in groundwater. Study conducted Harichandan et al. (2021) revealed that alteration of rock, dissolution, mineralization, leaching from rocks, percolation or seepage from active mines quarry/mine pits via rocks and human activities is found in the processes of concentration of ions and heavy metals in groundwater.
Summary and conclusion
The presented research has helped to establish factors that influence the chemical quality of groundwater within Enyigba Pb–Zn mining area of Ebonyi State, southeastern Nigeria. From detailed study, factor that influences groundwater, within the study area, differs from End-member. It reveals that silicate and carbonate weathering is the major process that influences groundwater, while plot of Ca2+/(HCO3− + SO42−) versus Na+/Cl− reveals that 92% of groundwater within the area falls within the natural state. The plot of Mg/Ca against Mg/Na reveals that 73% of groundwater is influenced by rock–water interaction. Diamond field plots reveal that groundwater falls within High Ca + Mg and SO4 + Cl. Parson plot showed that groundwater falls within Ca–Mg–Cl water type, plot of TDS against TH reveals that groundwater falls within soft-fresh and moderate–hard category. Chadba’s plot revealed that groundwater falls within Na+ + HCO3− water type. Gibbs plot showed a larger percentage of groundwater is not influenced by the three major factors that Gibbs proposes to influence groundwater. Soltan’s classification revealed that groundwater within the study area is of deep meteoric type. Ion exchange reveals that groundwater is influenced by mostly silicate weathering. And lastly, evaluation of groundwater seepage in heavy metals (metals loads versus pH) revealed that heavy metals were classified under acid, high metal, near-neutral high metal, acid low metal, and near-neutral low metal. From a detailed study, it was observed that groundwater is influenced by two factors anthropogenic and geogenic activities within the study area.
References
Adeyeye JA, Akinyemi OD, Awomeso JA, Bada BS, Akintan OB (2021) Geo-chemical investigation of groundwater salinity status in selected coastal areas of south western Nigeria. Sustain Water Res Manag. https://doi.org/10.1007/s40899-021-00541-9
Akakuru OC, Akudinobi BEB, Usman AO (2017) Organic and heavy metal assessment of groundwater sources around nigeria national petroleum corperation oil depot Aba, south-eastern nigeria. J Nat Sci Res 7(24):48–58
Akakuru OC, Akudinobi BEB (2018) Qualitative characterization of groundwater sources around nigeria national petroleum corperation oil depot aba, using multiple linear regressions modelling. Int J Adv Geosci 6(1):57–64
Akakuru OC, Akudinobi B, Opara AI, Onyekuru SO (2021) Hydrogeochemical facies and pollution status of groundwater resources of Owerri and environs, Southeastern Nigeria. Environ Monit Assess. https://doi.org/10.1007/s10661-021-09364-9
Akakuru OC, Akudinobi BE, Nwankwoala HO, Akakuru OU, Onyekuru SO (2021b) Compendious evaluation of groundwater in parts of Asaba, Nigeria for agricultural sustainability. Geosci J https://doi.org/10.1007/s12303-021-0010-x
Alsuhaimi AO, Almohaimidi KM, Momani KA (2019) Preliminary assessment for physicochemical quality parameters of groundwater in Oqdus Area, Saudi Arabia. J Saudi Soc Agric Sci 18(1):22–31. https://doi.org/10.1016/j.jssas.2016.12.002
American Public Health Association (APHA) (2012) Standards methods for the examination of water and wastewater, 22nd edn. American Public Health Association (APHA), New York, p 1360
Arumugam K, Elangovan K (2009) Hydrochemical characteristics and groundwater quality assessment in Tirupur Region, Coimbatore District, Tamil Nadu, India. Environ Geol 58:1509–1520. https://doi.org/10.1007/s00254-008-1652-y
Bear J (1979) Hydraulics of groundwater. McGraw-Hill International Book, New York
Chandrasekar N, Selvakumar S, Srinivas Y et al (2013) Hydro-geochemical assessment of groundwater quality along the coastal aquifers of southern Tamil Nadu, India. Environ Earth Sci 71(11):4739–4750. https://doi.org/10.1007/s12665-013-2864-3
Dikinya O, Areola O (2010) Comparative analysis of heavy metal concentration in secondary treated waste irrigated soil cultivated by diferent crops. Int J Environ Sci Technol 7(2):337–346
Ede CO, Nnabo PN (2015) Hydrogeochemical evaluation of groundwater quality of Abakaliki and its environs, South Eastern Nigeria. Int J Innov Sci Res 13(2):689–696
Egbueri JC, Mgbenu CN, Chukwu CN (2019) Investigating the hydrogeochemical processes and quality of water resources in Ojoto and environs using integrated classical methods. Model Earth Syst Environ. https://doi.org/10.1007/s40808-019-00613-y
Etteieb S, Cherif S, Tarhouni J (2017) Hydrochemical assessment of water quality for irrigation: a case study of the Medjerda River in Tunisia. Appl Water Sci. https://doi.org/10.1007/s13201-015-0265-3
Evanko CR, Dzombak, DA (1997) Remediation of metals-contaminated soils and groundwater. Technical evaluation report, pp 4–16
Eyankware MO (2019) Hydrogeochemical assessment of chemical composition of groundwater; a case study of the Aptian-Albian aquifer within sedimentary basin Nigeria. Water Conserv Manag 3(2):1–7
Eyankware MO, Obasi PN (2021) A holistic review of heavy metals in water and soil in Ebonyi SE. Nigeria; with emphasis on its effects on human, aquatic organisms and plants. World News Nat Sci 38:1–19
Eyankware MO, Nnajieze VS, Aleke CG (2018a) Geochemical assessment of water quality for irrigation purpose, in abandoned limestone quarry pit at Nkalagu area, Southern Benue Trough Nigeria. Environ Earth Sci 77:66. https://doi.org/10.1007/s12665-018-7232-x
Eyankware MO, Ogwah C, Okeke GC (2018b) Geochemical evaluation of groundwater origin using source rockdeduction and hydrochemical facies at Umuoghara Mining Area, Lower Benue Trough, SE Nigeria. Int Res J Earth Sci 6(10):1–11
Eyankware MO, Obasi PN, Omo-Irabor OO, Akakuru OC (2020a) Hydrochemical characterization of abandoned quarry and mine water for domestic and irrigation uses in Abakaliki, southeast Nigeria. Model Earth Syst Environ. https://doi.org/10.1007/s40808-020-00827-5
Eyankware MO, Aleke CG, Selemo AOI, Nnabo PN (2020b) Hydrogeochemical studies and suitability assessment of groundwater quality for irrigation at Warri and environs, Niger delta basin, Nigeria. Groundw Sustain Dev. https://doi.org/10.1016/j.gsd.2019.100293
Eyankware MO, Igwe EO, Onwe MO (2021) Geochemical study using geochemical modelling approach in Ojekwe region of southern Benue Trough, Nigeria. Int J Energy Water Resour. https://doi.org/10.1007/s42108-021-00163-z
Eyankware MO, Akakuru CO, Ulakpa ROE, Eyankware EO (2021b) Sustainable management and characterization of groundwater resource in coastal aquifer of Niger Delta Basin Nigeria. Sustain Water Resour Manag. https://doi.org/10.1007/s40899-021-00537-5
Ezeh HN, Anike OL, Egboka BCE (2007) The distribution of some heavy metals in soil around the derelict Enyigba mines and its implications. J Curr World Environ 2:99–106
Ficklin WH, Plumlee GS, Smith KS, McHugh JB (1992) Geo-chemical classification of mine drainages and natural drainages in mineralized areas. In: Kharaka YK, Maest AS (eds) Water–rock interaction: seventh international symposium on water–rock interaction. A.A Balkema, Rotterdam, pp 381–384
Friberg L, Nordberg GF, Vouk VB (eds) (1986) Handbook of the of metals, vol 2. Amsterdam, Elsevier, pp 130–184
Gaillardet J, Dupré B, Louvat P, Allègre CJ (1999) Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem Geol 159:3–30
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170(3962):1088–1090
Harichandan A, PatraS DAK, Sethy KM, Sahu SK, Ghosh M (2021) Suitability of groundwater quality for its drinking and agricultural use near Koira region of Odisha, India. Sustain Water Res Manag. https://doi.org/10.1007/s40899-021-00505-z
Henderson T (1985) Geochemistry of groundwater in two sandstone aquifer systems in the Northern Great Plains in parts of Montana and Wyoming. In: Regional aquifer-system analysis, US Geological Survey professional paper; 1402-C, pp C69–C72.
Ighalo JO, Adeniyi AG (2020) A comprehensive review of water quality monitoring and assessment in Nigeria. Chemosphere. https://doi.org/10.1016/j.chemosphere.2020.127569
Ighalo JO, Adeniyi AG, Adeniran AJ, Ogunniyi S (2020) A systematic literature analysis of the nature and regional distribution of water pollution sources in Nigeria. J Clean Prod. https://doi.org/10.1016/j.jclepro.2020.124566
Igwe EO, Ede CO, Nnabo PN, Ukpai SN (2020) Impact of heavy metals dispersion on water supplies around Oshiri and Ishiagu mine districts of Southern Benue Trough, Nigeria. Model Earth Syst Environ. https://doi.org/10.1007/s40808-020-00950-3
Igwe EO, Ede CO, Eyankware MO (2021) Heavy metals concentration and distribution in soils around Oshiri and Ishiagu lead—zinc mining areas, southeastern Nigeria. World Sci News 158:22–58
Kelepertsis A, Alexakis D, Skordas K (2006) Arsenic, antimony and other toxic elements in the drinking water of Eastern Thessaly in Greece and its possible effects on human health. Environ Geol 50:76–84. https://doi.org/10.1007/s00254-006-0188-2
Li P (2018) Mine water problems and solutions in China. Mine Water Environ. https://doi.org/10.1007/s10230-018-0543
Li XF, Wang PF, Feng CL, Liu DQ, Chen JK, Wu FC (2019) Acute Toxicity and hazardous concentrations of zinc to native freshwater organisms under different pH values in China. Bull Environ Contam Toxicol. https://doi.org/10.1007/s00128-018-2441-2
Meybeck M (1987) Global chemical weathering of surficial rocks estimated from river dissolved leads. Am J Sci 287:401–428
Nnabo PN (2015) Assessment of contamination of underground water sources in Enyigba Pb–Zn district, South Eastern Nigeria using metal enrichment and pollution indices. Int J Sci 4(9):47–56
Nnabo PN (2016) Surface water contamination by heavy metals from Enyigba Pb–Zn mine district, Southeastern Nigeria using metal enrichment and pollution indices. Int J Sci Technol 5(1):10–18
Nnabo PN (2011) Environmental impacts of lead/zinc mining in Enyigba area, SE of Abakaliki, SE Nigeria. Ph.D Thesis, Ebonyi State University, Abakaliki, Nigeria
Obasi PN, Akundinobi BEB (2020) Potential health risk and levels of heavy metals in water resources of lead–zinc mining communities of Abakaliki, southeast Nigeria. Appl Water Sci. https://doi.org/10.1007/s13201-020-01233-z
Obasi PN, MO, Akudinobi BBE, Nweke MO (2015) Hydrochemical investigation of water resources around Mkpuma Ekwaoku Mining District, Ebonyi State Southeastern Nigeria. Afr J Geo Sci Res 3(3):1–7
Obasi AI, Selemo AOI, Ogwah C, Afiukwa JN (2019) Field measurement of radionuclide concentrations in host rocks within some mines in Abakaliki-Ishiagu Areas, Southeastern Nigeria. World Appl Sci J 37(4):275–288
Obasi PN, Eyankware MO, Akudinobi BEB (2021) Characterization and evaluation of the effects of mine dischargeson surface water resources for irrigation: a case study of the Enyigba Mining District, Southeast Nigeria. Appl Water Sci 11:112. https://doi.org/10.1007/s13201-021-01400-w
Obiora SC, Chukwu A, Toteu SF, Davies TC (2016) Assessment of heavy metal contamination in soils around lead (Pb)-zinc (Zn) mining areas in Enyigba, southeastern Nigeria. J Geol Soc India 87:453–462
Ogwah C, Eyankware MO (2020) Investigation of hydrogeochemical processes in groundwater resources located around abandoned Okpara coal mine, Enugu SE. Nigeria. J Clean Was. https://doi.org/10.26480/cleanwas.01.2020.12.16
Okogbue CO, Ukpai NS (2013a) Geochemical evaluation of groundwater quality in Abakaliki area, Southeast Nigeria, Jordan. J Earth Environ Sci 5(1):1–8
Okogbue CO, Ukpai SN (2013b) Evaluation of trace element contents in groundwater in Abakaliki metropolis and around the abandoned mine sites in the southern part, southeastern Nigeria. Environ Earth Sci 70:3351–3362. https://doi.org/10.1007/s12665-013-2401-4
Okolo CC, Oyedotun TDT, Akamigbo FOR (2018) Open cast mining: threat to water quality in rural community of Enyigba in south-eastern Nigeria. Appl Water Sci. https://doi.org/10.1007/s13201-018-0849-9
Omo-Irabor OO, Eyankware MO, Ogwah C (2018) Integration of hydrogeochemical analytical methods and irrigation parameters in determination of groundwater quality at Ibinta, Southern Benue Trough Nigeria. FUPRE J Sci Ind Res 2(1):38–49
Opoke SU, Osayande AD (2018) Physicochemical, major anions and heavy metals in surface water and groundwater at Pb–Zn mining areas of Ebonyi State, Nigeria. J Appl Sci Environ Manag 22(7):1017–1020. https://doi.org/10.4314/jasem.v22i7.3
Rao NS, Rao GK (1991) Groundwater quality in Visakhapatnam urban area, Andhra Pradesh. Indian J Environ Health 33(1):25–30
Ravikumar P, Somashekar RK (2015) Principal component analysis and hydrochemical facies characterization to evaluate groundwater quality in Varahi river basin, Karnataka state, India. Appl Water Sci. https://doi.org/10.1007/s13201-015-0287-x
Sha L, Shuguang L, Chaomeng D, An T, Bo T, Gangfeng M, Roman SC, Sergey RC (2017) Heavy metal distribution and groundwater quality assessment for a coastal area on a Chinese Island. Pol J Environ Stud 26(2):733–745
Simsek C (2008) Assessment of natural radioactivity in aquifer medium bearing uranium ores in Koprubasi, Turkey. Environ Geol 55:1637–1646. https://doi.org/10.1007/s00254-007-1113-z
Skeppstrom K, Olofsson B (2007) Uranium and radon in groundwater. An overview of the problem. Eur Water 17(18):51–62
Soltan ME (1999) Evaluation of groundwater quality in Dakhla Oasis (Egyptian Western Desert). Environ Monit Assess 57:157–168
World Health Organization (2011) Guidelines for drinking-water quality [electronic resource]: incorporating 1st and 2nd addenda, recommendations, vol 1, 5th edn. WHO, Geneva, p 668
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Eyankware, M.O., Akakuru, O.C. & Eyankware, E.O. Interpretation of hydrochemical data using various geochemical models: a case study of Enyigba mining district of Abakaliki, Ebonyi State, SE Nigeria. Sustain. Water Resour. Manag. 8, 33 (2022). https://doi.org/10.1007/s40899-022-00613-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40899-022-00613-4