Abstract
We assessed the quality status of surface and ground waters used for drinking in Ikwo, Southeast Nigeria, using the physicochemical and bacteriological qualities, and antimicrobial susceptibility profiles following standard procedures. Overall. the mean values of the physicochemical qualities of the water samples revealed the following: colour (brown/dark-brown/colourless), odour (objectionable), temperature (28–30 °C), pH (6.30–7.50), electrical conductivity (12.94–12.99 µs cm−1), total dissolved solids (7.74–7.80 mg L−1), alkalinity (0.3–1.4 mg L−1), hardness (19–252 mg L−1), chloride (0.8–3.1 mg L−1), copper (0.01–0.72 mg L−1) and zinc (0.03–1.49 mg L−1). Only nitrate was not detected all through. Likewise, total heterotroph, coliform and Escherichia coli counts ranged from 1.16 to 6.96 × 103 cfu mL−1, 150 to 2400 MPN/100 mL and 45 to 345 CFU/100 mL, respectively. The antimicrobial susceptibility profiles showed that the E. coli isolates were relatively susceptible to gentamycin (58%) and levofloxacin (50%), with high percentages of the isolates displaying resistance against tetracycline (78%), norfloxacin (76%), nalidixic acid (76%), augmentin (68%), ampiclox (62%), doxycycline (62%) and amoxil (52%). While the physicochemical parameters were generally within the permissible limits of the WHO guidelines, reverse is the case for the bacteriological loads. Exceedance of bacteriological water quality criteria and prevalence of multidrug-resistant E. coli indicate high levels of microbial and drug pollutants in the waters, rendering them unfit for direct human ingestion without proper pretreatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water, the most abundant resource on earth, occupies about three quarters of the earth crust. It is essential for life’s sustenance, and almost all activities carried out by man spanning domestic, industrial, agricultural, recreational, transportation, commercial to energy purposes. It constitutes about 70% of the human body, and vital for physiological processes carried out daily by the body. This has promoted the continuous evaluation of water against future degradation that may affect the human body. The different uses of water and its great importance have resulted in great emphasis on its quality and ability to sustain its various usages (Vandas et al. 2002; Iskandar 2010).
Water is said to be polluted when it does not exist in its normal state of colourless, odourless, tasteless and sparking form. Running and stagnant surface waters on the earth’s surface or underground water beneath are exposed to contaminations by septic tanks, leakage of excreta, wastewaters, wastewaters from abattoirs and treatment plants, decontamination stations, hospitals, industrial and agricultural wastes (Ibe and Okplenye 2005; Ngele et al. 2014). Pollutions originating from these sources raise the levels of turbidity, suspended solids, biological oxygen demand, microbial pathogens and parasites in waters bodies (Figueras and Borrego 2010; Ichor et al. 2014). Ground waters including wells and boreholes are presumed to be the safest and purest sources of drinking water when compared to surface waters. They are found at approximately 100 m below the earth’s surface, resulting from seepage of water down into the ground and stored between soil particles, crevices or cracks in rocks wells (Ibe and Okplenye 2005).
The excessive demand for quality drinking water and other purposes have led people to source water from every available fount, especially in some developing regions where constant supply of safe water is lacking. Microbial pollution by animal and human feces is commonly used for considering water unfit for drinking due to the possibility of presence of pathogens. Water contamination is of global challenge affecting both developed and developing nations. Globally, 88% of diarrheal diseases results from ingestion of contaminated water and about 1.1 billion people of the world populations mainly from developing countries are exposed to unsafe drinking water, making about 250 Mio. people prone to water-borne diseases, and in turn leading to 10–20 Mio. mortality annually (Sarantuya et al. 2004; Kassa 2009). Recently, most of the outbreaks of diarrheal diseases have been traced to consumption of contaminated water worldwide. Therefore, determining the sanitary quality of drinking water through physicochemical and microbiological assessments remains a paramount way of monitoring the safety of the water, thereby protecting against illnesses and other diseases spread (Figueras and Borrego 2010).
Antimicrobials have been effectively used in the treatment of infectious diseases, especially those that have resulted from water-borne infections, but abuse due to improper antimicrobial therapy for an infection has resulted in increased resistance over time. Antimicrobial resistance affects health polices because of the increasing number of emerging and re-emerging bacterial species with changing patterns of resistance mechanisms that have been identified. Water-borne outbreaks attributable to high prevalence of multidrug resistant isolates have been documented (Ibiene et al. 2011; Titilawo et al. 2015b; Osunla and Okoh 2017). Despite increasing government and non-governmental interventions, the main drinking water supplies in many rural communities of Nigeria remain rain, surface and underground waters. However, biochemical pollutants from point and non-point sources could contaminate these sources (Amin et al. 2012).
Ikwo, like every other rural community, is faced with the challenge of getting safe water supplies for drinking and other domestic purposes. The people obtain their waters from the available sources including streams, rivers, boreholes and wells, without bearing in mind the risks of diseases associated with drinking from such contaminated sources. Previous investigations have emphasized on the physicochemical, bacteriological, heavy metal and nutrient loads of some rivers, ground waters, streams and springs across the region (Afiukwa and Eboatu 2013; Itumoh et al. 2013; Ngele et al. 2014; Omaka et al. 2014; Ani et al. 2016). However, to our knowledge, none included Ikwo community, and it is in this light that the present study investigated the physicochemical and bacteriological qualities of selected surface and ground waters used by Ikwo rural dwellers of Ebonyi State, Southeastern Nigeria, and also elucidated the antimicrobial susceptibility profiles of Escherichia coli isolates from the waters.
Materials and methods
Description of the study area
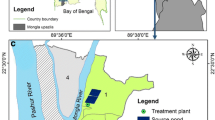
Ikwo (6° 03′ 11.38ʺ N; 8° 09′ 46.22ʺ E) is the largest Local Government Area in Ebonyi State, Souheastern Nigeria. The region borders with Abakaliki, Izzi and Ezza Local Government Areas as well as Cross River State. It plays host to few tertiary institutions of learning in the State. Its land area is approximately 500 km2 with a population of 359,000 (NPC 2006). The predominant occupation of the people is farming and the area is also rich in mineral resources. While Fig. 1 below presents the selected surface and ground water sampling locations, Table 1 highlights the locations and activities therein.
Sterilization of containers and collection of water samples
Detergent was used to wash the containers after which they were thoroughly rinsed and air-dried. The mouths of the containers were cleaned with cotton wool soaked with ethanol prior to collection of the water samples. Five (5) each of surface and ground water samples were collected from the selected locations over a 3-month sampling regime (April–June 2016). Surface water sample bottles were dipped into the water, and then allowed for little quantity of water to enter the sample containers to rinse the bottle after which they were dipped again into the sources of water to be collected. For the ground water samples, the mouths of the taps (hand pumps) were cleaned with cotton wool soaked with ethanol and flamed prior to sample collection. For convenience sake, surface water samples were labeled SW1–SW5 and ground waters GW1–GW5. Samples were placed on ice packs in portable cooler boxes and transported to the laboratory for analysis.
Physicochemical analysis
Colour and odour tests were performed using the sense organs as appropriate. Exactly, 100 mL of each water sample was put in a clean beaker, and then shaken vigorously for the appearance of frothing and allowed to settle. The beaker was then brought near bright light and observed for particulate matter. The beaker was also brought close to the nose for probable objectionable odour. While temperature, pH and conductivity were measured on site, total dissolved solids, alkalinity, hardness, nitrate, chloride, zinc and copper were carried out in the laboratory, following standard procedures (APHA 2012).
Bacteriological analysis
Heterotrophic bacteria, total coliforms and E. coli enumeration
While the total heterotrophic bacteria plate counts (HPC) were performed following the standard pour plate method, coliform counts were enumerated using multiple tube fermentation technique. In the former approach, water samples were serially diluted with sterile distilled water and then the third diluent (10–3) was inoculated into the petri dish and the medium cooled to 45 °C was poured into the petri dish and swirled gently. A positive plate was indicated by different forms of colony growth. After 24 h of incubation, the numbers of bacterial colony were counted and expressed as CFU mL−1. Similarly, the total coliforms were estimated using the standard most probable number (MPN) technique. This was achieved using multiple 5 × 5 tube regimen with MacConkey broth medium. The bottles were incubated and observed at the end of 24 and 48 h for presumptive and confirmatory tests, respectively. The coliforms, indicated by colour change and gas formation, were counted, estimated following the MPN interpretative chart and recorded as MPN/100 mL (USEPA 2009; Usharani et al. 2010).
In the same vein, isolation and enumeration of E. coli were performed using the membrane filtration technique. The filtration apparatus was sterilized before use and re-sterilized between samples using ethanol. In this procedure, 100 mL of the water samples was filtered through a 90-mm diameter, 0.45-μm pore-sized membrane filter papers (Millipore, Ireland). Using sterile forceps, the membrane filters were removed from the filtration cup, transferred to the petri dishes containing E. coli chromogenic medium (Conda Pronadisa, Spain) and incubated overnight at 44 °C. After incubation, characteristic blue colonies were enumerated and expressed as CFU/100 mL before storing on 50% glycerol for further analysis.
Antimicrobial susceptibility profiles of E. coli isolates
Exactly, 5 E. coli isolates from each sampling location, making a total of 50 were pooled together for the test, following the standardized Kirby–Bauer disk diffusion assay (Kirby-Bauer et al. 1966; CLSI 2015). Using a sterile loop, few colonies of overnight pure cultures were transferred to a micro-centrifuge tube containing 2 mL of 0.85% physiological sterile saline and gently vortexed for the preparation of inocula needed for the test. The turbidity of the resulting solution was adjusted to 0.5 McFarland standard solution. One hundred microliters of the suspension were then spread plated onto Mueller Hinton agar plates and left to dry for 15 min before placing antibiotic discs on them. A panel of 14 antibiotic multi-discs, comprising ciprofloxacin (10 µg), norfloxacin (10 µg), levofloxacin (10 µg), nalidixic acid (30 µg), streptomycin (30 µg), gentamycin (10 µg), erythromycin (30 µg), rifampicin (30 µg), chloramphenicol (30 µg), amoxil (20 µg), ampiclox (20 µg), augmentin (30 µg), tetracycline (10 µg) and doxycycline (10 µg) were employed. The plates were left on the bench for 30 min for antibiotic diffusion, then inverted and incubated at 37 °C for 24 h. After incubation, the zones of inhibition were observed, recorded and interpreted following the recommendations of Clinical and Laboratory Standards Institute (CLSI 2015).
Multiple antibiotics resistance phenotypes and indexing
Multi-antibiotic-resistant phenotypes (MARPs) for each sampling location were mapped out for isolates that showed resistance to three or more antimicrobials as previously described (Wose et al. 2010; Titilawo et al. 2015a). Likewise, the multiple antibiotic resistance indexing (MARI) was estimated using the mathematical expression:
where ‘a’ represents the number of antibiotics to which the isolate was resistant and ‘b’ the total number of antibiotics against which an individual isolate was tested (Blasco et al. 2008; Titilawo et al. 2015a).
Data analysis
Data analysis was conducted using the statistical package for social science (SPSS) software (version 24). Pearson correlations (r) analysis was performed to detect the possible correlations between the physicochemical and bacteriological qualities across all sampling locations. The one-way analysis of variance (ANOVA) was performed to determine variations in the antimicrobial susceptibilities and resistances against the isolates with respect to studied locations. Statistical significant level was set at P < 0.05.
Results and discussion
Physiochemical quality of the water samples
Water quality is measured by its physical and chemical characteristics (Rajeshwari and Saraswathi 2009). Of the 5 surface water samples, 2 (SW1 and SW4) were dark brown and 3 (SW2, SW3 and SW5) were brown. While 3 of the ground waters (GW2, GW3 and GW4) were colourless, 2 (GW1 and GW5) were brown. The results for odour showed that all the water samples were objectionable (Table 2). Generally, odours of the surface waters were observed to be more intense than the ground counterparts. Water meant for drinking should be clear and odourless (WHO 2008). The presence of colour and odour in water samples can be linked to the existence of organic, inorganic and biological contaminants in the water environments (Istifanus et al. 2013). A change in the colour, taste or odour of waters could indicate a public health concern. Hence, any water that looks, smells or tastes objectionable is not recommended for drinking (Washington Department of State 2018).
Temperature is one of the major physical and ecological factors with a strong impact on both the living and non-living components of the environment, thereby influencing organisms and the functioning of an ecosystem. Generally, it influences the quality of physicochemical and biological properties of water (Palamuleni and Akoth 2015). In the present study, the mean highest temperatures (30 °C) were obtained at SW1, SW2, SW3, GW4 and GW5, whereas the mean lowest (28 °C) was recorded at GW3. The observed temperature range (28–30 °C) falls within the acceptable limit for human consumption and aquatic life’s sustenance, and comparable to previous reports (Okonko et al. 2008; Dami et al. 2012; Ani et al. 2016). The fairly moderate temperatures noticed in our ground water samples was not surprising since they are stored underground with a relatively constant temperature throughout the year (Sundaram et al. 2009).
Hydrogen ion concentration (pH) of water is essential because many biological activities can occur only within a specific range; thus, any variation beyond the acceptable limit could be lethal to a particular organism (Trivede et al. 2010). The mean pH of the surface water samples analyzed was found within the range of 6.30–7.30, whereas it was between 7.00 and 7.50 for the ground waters. The peak value (7.50) was obtained at GW3 and the least (6.30) at SW4 (Table 2). Generally, most of the observed pH values fell within the European Union tolerance limit (between 6 and 9) in water meant for domestic use, recreational purpose and aquatic life (Chapman 1996). Similar pH ranges were obtained for borehole water samples (Eniola et al. 2007). The general drop in pH in some of the surface water samples could be linked to the reduced water volume and higher CO2 levels (Chigor et al. 2012). While the acidic pH could consequently result in gastrointestinal disorders such as ulcer, hyperacidity and burning sensations (Kalyana and Rupa 2014), those near neutrality could also allow the growth of most bacterial species (Ugbaja and Otokunefor 2015).
Electrical conductivity (EC) reflects the status of inorganic pollution and considered to be a good indicator for evaluating total dissolved solids and ionized species in water (WHO 2011; APHA 2012). In the current study, the mean ECs for all locations varied from 12.94–12.99 and 12.95–12.99 µs cm−1 in the surface and ground waters, respectively (Table 2), and extremely lower than the standard drinking water guideline of 250 µS cm−1 (WHO 2011). Hence, they were all within the limit of no risk. The studied water bodies are less impacted by sewage disposal, explaining the reason for more or less slowly and steadily same electrical conductance. Previous investigations indicate that sewage disposal and urban surface run-off tend to heighten the EC levels of the receiving water bodies due to the high presence of salts and ions in the sewage (Igbinosa and Okoh 2009; Suthar et al. 2010; Sibanda et al. 2013). Contrary to our findings were higher conductivities reported in other studies conducted in the developing world regions of India (Choudhory et al. 2011), Ethiopia (Tessema et al. 2014), Eritrea (Tesfamariam and Younis 2016), Iraq (Ewaid and Abed 2017) and South Africa (Zamxaka et al. 2004). High EC levels can be detrimental to aquatic life because of increased salinity in the stream and possible suffocation of the stream bottom, especially in the case of deep stream (DWAF 1996).
Total dissolved solids (TDS) is the sum total of all of the dissolved substances including hardness, alkalinity, chlorides, bromides, sulphates, silicates and other organic compounds, in a given water body (Sibanda et al. 2013). In the present investigation, both the surface and ground waters showed a similar trend, with the mean TDS concentrations ranging from 7.74 to 7.80 mg L−1 at GW1 and GW5, respectively (Table 2). Water having more than 500 mg L−1 is unpalatable for consumption (WHO 2011). The TDS of water samples were exceptionally lower than the desirable limit. The low level of occurrence of TDS may be likely due to less influence of domestic wastewater and industrial activities in the sparsely populated Ikwo region. The TDS levels obtained in our study concur with those reported elsewhere (Jindal and Sharma 2010; Gupta et al. 2017) and disagree with some investigations conducted previously (Ewaid and Abed 2017; Kora et al. 2017). High TDS values cause harmful effects such as severe central nervous system stimulation, irritations, dizziness and paralysis of the tongue, lips and face. The presence of synthetic organic chemicals even in low concentrations imparts offensive tastes, odours and colours to aquatic lives (Chang 2005).
Alkalinity in natural waters is owing to free hydroxyl ion and salt hydrolysis formed by weak acid and strong bases, and also to salt containing carbonates and bicarbonates or silicate and phosphate (Shinde et al. 2011; Ewaid and Abed 2017). The mean alkalinity values ranged from 0.3 to 0.6 mg L−1 in the surface waters; whereas, a slight increase was noticed in the ground waters varying from 0.8 to 1.4 mg L−1 (Table 2). The ten sites evaluated showed much lower values than the 200 mg L−1 WHO allowable limits for drinking water. Hence, no health consequence has been attributable to such alkalinity levels. On the other hand, low alkalinity values obtained in our study could be attributable to heavy downpour dilutions (Shinde et al. 2011). Our data exceeded those recently reported elsewhere (Taiwo et al. 2014; Ladokun and Oni 2015; Olorode et al. 2015). In the case of total hardness, the mean concentrations of surface water samples ranged from 186 to 252 mg L−1; whereas, ground water values varied from 19 to 31 mg L−1 and were below the highest desirable level of 500 mg L−1 recommended by the WHO. Our findings conform with previous investigations (Afiukwa and Eboatu 2013; Ukpong and Okon 2013; Omaka et al. 2014).
Nitrates (NO3−) are highly soluble in water and moveable in the environment, with high potentials for dissolving in runoff and flowing surface water and streams during rainfall events (Blanchard and Lerch 2000; Brainwood et al. 2004; Gutiérrez-Lucas et al. 2017). They are relatively non-toxic and normal individuals have low methemoglobin level (0.5–2.0%) in their blood (EPA 2007). Amazingly, nitrate was not detected in all the water samples analyzed (Table 2), therefore appearing not to be a serious water quality challenge. It was subsequently excluded from further analysis including the correlation test. Otherwise, the statistical tool would not compute it. All samples evaluated fell below the WHO 10 mg L−1 allowable limits and strongly tally with zero nitrate result of Ukpong and Okon (2013), and also with those reported to be within the acceptable level (Gutiérrez-Lucas et al. 2017; Ugbaja and Otokunefor 2015). Normally, oxidation of ammonia form of nitrogen from animal and human excreta to nitrite is a possible way by which nitrate enters into the ground water aquifer (Gerba and Smith 2005). Elevated concentrations of nitrate may cause eutrophication, methemoglobinemia and carcinogenic diseases (Jain et al. 2010). Chloride, being one of the major anions, is usually present in appreciable amounts in all natural waters. High level of chloride ions may cause impaired taste and corrosion of metal pipes (Choudhury et al. 2016). In the present study, the mean chloride levels ranged from 0.8 to 3.1 mg L−1 (Table 2). According to WHO, the level of chlorides in drinking water must not exceed 250 mg L−1 and this agrees with the chloride content of our samples. Higher concentration of chloride in surface than ground waters obtained in this study could be due to heavy influence by urine and feces of humans, domestic sewage and run off (WHO 2008).
Copper enters surface and ground water environments through industrial metal plating, domestic wastes and mineral leaching. It is an essential nutrient and a water pollutant required by the body for producing erythrocytes and strengthening immunity (Edward et al. 2013; USSG 2015; Vetrimurugan et al. 2017). In this study, the mean copper values ranged from 0.02 to 0.72 mg L−1 in the surface waters at SW5 and SW3, respectively, and 0.01–0.24 at GW3 and GW1 in the ground water, respectively (Table 2). The observed values fell below the WHO set limit of 2 mg L−1, an implication of no possibility of health threat associated with the metal (WHO 2008). The finding agrees with previous report on some metal contaminants (copper in this case) in the freshwater environments of Osun State, Southwest Nigeria (Titilawo et al. 2018). Highest mean concentration (0.72 mg L−1) was obtained at SW3, a location predominated by agricultural activities, thereby suggesting the possible influence of agrochemical run-offs from farms on the level of water contamination in the environment (Dawaki et al. 2013). Copper levels above 5 mg L−1 can impair the taste of water; whereas, concentration higher than 1 mg L−1 can result in the stain of sanitary wares and laundry materials (WHO 2008). The levels recorded in the studied locations could be as a result of corrosion of hand pump borehole metals, plumbing pipes in the homes, wearing away of natural deposits and accumulation of agricultural and domestic wastes (Idoko et al. 2012; Edward et al. 2013). High doses of copper cause detrimental effects such as irritations of nose, mouth and eyes, nausea, vomiting and diarrhea. While short-term exposure causes gastrointestinal distress, long-term exposure damages liver and kidney (Idoko et al. 2012; USEPA 2012; Edward et al. 2013; Vetrimurugan et al. 2017).
Zinc, an essential trace element for living organisms, is one of the highly mobile heavy metals in surface and ground waters because it is present as soluble compounds both at neutral and acidic pH. It enters aquatic milieus from sludge, mineral ores, industrial wastes, metal plating and plumbing. It aids the development of embryo, reproductive organs, normal body metabolism, immunity against diseases and wounds healing. Its deficiency may lead to low fertility, loss of taste, stunted growth and diminished sex hormones (Edward et al. 2013; USGS 2015; Vetrimurugan et al. 2017). In the present investigation, surface water zinc level was highest at SW3 (1.49 mg L−1) and lowest at SW2 (0.03 mg L−1); whereas, the levels of ground waters ranged from 0.04 to 0.09 mg L−1 (Table 2). They both fell within the WHO approved standard of 5 mg L−1 for drinking water (Oyekunle et al. 2011; Daso and Osibanjo 2012; USEPA 2012; Titilawo et al. 2018). Although zinc causes no ill health effects, but in very high doses, imparts undesirable tastes to water and becomes toxic to plants (WHO 2008; Idoko et al. 2012; USGS 2015).
Bacteriological analysis of the water samples
Total coliform (TC) and Escherichia coli are among the major indicators used to measure pathogenic pollution of waters (WHO 2000; Chen et al. 2015). Table 3 shows the mean bacteriological counts obtained in the surface and ground water samples. The mean total heterotrophic bacteria plate counts (HPC) of surface waters ranged from 1.48 to 6.96 × 103 CFU mL−1; whereas, the ground waters ranged between 1.16 and 6.24 × 103 CFU mL−1. For the total coliforms, mean counts were highest (2400 MPN/100 mL) at SW1, SW3 and SW5, and lowest at SW4 with 290 MPN/100 mL. Likewise, highest counts (460 MPN/100 mL) were obtained at GW4 and GW5, trailed by the least (150 MPN/100 mL) at GW1. While the mean E. coli counts ranged from 19 to 345 CFU/100 mL for surface waters, ground waters had 45–123 CFU/100 mL (Table 3). Total HPC has no health effect, as it measures variety of bacteria naturally present in the water environment. The lower the concentration of bacteria in drinking water, the better the water system management (USEPA 2012). However, treatment techniques should be designed towards regulating HPC concentrations in surface and ground waters to ˂ 500 CFU mL−1, taking cognizance of the fact that it is not a health-based standard though, but reflects the concern that at concentrations > 500 CFU mL−1, can interfere with total coliform and E. coli recovery methods (USEPA 2012).
Total coliforms counts are used to determine the level of domestic pollution in water samples. The relatively higher TC counts in surface than ground waters coincides with observations of Chandran et al. (2008). High coliform counts obtained in our study could be traced to the boreholes near pit latrines and open defecations within and around the locations. Other factors may include temperature rise, soils, biofilms, depths, and rusty pipes and electrical pumps (Palamuleni and Akoth 2015). Our coliform and E. coli counts obtained fell outside the WHO threshold limits and agree with the studies conducted elsewhere (Obi and Okocha 2007; Kassa 2009; Omari and Yeboah-Manu 2012; Uzoigwe and Agwa 2012; Ukpong and Okon 2013; Ugbaja and Otokunefor 2015).
Overall, with reference to the bacteriological status investigated, the entire water samples were found to be contaminated, with counts higher than the prescribed criteria. Ideally, potable waters should be ≤ 10 CFU/100 mL for total coliforms and devoid of E. coli in any given sample (WHO 2008). The data evidently point that excreta of animal and human origin mainly cause the bacterial proliferation in the water samples. Thus, the waters poses potential public health hazards and cannot be utilized for recreational purpose, let alone human consumption (Usharani et al. 2010). It has been generally established that higher levels of sewage contamination would concomitantly lead to increased number of coliforms in natural water bodies. Similar observations were reported in earlier studies (Sood et al. 2008; Lee et al. 2013; Rajkumar and Sharma 2013). The detection of E. coli in the water is an indicator of fecal contamination whose presence suggests the likelihood of the presence of other pathogens. High level of microbial pollution in river water is also a common challenge in urban and rural settings that often leads to occurrences of severe water- and food-borne diseases including gastroenteritis, typhoid fever, cholera and diarrhea (Chigor et al. 2012; Omalu.et al. 2012 Rajkumar and Sharma 2013).
Pearson correlation analysis
Table 4 shows the correlation coefficient matrix between the various physicochemical and microbiological qualities. At the 95% confidence limit, level of EC was significantly correlated with HPC (r = 0.44) and Cu was positively associated with Cl− (r = 0.36) and TC (r = 0.41). Only TA was found negatively correlated with TC (r = − 0.43). The finding is consistent with the reports of correlation of EC with the microbial indicators of fecal pollution studied elsewhere (Venkatesharaju et al. 2010; Liang et al. 2013). In the same vein, some highly significant positive correlations were observed at the 99% confidence limit. The TA was found positively correlated with TH (r = 0.69), Cl− with TC (r = 0.69), Cu with Zn (r = 0.67) and HPC (r = 0.46). Also, Zn showed moderate positive correlations with HPC (r = 0.49) and TC (r = 0.56) (Table 4). Conversely, some significant negative correlations were equally noted. The TH showed negative correlations with Cl− (r = − 0.58) and TC (r = − 0.61). Similarly, there existed moderate negative significant correlations between TA and Cl− (r = − 0.47) (Table 4). The TH positive significance with TA agrees with Olanrewaju et al. (2017). Metals, especially zinc generally correlates positively with E. coli concentrations, suggesting that metals and E. coli might have similar sources and causes, with stormwater runoff as the main source (Daly et al. 2013). The positively correlated metals might have originated from common sources and could displace each other in their environments and matrices possibly (Oyekunle et al. 2011). However, those that were negatively correlated could oppose one another in functions, or may contribute to the reduction in concentrations of each other in that milieu and may not depend on each other for effective operations (Dawaki et al. 2013; Olawusi-Peters et al. 2015).
No apparent correlation was noticeable among temperature, pH, TDS and E. coli in our analysis. Usharani et al. (2010) also recorded no obvious correlation between the fecal bacteria and physicochemical parameters surveyed. Although, no significant correlation existed between temperature, pH and TDS in the current investigation, yet the effect of temperature on water parameters by speeding up chemical reactions and reducing the solubility of gases cannot be overemphasized (Jonnalagadda and Mhere 2001). Similarly, the pH of an aquatic system is of vital importance of water quality indicator and the extent of pollution in the watershed area. The correlations between the physicochemical and bacteriological parameters agree with previous findings (Chigor et al. 2012; Sibanda et al. 2013), and certify the use of bacterial density as the basic criterion for the quality assessment of freshwaters. Correlation coefficients help in choosing treatments to halt contaminants in surface and ground waters for drinking and domestic purposes to safeguard the health of the consumers (Achuthan et al. 2005; Venkatesharaju et al. 2010).
Antibiotics susceptibility profiles
Water, being one of the most important microbial earth’s habitats, is a major means of disseminating microbes in nature and has been widely recognized as a significant reservoir of antibiotic resistance (Zhang et al. 2009; Rizzo et al. 2013). In our study, a total of 50 E. coli isolates, 5 each from surface and ground waters were profiled for their susceptibility against 14 conventionally employed antibiotics and the results are presented in Fig. 2. The E. coli isolates were relatively susceptible to gentamycin (58%) and levofloxacin (50%). Conversely, high percentages of the E. coli isolates were resistant against tetracycline (78%), norfloxacin (76%), nalidixic acid (76%), augmentin (68%), ampiclox (62%), doxycycline (62%) and amoxil (52%). Generally, ground water isolates exhibited higher percentage susceptibilities than the surface water isolates and vice versa for the resistances (Fig. 2). Our data are congruent with previous investigations on E. coli from freshwaters showing resistance against several antibiotics in the geographical districts of United States (Sayah et al. 2005), Poland (Osińska et al. 2017), China (Hu et al. 2008), India (Abhirosh et al. 2011), South Africa (Olaniran et al. 2009) and Nigeria (Titilawo et al. 2015b). Recently, multi-resistant E. coli to tetracycline and β-lactams have ben concurrently reported among drinking water isolates in Hangzhou city of China (Chen et al. 2017). In addition, resistance observed by the E. coli isolates to tetracycline and chloramphenicol was not surprising as these antibiotics have been of old and broadly used in human and veterinary medicines (Jiang et al. 2013; Maal-Bared et al. 2013; Pereira et al. 2013).
Susceptibility profiles of surface and ground water E. coli isolates against selected antimicrobials (n = 50). CPX ciprofloxacin, NB norfloxacin, LEV levofloxacin, NA Nalidixic acid, S streptomycin, CN gentamycin, E erythromycin, RD rifampicin, CH chloramphenicol, AMX amoxil, APX ampiclox, AU augmentin, T tetracycline, DXT doxycycline, GWSEC ground water-susceptible E. coli, SWSEC surface water-susceptible E. coli, GWIEC ground water-intermediate E. coli, SWIEC surface water-intermediate E. coli, GWREC ground water-resistant E. coli, SWREC surface water-resistant E. coli
The differences in the distribution of antimicrobial-resistant E. coli and sampling sites were analyzed statistically. The one-way ANOVA revealed no significant difference between E. coli susceptibility and resistance profiles across the sampling locations (P > 0.05). This concurs with the observation of Titilawo et al. (2015a) that the differences in resistance patterns of their E. coli isolates to selected antimicrobials were not statistically significant in relation to studied locations. Similar trends were previously reported among bacteria resistances to aminoglycosides, beta-lactams and quinolones (Amos et al. 2014).
Estimation of multiple antibiotic resistance phenotypes and indexes (MARPs and MARI)
According to EUCAST (2009), multidrug resistance is defined as an acquired non-susceptibility to at least one agent in three or more antimicrobial families. Table 5 reveals the multidrug resistance (MDR) patterns of the 50 E. coli isolates. The MARPs and MARI were determined for all the isolates derived from each site. Overall results showed that the isolates displayed resistance ranging from five (5) to eleven (11) antibiotics of the fourteen (14) antibiotics tested. The MDR resistance patterns observed among the surface and ground water isolates were in the order: five-drug (6), six-drug (11), seven-drug (11), eight-drug (11), nine-drug (6) and eleven-drug (5) resistances. Surprisingly, none had ten-drug resistance (Table 5). In the same vein, the multiple antibiotics resistance (MAR) index of isolates was evaluated. Data obtained showed that surface water isolates exhibited MAR index ranging from 0.36 to 0.79; whereas, the ground water isolates ranged from 0.36 to 0.64, both exceeding the permissible limit of 0.2, an indicative of high-risk contamination sources (Table 5). These findings corroborate previous investigations on the distribution of multidrug resistant E. coli isolates from surface waters conducted elsewhere (Akturk et al. 2012; Titilawo et al. 2015a; Zhang et al. 2015).
Conclusions
Water sources for human consumption are expected to be free from objectionable physical, chemical and microbial contaminants. The present study assessed the physicochemical and microbiological qualities of waters consumed by the inhabitants of Ikwo rural environment of Ebonyi state, Southeast Nigeria. Antimicrobial susceptibility of E. coli recovered from the waters was also profiled. The water sources complied with the physicochemical quality prescribed limits of no risk but exceeded the bacteriological drinking water quality evaluation criteria. The antimicrobial susceptibility profiles showed more E. coli isolates’ sensitivities to gentamycin, rifampicin and levofloxacin, suggesting that these antibiotics could be effectively used for the treatment of diseases associated with ingestion of contaminated waters. In the contrary, the resistance profiles of E. coli revealed high level of multi-resistance. While the MARPs showed resistance ranging from 5 to 11 antibiotics, MARI ranged from 0.36 to 0.79 exceeding the threshold limit of 0.2, indicating high-risk contamination sources and potentially posing a public health threat. Hence, the study advocates provision of safe drinking water, cleanliness and hygiene, and mobilization of onsite treatment interventions of drinking waters before ingestion to safeguard the households from possible occurrence of microbial water-borne related diseases. Also, strategic monitoring, awareness and surveillance approaches should be encouraged to protect the waters from further contamination. There is equally the need to educate the populace on the inappropriate and excessive usage of antibiotics as it may contribute to increased occurrence of antimicrobial resistance.
References
Abhirosh, C., Sherin, V., Thomas, A. P., Hatha, A. A. M., & Mazumder, A. (2011). Potential public health significance of faecal contamination and multidrug-resistant Escherichia coli and Salmonella serotypes in a lake in India. Public Health, 125, 377–379.
Achuthan, N. G., Abdullah, I. M., & Mahamoud, M. F. (2005). Physiochemical parameters and correlation coefficients of ground waters of North-East Libya. Pollution Research, 24, 1–6.
Afiukwa, J. N., & Eboatu, A. N. (2013). Analysis of spring water quality in Ebonyi South Zone and its health impact. American Journal of Scientific and Industrial Research, 4(2), 231–237.
Akturk, S., Dincer, S., & Toroglu, S. (2012). Determination of microbial quality and plasmid mediated multidrug resistant bacteria in fountain drinking water sources in Turkey. Journal of Environment Mabel Varghese Sciences, 33, 1127–1136.
American Public Health Association. (2012). Standard methods for the examinations of water and wastewater (22nd ed.). Washington, DC: American Public Health Association.
Amin, R., Ali, S. S., Anwar, Z., & Khattak, J. Z. K. (2012). Microbial analysis of drinking water and water distribution system in new urban Peshawar. Current Research Journal of Biological Science, 4(6), 731–737.
Amos, G. C. A., Zhang, L., Hawkey, P. M., Gaze, W. H., & Wellington, E. M. (2014). Functional metagenomic analysis reveals rivers are a reservoir for diverse antibiotic resistance genes. Veterinary Microbiology, 171, 441–447.
Ani, C., Okogwu, O. I., Nwonumara, G. N., Nwani, C. D., & Nwinyimagu, A. J. (2016). Evaluation of physicochemical parameters of selected rivers in Ebonyi State, Southeast, Nigeria. Greener Journal of Biological Sciences, 6(2), 34–41.
Blanchard, P. E., & Lerch, R. N. (2000). Watershed vulnerability to losses of agricultural chemicals: Interactions of chemistry, hydrology and land-use. Environmental Science and Technology, 34, 3315–3322.
Blasco, M. D., Esteve, C., & Alcaide, E. (2008). Multiresistant waterborne pathogens isolated from water reservoirs and cooling systems. Journal of Applied Microbiology, 105, 469–475.
Brainwood, M. A., Burgin, S., & Maheshwari, B. (2004). Temporal variations in water quality of farm dams: Impacts of land use and water sources. Agricultural Water Management, 70, 151–175.
Chandran, A., Hatha, A. A. M., Varghese, S., & Sheeja, K. M. (2008). Prevalence of multiple drug resistant Escherichia coli serotypes in a tropical estuary, India. Microbes and Environment, 23, 153–158.
Chang, H. (2005). Spatial and temporal variations of water quality in the Han River and its tributaries, Seoul, Korea, 1993–2002. Water, Air and Soil Pollution, 161, 267–284.
Chapman, D. (1996). Water quality assessments: A guide to the use of biota, sediments and water in environmental monitoring (2nd ed.). London: UNESCO, World Health Organization, United Nations Environment Programme.
Chen, Y., Wang, Y., Wei, Y., Zheng, X., & Wang, G. (2015). Evolution and standard comparison of indicator microorganisms for different surface water. Acta Scientiae Circumstantiae, 35, 337–351.
Chen, Z., Yu, D., He, S., Ye, H., Zhang, L., Wen, Y., et al. (2017). Prevalence of antibiotic-resistant Escherichia coli in drinking water sources in Hangzhou City. Frontiers in Microbiology, 8, 1133.
Chigor, V. N., Umoh, V. J., Okuofu, C. A., Ameh, J. B., Igbinosa, E. O., & Okoh, A. I. (2012). Water quality assessment: Surface water sources used for drinking and irrigation in Zaria, Nigeria are a public health hazard. Environmental Monitoring and Assessment, 184, 3389–3400.
Choudhory, R., Rawrani, P., & Vishwakarma, M. (2011). Comparative study of drinking water quality parameters of three manmade reservoirs i.e. Kolar, Kaliasote and Kerwa Dam. Current World Environment, 6(1), 145–149.
Choudhury, S., Keot, A., Das, H., Das, M., Baishya, C., Sarma, A., et al. (2016). Preliminary physicochemical and microbiological analysis of Bahini river water of Guwahati, Assam, India. International Journal of Current Microbiology and Applied Sciences, 2, 684–692.
Clinical and Laboratory Standards Institute. (2015). Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria, M45 (3rd ed.). Wayne: Informational Supplement.
Daly, E., Kolotelo, P., Schang, C., Osborne, C. A., Coleman, R., Deletic, A., et al. (2013). Escherichia coli concentrations and loads in an urbanised catchment: The Yarra River, Australia. Journal of Hydrology, 497, 51–61.
Dami, A., Ayuba, H. K., & Amukali, O. (2012). Physicochemical and bacteriological analysis of the surface water used for domestic purposes in Okpai and Beneku, Delta State, Nigeria. Global Journal of Human Social Science Geography and Environmental Geosciences, 12(13), 31–36.
Daso, A. P., & Osibanjo, O. (2012). Water quality issues in developing countries—A case study of Ibadan Metropolis, Nigeria. Water Quality Monitoring and Assessment. In Tech. ISBN 978-953-51-0486-5. Retrieved June 10, 2016, from https://www.intechopen.com/books/water-quality-monitoring-andassessment/water-quality-issues-in-developing-countries-a-case-study-of-ibadan-metropolis-nigeria.
Dawaki, M. U., Dikko, A. U., Noma, S. S., & Aliyu, U. A. (2013). Pollution as a threat factor to urban food security in metropolitan Kano, Nigeria. Food and Energy Security, 2(1), 20–33.
Department of Water Affairs and Forestry. (1996). South African water quality guidelines, aquatic ecosystems (1st ed., Vol. 7). Pretoria: DWAF.
Edward, J. B., Idowu, E. O., Oso, J. A., & Ibidapo, O. R. (2013). Determination of heavy metal concentration in fish samples, sediment and water from Odo-Ayo River in Ado-Ekiti, Ekiti-State, Nigeria. International Journal of Environmental Monitoring and Analysis., 1(1), 27–33.
Eniola, K. I. T., Obafemi, D. Y., Awe, S. F., Yusuf, I. I., & Falaiye, O. A. (2007). Effects of containers and storage conditions on bacteriological quality of borehole Water. Nigerian Journal of Microbiology, 21, 1578–1585.
Environmental Protection Agency. (2007). Effect of treatment on nutrient availability (pp. 1–45). Retrieved May 24, 2018, from https://www.epa.gov/ogwdw/disinfection/tcr/pdfs/issuepaper_tcr_treatment-nutrients.pdf.
European Committe on Antimicrobial Suceptibility Testing. (2009). European Centre for disease prevention and control/European medicines agency. ECDC/EMEA Joint Technical Report. The Bacterial Challenge: Time to react. ECDC/EMEA Stockholm, Sweden & London, United Kingdom. Retrieved September 11, 2017 from https://www.eucast.org.
Ewaid, S. H., & Abed, S. A. (2017). Water quality index for Al-Gharraf River, Southern Iraq. Egyptian Journal of Aquatic Research, 43, 117–122.
Figueras, M. J., & Borrego, J. J. (2010). New perspectives in monitoring drinking water microbial quality. International Journal of Environmental Research and Public Health, 7, 4179–4202.
Gerba, C. P., & Smith, J. E., Jr. (2005). Sources of pathogenic microorganisms and their fate during land application of wastes. Journal of Environmental Quality, 34, 42–48.
Gupta, N., Pandey, P., & Hussain, J. (2017). Effect of physicochemical and biological parameters on the quality of river water of Narmada, Madhya Pradesh, India. Water Science, 31, 11–23.
Gutiérrez-Lucas, L. R., Chávez-Ramírez, M. L., & Arregui, L. (2017). Physico-chemical and microbiological analysis of water of the “Presa De Los Patos” in the Desierto De Los Leones National Park, Mexico. Advances in Biological Chemistry, 7, 122–138.
Hu, J., Shi, J., Chang, H., Li, D., Yang, M., & Kamagata, Y. (2008). Phenotyping and genotyping of antibiotic-resistant Escherichia coli isolated from a natural river basin. Environmental Science and Technology, 42, 3415–3420.
Ibe, S. N., & Okplenye, J. I. (2005). Bacteriological analysis of borehole water in Uli Nigeria. African Journal of Applied Zoology and Environmental Biology, 7, 116–119.
Ibiene, A. A., Okonko, I. O., & Agbeyi, E. V. (2011). Multidrug resistant (MDR) bacteria isolated from different drinking water sources. New York Science Journal, 4(12), 50–56.
Ichor, T., Umeh, E. U., & Duru, E. E. (2014). Microbial contamination of surface water sources in rural areas of guma local government area of Benue State, Nigeria. Journal of Medical Sciences and Public Health, 2(2), 43–51.
Idoko, O. M., Ologunorisa, T. E., & Okoya, A. A. (2012). Temporal variability of heavy metals concentration in rural groundwater of Benue State, Middle Belt, Nigeria. Journal of Sustainable Development, 5(2), 1–16.
Igbinosa, E. O., & Okoh, I. A. (2009). Impact of discharge wastewater effluents on the physico-chemical qualities of a receiving watershed in a typical rural community. International Journal of Environmental Science and Technology, 6(2), 175–182.
Iskandar, M. B. (2010). The effectiveness of biofilter as a treatment for domestic wastewater. University Malaysia Pahang, thesis.
Istifanus, Y. C., Elisha, K., Ishaku, Z., & Ephraim, D. A. (2013). Physicochemical analysis of ground water of selected areas of Dass and Ganjuwa Local Government Areas, Bauchi State, Nigeria. World Journal of Analytical Chemistry, 1(4), 73–79.
Itumoh, E. J., Uraku, A. J., Omaka, O. N., & Nwabue, F. I. (2013). Trace metal toxicity in our environment: Case studies of influx of metals in soils, crops, waters and air in Ebonyi State. Global Journal of Bioscience and Technology, 2, 33–36.
Jain, C. K., Bandyopadhyay, A., & Bhadra, A. (2010). Assessment of groundwater quality for drinking water purposes, District Nainital, Uttarakhand, India. Environmental Monitoring and Assessment, 166, 663–676.
Jiang, L., Hu, X., Xu, T., Zhang, H., Sheng, D., & Yin, D. (2013). Prevalence of antibiotic resistance genes and their relationship with antibiotics in the Huangpu River and the drinking water sources, Shanghai, China. Science of the Total Environment, 458–460, 267–272.
Jindal, R., & Sharma, C. (2010). Studies on water quality of Sutlej River around Ludhiana with reference to physicochemical parameters. Environmental Monitoring and Assessment, 174, 417–425.
Jonnalagadda, S. B., & Mhere, G. (2001). Water quality of the Odzi River in the eastern highlands of Zimbabwe. Water Research, 35, 2371–2376.
Kalyana, R. B., & Rupa, K. G. (2014). Study on determination of physicochemical parameters of ground water in industrial area of Pydibheemavaram, Vizianagaram District, Andhrapradesh, India. Austin Journal of Public Health and Epidemiology, 1(2), 1–2.
Kassa, K. (2009). Physicochemical and bacteriological quality assessment of drinking water from source to households distribution point in Deberezei Town, Ethiopia. Masters thesis, Addis Abba University, Ethiopia.
Kirby-Bauer, W. M., Sherris, J. C., & Turck, M. (1966). Antibiotic susceptibility testing by single disc method. American Journal of Clinical Pathology, 45, 4.
Kora, A. J., Rastogi, L., Kumar, S. J., & Jagatap, B. N. (2017). Physicochemical and bacteriological screening of Hussain Sagar Lake: An urban wetland. Water Science, 31, 24–33.
Ladokun, O. A., & Oni, S. O. (2015). Physico-chemical and microbiological analysis of potable water in Jericho and Molete areas of Ibadan metropolis. Advances in Biological Chemistry, 5, 197–202.
Lee, G. C., Jheong, W. H., Kim, M. J., Choi, D. H., & Baik, K. H. (2013). A 5-year survey (2007–2011) of enteric viruses in Korean aquatic environments and the use of coliforms as viral indicators. Microbiology and Immunology, 57, 46–53.
Liang, Z., He, Z., Zhou, X., Powell, C. A., Yang, Y., He, L. M., et al. (2013). Impact of mixed land-use practices on the microbial water quality in a subtropical coastal watershed. Science of the Total Environment, 449, 426–433.
Maal-Bared, R., Bartlett, K. H., Bowie, W. R., & Hall, E. R. (2013). Phenotypic antibiotic resistance of Escherichia coli and E. coli O157 isolated from water, sediment and biofilms in an agricultural watershed in British Columbia. Science of the Total Environment, 443, 315–323.
National Bureau of Statistics. (2006). Retrieved June 28, 2016, from www.geo.com/cntry/453nigreria.
Ngele, S. O., Itumoh, E. J., Onwa, N. C., & Alobu, F. (2014). Quality assessment of selected groundwater samples in Amike-Aba, Abakaliki Ebonyi State, Nigeria. Canadian Journal of Pure and Applied Science, 8(1), 2801–2805.
Obi, C. N., & Okocha, C. O. (2007). Microbiological and physico-chemical analysis of selected borehole waters in World Bank Housing Estate, Umuahia, Abia State, Nigeria. Journal of Engineering and Applied Science, 2(5), 920–929.
Okonko, I. O., Adejoye, O. D., & Shittu, O. B. (2008). Microbiological and physicochemical analysis of different water samples used for domestic purposes in Ojota, Lagos State, Nigeria. African Journal of Biotechnology, 7(3), 617–621.
Olaniran, A. O., Naicker, K., & Pillay, B. (2009). Antibiotic resistance profiles of Escherichia coli isolates from river sources in Durban, South Africa. World Journal of Microbiology and Biotechnology, 25, 1743–1749.
Olanrewaju, A. N., Ajani, E. K., & Kareem, O. K. (2017). Physicochemical Status of Eleyele Reservoir, Ibadan, Nigeria. Journal of Aquaculture Research and Development, 8, 512.
Olawusi-Peters, O. O., Aguda, O. E., & Okoye, F. O. (2015). Heavy metals (Pb, Cu, Fe, and Zn) level in shellfish (Etheria elliptica), water and sediments of river Ogbese, Ondo State, Nigeria. International Journal of Animal and Veterinary Sciences, 9(3), 331–334.
Olorode, O. A., Bamigbola, E. A., & Ogba, O. M. (2015). Comparative studies of some river waters in port Harcourt based on their physico-chemical and microbiological analysis, Niger Delta Region of Nigeria. International Journal of Basic and Applied Science, 3, 29–37.
Omaka, O. N., Nwabue, F. I., Itumoh, E. J., Oroke, E. C., & Igwe, D. O. (2014). Physiological parameters and nutrients variations of streams and rivers in Abakaliki, Ebonyi State Nigeria. Global NEST Journal, 16(1), 114–123.
Omalu, I. C. J., Mohammed, A. Z., Olamide, P. I., Ayanwale, V. A., Adeniran, L., & Gbise, S. (2012). Bacteriological and physicochemical analysis of sachet water in North Central Nigeria. Journal of Pharmaceutical and Biomedical Sciences, 2(8), 1–4.
Omari, S., & Yeboah-Manu, D. (2012). The study of bacterial contamination of drinking water sources: A case study of Mpraeso, Ghana. Internet Journal of Microbiology, 10(1), 6–11.
Osińska, A., Korzeniewska, E., Harnisz, M., & Niestępski, S. (2017). The prevalence and characterization of antibiotic-resistant and virulent Escherichia coli strains in the municipal wastewater system and their environmental fate. Science of the Total Environment, 577, 367–375.
Osunla, C. A., & Okoh, A. O. (2017). Vibrio pathogens: A public health concern in rural water resources in sub-Saharan Africa. International Journal of Environmental Research and Public Health, 14(10), 1188.
Oyekunle, J. A. O., Ogunfowokan, A. O., Akanni, M. S., & Torto, N. (2011). Seasonal mean levels of heavy metals in water and associated sediments from Ajawere river in Oke‐Osun farm settlement, Osogbo, Nigeria. In Proceedings of the environmental management conference (pp. 350–363). Abeokuta: Federal University of Agriculture.
Palamuleni, L., & Akoth, M. (2015). Physicochemical and microbial analysis of selected borehole water in Mahikeng, South Africa. International Journal of Environmental Research and Public Health, 12, 8619–8630.
Pereira, A., Santos, A., Tacao, M., Alves, A., Henriques, I., & Correia, A. (2013). Genetic diversity and antimicrobial resistance of Escherichia coli from Tagus estuary (Portugal). Science of the Total Environment, 461–462, 65–71.
Rajeshwari, C. V., & Saraswathi, B. (2009). Assessment of water quality of Rivers Tungabhadra and Hundri, India. Pollution Research, 28(3), 499–505.
Rajkumar, B., & Sharma, G. D. (2013). Seasonal bacteriological analysis of Barak River, Assam, India. Applied Water Science, 3, 625–630.
Rizzo, L., Manaia, C., Merlin, C., Schwartz, T., Dagot, C., Ploy, M. C., et al. (2013). Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: A review. Science of the Total Environment, 447, 345–360.
Sarantuya, J. J., Nishi, J., Wakimoto, N., Erdene, S., Nataro, J. P., Sheikh, J., et al. (2004). Typical enteroaggregative Escherichia coli is the most prevalent pathotype among E. coli strains causing diarrhea in Mongolian children. Journal of Clinical Microbiology, 42, 133–139.
Sayah, R. S., Kaneene, J. B., Johnson, Y., & Miller, R. (2005). Patterns of antimicrobial resistance observed in Escherichia coli isolates obtained from domestic- and wild-animal fecal samples, human septage, and surface water. Applied and Environmental Microbiology, 71, 1394–1404.
Shinde, S. E., Pathan, T. S., Raut, K. S., & Sonawan, D. L. (2011). Studies on the physicochemical parameters and correlation coefficient of Harsool savani Dam, District Aurangabad, India. Middle East Journal of Scientific Research, 8, 544–554.
Sibanda, T., Chigor, V. N., Koba, S., Obi, C. L., & Okoh, A. I. (2013). Characterisation of the physicochemical qualities of a rural- based river: ecological and public health implications. International Journal of Environmental Science and Technology, 11, 1771–1780.
Sood, A., Singh, K. D., Pandey, P., & Sharma, S. (2008). Assessment of. bacterial indicators and physicochemical parameters to. investigate pollution status of Gangetic river system of Uttarakhand (Nigeria). Ecological Indicators, 8, 709–717.
Sundaram, B., Feitz, A., Caritat, P. D., Plazinska, A., Brodie, R., Coram, J., & Ransley, T. (2009). Groundwater Sampling and Analysis - A Field Guide. Geoscience Australia, Record 2009/27, Canberra, Australia.
Suthar, S., Sharma, J., Chabukdhara, M., & Nema, A. K. (2010). Water quality assessment of river Hindon at Ghaziabad, India: Impact of industrial and urban wastewater. Environmental Monitoring and Assessment, 165, 103–112.
Taiwo, A. G., Adewunmi, A. R., Ajayi, J. O., Oseni, O. A., & Lanre-lyanda, Y. A. (2014). Physico-chemical and microbial analysis of the impact of abatoir effluents on Ogun River course. International Journal of ChemTech Research, 6, 3083–3090.
Tesfamariam, Z., & Younis, M. H. Y. (2016). Assessment of physicochemical parameters and levels of heavy metals concentrations in drinking water of Asmara city, Eritrea III. American Journal of Research Communication, 4(8), 30–44.
Tessema, A., Mohammed, A., Birhanu, T., & Negu, T. (2014). Assessment of physico-chemical water quality of Bira Dam, Bati Wereda, Amhara Region, Ethiopia. Journal of Aquaculture Research and Development, 5, 267.
Titilawo, Y., Adeniji, A., Adeniyi, M., & Okoh, A. (2018). Determination of levels of some metal contaminants in the freshwater environments of Osun State, Southwest Nigeria: A risk assessment approach to predict health threat. Chemosphere, 21, 834–843.
Titilawo, Y., Obi, L., & Okoh, A. (2015a). Antimicrobial resistance determinants of Escherichia coli isolates recovered from some rivers in Osun State, Southwestern Nigeria: Implications for public health. Science of the Total Environment, 523, 82–94.
Titilawo, Y., Sibanda, T., Obi, L., & Okoh, A. (2015b). Multiple antibiotic resistance indexing of Escherichia coli to identify high-risk sources of faecal contamination of water. Environmental Science and Pollution Research, 22(14), 10969–10989.
Trivede, P., Bajpai, A., & Thareja, S. (2010). Comparative study of seasonal variations in physicochemical characteristics in drinking water quality of Kanpur, India with reference to 200 MLD filtration plant and groundwater. Natural Science, 8, 11–17.
Ugbaja, V. C., & Otokunefor, T. V. (2015). Bacteriological and physicochemical analysis of groundwater in selected communities in Obio Akpor, Rivers State, Nigeria. British Microbiology Research Journal, 7(5), 235–242.
Ukpong, E. C., & Okon, B. B. (2013). Comparative analysis of public and private borehole water supply in Uruan Local Government of Akwa Ibom State. International Journal of Applied Science and Technology, 3, 76–91.
United States Environmental Protection Agency. (2009). Method 1603: Escherichia coli in water by membrane filtration using modified membrane—Thermotolerant Escherichia coli agar (modified mTEC). USEPA Office of Water (4303 T) (p. 42). Washington, DC: USEPA.
United States Environmental Protection Agency (2012). USEPA. Recreational water quality criteria. Office of Water 820-F-12-058. Health and Ecological Criteria Division.
United States Geological Survey. (2015). National field manual for the collection of water-quality data, techniques of water-resources investigations. Book 9. Handbooks for water resources investigations (p. 1539). Reston, VA: United States Geological Survey.
Usharani, K., Umarani, K., Ayyasamy, P. M., Shanthi, K., & Lakshmanaperumalsamy, P. (2010). Physicochemical and bacteriological characteristics of Noyyal River and groundwater Quality of Perur, India. Journal of Applied Sciences and Environmental Management, 14(2), 29–35.
Uzoigwe, C. I., & Agwa, O. K. (2012). Microbiological quality of water collected from boreholes near refuse dumpsites in Port Harcourt, River State, Nigeria. Journal of Biotechnology, 11(13), 3135–3139.
Vandas, S. J., Winter, T. C., & Battaglin, W. A. (2002). Water and the Environment. American Geosciences Institute Environmental Awareness Serie, 5, 20–23.
Venkatesharaju, K., Ravikumar, P., Somashekar, R. K., & Prakash, K. L. (2010). Physicochemical and bacteriological investigation on the river Cauvery of Kollegal stretch in Karnataka. Kathmandu University Journal of Science, Engineering and Technology, 6, 50–59.
Vetrimurugan, E., Brindha, K., Elango, L., & Ndwandwe, O. M. (2017). Human exposure risk to heavy metals through groundwater used for drinking in an intensively irrigated river delta. Applied Water Science, 7, 3267–3280.
Washington Department of State. (2018). Fact Sheet Color, taste and odor problems in drinking water 331–286 Revised February 2018. Retrieved April 9, 2018, from www.doh.wa.gov/drinkingwater.
World Health Organization. (2000). Monitoring bathing waters—A practical guide to the design and implementation of assessments and monitoring program. London: F&FN Spon.
World Health Organization. (2008). Guidelines to drinking water quality (3rd ed., Vol. 1, pp. 1–666). Geneva: WHO.
World Health Organization. (2011). Guidelines for drinking water quality (4th ed.). Geneva: WHO.
Wose, K. C. N., Ateba, N., & Kawadza, T. D. (2010). Antibiotic resistance profiles of Escherichia coli isolated from different water sources in the Mmabatho locality, North-West Province, South Africa. Research Letters, 106(1–2), 44–49.
Zamxaka, M., Pironcheva, G., & Muyima, N. Y. O. (2004). Microbiological and physicochemical assessment of the quality of domestic water sources in selected rural communities of the Eastern Cape Province, South Africa. Water SA, 30(3), 333–340.
Zhang, S. H., Lv, X., Han, B., Gu, X., Wang, P. F., Wang, C., et al. (2015). Prevalence of antibiotic resistance genes in antibiotic-resistant Escherichia coli isolates in surface water of Taihu Lake Basin, China. Environmental Science and Pollution Research, 22, 11412–11421.
Zhang, X. X., Zhang, T., & Fang, H. H. (2009). Antibiotic resistance genes in water environment. Applied Microbiology and Biotechnology, 82, 397–414.
Acknowledgements
The authors are very grateful to their respective institutions for the enabling environments to conduct this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Titilawo, Y., Nwakpa, F., Bankole, S. et al. Quality audit of drinking water sources in Ikwo rural setting of Ebonyi State, Southeastern Nigeria. Int J Energ Water Res 4, 321–334 (2020). https://doi.org/10.1007/s42108-020-00062-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42108-020-00062-9