Abstract
Water contamination with faecal matter is usually the main cause of microbial waterborne diseases. Such diseases are an alarming situation for small cities in developing countries like India. In this research, to check the microbiological status of drinking water in Solan, Himachal Pradesh (India), water samples were collected from baories/stepwells (n = 14), handpumps (n = 9), and the municipal water distribution system (MWDS) (n = 2) in alternative months of the year (covering three main seasons). In 6 months, 150 samples were collected, and they were all examined for the presence of total coliforms and other bacterial pathogens. The associations between the isolates’ ecological and seasonal prevalence were also examined. The coliforms were detected by the Most Probable Number (MPN) method, whose range was noticed from the 2–540/100-ml MPN index. The colony forming unit (CFU) count for different samples at the base log 10 value ranged from 3.03 to 6.19. Different genera isolated and identified were Escherichia coli, Salmonella enteric subsp. enterica, Pseudomonas spp., Klebsiella spp., and Staphylococcus aureus. Overall, 74% of the isolates identified in water samples were from the Enterobacteriaceae family. E. coli was about 42.67% (n = 102), followed by Salmonella enterica subsp. enterica 20.92% (n = 50), Staphylococcus aureus 13.38% (n = 32), Pseudomonas spp. 12.55% (n = 30), and Klebsiella spp. 10.46% (n = 25) amongst the total of 239 isolates. The seasonal impact and the dependency of the occurrence of bacteria on one another were determined to be insignificant in the Spearman correlation test. These results showed that external factors (anthropogenic activities) are mainly responsible for the presence of these bacteria in water resources. The occurrence of bacterial isolates has been noticed in all water samples, irrespective of collecting site or season.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water means life to all living forms, and drinking clean water is important for human health. Due to anthropogenic activities, drinking water is getting polluted and contaminated day by day, which leads to the occurrence of many infectious and life-threatening diseases (Qadri & Faiq, 2020). In addition to the scarcity of potable water (Sharma et al., 2008), waterborne diseases is another major issue to be resolved in developing countries like India. Lack of safe drinking water exposes people to the threat of diseases such as diarrhoea, dysentery, cholera, gastroenteritis, typhoid, and chemical intoxication (Agbabiaka et al., 2010; Gumbo et al., 2015). Amongst waterborne diseases alone, diarrhoea is taking about 1 million lives worldwide annually (Levallois & Villanueva, 2019).
The United Nations General Assembly recently proclaimed 2005–2015 as the worldwide decade for action, “Water for Life” (WHO, 2011). Water, cleanliness, and health are interconnected (Pouramin et al., 2020). All the pathogens present in water are difficult to analyse, so there must be some indicators to be detected for public health protection. All mammal faeces are known to contain Escherichia coli at concentrations of 10 log 9−1 (Edberg et al., 2000). According to World Health Organization (WHO) and Food and Agricultural Organization (FAO) allowable limit for potable water is 1.0 × 102 CFU/ml (Fardami et al., 2019). For water treatment safety, E. coli was chosen as a biological indicator in the 1890s, and later on, various methods for coliforms detection were developed, which became part of drinking water regulations (Edberg et al., 2000).
Solan is situated at 30.92°N, 77.12°E (lower hills) of H.P. The main source of drinking water is Ashwani Khud and the Giri River, whose supply is not sufficient for the whole town and its nearby areas, especially during the summer (Chauhan & Bhardwaj, 2017). Therefore, people are mostly dependent on local boreholes (natural surface and underground water sources) and handpumps for potable water (Singh et al., 2021). These are the main water sources used by native people for domestic activities. Variations in seasonal precipitation, interflow, runoff, groundwater flow, and piped in and outflows have a significant impact on river flow and, consequently, the amount of pollutants in the water resources (Pandit et al., 2020). E. coli 0157 caused an outbreak of gastroenteritis amongst 5 staff members and 42 hostel students of Government Girls College, Solan District, in June–July 2015 (Chawla., 2017). Bacterial pathogens like E. coli and Vibrio spp. have been identified in faecal samples of diarrhoea patients at Solan regional hospital, as reported in previous studies (Thakur et al., 2018). Maximum numbers of E. coli presence in water resources are noticed during the rainy season (Singh et al., 2021). The National Health Profile (NHP) has reported 2.34 percent (3,14,463) of diarrhoeal disease cases in Himachal Pradesh out of 1,34,16,748 cases in the country in 2017, which was reduced to 2.27 percent (3,00,183) out of around 1.3 crore cases (1,31,94,775) in 2018. The objective of the study was the investigation of seasonal effect on the microbial density and diversity in water samples from different water resource sites of the Solan City.
Materials and methods
Study area
Solan is a small city and district headquarter of Solan District of Himachal Pradesh in India. It is also known as Mushroom City of India. The location of city is between 30°50′30″ to 30°52′00″N latitude and 77°08′30″ to 77°11′30″ longitudes, spread over an area of 210 km2. The district receives average annual rainfall about 1200 mm, mostly during monsoon season (July to September). Snowfall also occurs at higher reaches in winters with mild winter showers (Chauhan & Bhardwaj, 2017).
Sample collection procedure
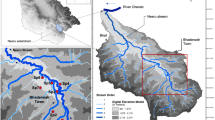
A total of 25 samples were collected, consisting of 14 samples from baories (stepwells), 9 samples from handpumps, and 2 samples from municipal water distribution systems (before and after treatment). The GPS locations of sampling sites are shown in Fig. 1 (Supplementary Table S1). The water samples collected from baories are labelled as B1, B2, B3,… B14; from handpumps, H1, H2, H3,… H9; and from the municipal water distribution system (MWDS), T1 and T2. Sampling was done on alternate months of the year to cover different seasons (winter, summer, and monsoon), i.e. from November 2018 to September 2019. For the microbiological analysis, 500 ml of water was collected in narrow-mouthed, clean, sterilized neutral glass bottles (autoclavable) of 500-ml capacity (Nienie et al., 2017) (Supplementary Fig. S1). Sampling bottles were treated with 0.5 ml of 10% sodium thiosulfate prior to sterilisation to neutralize the residual chlorine (SD & Panda, 2012). Immediate after collection, the samples were transported to the laboratory in ice bags and, until processing, kept in a refrigerator at 4 °C (APHA, 2017).
Assessment of total coliforms and other pathogens
Coliform analysis was done by performing the Most Probable Number (MPN) test (Abdelrahman & Eltahir, 2011). The MPN test consists of a preliminary test, a confirmatory test, and a complete test. Pure culture techniques of microbiology, i.e. serial dilution followed by spread plate and streak plate methods, were performed for the isolation and identification of other pathogens in water (Haque et al., 2019). These plates were incubated for 24 to 48 h and observed for bacterial growth. Colonies were counted on the colony counter, and colony forming units (CFU) were calculated for each sample. Further, bacterial colonies with specific characteristics were streaked on different media, like MacConkey agar, Deoxycholate Citrate agar (DCA), Mannitol Salt Agar (MSA), Eosine-Methylene-Blue (EMB) agar, and Thiosulfate-Citrate-Bile Salts-Sucrose (TCBS) agar (HiMedia). Pure cultures were identified by Gram’s staining and biochemical characterization according to Bergey’s Manual of Determinative Bacteriology (Yadav et al., 2019). Sub-culturing was performed every 15 days for the preservation of isolates.
Bacterial identification using 16SrRNA sequencing
For genomic DNA extraction, 5 ml of overnight grown culture was spun at 13,000 rpm for 1 min and pellet was dissolved in 500 µl of extraction buffer (0.5 M EDTA, 5 M NaCl, 10 mM Tris HCl, pH 8.0) and 50 µl 10% SDS. Five microliters of 20 mg/ml proteinase-K was added and incubation for 2–4 h in water bath at 65 °C was given until clear solution occurred. Further extraction was followed with phenol-CHCl3-isoamyl alcohol (25:24:1) and ethanol precipitation. To the lysate, equal volume of PCI was added, mixed well, and centrifuged at 10,000 rpm for 5 min. Upper aqueous layer was collected carefully in new Eppendorf. Of the 5 M sodium acetate, 1/10 µl of 5M sodium acetate and 2.5 volume of absolute ethanol to the collected aqueous phase were then added to it and the mixture was incubated overnight at −20 °C. Centrifugation at 12,000 rpm was done for 20 min at room temperature and supernatant was discarded. The DNA pellet was washed with 1 ml ethanol (70–100%) and centrifuged at 12,000 rpm for 5 min. The supernatant was discarded and DNA pellet was air dried and then dissolved in 80 µl TE buffer and stored at −20 °C for further use (Chakraborty et al., 2018).
Polymerase chain reaction (PCR) amplification was performed using a 16SrRNA characterization set of forward and reverse primers, i.e. F: (5′ ACGGTCTTGCTGTCACTTATA3′) and R: (5′ TACACATATGTTCTTCCCTAATAA 3′). The PCR mixture reaction consist of 5 µl of 5 × buffer, 2 µl MgCl2, 0.5 µl of each deoxynucleoside triphosphate, 0.25 µl of Taq DNA polymerase (Thermo Scientific, Lithuania), and 0.5 µl of each primer pair (Thermo Scientific, Lithuania). The reactions were run in a thermal cycler (Applied Biosystems Thermal Cycler, version 2.09) with the following cycling conditions: 94 °C for 3 min, 35 cycles of denaturation at 94 °C for 20 s, annealing for 30 s at 52 °C, extension at 72 °C for 30 s, and final extension at 72 °C for 7 min. PCR products were visualized after electrophoresis in 1.5% agarose gels in 1 × TAE buffer and ethidium bromide staining (Varela et al., 2015). The amplified products were purified using the Gene Jet PCR Purification kit (Thermo Scientific, Lithuania). The PCR product was sequenced using Sanger sequencing method at Eurofins Genomics (Applied Biosystems, India).
Statistical analysis
Statistical analysis was done using the Statistical Package for Social Science (SPSS software Version 20). To explain spatial and temporal variations between different water sources and seasons, the values (mean and SD) were computed, analysed, and presented as mean standard deviation (Naseema et al., 2013). Each experiment was set up in triplicate for all analyses of water samples. A statistical analysis of the data (Spearman’s rank-order correlation) was applied to know whether there was a relationship between bacterial parameters seasonally.
Results and discussion
MPN index per 100 ml of water sample
The overall range of coliforms/100 ml observed was 2–540 (Table 1). The MPN index value for most of the sampling sites was highest during the rainy season, followed by summer and winter. Metallic sheen-coloured E. coli was observed on eosine-methylene-blue agar (EMB agar) (Supplementary Fig. S2).
The highest MPN value was recorded for the water samples collected from Shoolini Mata Mandir Baori (B1) in the rainy season, followed by water samples collected from MWDS (T1) before chlorination treatment in the summer season. The water samples of both these sites were recorded with unacceptable MPN values in all the seasons. The MPN results were beyond the acceptable limit for all the water samples collected in the month of July (rainy season), whereas the water samples with low MPN values were recorded in the winter season. The WHO’s acceptable MPN index/100 ml of water is 2.2, whereas it is 0/100 ml for potable water, according to the Environmental Protection Agency (EPA) (Alemayehu & Mulugeta, 2017).
The high MPN value for maximum water samples confirms the faecal contamination of water resources (Lukubye & Andama, 2017). The presence of Escherichia coli is conclusive proof of faecal contamination. All the water samples did not fulfil the criteria for drinking water except Shilli road baori, Kathed baori, and Basaal baori and amongst handpumps: Meridean, Kotho, Shilli road, and Chambaghat in winter season only. Hence, the water from all these resources is not suitable for drinking until it is treated well, e.g. boiled, chlorinated, and filtered.
The high value of the MPN index during the monsoon season is due to the rain event and the favourable temperature for microorganism growth (An & Breindenbach, 2005). Rainwater flowing through the surrounding populated areas where sewage disposal systems are not well planned will carry the microbial load and contaminate the surface water (baories water) easily by mixing with it. The low MPN value in the winter is due to the fact that low temperatures in hilly areas are quite unfavourable for microbial growth (Koloren & Kaya, 2012). The MPN index value has also been found to be significantly high for handpump water samples that are located in areas that are heavily populated and where chances of sewage leakage from septic tanks are also high (Goyal et al., 1977; Bain et al., 2014). The presence of Escherichia coli is conclusive proof of faecal contamination. Human waste produces chemical impurities like chloride and nitrate as well as microbial contamination. Through the medium, these pollutants move and eventually come into touch with the groundwater (Pujari et al., 2007). Surface water sources are no doubt more likely to be contaminated than groundwater sources. Surface water quality is frequently affected by environmental and climatic circumstances, as well as the presence of animal and/or human faeces within the catchment region (Koloren & Kaya, 2012). Drinking water must be devoid of coliforms and faecal coliforms (WHO, 2011). Stagnation, a low level of residual chlorine, belated or inappropriate checks, and the dechlorination property of the desalination system could all contribute to elevated MPN values in some samples (Yari et al., 2018).
Isolation and identification of bacterial isolates
Screening of bacterial isolates was done by serial dilution followed by the spread plate method, and after incubation, plates were observed for colony forming units. The mean value range of CFU for the winter season was 3.03–5.72, and the standard deviation was 0.04–1.30 (Table 2). The standard deviation for the summer season was 0.05–0.76, with a mean value of 4.18–6.19. The mean CFU range for the rainy season is 3.03–5.53, with a standard deviation of 0.07–1.33. The CFU values were high for the summer season and rainy season as compare to winter season. The CFU enumerates all coliforms as well as non-coliforms present in samples. Highest CFU value (6.19) was recorded for Shilli road baori during summer season and lowest CFU value (3.03) was recorded for Forest road handpump in rainy season. All the water samples were exceeding the permissible CFU value for drinking water according to WHO drinking water guideline which is zero colony forming units (cfu/100 ml) (Lapworth et al., 2020). The bacteria were further isolated and identified based on Gram’s staining and biochemical and molecular characterization shown in Tables 3 and 4, respectively (Supplementary Table S2 and Supplementary Fig. S3).
A difference in the density of the bacterial CFU count was noticed, which was highest for the winter and summer seasons (for 19 sampling sites), followed by the summer and rainy (monsoon) seasons (12 water sampling sites), and the minimum difference was found for the winter and rainy seasons (07 sites) (Table 2). However, during the summer, the increased concentration of viable counts (VC) may be due to lower water levels, a high concentration of organic matter, a lower number of bacterivores, and optimal growth-supporting nutrients that stimulate bacterial development. A greater load of indicator bacteria is likewise linked to a higher temperature. When water temperatures rose above 15 °C, the occurrence of total coliforms increased considerably. The temperature is a significant controlling element in bacterial development. Bacterial activity is noticed highest above 15 °C and lowest below this. The variation in growth pattern is also influenced by the environmental factors around and the microbial response (Yadav et al., 2019). There was not a significant variation in VC counts across seasons (p < 0.05), but differences between locations were minor (Haque et al., 2019). Point sources (municipal sewage), non-point sources (contaminated runoff from various places), on-site septic tanks, and latrines are all possible pathogen sources in surface waterways. On-site sanitation, which is becoming more popular in India, may be to blame for significant bacterial pollution in drinking water sources (Suthar et al., 2009).
Based on morphological, biochemical characterization, and molecular sequencing, a total of five genera were identified: E. coli (WI-1), Klebsiella spp. (WI-2), Salmonella enterica subsp. enterica (WI-3), Pseudomonas spp. (WI-4), and Staphylococcus aureus (WI-5). The occurrence rate of these isolates is given as follows: out of 150 samples, a total of 239 isolates were screened, amongst which E. coli accounted for about 42.67% (n = 102) followed by Salmonella enterica subsp. enterica 20.92% (n = 50), Staphylococcus aureus 13.38% (n = 32), Pseudomonas spp. 12.55% (n = 30), and Klebsiella spp. 10.46% (n = 25) shown in Fig. 2. E. coli has been isolated and identified from most of the sampling sites, independent of the type of site (baories, handpumps, and MWDS) (Supplementary Table S3). Overall, the Enterobacteriaceae occurrence rate was 74%. Non-coliforms were present due to certain anthropologic activities. The septic pit system is widely employed in this area because of space constraints, overpopulation, and a lack of a sufficient drainage network, and seepage from these subterranean pits into surrounding areas may have contaminated the handpump water sources. Total coliforms and faecal coliforms in 100 ml of water must both be below detectable levels, according to the WHO standard for public drinking water (Ye et al., 2013). In developing regions poor water quality, hygiene, and sanitation cause approximately 1.7 million deaths worldwide each year, primarily due to infectious diarrhoea. Nine out of ten of these deaths are in children, and almost all of them occur in developing countries. Rotavirus, Campylobacter jejuni, enterotoxigenic Escherichia coli, Shigella spp., and Vibrio cholerae O1 are major enteric pathogens in these children (Ashbolt, 2004).
The existence of such pathogenic microbes in the potable water samples under investigation is significant for public health. This could be due to poor sanitary procedures in the area. It could also be related to runoff and floods into water sources from contaminated surface water. The existence of these organisms could also be attributable to the water source’s receptivity, which allows particles from the environment to easily enter, or to pulling water from sources with polluted equipment. Faecal bacteria should not be present in any water used for drinking or cleaning (Fardami et al., 2019).
Out of a total of 150 samples, 84 were collected from baories, amongst which the predominance of occurrence was E. coli (63/84), followed by Salmonella enteric subsp. enterica (26/84), Staphylococcus aureus (21/84), Pseudomonas spp. (13/84), and Klebsiella spp. (10/84), whereas the occurrence rate of these isolates from handpumps is E. coli (29/54), followed by Salmonella enteric subsp. enterica (18/54), Pseudomonas spp. (17/54), Staphylococcus aureus (5/54), and Klebsiella spp. (11/54). Saprophytes, such as Pseudomonas spp., were found in a substantial number of handpump water samples. P. aeruginosa detection has been proposed as a way to determine the hygienic condition of potable water (Mukhopadhyay et al., 2012). According to Fig. 3, there were 12 water samples collected from MWDS, with the most common being of E. coli (10/12), followed by Salmonella enteric subsp. enterica (6/12), Staphylococcus aureus (4/12), Klebsiella spp. (4/12), and Pseudomonas spp. (4/12). On the basis of this observation, the rate of prevalence of these pathogens is high in MWDS as compared to baories and handpumps, which is an alarming situation for the residing population.
Statistical results
Irrespective of the seasons, E. coli was present in high numbers in all bodies of water, followed by Salmonella enterica subsp. enterica, Staphylococcus aureus, Klebsiella spp., and Pseudomonas spp. Table 5 reveals the overall prevalence rate of bacteria with respect to the resources site, and their interdependence rate is shown in Tables 6, 7, and 8.
In the Spearman correlation test, the dependence of the occurrence of bacteria upon one another was found to be negligible. In the winter season, Pseudomonas spp. have shown a positive relationship with the occurrence of Staphylococcus aureus. In the summer season, a significant relationship could be seen between E. coli with Pseudomonas spp. and Staphylococcus aureus, whilst in the rainy season, Klebsiella spp. with Pseudomonas spp. and E. coli with Salmonella enterica subsp. enterica have shown a significant relationship. These results showed that external factors (anthropogenic activities) are mainly responsible for the presence of these bacteria in water resources.
Conclusions
According to the study’s findings, the summer as well as rainy season showed the most contamination of water supplies. Water samples collected from the municipal water distribution system (MWDS-T1) and Shoolini Mata Mandir Baori (B1) were found most contaminated with E. coli, Klebsiella, Staphylococcus aureus, Salmonella enterica, and Pseudomonas spp. Faecal matter is more frequently mixed with water during the rainy season due to overcrowding and inadequate waste disposal systems. The temperature range in this hill region between 30 and 35 °C is ideal for mesophile growth and results in the highest CFU values, which is the most beneficial factor for microbial growth in the summer. Overall dominance amongst the identified genera was observed for E. coli, followed by Salmonella enterica and a few Klebsiella spp.; hence, Enterobacteriaceae were present in high percentage. In reality, regardless of the season, the unsatisfactory MPN and VC results were found in almost every water sample. These water supplies have a predominance of pathogenic bacteria because they have not been properly investigated or treated. The results of this study indicate that these water resources should be regularly inspected for contamination. The local residents should be informed about the pathogen presence and the pathogenicity they cause so that they can be cautious and take precautions when using the water.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request. Table 2 is representing the data related to the CFU count. If required in a simple form, then it is attached to this submission.
References
Abdelrahman, A. A., & Eltahir, Y. M. (2011). Bacteriological quality of drinking water in Nyala, South Darfur, Sudan. Environmental monitoring and assessment, 175(1), 37–43. https://doi.org/10.1007/s10661-010-1491-7
Agbabiaka, T. O., & Sule, I. O. (2010). Bacteriological assessment of selected borehole water samples in Ilorin metropolis. International Journal of Applied Biological Research, 2(2), 31–37.
Alemayehu, G. K., & Mulugeta, K. A. (2017). Microbiological quality and safety of street vended meat sauce in Bahir Dar Town. Journal of Microbiology and Antimicrobials, 9(2), 14–22. https://doi.org/10.5897/JMA2015.0358
An, Y. J., & Breindenbach, G. P. (2005). Monitoring E. coli and total coliforms in natural spring water as related to recreational mountain areas. Environmental monitoring and assessment, 102(1), 131–137. https://doi.org/10.1007/s10661-005-4691-9
APHA-AWWA-WPCF. (2017). Standard methods for the examination of water and wastewater (23rd ed.). APHA.
Ashbolt, N. J. (2004). Microbial contamination of drinking water and disease outcomes in developing regions. Toxicology, 198(1–3), 229–238. https://doi.org/10.1016/j.tox.2004.01.030
Bain, R., Cronk, R., Wright, J., Yang, H., Slaymaker, T. & Bartram, J. (2014). Fecal contamination of drinking-water in low-and middle-income countries: A systematic review and meta-analysis. PLoS medicine, 11(5), e1001644. https://doi.org/10.1371/journal.pmed.1001644
Chakraborty, A. K., Poira, K., Saha, D., Halder, C., Das, S., & Nandi, S. K. (2018). Multidrug-resistant bacteria with activated and diversified MDR genes in Kolkata water: Ganga action plan and heterogeneous phyto antibiotics tackling superbug spread in India. American Journal of Drug Delivery and Therapeutics, 5(1), 2.
Chauhan, S., & Bhardwaj, S. K. (2017). Inventorization of water resources in Solan block of Himachal Pradesh. India. Current World Environment, 12(2), 456.
Chawla, S. (2017). Role of Integrated Disease Surveillance Programme in eliciting an E. coli 0157:H7 gastroenteritis outbreak at Tribal Girls Hostel, Solan, HP. Scholars Journal of Applied Medical Sciences, 5(3A), 717–722.
Edberg, S. C. L., Rice, E. W., Karlin, R. J., & Allen, M. J. (2000). Escherichia coli: The best biological drinking water indicator for public health protection. Journal of applied microbiology, 88(S1), 106S-116S.https://doi.org/10.1111/j.1365-2672.2000.tb05338.x
Fardami, A. Y., Mamuda, B. & Kangiwa, I. A., (2019). Bacteriological analysis of drinking water in Zamfara North Senatorial District, Nigeria. Microbiology Research Journal International, 27(5), 1–10. https://doi.org/10.9734/MRJI/2019/v27i530110
Goyal, S. M., Gerba, C. P., & Melnick, J. L. (1977). Occurrence and distribution of bacterial indicators and pathogens in canal communities along the Texas coast. Applied and Environmental Microbiology, 34(2), 139–149. https://doi.org/10.1128/aem.34.2.139-149.1977
Gumbo, A., Bangure, D., Gombe, N. T., Tshimanga, M., Hwalima, Z., & Dube, I. (2015). Staphylococcus aureus food poisoning among Bulawayo City Council employees, Zimbabwe, 2014. BMC Research Notes, 8(1), 1–6.
Haque, M. A., Jewel, M. A. S., & Sultana, M. P. (2019). Assessment of physicochemical and bacteriological parameters in surface water of Padma River. Bangladesh. Applied Water Science, 9(1), 1–8. https://doi.org/10.1007/s13201-018-0885-5
Koloren, Z., & Kaya, D. (2012). Fecal pollution in rural water supplies of Ordu, at the Mid-Black Sea Coast of Turkey: The effect of climate and environmental elements. EnergEducSciTechnol Part a: Energy Science Research, 28(2), 869–878.
Lapworth, D. J., MacDonald, A. M., Kebede, S., Owor, M., Chavula, G., Fallas, H., Wilson, P., Ward, J. S. T., Lark, M., Okullo, J. J. E. R. L., & Mwathunga, E. (2020). Drinking water quality from rural handpump—Boreholes in Africa. Environmental Research Letters, 15(6), 064020. https://doi.org/10.1088/1748-9326/ab8031
Levallois, P., & Villanueva, C. M. (2019). Drinking water quality and human health: An editorial. International journal of environmental research and public health, 16(4), 631. https://doi.org/10.3390/ijerph16040631
Lukubye, B., & Andama, M. (2017). Bacterial analysis of selected drinking water sources in Mbarara Municipality, Uganda. Journal of Water Resource and Protection, 9(8), 999–1013. https://doi.org/10.4236/jwarp.2017.98066
Mukhopadhyay, C., Vishwanath, S., Eshwara, V. K., Shankaranarayana, S. A., & Sagir, A. (2012). Microbial quality of well water from rural and urban households in Karnataka, India: A cross-sectional study. Journal of Infection and Public Health, 5(3), 257–262. https://doi.org/10.1016/j.jiph.2012.03.004
Naseema, K., Masihur, R., & Husain, K. A. (2013). Study of seasonal variation in the water quality among different ghats of river Ganga, Kanpur, India. Journal of Environmental Research and Development, 8(1), 1.
Nienie, A. B., Sivalingam, P., Laffite, A., Ngelinkoto, P., Otamonga, J. P., Matand, A., Crispin, K., Mulaji, J. I., Mubedi, P. T., & Mpiana, J. P. (2017). Seasonal variability of water quality by physicochemical indexes and traceable metals in suburban area in Kikwit, Democratic Republic of the Congo. International Soil and Water Conservation Research, 5(2017), 158–165. https://doi.org/10.1016/j.iswcr.2017.04.004
Pandit, J., & Bhardwaj, S. K. (2020). Quality characterization of surface water sources using water quality index in urban areas of Solan District of Himachal Pradesh. International Research Journal of Pure & Applied Chemistry, 21(24), 1–12. https://doi.org/10.9734/IRJPAC/2020/v21i2430329
Pouramin, P., Nagabhatla, N., & Miletto, M. (2020). A systematic review of water and gender interlinkages: Assessing the intersection with health. Frontiers in Water, 2, 6. https://doi.org/10.3389/frwa.2020.00006
Pujari, P. R., Nanoti, M., Nitnaware, V. C., Khare, L. A., Thacker, N. P., & Kelkar, P. S. (2007). Effect of on-site sanitation on groundwater contamination in basaltic environment: A case study from India. Environmental Monitoring and Assessment, 134, 271–278.
Qadri, R. & Faiq, M. A. (2020). Freshwater pollution: Effects on aquatic life and human health. Fresh water pollution dynamics and remediation, 15–26. https://doi.org/10.1007/978-981-13-8277-2-2
SD, T., & Panda, A. K. (2012). Laboratory investigation of drinking water sources of Kangra. Himachal Pradesh. J. Commun. Dis, 44(2), 103–108.
Sharma, K. D., Ghosh, N. C., & Singh, R. D. (2008). Scarcity of water in India. Current Science-Bangalore-, 94(12), 1551.
Singh, A. K., Bhardwaj, S. K., & Devi, S. (2021). Microbiological status of drinking water sources and its relationship with human health in Solan. India. Environmental Monitoring and Assessment, 193(1), 32.
Suthar, S., Chhimpa, V., & Singh, S. (2009). Bacterial contamination in drinking water: A case study in rural areas of northern Rajasthan. India. Environmental Monitoring and Assessment, 159(1), 43–50.
Thakur, N., Changotra, H., Shrivastava, R., Grover, N., & Vashistt, J. (2017). Estimation of Vibrio species incidences and antibiotic resistance in diarrhea patients. Asian Journal of Pharmaceutical and Clinical Research, 11(1), 369–373. https://doi.org/10.22159/ajpcr.2018.v11i1.22088
World Health Organization. (2011). Guideline for drinking water quality — (3rd ed.). Recommendation WHO.
Yadav, N., Singh, S., & Goyal, S. K. (2019). Effect of seasonal variation on bacterial inhabitants and diversity in drinking water of an office building, Delhi. Air, Soil and Water Research, 12, 1178622119882335. https://doi.org/10.1177/1178622119882335
Yari, A. R., Mohammadi, M. J., Geravandi, S., Doosti, Z., Matboo, S. A., Jang, S. A., & Nazari, S. (2018). Assessment of microbial quality of household water output from desalination systems by the heterotrophic plate count method. Journal of Water and Health, 16(6), 930–937. https://doi.org/10.2166/wh.2018.082
Ye, B., Yang, L., Li, Y., Wang, W., & Li, H. (2013). Water sources and their protection from the impact of microbial contamination in rural areas of Beijing, China. International Journal of Environmental Research and Public Health, 10(3), 879–891. https://doi.org/10.3390/ijerph10030879
Varela, A. R., Manageiro, V., Ferreira, E., Guimarães, M. A., da Costa, P. M., Caniça, M., & Manaia, C. M. (2015). Molecular evidence of the close relatedness of clinical, gull and wastewater isolates of quinolone-resistant Escherichia coli. Journal of Global Antimicrobial Resistance, 3(4), 286–289.
Acknowledgements
All authors are thankful to the Vice Chancellor, Shoolini University of Biotechnology and Management Sciences, Solan for providing the necessary facilities.
Author information
Authors and Affiliations
Contributions
Conceptualization: Mamta Devi Sharma, Saurabh Kulshrestha; Methodology: Mamta Devi Sharma, Payal Gupta; Formal analysis and investigation: Mamta Devi Sharma, Ranjana Panwar; Writing -original draft preparation: Mamta Devi Sharma, Pradeep Kumar, Saurabh Kulshrestha; Writing - review and editing: Mamta Devi Sharma, Sewa Singh, Saurav Chauhan, Pradeep Kumar; Resources, Funding and Supervision: Saurabh Kulshrestha
Corresponding author
Ethics declarations
Ethical responsibilities of authors
All authors have read, understood, and have complied as applicable with the statement on “Ethical responsibilities of Authors” as found in the Instructions for Authors and are aware that with minor exceptions, no changes can be made to authorship once the paper is submitted.
Ethical approval
The paper does not contain any ethically questionable information.
Consent to participate
The authors mutually agreed that they participated in the preparation of the manuscript.
Consent for publication
The authors declare that the manuscript is their intellectual property and that they want to publish it in the Journal “Environmental Monitoring and Assessment”.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharma, M.D., Gupta, P., Chauhan, S. et al. Seasonal impact on microbiological quality of drinking water in Solan City of Himachal Pradesh, India. Environ Monit Assess 195, 930 (2023). https://doi.org/10.1007/s10661-023-11510-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-023-11510-4